Abstract
We have developed an in vitro model for studying vascular injury. After 7–10 days in a three-dimensional collagen gel culture, capillary-like tubes were formed in the collagen gels. We injured these capillary-like tubes with a laser microdissection system or a scrape method with razors and then examined the collagen gel culture by phase contrast and electron microscopy. After laser injury, profuse necrotic cells were observed around the injured capillary-like tubes and within the tubular lumen compared to the razor injury. We then isolated total RNA from these cultures and prepared cDNA for investigations by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR). Quantitative real time RT-PCR revealed the up-regulation of transcription factor early growth response-1 (Egr-1) after both laser and razor injury, accompanied by the up-regulation of fibroblast growth factor-2 (FGF-2), a proangiogenic factor downstream of Egr-1. The effective laser energy is concentrated on the minute focal spot only. These methods provide a useful in vitro model for studying vascular injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis, the development of vessels from preexisting vessels, is essential for wound healing (Nissen et al. 1998). Several techniques have been developed for in vitro studies on angiogenesis. Folkman and Haudenschild (1980) and Montesano et al. (1983) found that the formation of capillary-like tubes could be induced by embedding endothelial cells in collagen gels. Later, angiogenesis was induced in tissues with the use of fibrin and collagen gels (Montesano et al. 1985), matrigel, collagen, fibrin and plasma clot (Nicosia and Ottinetti 1990a). Among these various techniques, the culture of blood vessels in collagen gels has been used widely and effectively (Mori et al. 1988; Nicosia and Ottinetti 1990b; Akita et al. 1997a, b; Fujita et al. 2002, 2004; Suda et al. 2004). In our previous experiment using collagen gels, the morphological and histochemical structures of the tubes formed in the gels were similar to the capillary-like structures of blood capillaries observed in vivo (Akita et al. 1997a, b). Reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemical investigations also detected the expression of fibroblast growth factor (FGF) -2 in the collagen gel cultures (Akita et al. 2000). FGF-2, a potent angiogenic molecule in vivo and in vitro, stimulates endothelial cell growth (Moscatelli et al. 1986; Presta et al. 1986), smooth muscle cell growth, wound healing and tissue repair (Basilico and Moscatelli 1992; Schwartz and Liaw 1993). Fahmy et al. (2003) recently reported that transcription factor Egr-1 supported FGF-2-dependent angiogenesis during neovascularization and tumor growth. FGF-2 is assumed to function as a proangiogenic factor downstream of Egr-1.
The scrape assay method is popularly used as an in vitro model of the wound healing of vascular injury (Ku and D’Amore 1995; Unger et al. 1998; van Aalst et al. 2004). This is a method for quantifying the number or migration distance of newly migrated endothelial cells scraped from postconfluent monolayer cultures with rubber policemen or razors. In this study we cultured aortic explants from mice in collagen gels and injured the capillary-like tubes formed in the gels with the use of a laser microdissection system or a scrape method with razors. After inducing the injury we observed the morphology of the capillary-like tubes and quantified the expressions of transcription factors Egr-1 and FGF-2 by quantitative real time RT-PCR. Quantitative real time RT-PCR revealed the up-regulation of Egr-1 after both laser and razor injury, accompanied by the up-regulation of FGF-2. These methods provide a useful in vitro model for studying vascular injury.
Materials and methods
Collagen gel culture and injury
This culture technique has already been described by Akita et al. (1993, 1997a, b). Thoracic aortae were obtained from 1-month-old ICR male mice (n=15). After separating the tunica adventitia from the aorta under a stereoscopic microscope, the blood vessel specimens were cut into small pieces of about 2 mm in length. Four pieces were placed at the bottom of each tissue culture plate (Petri PERM 50 hydrophilic, In Vitro Systems & Services GmbH, Goettingen, Germany), overlaid with an even layer of reconstituted collagen solution (0.3% Cellmatrix type IA, Nitta Gelatin, Tokyo, Japan) and allowed to gel at 37°C for about 10 min. After the gels had formed, they were overlaid with Ham’s F-12 medium (Invitrogen Corp., Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 1% non-essential amino acids, 2 mM l-glutamine, 100 Units/ml penicillin and 100 mg/ml streptomycin (Invitrogen Corp., Carlsbad, CA, USA), and cultured for 10 days in an incubator (95% air/5% CO2). The culture medium was replaced halfway through the incubation, at 1 week. Capillary-like tubes were observed with FITC-conjugated endothelial cell-specific tomato lectin (Lycopersicon esculentum; EY Labo, CA, USA), a lectin that selectively binds to fucose residues on the endothelial cell surface (Hoffmann et al. 1998).
