Abstract
Prebiotic oligosaccharides are receiving immense attention due to their nutraceutical and therapeutic potential associated with the food and pharmaceutical industry. Owing to increasing demand for prebiotic oligosaccharides, various strategies of their production are being pursued. Fungal enzymes, mainly fructosyltransferase, inulinase, mannanase, cellulases, galactosidase, and xylanase, are predominantly involved in the synthesis of various oligosaccharides such as fructooligosaccharides (FOS), inulooligosaccharides (IOS), mannooligosaccharides (MOS), cellooligosaccharides (COS), galactooligosaccharides (GOS), and xylooligosaccharides (XOS) through biotransformation of their respective precursor raw sugars. This chapter highlights modern approaches and production strategies of nutritional oligosaccharides using fungal enzymes and their nutritional values.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oligosaccharides
- Prebiotics
- Fructooligosaccharides
- Inulooligosaccharides
- Mannooligosaccharides
- Cellooligosaccharides
- Galactooligosaccharides
- Xylooligosaccharides
1 Introduction
Nutritional dependencies of the modern lifestyle are major socioeconomic concerns in our day-to-day life. Incorporation of functional food ingredients as a part of daily diet may play crucial role in the well-being. Functional foods can be a better preventive measure for mitigating chronic health disorders, such as irritable bowel disease (IBD), inflammatory disorders, indigestion, colon cancer, gut epithelial dysfunction, etc. [1]. Prebiotic dietary fibers are selectively utilized by probiotic gut microflora which provide important health benefits to the host [2]. In a first, a study evidenced lactulose as a bifidogenic factor [3]. After several years, some non-digestible oligosaccharides were found as promising bifidogenic components [4, 5]. The term “prebiotic” was coined by Gibson and Roberfroid, and they defined prebiotics as non-digestible food components that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving the host health [6].
Due to upsurge in their consumption all over the globe, the prebiotic market in 2015 was estimated to be $200 million with a growth rate of 15% per year and is expected to reach $8.5 billion by the year 2024 [7]. Nutraceutical oligosaccharides are considered as non-digestible carbohydrates that help attain balanced microbial composition in the host gut and are responsible for a number of health benefits such as reduced risk of colon cancer, improved mineral absorption, antioxidant activities, inhibition in pathogenic microbes in the gut, generation of immunomodulatory compounds, reduction of neoplastic lesions and cancer risk, improvement of bowel movement, stimulation of immune system, improvement of gastrointestinal and urinary tract health, anti-inflammatory effects, reduction of blood pressure, maintenance of vision, antibacterial and antiviral activities, and reduction of osteoporosis and lipid levels [8, 9]. They also help in improving gut barrier function and gut-brain signaling in depressive disorders [10].
Among the functional food components, prebiotics and probiotics constitute a focus on contemporary advancements in human nutrition. The market of prebiotics in India is still emerging. A few fructooligosaccharide-based products are available in Indian market, and these continue to draw more attention by the health-conscious individual. All prebiotics are considered in the group of dietary fibers, but some dietary fibers do not exhibit prebiotic activity. Major prebiotics are fructooligosaccharides (FOS), inulin and inulooligosaccharides (IOS), galactooligosaccharides (GOS), xylooligosaccharides (XOS), mannooligosaccharides (MOS), and cellooligosaccharides (COS). In addition to these, maltooligosaccharides (MaOS), isomalto-oligosaccharides (IMaOS), human milk oligosaccharides (HMOS), pectin-derived oligosaccharides (POS), agarooligosaccharides (AOS), lactulose, and lactosucrose are also being evaluated for the purpose (Table 12.1). Among these, inulin, IOS, FOS, and GOS are the most popular prebiotics and are commonly used in food and feed, confectionary, and animal feed industries [21].
2 Production of Prebiotic Oligosaccharides
Although prebiotic oligosaccharides are found in various natural sources (Table 12.1), the quantity and composition vary with respect to environmental conditions. The production of oligosaccharides may be done either by chemical synthesis by transglycosylation or by biocatalysis of raw sugars using various enzymes. Chemical synthesis of oligosaccharides may be attained by thioglycoside glycosylation, ortho-trichloroacetimidate method, orthoester method, condensation of tritylcyanothylidene, modified method of Koenigs-Knorr, etc. Chemical methods of oligosaccharide synthesis have disadvantages due to the uncontrolled stereochemistry and non-specificity [22].
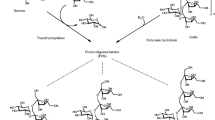
Due to these limitations of chemical methods, oligosaccharide generation involving enzymatic bioprocesses is a very promising alternative. Several fungal enzymes such as fructosyltransferase, inulinase, mannanase, xylanase, β-galactosidase, and cellulase are being utilized for the generation of FOS, IOS, MOS, XOS, GOS, and COS, respectively (Fig. 12.1). Most of the prebiotic oligosaccharides are generated using enzymes belonging to three kinds of enzymes, viz., glycoside hydrolase (GH), glycosyltransferase (GT), and transglycosylase (TG) [23].
GH enzymes are involved in the hydrolysis of acetal linkages between two carbohydrates or between a carbohydrate and a non-carbohydrate moiety. GT enzymes catalyze the transfer of glycosidic moiety from activated donor to acceptor residue. GTs are sub-classified as inverting or retaining on the basis of the stereochemistry of the glycosidic bond (α/β) in reaction product is maintained or altered. TGs are involved in similar catalytic reaction as the GHs, but the hydrolase versus transferase ratio differs in different TGs. The mechanism and biological role of TGs is still undistinguishable [24]. The production of different types of oligosaccharides is commercially achieved using several enzymes based on their type of glycosidic linkage formation in the product oligosaccharide. The prebiotic and other nutritional properties or oligosaccharides depend upon the three-dimensional stereochemistry of the glycosidic bond, which could not be controlled by chemical catalysis. Enzymatic synthesis of oligosaccharides displays some properties such as selectivity, specificity, and energy minimized catalysis with high turnover number [24]. Furthermore, several enzyme engineering approaches such as immobilization, genetic engineering, codon optimization, and mutagenesis have also been regularly utilized to improve catalytic properties of enzymes.
Oligosaccharides have been marketed since the 1980s as low-calorie agents and recently have gained interest in the pharmaceutical and food industry as functional sweeteners and prebiotic enriching population of Bifidobacteria. Currently, they have an approximated value of $ 200 per kg, and recently, inulin has been proposed as a feedstock for production of oligosaccharides through selective hydrolysis by action of endo-inulinase [25]. The influences of probiotic or pre-diet on the zebrafish gut-brain axis have reported. Zebrafish (Danio rerio) fed with probiotic were found with increased levels of serotonin and brain-derived neurotrophic factor [26]. Lactobacillus rhamnosus IMC 501® (a probiotic strain) showed health-promoting properties such as decreased DNA damage, less oxidative stress, and increased immune response including hepatic stress tolerance in zebrafish [27]. Some of the commercially available oligosaccharides are listed in Table 12.2. Details of some of the nutritionally important oligosaccharides and enzymes involved in their generation are presented in the following sections.
