Abstract
In the corn crop industry, of all the biomass produced, about 80% are residues. The so-called corn fibers, one of the most important residues of the corn processing industry, represent about 9% of the corn kernel weight, being a low value material that could, potentially, be used for making higher added value products. This work aimed to extract the hemicelluloses present in the corn fiber via alkaline extraction, with their subsequent functionalization for production of carboxymethyl xylans in mild conditions. The corn fibers were characterized for their contents of carbohydrates, lignin, extractives, total uronic acids, acetyl groups, and ash. Their arabinoxylans were extracted by 2–18% (w/v) sodium hydroxide at room temperature, for 5 h at 10% consistency, precipitated with ethanol, washed and then vacuum dried. The resulting extract was characterized by FT-IR, viscosity, arabinoxylan content and purity. It was demonstrated that CCE treatment provides a high purity and little degraded xylan, but the extraction yields are relatively low, in the range of 4.0–23.9% wt/wt depending upon extraction conditions. The use of corn fiber arabinoxylans to obtain hemicellulosic derivatives through chemical modification reactions was also evaluated. The arabinoxylans were derivatized by carboxymethylation with sodium monochloroacetate in a 2-propanol alkaline medium using different proportions of alcohol and alkali. The product carboxymethyl xylan was characterized by degree of substitution, FT-IR, DSC, and yield, and showed high degree of substitution, yield and enthalpy of fusion. This work proved the feasibility of producing hemicellulosic derivatives from corn fibers, which excludes the use of extreme conditions of solvents and temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of renewable resources is an attractive alternative to reduce the demand for fossil-based materials. Biomass residues, mainly from plant sources, are the major renewable raw materials for obtaining biofuels, chemicals, and biomaterials. Among all renewable materials, lignocellulosic biomasses are the most abundant as they come from lower plants, forests, and agroindustry residues (Konduri and Fatehi 2016; Silva et al. 2009).
The main lignocellulosic materials present in plant cell walls are cellulose (40–50%), hemicelluloses (25–35%) and lignin (15–25%), in addition to other materials such extractives, inorganics, pectins, proteins etc. The hemicelluloses are the second most abundant polysaccharides found in nature, after cellulose and its relevance is increasing for the development of biopolymers and chemically modified functional materials, which may be used in films, coatings and hydrogels (Peng et al. 2015; Viana and Cruz 2016; Ren et al. 2008).
Compared to cellulose, hemicelluloses have a lower degree of polymerization (DP), with amorphous and branched chains, containing pentoses, hexoses, and acids, presenting lower thermal and chemical stabilities than those from cellulose (Hilpmann et al. 2016).
Xylans present the major contribution to hemicelluloses from hardwood (15–30%), grasses and grains (20–35%) and softwood (7–12%). When compared to cellulose and starch, xylans show limited industrial applications, due mainly to their high solubility in water (Gomes et al. 2015), although it may be overcome by the final product properties and usage. There are possible potential uses of the xylans if they are produced in their insoluble form (hydrogels), such as biopolymer films and drug release products, additives for viscosity improvement, hydrophobic barriers, packaging films, paper additives, emulsifiers for cosmetics and food (Gomes et al. 2015; Erbringerová 2006).
Various biomass residues were recently investigated in terms of xylans content, as barley straw, sugarcane leaves, corn cubs and straw, cotton rods, rice husks and straw, sugarcane bagasse, Plantago ovata, etc. These studies showed that the xylans mass percentage varied from 9.2 to 32% (Deutschmann and Dekker 2012). The physical properties of xylans vary according to the raw material used, the substituents, and the substitution pattern of polymers, the source of the material and the method of extraction and purification (Deutschmann and Dekker 2012).
Corn is the third major crop after rice and wheat plants, being one of the grains with the highest volume produced in the world, with approximately 960 million tons in 2013. The United States, China, Brazil, and Argentina are the greatest producers, representing 70% of world total production (Deutschmann and Dekker 2012).