One of newly formed ten capillary-like tubes per one aortic explant was injured by a laser microdissection system (337 nm pulsed nitrogen laser; P.A.L.M. Microlaser Technologies A.G., Bernried, Germany) or a scrape method with razors (Surgical Blade Steinless Steel No.23, Feather, Japan) under a phase contrast microscope. The cultures were examined by phase and electron microscopy after the injury.
Electron microscopy
At 30 min after injury, the cultured materials (laser; three plates, razor; three plates: each plate consists of four aortic explants) were fixed once in 0.1 M phosphate buffer (pH 7.2) containing 2.5% glutaraldehyde for 1 h and once in 0.1 M phosphate buffer (pH 7.2) containing 1% OsO4 for another hour. The specimens were then dehydrated with ethanol, embedded in epoxy resin, cut into ultrathin sections, and stained with uranyl acetate and lead citrate. The stained ultrathin sections were observed under a transmission electron microscope (H-7000; Hitachi, Tokyo, Japan).
Quantitative real time RT-PCR
Total RNA was isolated by TRIZOL (Synovis Life Technologies, Minn, USA) from each culture plate (n=35) without the aortic explant. The first-strand cDNA synthesis from the template RNA was performed by Super Script III(Invitrogen Corp., Carlsbad, CA, USA)according to the manufacturer’s instructions. Samples were collected before and after laser or razor injury (30 and 90 min, 18 h) and analyzed by quantitative real-time RT-PCR. Quantitative real-time PCR was performed using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, CA, USA) according to the manufacturer’s instructions. TaqMan Gene expression assays primers and probes were used for the mouse target genes Egr-1 and FGF-2 and for the housekeeping gene GAPDH (assay IDs Mm00656724_m1, Mm00433287_m1, and Mm99999915_g1, respectively: Applied Biosystems). The cycling parameters for one-step RT-PCR included initial incubations for 10 min at 95°C, respectively, followed by 40 PCR cycles, each consisting of 15 s of denaturation at 95°C, followed by 1 min of annealing/extension at 60°C on ABI Prism 7900HT. All experiments were performed in triplicate. CT values were analyzed with Microsoft Excel using the comparative cycle threshold (ΔCT) method recommended by the manufacturer of the detection system (Applied Biosystems).
Results
Light and electron microscopy
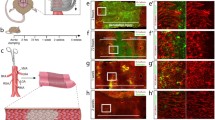
The newly formed capillary-like tubes began appearing after 7–10 days of culture in the collagen gels. These capillary-like tubes were strongly positive for FITC-conjugated endothelial cell-specific tomato lectin (Fig. 1). Figure 2 shows phase contrast micrographs of the capillary-like tubes before and after the laser injury. Figure 3 shows phase contrast micrographs after the razor injury. Both injured sites were detectable by the phase contrast microscope. A pulsed ultra-violet (UV) laser of high beam quality is interfaced into the microscope and focused through an objective to a beam spot size of less than 50 μm in diameter. The effective laser energy was concentrated on the minute focal spot only. However, concentration of razor to the specified objective is difficult and injured size was become larger (more than 250 μm) than laser.
Thirty minutes after razor injury. Phase contrast micrographs of the capillary-like tubes. The arrow indicates the edge of capillary-like tube by cutting with razor. Injured site is shown by an asterisk. After razor injury, collagen gels are contracted, and the wound is opened. To the bottom right is an aortic explant. Scale bar=250 μm
Transmission electron microscopy revealed profuse necrotic cells around the capillary-like tubes and in the tubular lumen after the laser injury compared to the razor injury (Fig. 4).
Transmission electron micrographs of the capillary-like tubes. Scale bar=5 μm a 30 min after laser injury. Necrotic cells (arrows) were observed around the injured capillary-like tube end and in the tubular lumen. b A capillary-like tube before laser injury as a control. Necrotic cells were not observed. c 30 min after razor injury. Electron micrograph shows the injured capillary-like tube end. Arrows indicate some necrotic (or apoptotic) cells in the tubular lumen. Erythrocytes were seen, although the origin was obscure.