3 Types of Prebiotic Oligosaccharides
3.1 Fructooligosaccharides (FOS) and Inulooligosaccharides (IOS)
Fructooligosaccharides (FOS) are considered to be the most important among various prebiotics due to their nutraceutical properties such as hypolipidemic (cholesterol-lowering) and enhanced calcium absorption [28, 29]. FOS consist of a series of oligosaccharides that are composed of 1-kestose (GF2), nystose (GF3), and 1F-β-fructofuranosyl nystose (GF4), in which two, three, and four fructosyl units are bound at the β-2,1 position of glucose, respectively. FOS are obtained either by extraction from various plant materials or by enzymatic synthesis from different substrates. Enzymatically, these can be obtained either from sucrose using FTase or from inulin hydrolysis by endo-inulinase [11]. Increasing demand for an alternative healthy sweetener and multifunctional fructooligosaccharides has prompted investigators to explore microorganisms for inulinase and FTase production and to develop bioprocesses for the production of high-fructose syrup [8].
Current commercial production of FOS is carried out by fructofuranosidase (FFase, EC 3.2.1.26) or fructosyltransferase (FTase, EC 2.4.1.9) using sucrose as the raw material [30, 31]. Fructosyltransferase (FTase; EC 2.4.1.9) hydrolyzes sucrose and transfers fructosyl group to an acceptor molecule to generate fructooligosaccharides (FOS) along with glucose and fructose [32]. Hidaka et al. [33] reported that Aspergillus niger ATCC 20611 produces β-fructofuranosidase (FopA) with high transfructosylation activity. A. niger ATCC 20611 is being exploited at industrial scale for FOS production in the last two decades as commercial FOS producer [34]. Recently, genetically modified Pichia, expressing plant fructosyltransferase, was designed for industrial production of FOS [35]. Another recent report describes neo-series FOS production using β-fructofuranosidase (FFase) derived from Xanthophyllomyces dendrorhous. For industrial application, the gene encoding FFase has been cloned in Pichia, and the expressed enzyme was immobilized on polyvinyl alcohol matrix and used for continuous generation of FOS [36]. A cold-active FTase from Aspergillus tamarii generated a maximum of 55% (325 g/L) FOS from sucrose under optimized biotransformation parameters [37]. Another fungus Penicillium citrinum produced FTase units in fermentation media containing banana peel (6.9 U/mL) and sugarcane molasses (7.3 U/mL) [38].
Prebiotic fructooligosaccharides can also be obtained by one-step hydrolysis of inulin by endo-acting inulinases (endo-inulinases). Inulin serves as a storage polysaccharide in many plants of Composite and Gramineae. It consists of β-(2–1)-D-fructosyl-fructose links terminated by a sucrose residue [8, 39, 40]. This fructan is a potential substrate for generation of high fructose syrup (HFS) and prebiotic inulooligosaccharides (IOS). Inulin is acted upon by two types of inulinases, i.e., endo-inulinase (2,1-β-D-fructanfructanohydrolase, EC 3.2.1.7) and exoinulinase (β-D-fructanfructohydrolase, EC 3.2.1.80). Endo-inulinases liberate IOS as the main product [8], while exoinulinases hydrolyze the terminal linkages to yield fructose as the main product. Pertaining to the high demand of FOS, their cost-effective production is assuming greater challenges. In this context, development of an enzyme-based process using microbial transferases and hydrolases can help achieving the target of producing FOS using cost-effective indigenous technology. High fructose syrup can be biotransformed into value added products such as ethanol and single-cell protein, while IOS are indicated in nutraceutical industry as prebiotics [41]. Bhalla et al. [42] reported FOS generation using Saccharomyces cerevisiae isolated from local fermented beverage called Chaang. It was selected after screening for high invertase activity. Highest yield was obtained from 250 mg sucrose concentration and 2.5 U of invertase in 1 ml reaction at pH 5.5 and 40 °C. Production of an extracellular, thermostable inulinase was carried out by Aspergillus tubingensis CR16 using wheat bran and corn steep liquor (CSL) under solid-state fermentation (SSF). The fungus produced 1358.6 ± 0.8 U/g inulinase after parametric optimization which was fivefold higher [43]. Bacillus safensis AS-08 grown on dahlia inulin produced inulinases which hydrolyzed inulin to mixture of fructooligosaccharides [44].
A. niger NK-126 showed high inulinase activity on dandelion tap root extract (52.3 U/ml) and produced a mixture of fructose and FOS from chicory inulin [45]. In silico studies of Singh and Shukla [46] have shown that exo- and endo-inulinases from Penicillium sp. TN-88 have different arrangement of amino acids in the active site for recognition of substrate. Dilipkumar et al. [47] have used sugarcane press mud for the production of inulinase in solid-state fermentation (SSF). The optimized medium with sugarcane juice at 20% (v/v) and casein peptone at 2% (w/v) was found to be optimal at an initial pH 7.0 and incubation temperature 35 °C for 48 h. The produced inulin-type FOS (kestose and neokestose) and levan were characterized by Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR) analysis. The study revealed that the levansucrase could form FOS from sucrose [48]. Aspergillus fumigatus NFCCI 2426 was found to produce 68 U/ml of FTase activity on a medium containing 20% w/v sucrose. The enzyme was partially purified (acetone precipitation followed by DEAE-Cellulose anion exchange chromatography) and immobilized in calcium alginate for continuous transfructosylation of food grade sucrose to generate FOS (GF5, GF4, GF3, GF2) and gluco-fructose (our unpublished results). The research in the field of FOS is gaining momentum among researchers because of their tremendous potential as nutraceuticals. Purama et al. [49] summarized pathways of colorectal cancer (CRC) inhibition by FOS. These oligos also play crucial role in immune health maintenance by increasing the concentration of interleukins and IgG. They inhibit cancer cell growth by activating caspase pathway for apoptotic death of CRC cells [49].
3.2 Galactooligosaccharides (GOS) and Other Lactose-Derived Oligosaccharides
GOS are non-digestible oligosaccharides composed of 2–8 galactose moieties linked by β (1 → 4) and β (1 → 6) bonds with a terminal glucose residue, while β (1 → 2) and β (1 → 3) glycosidic linkages may be found in some GOS [24]. GOS are usually produced by the transgalactosylation catalyzed by β-galactosidase (βG; EC 3.2.1.23) using lactose-rich solutions. The βG catalyzes transgalactosylation in a similar way to the fructosyltransferase in a kinetically controlled manner [50]. The transgalactosylation reaction proceeds through the release of galactose from lactose followed by its transfer to another lactose molecule to generate trisaccharide (GOS 3). Subsequent formation of GOS 4, 5, and 6 occurs with GOS 3, 4, and 5 as an acceptor molecule. βGs are obtained from a variety of microorganisms mainly from Aspergillus spp. (A. niger and A. oryzae), yeasts (Kluyveromyces spp.), and bacteria (Bacillus circulans) as prominent producers.
GOS are frequently used as functional food ingredient in dairy products, bakery, and beverages. Recently, a novel β-galactosidase from the fungus Thermothielavioides terrestris was heterologously expressed for the production of GOS. The recombinant enzyme resulted in 19.4% and 14.8% GOS yield from lactose solutions and acid whey, respectively [51]. On the other hand, bacterial galactosidase (GH family 42) from Pantoea anthophila resulted in 40% GOS yield corresponding to 86% lactose conversion from 400 g/L lactose. The lactose hydrolysate was composed of 14% GOS, 5.7% 6-galactobiose, 20.2% allolactose, 46.7% mixed monosugars (glucose and galactose), and 13.3% residual lactose [52]. Another acidic β-galactosidase from A. oryzae (ENZECO), having optimum pH 4.5, produced a maximum of 26.73% GOS (DP 3 and 4) from lactose solution [53]. β-Galactosidase from A. oryzae (≥8 IU mg−1; Sigma Chemicals Co, USA) was utilized to generate GOS from whey powder substrate. Maximum yield of 62 g L−1 of GOS was obtained from 40% sweet whey powder bioconversion. Also, the immobilization of this enzyme on a synthetic methacrylic immobilization carrier (Lifetech ECR8409) resulted in 2.5-fold enhanced GOS productivity and recyclability over 4 cycles [54]. Commercial β-galactosidase preparations sourced from Aspergillus aculeatus, A. oryzae, A. niger, B. circulans, and Kluyveromyces lactis have been employed for GOS production from lactose and lactulose solutions. Among all the tested enzyme preparations, fungal lactase sourced from A. oryzae yielded maximum GOS (0.29 g g−1 from lactose and 0.38 g g−1 from lactulose) [55].