The corn has many foods uses, but its industrial application is wider as, for example, in the production of thickener and binds, oils and ethanol (Sindimilho 2005). The corn grain is a fruit, called caryopsis, with a dry basis composition of starch (61–78%), proteins (6–12%), fiber (2–4%), oil (3–6%) and minerals (1–4%), distributed in a heterogeneous way on the four main physical structures of the grain: endosperm, germ, pericarp (husk) and tip. The pericarp represents, on average, 5% of the total mass of the grain, witch cell layers composed of cellulose and hemicelluloses, although some content of lignin is also found on it (Paes 2007). The corn pericarp can be generally called corn fiber due to its chemical composition be similar to other plant fibers. The corn fiber is an agroindustry residue obtained from the starch or corn syrup production. The corn fiber has a low market price, due to its high content of lignocellulosic components, and is commonly used for livestock breeds.
The xylans have recently being applied for the development of new biopolymeric materials and functional polymers produced by chemical modifications, as addition, substitution and degradation (Petzold et al. 2006; Pen et al. 2012).
Among all chemical modifications, the carboxymethylation is the most versatile and industrial attractive technique to synthesize high value-added products from biobased polymers. This is explained by the reactions carried out under low temperatures and solvent concentrations (Konduri and Fatehi 2016). Thus, carboxymethylation represents a useful way to produce soluble derivatives with anionic functions from hemicelluloses, when carried out in different suspension media.
The carboxymethyl xylan is an anionic polymeric ether derived from the xylan, in which the hydroxide groups from xylan are partially substituted by carboxymethyl groups. Total substitution is not possible because of the branched d-xylopyranose units. The degree of substitution (DS) reaches variable values and the final product usually contains non-substituted, mono- or di-substituted monomers in the xylan chain.
Several studies propose that the modified xylans have many applications as emulsifying agents, binding agents for drugs and metals, paper production, medical uses, packaging films, dispersants etc., but no industrial uses have yet been reported (Alekhina et al. 2014; Erbringerová 2006; Konduri and Fatehi 2016; Nascimento et al. 2013; Peng et al. 2015; Velkova et al. 2015). This work aimed at investigating the alkaline extraction of xylans from corn fibers and the production of soluble hemicellulosic derivatives via homogeneous and heterogeneous carboxymethylation of the extract.
Experimental
Corn fiber characterization
The corn fibers were crushed in a mil (IKA A11) and the accept was collected after sieving at 40 and 60 mesh. The fractions retained on these two sieves were air-conditioned at 23 °C and 50% of humidity.
The quantitative determination of the main sugars of the corn fiber carbohydrates was carried out by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The samples were treated with H2SO4 72% at 30 °C for 1 h, followed by autoclaving at 120 °C for 1 h. The chromatographic separation was performed in a DIONEX ICS-3000, equipped with a CarboPac PA1 (250 × 4 mm) column. For all separations, an isocratic elution of NaOH 0.001 mol L−1 and an eluent flux of 1 mL min−1 during 45 min were applied (Wallis et al. 1996). The residue after hydrolysis was considered as Klason lignin according to TAPPI standard T222 om-11. The soluble lignin was determined by the measurement of absorbance at 205 nm on the filtrate from the acid hydrolysis (Goldschimid 1971). The uronic acids hydrolyzed corn fibers were measured by colorimetry at 450 nm by color development with 3,5-dimethylphenol (Scott 1979). The acetyl group content was measured by quantifying the sodium acetate produced by hydrolysis of corn fibers with oxalic acid after pH adjustment. The measurement was carried out by high-performance liquid chromatography with UV detection, as described by Sólar et al. (1987). The extractives content was determined after Soxhlet extraction with the sequence of extraction media: ethanol:toluene, ethanol, and hot water, as described at TAPPI standard T264 om-07. The ash content was determined after calcination of the samples at 575 °C (TAPPI T211 om-12). All values were reported as an average of two independent replicates.