Quantitative real time RT-PCR
The expression of Egr-1 increased after both laser and razor injury, and this increase reached a significant level after 90 min (laser; P<0.05 and razor; P<0.01). At 18 h, however, the expression of Egr-1 was decreased (Fig. 5a). The expression of FGF-2 increased with time. After both laser and razor injury, the expression of FGF-2 increased significant level after 90 min (razor; P<0.01) and after 18 h (laser; P<0.05) (Fig. 5b).
Quantitative RT-PCR analysis of the (a) Egr-1 and (b) FGF-2 genes after laser or razor injury (laser =gray column, razor = black column). Egr-1 and FGF-2 mRNAs expressions, measured by real-time RT-PCR as ratios of Egr-1 mRNA to GAPDH mRNA and FGF-2 mRNA to GAPDH mRNA at different time points. Student’s t-test for unpaired data was used for statistical analysis. Data represent the mean (±SE) of five dishes containing 20 aortic explants. There was a significant difference (* P<0.01, ** P<0.05).
Discussion
The present collagen gel culture has many advantages over the monolayer culture. Compared to the environments of monolayer cultures, the environment of the three-dimensional culture using collagen gels is much closer to the actual condition in vivo. Once the injury is applied, the healing of the tubes can be observed with time laps in vitro, and transcription factors and other angiogenic factors can be analyzed simultaneously. Compared to the present scrape method with razors, the effective laser energy is concentrated on the minute focal spot only. The laser method also makes it possible to select a specific capillary-like tube to injure by laser. It is also possible to perform microsurgery on the specific site. The main disadvantage of the present laser method is the need for specialized equipment with which to apply the laser. The principle of laser cutting is a locally restricted ablative photodecomposition process without heating of adjacent material (Srinivasan, 1986). He noted the excimer wavelength of 193 nm has been found to be particularly suitable for surgery on the cornea since the morphology of the cut is superior to those obtained when radiation at other wavelength in the UV (e.g., 248 nm). In the present study, we used 337 nm pulsed nitrogen laser. Therefore, it is more likely to cause a thermal damage. The differences obtained by electron microscopy and quantitative real time RT-PCR between laser and razor injury may attribute the thermal effect. Another possibility is the difference of the injured size between laser and razor injury, but otherwise it is a fact that both laser and razor injury causes the up-regulation of Egr-1 and FGF-2 genes.
The transcription factor Egr-1 is an immediate early response gene known to regulate a number of pathophysiologically relevant genes involved in the growth, differentiation, immune response, wound healing, and blood clotting of the vasculature (Pendurthi and Rao 2000). According to Khachigian et al. (1996), Egr-1 is rapidly activated by multiple extracellular agonists (such as growth factors and cytokines) and environmental stresses (such as hypoxia, fluid shear stresses and vascular injury). Once activated, Egr-1 controls the expression of a diverse array of proangiogenic genes such as encoding growth factors, cytokines, receptors, adhesion molecules and proteases (Gashler and Sukhatme 1995; Khachigian and Collins 1997). Egr-1 in turn can bind and activate the promoters of many genes whose products influence vascular repair as angiogenic factors (Fahmy and Khachigian 2002).
Egr-1 stimulates the production of platelet-derived growth factor-AB (PDGF-AB), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), FGF-2 (Biesiada et al. 1996) and transforming growth factor-β1 (TGF-β1) and its receptor (Liu et al. 1999; Du et al. 2000; Houston et al. 2001). Fahmy et al. (2003) recently reported that Egr-1 supports FGF-dependent angiogenesis and the cellular growth and neovascularization of the microvascular endothelium. According to Cancilla et al. (2001), fibroblast growth factors (FGFs) are a family of at least 21 heparin-binding proteins involved in numerous biological processes, including angiogenesis. FGF-2, a potent angiogenic molecule in vivo and in vitro, stimulates endothelial cell growth (Moscatelli et al. 1986; Presta et al. 1986), smooth muscle cell growth, wound healing and tissue repair (Basilico and Moscatelli 1992; Schwartz and Liaw 1993). The present study revealed the up-regulation of FGF-2 following the up-regulation of Egr-1 after both laser and razor injury. In our previous experiment we confirmed the expressions of FGF-9 (Akita et al. 2000; Nagatoro et al. 2003) and FGF-7 (Akita et al. 2004) during angiogenesis in collagen gels. Pilcher et al. (1997) and Miyagi et al. (1998) reported that FGF-9 relates to the expression of matrix metalloproteinases (MMPs) and their inhibitors. The MMPs play a key role in the processes of endothelial cell migration and matrix remodeling during angiogenesis (Haas et al. 1998). Little is still known about the angiogenic roles of FGF-7 and the key transcription factors regulating angiogenesis and vascular injury in general. Further studies on the relationship between FGF-9 and FGF-7, including their associations with FGF-2 and transcription factors, will be needed for a fuller understanding of the mechanisms and crucial role of angiogenesis in wound healing.