3.3 Cellooligosaccharides (COS)
Cellulose is the most abundant structural polysaccharide on the earth. Cellulose (poly β-1,4-glucopyranose) is composed of closely knit linear chains of anhydro-glucose monomers linked by β-1,4-glycosidic linkages at the C1 and C4 positions. Cellooligosaccharides (COS) display the same chemical structure as cellulose but have DP in the range of 2–6. Although cellulose is insoluble in water, due to smaller DP, COS are fairly soluble in water at room temperature. COS are generated by depolymerization and glycosylation of cellulose polymer. Glycosidic linkages other than β-1,4 glycosidic bonds such as α-1,4, α/β-1,3, α/β-1,6, and α/β-1,2-glycosidic linkages may be found in COS [56]. COS are mainly derived by controlled de-polymerization from plant cellulose. COS can be obtained by acid or enzymatic hydrolysis of plant cellulose. Partial hydrolysis of cellulosic biomass is based on the protonation of glycosidic bond by acidic or enzymatic catalysis. Among the entire COS synthesis approaches, enzymatic de-polymerization of cellulose is the most promising, eco-friendly, and economical approach.
COS synthesis by enzymatic catalysis is generally done either from sucrose and glucose biotransformation employing a catalytic cascade involving three glycoside phosphorylases, namely, sucrose phosphorylase (ScP; GH 13 family), cellobiose phosphorylase (CbP; GH 94 family), and cellodextrin phosphorylase (CdP; GH 94 family) [57,58,59] or by hydrolyzing cellulose using cellulases [60]. Cellulase consortium predominantly contains three enzymes: (1) endo-1,4-glucanase (EG) (EC 3.2.1.4), responsible for the endo-hydrolysis of cellulose polymer at internal amorphous sites generating cello-oligomers (high DP); (2) cellobiohydrolases (CBH) (EC 3.2.1.91) or exo-1,4-glucanases, catalyze the exo-hydrolysis of crystalline cellulose at the reducing end to generate cellobiose and short-chain COS; and (3) β-glucosidases (βG) (E.C. 3.2.1.21), which hydrolyze the produced cellobiose to glucose monomers [61]. A controlled enzymatic hydrolysis of cellulose using different commercial cellulase combinations (CBH from Trichoderma longibrachiatum and EG from Thermothelomyces thermophila) has been demonstrated to generate cellobiose. Cellulase combination (CBHI/EG5) in a ratio of 80:20 led to the generation of optimal yields (49.7% w/w) of cellobiose with the cellobiose/glucose ratio of 9.4 [60]. Details on the production of COS from lignocellulosic biomass and their prebiotic applications have been compiled by Avila et al. [62]. Production of cellobiose from organosolv-pretreated birch lignocellulose hydrolysis using an optimum combination of cellulases (GH family 5 endoglucanase sourced from Talaromyces emersonii, GH family 6 cellobiohydrolase from Podospora anserina, GH family 7 cellobiohydrolase, and GH family 7 endoglucanase from T. thermophila with an accessory enzyme lytic polysaccharide monooxygenase from T. thermophila) led to production of cellobiose (22.3%) [63]. Zhou et al. [64] demonstrated an effective strategy to produce purified COS by simultaneous production of sugar monoesters to remove monosugars. This strategy resulted in an improvement in COS production from 33.3 to 74.3%. On the other hand, controlled synthesis of ammonia- pretreated wheat straw depolymerization had been achieved using some of the adsorbed activities of cellulase cocktail obtained from A. niger. Cellulase adsorption (majorly exocellulase) on cellulosic biomass favored cellobiose synthesis, while the unadsorbed liquid fraction predominantly produced other COS [65]. Selective removal of β-glucosidase activity from cellulase pool resulted in a 36% increased COS production from corncob residue with a yield of 51.78% than single-stage hydrolysis [66].
3.4 Mannooligosaccharides (MOS)
Mannans, consisting of D-mannose linked by β-1,4 mannosidic linkages, are found in coffee beans, locust bean gum (LBG), guar gum (GG), palm kernel cake (PKC), konjac gum (KG), ivory nuts, sugar beets, soybeans, etc. Mannans are of four types based on their glycosidic linkage and branching patterns [67, 68]. Oligomers of mannose, mannooligosaccharides (MOS), are classified into alpha or beta types. The α-MOS are mainly obtained by hydrolysis of yeast cell wall, while β-MOS are mainly produced by hydrolysis of plant mannans by chemical (acid/alkaline), physical (ultrasonic), or enzymatic (mannanase) means [69].
β-MOS are generated using enzyme consortia consisting of β-1, 4-mannanase (EC 3.2.1.78), β-mannosidase (EC3.2.1.25), α-galactosidase (EC 3.2.1.22), and β-glucosidase (EC 3.2.1.21) [15, 70]. According to the sequence similarity database of catalytic sequences, they are grouped into glycoside hydrolase (GH) families – 5, 26, 113, and 134 (http://www.cazy.org/) [71]. Several reports describe the production of MOS from agro-industrial wastes using microbial β-mannanases [72]. Among these, fungal mannanases are for high-yield generation of MOS. Many fungal sources have been reported to produce β-mannanases that belong to family GH-5 and GH-26. Mannanases from Yunnania penicillata, Aspergillus nidulans, A. niger, A. oryzae, and Rhizomucor miehei have been reported to produce MOS [67, 71, 73,74,75,76]. Li and co-workers [77] engineered R. miehei β-mannanase and heterologously expressed in Pichia pastoris. This engineered mannanase produced 34.8 g MOS per 100 g dry palm kernel cake with 80.6% hydrolysis yield. Additionally, Penicillium oxalicum β-mannanase was employed to generate MOS from copra meal and coffee rests. Their hydrolyzed products were composed of mannose, M2, M4, and M6. Recombinant mannanase (1625 U/mL) was utilized for the production of MOS from copra meal and palm kernel meal. Copra meal generated M2 as major product, while mannose and M2 were the major products with smaller amounts of M3 in case of palm kernel meal [78]. β-Mannanase from Talaromyces trachyspermus generated mainly mannose and M2 from coffee waste and M2, M3, and M4 from locust bean gum [79].
Among microbial sources, mannanases (mainly GH5 and GH26) from filamentous fungus A. niger are the most widely studied for MOS production. Commercial production of MOS requires robust enzyme which is suitable for industrial applications [76]. A codon-optimized mannanase (AnMan26) from A. niger was expressed in Pichia pastoris, and titers to the tune of 22,100 U mL−1 were obtained in a 5-L fermenter. It had maximum specific activity toward locust bean gum and produced mannooligosaccharides from locust bean galactomannan (LBG). Moreover, it also resulted in the production of high DP MOS (1.8 × 103 Da) from partial hydrolysis of fenugreek gum [76]. β-Mannanases from A. oryzae, A. quadrilineatus, Aspergillus terreus, and thermophilic Malbranchea cinnamomea were characterized and used to produce MOS from LBG, guar gum, palm kernel cake, and copra meal [67, 80,81,82].
3.5 Xylooligosaccharides (XOS)
Xylan is a low molecular weight (DP 80–200) plant polysaccharide mainly found in the form of cell wall hemicellulose. Xylans are branched polymers of (1 → 4) linked β-D-xylopyranosyl backbones. Branched chains may be substituted with ferulic acid, acetyl, 4-O-methyl glucuronic acid, p-coumaric acid, or an arabinose side group [8, 83, 84]. Plant hemicelluloses having xylose and arabinose with traces of uronic acid (glucuronic acid and 4-O-methyl derivative) are termed as arabinoxylans, while glucose linked xylan are termed as gluco-xylans [85, 86]. Xylooligosaccharides are (1 → 4) linked β-D-xylopyranose oligomers (DP 2–7) with varying properties such as degree of polymerization and structural properties depending upon the raw source used. Xylan may be extracted from plant cell wall using water [87], acid treatment [88], alkali [89, 90], dimethyl sulfoxide (DMSO) [91, 92], or hot and cold water under pressure [93]. Xylooligosaccharides can be produced by enzymatic hydrolysis of β-1,4-xylosidic bonds of xylan by endo-1,4-β-D-xylanases (EC 3.2.1.8). Endo-xylanases mainly belong to glycoside hydrolase (GH) families 10 and 11, while some xylanases also belong to other GH families (5, 7, 8, 16, 26, 30, 43, 52, and 62) [94]. As an emerging prebiotic, XOS exhibit health benefitting properties such as bifidogenic potential [95] and increased calcium absorptivity, minimize colon cancer risk, and confer immune-regulatory properties [96]. They also display some other medicinal properties such as antioxidant, anti-allergic, anti-inflammatory, and cytotoxic properties [94, 97]. Several fungal strains such as Paecilomyces variotii, A. terreus, A. fumigatus, Penicillium glabrum, Sorangium cellulosum, Thermomyces lanuginosus, and M. cinnamomea have been reported to produce high titers of xylanases [98,99,100,101,102,103]. Xylanase secreted by P. variotii resulted in the generation of XOS composed of xylobiose (X2 14%), xylotriose (X3 27%), and xylotetrose (X4 23%), together with a small amount of xylopentaose (X5 18%) and xylohexose (X6 13%) and xylose (X1 0.8%) in 0.5 h hydrolysis of 1% w/v beechwood at 55 °C [94].
Brenelli et al. [104] described an interesting approach using slight acetylation followed by hydrothermal pretreatment for improved XOS production from sugarcane straw (SS) catalyzed by Aspergillus nidulans xylanase (GH 10). The pretreatment strategy promoted 81.5% hemicellulose solubilization and resulted in XOS (X2, X3) yield up to 9.8%. Xylanase from T. lanuginosus produced XOS composed of X2 (66.46%), X3 (25.10%), and small amount of xylose (4.97%) from beechwood xylan [105]. Commercial xylanase from T. longibrachiatum produced 44.43% XOS from Brewers’ spent grain over 12 h hydrolysis [106]. Endoxylanase from Streptomyces thermovulgaris was utilized to produce XOS (10.66%) from pretreated corn cobs [107]. Other raw sources such as coconut husk, finger millet seed coat, rice bran, sugarcane bagasse, wheat straw, etc. have been utilized for XOS production using bacterial and fungal xylanases [108,109,110,111]. Corn cob xylan (2% w/v) treated with partially purified T. lanuginosus xylanase for 8 h at 45 °C yielded 6.9 mg/ml of XOS (X2, X3) [112].
3.6 Maltooligosaccharides (MaOS)
Maltooligosaccharides (MaOS) are composed of 2–10 units of α-1,4-linked glucopyranose monomers. MaOS are generally produced from starch by the catalytic action of α-amylase (EC 3.2.1.1) [113]. MaOS generating amylases have been described from several bacterial species [114] and also from a few fungal species such as A. niger and A. nidulans [56, 115]. Kazim et al. [115] characterized the MaOS generating ability of an amylase (AmyG) sourced from A. nidulans. The AmyG generated DP3 to DP6 MaOS from starch hydrolysis. In another study, high concentration (1 μM) of A. niger amylase has been applied to hydrolyze corn starch, potato starch, and wheat starch for 12 h to generate MaOS (DP 1–3). The enzyme treatment produced a maximum of 16 mg/mL MaOS from potato starch while 14 mg mL−1 from corn and wheat starch with trace amounts of DP 4 MaOS [56].
On the other hand, some reports of MaOS generating amylosucrase (EC 2.4.1.4, ASase) are also available. This enzyme exhibits glucosyltransferase activity and catalyzes MaOS synthesis using sucrose as the substrate. A comparative study on two-step and one-step production strategies of MaOS (DP 3–6) using bacterial amylases has been carried out by Zhu et al. [113].
4 Nutritional Aspects of Prebiotic Oligosaccharides
Pro-health properties of oligosaccharides made them a very important ingredient among functional foods. Most of the oligosaccharides are known to exhibit multifarious nutritional benefits through modulation of the gut microbiota towards healthy-gut environment [116, 117] and imparting antioxidant, immune-booster effects, and high mineral (especially calcium and magnesium) absorptivity through gut epithelium [118]. Moreover, they also help in reduction of some metabolic disorders such as cardiovascular diseases (CVD) [119], inflammable Bowel’s disease (IBD), major depressive disorder (MDD) [120], and obesity [121, 122]. These properties have highlighted oligosaccharides as an important nutraceutical additive in food and feed industry, juice and beverage industry, cosmetic applications, medicinal applications, animal feed and livestock applications, etc. Recently, fructooligosaccharides and inulin (dried Jerusalem artichoke tubers) have been shown to produce positive effect on the pork quality and fatty acid profile. Improved antioxidant status, water-holding capacity, and a reduced shear force was observed. Furthermore, prebiotic addition in the pig diet improved the quality and shelf-life of the pork [122]. Prebiotic-enriched diet also contributed to a better weight gain and inhibition in diarrhea in piglets [123]. Inulin oligosaccharides may reduce the activity and expression of fat-generating enzymes in liver and inhibit the fatty acid synthesis and, therefore, can be used in fatteners [124]. XOS catalyzed from P. variotii xylanase exhibited potent antioxidant activity toward DPPH free radicals [94]. Gao et al. [118] determined the effect of GOS on the colonic mucosa of LPS-challenged piglets. GOS consumption resulted in reduction of reactive oxygen species (ROS) and malondialdehyde (MDA) and improvement in total antioxidant capacity in the injured piglets. Also, enhanced production of total short-chain fatty acids (SCFAs) was observed in LPS-challenged suckling piglets. Additionally, GOS significantly played role in immune modulation via reduced production of inflammatory molecules, interleukin 1β (IL-1β), interleukin 6 (IL-6), myeloid differentiation primary response 88 (MyD88), tumor necrosis factor-α (TNF-α), and cluster of differentiation 14 (CD14) in injured piglets. Recently, GOS have also been demonstrated to be beneficial in modulating gut microbiome of lactose-intolerant patients [116]. Probiotic growth promotion, anticancer, and antioxidant potential of MOS produced using fungal mannanase has been investigated by Jana et al. [125]. Another study described the evaluation of MOS-enriched diet for 60 days over the white leg Litopenaeus vannamei shrimp. MOS diet amended the productivity by 30% improved survival of shrimps. Next-generation sequencing suggested that MOS improved the Actinobacteria (28%) as predominant gut microbiota and inhibition in opportunistic pathogens such as Bergeyella, Vibrio, Aeromonas, and Shewanella [126]. Prebiotic potential of birch- and spruce-derived COS against Lactobacillus and Bifidobacterium has been demonstrated. Growth rate and cell density of probiotic strains was improved in the medium comprising COS as sole carbon source [63]. Similar to other oligosaccharides, MaOS are important functional food ingredient with low sweetness and osmolality and high water-holding capacity which may be utilized as sucrose substitute [127,128,129]. They also exhibit immunomodulatory properties and participate in improved colonic microbiome with the reduction in pathogenic microbes [113, 130].