The present study revealed the up-regulation of Egr-1 after both laser and razor injury, accompanied by the up-regulation of FGF-2. The effective laser energy could be concentrated on the minute focal spot only compared to the razor, although thermal effect of laser should be considered. The present methods using capillary-like tubes have many advantages over the conventional scrape assay method using monolayer culture, and provides a useful tool as an in vitro model for studying angiogenesis and the wound healing of vascular injury.
References
Akita M, Murata E, Kaneko K, Ghaida J, Merker HJ (1993) Cell shape and arrangement of cultures aortic smooth muscle cells grown on collagen gels. Cell Tissue Res 274:91–95
Akita M, Murata E, Merker HJ, Kaneko K (1997a) Formation of new capillary-like tubes in a three-dimensional in vitro model (aorta/collagen gel). Ann Anat 179:127–136
Akita M, Murata E, Merker HJ, Kaneko K (1997b) Morphology of capillary-like structures in a three-dimensional aorta/collagen gel culture. Ann Anat 179:137–147
Akita M, Fujita K, Murata E, Tanaka K, Nagano T, Kaneko K (2000) Expression of FGFs during angiogenesis in collagen gel culture. J Submicro Cytol Pathol 32:430
Akita M, Fujita K, Tanaka K, Asami Y, Murata E, Kaneko K (2004) Remodeling of small blood vessels after laser injury in vitro. Cardiovasc Pathol 13 (Suppl 3):163 (Abstract of the XIIIth International Vascular Biology Meeting, 2004 in Toronto, Canada)
Basilico C, Moscatelli D (1992) The FGF family of growth factors and oncogenes. Adv Cancer Res 59:115–165
Biesiada E, Razandi M, Levin ER (1996) Egr-1 activates basic fibroblast growth factor transcription. Mechanistic implications for astrocyte proliferation. J Biol Chem 271:18576–18581
Cancilla B, Davies A, Cauchi JA, Risbridger GP, Bertram JF (2001) Fibroblast growth factor receptors and their ligands in the adult rat kidney. Kidney Int 60:147–155
Du B, Fu C, Kent KC, Bush H Jr, Schulick AH, Kreiger K, Collins T, McCaffrey TA (2000) Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-beta type II receptor. J Biol Chem 275:39039–39047
Fahmy RG, Khachigian LM (2002) Antisense Egr-1 RNA driven by the CMV promoter is an inhibitor of vascular smooth muscle cell proliferation and regrowth after injury. J Cell Biochem 84:575–582
Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM (2003) Transcription factor Egr-1supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med 9:1026–1032
Folkman J, Haudenschild C (1980) Angiogenesis in vitro. Nature (Lond.) 288:551–556
Fujita K, Asami Y, Murata E, Akita M, Kaneko K(2002) Effects of thalidomide, cytochrome P-450 and TNF-alpha on angiogenesis in a three-dimensional collagen gel-culture. Okajimas Folia Anat Jpn 79:101–106
Fujita K, Asami Y, Tanaka K, Akita M, Merker HJ(2004)Anti-angiogenic effects of thalidomide: expression of apoptosis-inducible active-caspase-3 in a three-dimensional collagen gel culture of aorta. Histochem Cell Biol 122:27–33
Gashler A, Sukhatme VP (1995) Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 50:191–224
Haas TL, Davis SJ, Madri JA (1998) Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem 273:3604–3610
Hoffmann S, Spee C, Murata T, Cui JZ, Ryan SJ, Hinton DR (1998) Rapid isolation of choriocapillary endothelial cells by Lycopersicon esculentum-coated Dynabeads. Graefes Arch Clin Exp Ophthalmol 236:779–784
Houston P, Campbell CJ, Svaren J, Milbrandt J, Braddock M (2001) The transcriptional corepressor NAB2 blocks Egr-1-mediated growth factor activation and angiogenesis. Biochem Biophys Res Commun 283:480–486
Khachigian LM, Lindner V, Williams AJ, Collins T (1996) Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science 271:1427–1431
Khachigian LM, Collins T (1997) Inducible expression of Egr-1-dependent genes. A paradigm of transcriptional activation in vascular endothelium. Circ Res 81:457–461