5 Conclusions and Future Prospects
Oligosaccharides, particularly FOS and GOS, are most explored prebiotics, while nutraceutical propertied of MOS, COS, XOS, and MaOS are being explored toward disease alleviation. Fungal enzymes (either hydrolases or transferases) are a good source for commercial preparation of these oligosaccharides from raw agro-waste sources. Production yield, biological properties, and the ease of commercialized production of nutraceutical oligosaccharides are majorly dependent on the source and catalytic properties of these fungal enzymes. Industrial process requires improved catalytic properties such as stability over a broad range of pH and temperature, high product yield, utilization of waste biomass as substrate, high shelf life, etc. Fungal enzymes are suitable with respect to these parameters; therefore, they are being a hot-spot for applied research on oligosaccharides for functional food industries.
References
Ibañez E, Cifuentes A (2013) Benefits of using algae as natural sources of functional ingredients. J Sci Food Agric 93:703–709
de Borba GD, Cinelli LP, Simas NK, Pessoa A, Sette LD (2019) Marine prebiotics: polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem 280:175–186
Petuely F (1957) Bifidusflora bei flaschenkindern durch bifidogene substanzen (Bifidusfaktor). Z Kinderheilkunde 79:174–179
Yazawa K, Tamura Z (1982) Search for sugar sources for selective increase of bifidobacteria. Bifidobacteria Microflora 1:34–44
Yazawa K, Imai K, Tamura Z (1978) Oligosaccharides and polysaccharides specifically utilizable by bifidobacteria. Chem Pharm Bull 26:3306–3311
Gibson GR, Roberfroid M (1995) Dietary modulation of the human colonic microbiota -introducing the concept of prebiotics. J Nutr 125:1401–1412
Martins GN, Ureta MM, Tymczyszyn EE, Castilho PC, Gomez-Zavaglia A (2019) Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis Front Nutr 6:78
Kango N, Jain SC (2011) Production and properties of microbial inulinases: recent advances. Food Biotechnol 25:165–212
Singh SP, Jadaun JS, Narnoliya LK, Pandey A (2017) Prebiotic oligosaccharides: special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl Biochem Biotechnol 183:613–635
Choukade R, Kango N (2021) Production, properties and applications of fructosyltransferase: a current appraisal. Crit Rev Biotechnol 41:1178–1193
Choukade R, Kango N (2020) Applications of fungal inulinases. In: Zaragoza O, Casadevall A (eds) Encyclopedia of mycology, reference module in life sciences. Elsevier, pp 337–347
Itaya NM, Asega AF, Carvalho MAM, Rita de Cássia L (2007) Hydrolase and fructosyltransferase activities implicated in the accumulation of different chain size fructans in three Asteraceae species. Plant Physiol Biochem 45:647–656
Sangeetha PT, Ramesh MN, Prapulla SG (2005) Recent trends in the microbial production, analysis and application of fructooligosaccharides. Trends Food Sci Technol 16:442–457
Garcia Diaz T, Ferriani Branco A, Jacovaci FA, Cabreira Jobim C, Pratti Daniel JL, Iank Bueno AV, Ribeiro MG (2018) Use of live yeast and mannoligosaccharides in grain-based diets for cattle: ruminal parameters, nutrient digestibility, and inflammatory response. PLoS One 13:e0207127
Jana UK, Kango N, Pletschke B (2021) Hemicellulose-derived oligosaccharides: emerging prebiotics in disease alleviation. Front Nutr 8:670817
Moure A, Gullón P, Domínguez H, Parajó JC (2006) Advances in the manufacture, purification and applications of xylooligosaccharides as food additives and nutraceuticals. Process Biochem 41:1913–1923
Voragen AG (1998) Technological aspects of functional food-related carbohydrates. Trends in Food Sci Technol 9:328–335
Intanon M, Arreola SL, Pham NH, Kneifel W, Haltrich D, Nguyen TH (2014) Nature and biosynthesis of galactooligosaccharides related to oligosaccharides in human breast milk. FEMS Microbiol Lett 353:89–97
Martínez-Villaluenga C, Frías J (2014) Production and bioactivity of oligosaccharides in plant foods. In: Moreno FJ, Sanz ML (eds) Food oligosaccharides: production, analysis and bioactivity. John Wiley & Sons, Ltd., pp 35–54
Holck J, Hjernø K, Lorentze A, Vigsnæs LK, Hemmingsen L, Licht TR, Mikkelsen JD, Meyer AS (2011) Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process Biochem 46:1039–1049
Vandenplas Y, Zakharova I, Dmitrieva Y (2015) Oligosaccharides in infant formula: more evidence to validate the role of prebiotics. Br J Nutr 113:1339–1344
Rajagopalan G, Krishnan C (2019) Functional oligosaccharides: production and action. In: Rathinam NK, Sani RK (eds) Next generation biomanufacturing technologies. ACS Publications, American Chemical Society, Washington, DC, pp 155–180
Benkoulouche M, Fauré R, Remaud-Siméon M, Moulis C, André I (2019) Harnessing glycoenzyme engineering for synthesis of bioactive oligosaccharides. Interface Focus 9:20180069
Vera C, Illanes A, Guerrero C (2020a) Enzymatic production of prebiotic oligosaccharides. Curr Opin Food Sci 37:160–170
Flores-Maltos DA, Mussatto SI, Contreras-Esquivel JC, Rodríguez-Herrera R, Teixeira JA, Aguilar CN (2016) Biotechnological production and application of fructooligosaccharides. Crit Rev Biotechnol 36:259–267
Borrelli L, Coretti L, Dipineto L, Bovera F, Menna F, Chiariotti L, Nizza A, Lembo F, Fioretti A (2017) Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci Rep 7:6269
Gioacchini G, Giorgini E, Olivotto I, Maradonna F, Merrifield DL, Carnevali O (2014) The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress. Zebrafish 11:98–106
Dominguez AL, Rodrigues LR, Lima NM, Teixeira JA (2014) An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol 7:324–337
Maiorano AE, Piccoli RM, da Silva ES, Rodrigues MFDA (2008) Microbial production of fructosyltransferases for synthesis of prebiotics. Biotechol Lett 30:1867–1877
Ghazi I, Fernandez-Arrojo L, Garcia-Arellano H, Ferrer M, Ballesteros A, Plou FJ (2007) Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J Biotechnol 128:204–211
Kurakake M, Masumoto R, Maguma K, Kamata A, Saito E, Ukita N, Komaki T (2010) Production of fructooligosaccharides by β-fructofuranosidases from Aspergillus oryzae KB. J Agric Food Chem 58:488–492
Ganaie MA, Gupta US, Kango N (2013) Screening of biocatalysts for transformation of sucrose to fructooligosaccharides. J Mol Catal B Enzym 97:12–17
Hidaka H, Hirayama M, Sumi N (1988) A fructooligosaccharide-producing enzyme from Aspergillus niger ATCC 20611. Agric Biol Chem 52:1181–1187
Trollope KM, Van Wyk N, Kotjomela MA, Volschenk H (2015) Sequence and structure-based prediction of fructosyltransferase activity for functional subclassification of fungal GH32 enzymes. FEBS J 282:4782–4796
Hernández L, Menéndez C, Pérez ER, Martínez D, Alfonso D, Trujillo LE, Ramírez R, Sobrino A, Mazola Y, Musacchio A, Pimentel E (2018) Fructooligosaccharides production by Schedonorus arundinaceus sucrose:sucrose 1-fructosyltransferase constitutively expressed to high levels in Pichia pastoris. J Biotechnol 266:59–71
Míguez N, Gimeno-Pérez M, Fernández-Polo D, Cervantes FV, Ballesteros AO, Fernández-Lobato M, Ribeiro MH, Plou FJ (2018) Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by entrapment in polyvinyl alcohol and its application to neo-fructooligosaccharides production. Catalysts 8:201
Choukade R, Kango N (2019) Characterization of a mycelial fructosyltransferase from aspergillus tamarii NKRC 1229 for efficient synthesis of fructooligosaccharides. Food Chem 286:434–440
Jayalakshmi J, Sadiq AM, Sivakumar V (2021) Microbial enzymatic production of fructooligosaccharides from sucrose in agricultural harvest. Asian Jr. of Microbiol. Biotech Env Sc 23:84–88
Chi Z, Chi Z, Zhang T, Liu G, Yue L (2009) Inulinase-expressing microorganisms and applications of inulinases. Appl Microbiol Biotechnol 82:211–220
Liu GL, Chi Z, Chi ZM (2013) Molecular characterization and expression of microbial inulinase genes. Crit Rev Microbiol 39:152–165
Rawat HK, Soni H, Treichel H, Kango N (2017) Biotechnological potential of microbial inulinases: recent perspective. Crit Rev Food Sci Nutr 57:3818–3829
Bhalla TC, Thakur B, Savitri N, Thakur N (2017) Invertase of Saccharomyces cerevisiae SAA-612: production, characterization and application in synthesis of fructo-oligosaccharides. LWT Food Sci Technol 77:178–185
Trivedi S, Divecha J, Shah A (2012) Optimization of inulinase production by a newly isolated Aspergillus tubingensis CR16 using low cost substrates. Carbohydr Polym 90:483–490
Singh RS, Singh RP (2014) Response surface optimization of endoinulinase production from a cost effective substrate by Bacillus safensis AS-08 for hydrolysis of inulin. Biocatal Agric Biotechnol 3:365–372
Kango N (2008) Production of inulinase using tap roots of dandelion (Taraxacum officinale) by Aspergillus niger. J Food Eng 85:473–478
Singh PK, Shukla P (2012) Molecular modeling and docking of microbial inulinases towards perceptive enzyme–substrate interactions. Indian J Microbiol 52:373–380
Dilipkumar M, Rajasimman M, Rajamohan N (2014) Utilization of copra waste for the solid state fermentative production of inulinase in batch and packed bed reactors. Carbohydr Polym 102:662–668
Xavier JR, Ramana KV (2016) Optimization of Levan production by cold-active Bacillus licheniformis ANT 179 and fructooligosaccharide synthesis by its levansucrase. Appl Biochem Biotechnol 181:986–1006
Purama RK, Raman M, Ambalam P, Pithva S, Kothari C, Doble M (2018) Prebiotics and probiotics in altering microbiota: implications in colorectal cancer. In: Chatterjee S, Jungraithmayr W, Bagchi D (eds) Immunity and inflammation in health and disease. Academic Press, Elsevier, pp 403–413
Vera C, Guerrero C, Aburto C, Cordova A, Illanes A (2020b) Conventional and non-conventional applications of β-galactosidases. Biochim Biophys Acta - Proteins Proteomics 1868:140271
Zerva A, Limnaios A, Kritikou AS, Thomaidis NS, Taoukis P, Topakas E (2021) A novel thermophile β-galactosidase from Thermothielavioides terrestris producing galactooligosaccharides from acid whey. New Biotechnol 63:45–53
Yañez-Ñeco CV, Cervantes FV, Amaya-Delgado L, Ballesteros AO, Plou FJ, Arrizon J (2021) Synthesis of β (1 → 3) and β (1 → 6) galactooligosaccharides from lactose and whey using a recombinant β-galactosidase from Pantoea anthophila. Electron J Biotechnol 49:14–21
Cinar K, Gunes G, Gulec HA (2020) Enzymatic synthesis of prebiotic carbohydrates from lactose: kinetics and optimization of transgalactosylation activity of β-galactosidase from Aspergillus oryzae. J Food Process Eng 2020:e13435
Simović M, Milivojević A, Ćorović M, Banjanac K, Bezbradica D (2019) Whey valorization using transgalactosylation activity of immobilized β-galactosidase. Int J Food Sci Technol 54:3074–3082
Guerrero C, Vera C, Conejeros R, Illanes A (2015) Transgalactosylation and hydrolytic activities of commercial preparations of β-galactosidase for the synthesis of prebiotic carbohydrates. Enzym Microb Technol 70:9–17
Chen P, Shrotri A, Fukuoka A (2021) Synthesis of cello-oligosaccharides by depolymerization of cellulose: a review. Appl Catal A Gen 621:118177
Puchart V (2015) Glycoside phosphorylases: structure, catalytic properties and biotechnological potential. Biotechnol Adv 33:261–276
Zhong C, Ukowitz C, Domig K, Nidetzky B (2020a) Short-chain cello-oligosaccharides: intensification and scale-up of their enzymatic production and selective growth promotion among probiotic bacteria. Agric Food Chem 68:8557–8567
Zhong C, Duic B, Bolivar JM, Nidetzky B (2020b) Three-enzyme phosphorylase cascade immobilized on solid support for biocatalytic synthesis of cello-oligosaccharides. ChemCatChem 12:1350–1358
Hrůzová K, Matsakas L, Karnaouri A, Norén F, Rova U, Christakopoulos P (2021) Valorization of outer tunic of the marine filter feeder Ciona intestinalis towards the production of second-generation biofuel and prebiotic oligosaccharides. Biotechnol Biofuels 14:32
Kango N, Jana UK, Choukade R (2019) Fungal enzymes: sources and biotechnological applications. In: Satyanarayana T, Deshmukh S, Deshpande M (eds) Advancing Frontiers in Mycology & Mycotechnology. Springer, Singapore, pp 515–538
Ávila PF, Silva MF, Martins M, Goldbeck R (2021) Cello-oligosaccharides production from lignocellulosic biomass and their emerging prebiotic applications. World J Microbiol Biotechnol 37:73
Karnaouri A, Matsakas L, Krikigianni E, Rova U, Christakopoulos P (2019) Valorization of waste forest biomass toward the production of cello-oligosaccharides with potential prebiotic activity by utilizing customized enzyme cocktails. Biotechnol Biofuels 12:1–19
Zhou P, Liu C, Wang W, Wang F, Nie K, Deng L (2020) The effectively simultaneous production of cello-oligosaccharide and glucose mono-decanoate from lignocellulose by enzymatic esterification. Appl Biochem Biotechnol 192:600–615
Birhade S, Pednekar M, Sagwal S, Odaneth A, Lali A (2017) Preparation of cellulase concoction using differential adsorption phenomenon. Prep Biochem Biotechnol 47:520–529
Chu Q, Li X, Xu Y, Wang Z, Huang J, Yu S, Yong Q (2014) Functional cello-oligosaccharides production from the corncob residues of xylo-oligosaccharides manufacture. Process Biochem 49:1217–1222