Ku PT, D’Amore PA (1995) Regulation of basic fibroblast growth factor (bFGF) gene and protein expression following its release from sublethally injured endothelial cells. J Cell Biochem 58:328–343
Liu C, Yao J, de Belle I, Huang RP, Adamson E, Mercola D (1999) The transcription factor EGR-1 suppresses transformation of human fibrosarcoma HT1080 cells by coordinated induction of transforming growth factor-beta1, fibronectin, and plasminogen activator inhibitor-1. J Biol Chem 274:4400–4411
Miyagi N, Kato S, Terasaki M, Shigemori M, Morimatsu M (1998) Fibroblast growth factor-2 and 9 regulate proliferation and production of matrix metalloproteinases in human gliomas. Int J Oncol 12:1085–1090
Montesano R, Orci L, Vassalli P (1983) In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol 97:1648–1652
Montesano R, Mouron P, Orci L (1985) Vascular outgrowths from tissue explants embedded in fibrin or collagen gels: a simple in vitro model of angiogenesis. Cell Biol Int Rep 9:869–875
Mori M, Sadahira Y, Kawasaki S, Hayashi Y, Notohara K, Awai M (1988) Capillary growth from reversed rat aortic segments cultured in collagen gel. Acta Pathol Jpn 38:1503–1512
Moscatelli D, Presta M, Rifkin DB (1986) Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci USA 83:2091-2095
Nagatoro T, Fujita K, Murata E, Akita M (2003) Angiogenesis and fibroblast growth factors (FGFs) in a three-dimensional collagen gel culture. Okajimas Folia Anat Jpn 80:7–14
Nicosia RF, Ottinetti A (1990a) Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol 26:119–128
Nicosia RF, Ottinetti A (1990b) Growth of microvessels in serum-free matrix culture of rat aorta: a quantitative assay of angiogenesis in vitro. Lab Invest 63:115–122
Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA (1998) Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 152:1445–1452
Pendurthi UR, Rao LV (2000) Suppression of transcription factor Egr-1 by curcumin. Thromb Res 15; 97:179–189
Pilcher BK, Gaither-Ganim J, Parks WC, Welgus HG (1997) Cell type-specific inhibition of keratinocyte collagenase-1 expression by basic fibroblast growth factor and keratinocyte growth factor. A common receptor pathway. J Biol Chem 272:18147–18154
Presta M, Moscatelli D, Joseph-Silverstein J, Rifkin DB (1986) Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis, and migration. Mol Cell Biol 6:4060–4066
Schwartz SM, Liaw L (1993) Growth control and morphogenesis in the development and pathology of arteries. J Cardiovasc Pharmacol 21 (Suppl 1): 31–49
Srinivasan R (1986) Ablation of polymers and biological tissue by ultraviolet lasers. Science 234:559–565
Suda H, Asami Y, Murata E, Fujita K, Akita M (2004) Immuno-histochemical expression of alpha1, alpha2 and alpha3 integrin subunits during angiogenesis in vitro. Histol Histopathol 19:735–742
Unger GM, Bellrichard RL, Trinh BI, Sammak PJ (1998) Quantitative assessment of leading edge adhesion: reattachment kinetics modulated by injury-derived intracellular calcium predict wound closure rates in endothelial monolayers. J Cell Physiol 174:217–231
van Aalst JA, Burmeister W, Fox PL, Graham LM (2004) Alpha-tocopherol preserves endothelial cell migration in the presence of cell-oxidized low-density lipoprotein by inhibiting changes in cell membrane fluidity. J Vasc Surg 39:229–237
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (No. 16591796) from the Japan Society for the promotion of science (to M. A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, K., Komatsu, K., Tanaka, K. et al. An in vitro model for studying vascular injury after laser microdissection. Histochem Cell Biol 125, 509–514 (2006). https://doi.org/10.1007/s00418-005-0106-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0106-9