Jana UK, Suryawanshi RK, Prajapati BP, Kango N (2020) Prebiotic mannooligosaccharides: synthesis, characterization, and bioactive properties. Food Chem 342:128328
Singh S, Ghosh A, Goyal A (2018) Manno-oligosaccharides as prebiotic-valued products from agro-waste. In: Varjani S, Parameswaran B, Kumar S, Khare S (eds) Biosynthetic technology and environmental challenges, Energy, environment, and sustainability. Springer, Singapore, pp 205–221
Dhawan S, Kaur J (2007) Microbial mannanases: An overview of production and applications. Crit Rev Biotechnol 27:197–216
Wang J, Zeng D, Liu G, Wang S, Yu S (2014) Truncation of a mannanase from Trichoderma harzianum improves its enzymatic properties and expression efficiency in Trichoderma reesei. J Ind Microbiol Biotechnol 41:125–133
Srivastava PK, Kapoor M (2017) Production, properties, and applications of endo-β-mannanases. Biotechnol Adv 35:1–19
Rungruangsaphakun J, Keawsompong S (2018) Optimization of hydrolysis conditions for the mannooligosaccharides copra meal hydrolysate production. 3 Biotech 8:169
Li YO, Komarek AR (2017) Dietary fibre basics: health, nutrition, analysis, and applications. Food Qual Saf 1:47–59
Von Freiesleben P, Moroz OV, Blagova E, Wiemann M, Spodsberg N, Agger JW, Davies GJ, Wilson KS, Stålbrand H, Meyer AS, Krogh KBRM (2019) Crystal structure and substrate interactions of an unusual fungal non-CBM carrying GH26 endo-β-mannanase from Yunnania penicillata. Sci Rep 9:2266
Von Freiesleben P, Spodsberg N, Blicher TH, Anderson L, Jørgensen H, Stålbrand H, Meyer AS, Krogh KBRM (2016) An Aspergillus nidulans GH26 endo-β-mannanase with a novel degradation pattern on highly substituted galactomannans. Enzym Microb Technol 83:68–77
Wang NN, Liu J, Li YX, Ma JW, Yan QJ, Jiang ZQ (2021) High-level expression of a glycoside hydrolase family 26 β-mannanase from Aspergillus niger in Pichia pastoris for production of partially hydrolysed fenugreek gum. Process Biochem 100:90–97
Li Y, Yi P, Liu J, Yan Q, Jiang Z (2018) High-level expression of an engineered β-mannanase (mRmMan5A) in Pichia pastoris for manno-oligosaccharide production using steam explosion pretreated palm kernel cake. Bioresour Technol 256:30–37
Harnpicharnchai P, Pinngoen W, Teanngam W, Sornlake W, Sae-Tang K, Manitchotpisit P, Tanapongpipat S (2016) Production of high activity aspergillus Niger BCC4525 β-mannanase in Pichia pastoris and its application for mannooligosaccharides production from biomass hydrolysis. Biosci Biotechnol Biochem 80:2298–2305
Suzuki K, Michikawa M, Sato H, Yuki M, Kamino K, Ogasawara W, Fushinobu S, Kaneko S (2018) Purification, cloning, functional expression, structure, and characterization of a thermostable β-mannanase from Talaromyces trachyspermus B168 and its efficiency in production of mannooligosaccharides from coffee wastes. J Appl Glycosci 65:13–21
Ahirwar S, Soni H, Rawat HK, Ganaie A, Pranaw K, Kango N (2016) Production optimization and functional characterization of thermostable β-mannanase from Malbranchea cinnamomea NFCCI 3724 and its applicability in mannotetraose (M4) generation. J Taiwan Inst Chem Eng 63:344–353
Soni H, Rawat HK, Pletschke BI, Kango N (2016) Purification and characterization of β-mannanase from Aspergillus terreus and its applicability in depolymerization of mannans and saccharification of lignocellulosic biomass. 3 Biotech 6:136
Suryawanshi RK, Kango N (2021) Production of mannooligosaccharides from various mannans and evaluation of their prebiotic potential. Food Chem 334:127428
Linares-Pasten JA, Aronsson A, Karlsson EN (2018) Structural considerations on the use of endo-xylanases for the production of prebiotic xylooligosaccharides from biomass. Curr Protein Pept Sci 19:48–67
Palaniappan A, Antony U, Emmambux MN (2021) Current status of xylooligosaccharides: production, characterization, health benefits and food application. Trends Food Sci Technol 111:506–519
Muralikrishna G, Subba Rao MVSST (2007) Cereal non-cellulosic polysaccharides: structure and function relationship-An overview. Crit Rev Food Sci Nutr 47:599–610
Scheller HV, Ulvskov P (2010) Hemicelluloses. Ann. Rev. Plant Biol 61:263–289
Palaniappan A, Yuvaraj SS, Sonaimuthu S, Antony U (2017a) Characterization of xylan from rice bran and finger millet seed coat for functional food applications. J Cereal Sci 75:296–305
Wen P, Zhang T, Wang J, Lian Z, Zhang J (2019) Production of xylooligosaccharides and monosaccharides from poplar by a two-step acetic acid and peroxide/acetic acid pretreatment. Biotechnol Biofuels 12:87
de Mattos NR, Colodette JL, de Oliveira CR (2019) Alkaline extraction and carboxymethylation of xylans from corn fiber. Cellulose 26:2177–2189
Khat-Udomkiri N, Sivamaruthi BS, Sirilun S, Lailerd N, Peerajan S, Chaiyasut C (2018) Optimization of alkaline pretreatment and enzymatic hydrolysis for the extraction of xylooligosaccharide from rice husk. AMB Express 8:115–124
de Carvalho DM, Martínez-Abad A, Evtuguin DV, Colodette JL, Lindstrom ME, Vilaplana F, Sevastyanova O (2017) Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw. Carbohydr Polym 156:223–234
Fu G, Yue P, Hu Y, Li N, Shi Z, Peng F (2018) Fractionation of DMSO-extracted and NaOH-extracted hemicelluloses by gradient ethanol precipitation from neosinocalamus affinis. Int J Pol Sci 2018:1–8
Kilpeläinen P, Leppänen K, Spetz P, Kitunen V, Ilvesniemi H, Pranovich A, Willför S (2012) BIOREFINERY. Pressurised hot water extraction of acetylated xylan from birch sawdust. Nord Pulp Paper Res J 27:680–688
Abdella A, Ramadan S, Hamouda RA, Saddiq AA, Alhazmi NM, Al-Saman MA (2021) Paecilomyces variotii xylanase production, purification and characterization with antioxidant xylo-oligosaccharides production. Sci Rep 11:16468
Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 8:92
Sheu WHH, Lee IT, Chen W, Chan YC (2008) Effects of xylooligosaccharides in type 2 diabetes mellitus. J Nutr Sci Vitaminol 54:396–401
Akhtar MS, Swamy MK (eds) (2018) Anticancer plants: natural products and biotechnological implements. Springer Nature, Singapore
Amir A, Arif M, Pande V (2013) Purification and characterization of xylanase from Aspergillus fumigatus isolated from soil. Afr J Biotechnol 12:3049–3057
Basotra N, Joshi S, Satyanarayana T, Pati PK, Tsang A, Chadha BS (2018) Expression of catalytically efficient xylanases from thermophilic fungus Malbranchea cinnamomea for synergistically enhancing hydrolysis of lignocellulosics. Int J Biol Macromol 108:185–192
Elgharbi F, Hmida-Sayari A, Zaafouri Y, Bejar S (2015) Expression of an Aspergillus niger xylanase in yeast: application in breadmaking and in vitro digestion. Int J Biol Macromol 79:103–109
Knob A, Beitel SM, Fortkamp D, Terrasan CRF, De Almeida AF (2013) Production, purification, and characterization of a major Penicillium glabrum xylanase using brewer’s spent grain as substrate. Biomed Res Int 2013:728735
Kumar V, Shukla P (2018) Extracellular xylanase production from T. lanuginosus VAPS24 at pilot scale and thermostability enhancement by immobilization. Process Biochem 71:53–60
Wang SY, Hu W, Lin XY, Wu ZH, Li YZ (2012) A novel cold-active xylanase from the cellulolytic myxobacterium Sorangium cellulosum So9733-1: gene cloning, expression, and enzymatic characterization. Appl Microbiol Biotechnol 93:1503–1512
Brenelli LB, Figueiredo FL, Damasio A, Franco TT, Rabelo SC (2020) An integrated approach to obtain xylo-oligosaccharides from sugarcane straw: from lab to pilot scale. Bioresour Technol 313:123637
Guido ES, Silveira JT, Kalil SJ (2019) Enzymatic production of xylooligosaccharides from beechwood xylan: effect of xylanase preparation on carbohydrate profile of the hydrolysates. Int Food Res J 26:713–721
Amorim C, Silverio SC, Silva SP, Coelho E, Coimbra MA, Prather KL, Rodrigues LR (2018) Single-step production of arabino-xylooligosaccharides by recombinant Bacillus subtilis 3610 cultivated in brewers’ spent grain. Carbohydr Polym 199:546–554
Seesuriyachan P, Kawee-ai A, Chaiyaso T (2017) Green and chemical-free process of enzymatic xylooligosaccharide production from corncob: enhancement of the yields using a strategy of lignocellulosic destructuration by ultra-high pressure pretreatment. Bioresour Technol 241:537–544
Faryar R, Linares-Pasten JA, Immerzeel P, Mamo G, Andersson M, Stålbrand H, Mattiasson B, Karlsson EN (2015) Production of prebiotic xylooligosaccharides from alkaline extracted wheat straw using the K80R-variant of a thermostable alkali-tolerant xylanase. Food Bioprod Process 93:1–10
Jnawali P, Kumar V, Tanwar B, Hirdyani H, Gupta P (2018) Enzymatic production of xylooligosaccharides from brown coconut husk treated with sodium hydroxide. Waste Biomass Valorization 9:1757–1766
Palaniappan A, Balasubramaniam VG, Antony U (2017b) Prebiotic potential of xylooligosaccharides derived from finger millet seed coat. Food Biotechnol 31:264–280
Reddy SS, Krishnan C (2016) Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT-Food Sci Technol 65:237–245
Khangwal I, Nath S, Kango N, Shukla P (2020) Endo-xylanase induced xylooligosaccharide production from corn cobs, its structural features, and concentration-dependent antioxidant activities. Biorefin, Biomass Convers. https://doi.org/10.1007/s13399-020-00997-3
Zhu F, Prosser C, Zhu Y, Otter D, Hemar Y (2018) Enzymatic formation of galactooligosaccharides in goat milk. Food Biosci 26:38–41
Sihui P, Ning D, Junyan R, Zhengbiao G, Caiming L, Yan H, Li C, Tod PH, Zhaofeng L (2017) Maltooligosaccharide-forming amylase: characteristics, preparation, and application. Biotechnol Adv 35:619–632
Kazim ARS, Jiang Y, Li S, He X (2021) Aspergillus nidulans AmyG functions as an intracellular a-amylase to promote a-glucan synthesis. Microbiol Spectr 9:e00644–e00621
Azcarate-Peril MA, Roach J, Marsh A, Chey WD, Sandborn WJ, Ritter AJ, Savaiano DA, Klaenhammer TR (2021) A doubleblind, 377-subject randomized study identifies Ruminococcus, Coprococcus, Christensenella, and Collinsella as long-term potential key players in the modulation of the gut microbiome of lactose intolerant individuals by galacto-oligosaccharides. Gut Microbes 13:1957536
Wu Y, Pan L, Shang QH, Ma XK, Long SF, Xu YT, Piao XS (2017) Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim Feed Sci Technol 230:126–135
Gao J, Li X, Zhang G, Sadiq FA, Simal-Gandara J, Xiao J, Sang Y (2021) Probiotics in the dairy industry-advances and opportunities. Compr Rev Food Sci Food Saf 20:3937–3982
Wu H, Chiou J (2021) Potential benefits of probiotics and prebiotics for coronary heart disease and stroke. Nutrients 13:2878
Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Monserrat J, Lahera G, Mora F, Rodriguez-Quiroga A, Fernandez-Rojo S, Quintero J, Alvarez-Mon M (2021) Exploring the role of nutraceuticals in major depressive disorder (MDD): rationale, state of the art and future prospects. Pharmaceuticals 14:821
Beam A, Clinger E, Hao L (2021) Effect of diet and dietary components on the composition of the gut microbiota. Nutrients 13:2795
Grela ER, Swiatkiewicz M, Florek M, Bakowski M, Skiba G (2021) Effect of inulin source and a probiotic supplement in pig diets on carcass traits, meat quality and fatty acid composition in finishing pigs. Animals 11:2438
Chlebicz-Wójcik A, Śliżewska K (2020) The effect of recently developed synbiotic preparations on dominant fecal microbiota and organic acids concentrations in feces of piglets from nursing to fattening. Animals 10:1999
Birmani MW, Nawab A, Ghani MW, Li G, Xiao M, An L (2019) A review: role of inulin in animal nutrition. J Food Technol Res 6:18–27
Jana UK, Suryawanshi RK, Prajapati BP, Soni H, Kango N (2018) Production optimization and characterization of mannooligosaccharide generating β-mannanase from Aspergillus oryzae. Bioresour Technol 268:308–314
Gainza O, Romero J (2020) Effect of mannan oligosaccharides on the microbiota and productivity parameters of Litopenaeus vannamei shrimp under intensive cultivation in Ecuador. Sci Rep 10:2719
Ben Ali M, Mhiri S, Mezghani M, Bejar S (2001) Purification and sequence analysis of the atypical maltohexaose-forming alpha-amylase of the Bacillus stearothermophilus US100. Enzym Microb Technol 28:537–542
Chen X, Wang J, Li Q (2015) Simultaneous determination of maltooligosaccharides in beer using HPLC-ELSD and their influence on beer foam stability. J Am Soc Brew Chem 73:78–83
Zheng CJ, Liu R, Xue B, Luo J, Gao L, Wang Y, Ou S, Li S, Peng X (2017) Impact and consequences of polyphenols and fructooligosaccharide interplay on gut microbiota in rats. Food Funct 8:1925–1932
Crittenden RG, Playne M (1996) Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol 7:353–361
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Choukade, R., Kango, N. (2023). Fungal Enzyme-Based Nutraceutical Oligosaccharides. In: Satyanarayana, T., Deshmukh, S.K. (eds) Fungi and Fungal Products in Human Welfare and Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-19-8853-0_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-8853-0_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8852-3
Online ISBN: 978-981-19-8853-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)