Alkaline extraction of corn fibers
The corn fibers were extracted by alkali in a beaker of 250 mL of capacity with a defined ratio mass of fibers/volume of NaOH of 1:10, at room temperature and magnetic stirring for 5 h.
The NaOH solution concentration varied from 2 to 18% (m/v). The extract was centrifuged for 20 min at 8000 rpm, and the alkaline supernatant was collected and acidified by a HNO3 solution (8 mol L−1) to pH around 5, being further precipitated with ethanol.
The resulting suspension was centrifuged again at 4000 rpm for 10 min. The precipitate was purified by three washing procedures with 10 mL of methanol merged with the centrifugation procedure described above. The purified material was vacuum dried in a desiccator for 48 h, milled, weighted and conditioned in hermetically closed bottles at room temperature for further characterization.
Arabinoxylan characterization
The purity and yield of arabinoxylans found by the HPAEC-PAD method were calculated by Eqs. 1 and 2.
where mmo is the mass of organic matter extracted (g), TP (%) is the percentage content of arabinoxylans determined and mes is the total dry mass extracted (g).
where mp is the mass related to the arabinoxylans found in the dry mass of xylans extracted (g) and mi is the mass related to the arabinoxylans which would have been extracted according to the content of arabinoxylans originally present in the corn fibers (g).
The arabinoxylan viscosity was measured according to the TAPPI standard T230 om-94: 0.15 g of absolutely dried xylans were weighted in a flask with 25 mL of water. The flask was tightly sealed and the mixture was stirred for 5 min in a horizontal shaker. After stirring, 25 mL of ethylenediamine solution was added to the flask and the system was again stirred for 15 min. After this period, the viscosity was measured in a VISCOMAT II viscometer.
Xylan carboxymethylation
The extracted arabinoxylans were carboxymethylated according to the method described of Heinze and Pfeiffer (1999), Heinze et al. (2004) and optimized by Petzold et al. (2006). The carboxymethylation was carried out in a homogeneous and in a heterogeneous reaction media.
Heterogeneous activation
The synthesis of the carboxymethyl xylan (CMX) through heterogeneous activation was carried out with 5 g (37.8 mmol) of anhydrous xylan (AXU) suspended in 150 mL of 2-propanol, followed by the addition of 10 mL of NaOH 15% (w/v) (1.51 g, 37.8 mmol) as seen in Table 1 (sample 1). The mixture was vigorously stirred, at room temperature for 1 h. After this period, 4.39 g (37.8 mmol) of sodium monochloroacetate (SMCA) were added, at 55 °C for 5 h. The product was filtered, suspended in methanol 80% (v/v), neutralized with diluted acetic acid and washed six times with ethanol aliquots of 20 mL, merged by centrifugation at 4000 rpm for 5 min. After this procedure, the precipitate was dried in a desiccator. Different ratios of AXU:SMCA:NaOH were tested in order to evaluate the influence of the stoichiometric proportions in the DS, as presented in Table 1.
Homogeneous activation
The synthesis of CMX through homogeneous activation was carried out as follows: 5 g (37.8 mmol) of AXU were dissolved in 25 mL of NaOH 25% (w/v) (6.25 g; 156.2 mmol) solution, followed by the addition of 35 mL of 2-propanol. The mixture was vigorously stirred for 3 h at 30 °C. After this period, 4.39 g (37.8 mmol) of SCMA were added to the reaction media and temperature was raised to 75 °C for 70 min. The resulting mixture was neutralized with acetic acid 65% (v/v) followed by the polymer precipitation with ethanol. The precipitate was washed twice with 50 mL of ethanol 65% (v/v) and was washed six times with 20 mL of ethanol merged with centrifugation at 4000 rpm for 5 min. Then, the precipitate was dried in a desiccator.
The procedure described above was performed for samples 12–16, but without 2-propanol as a suspension media. The molar ratios applied are showed in Table 2.
Carboxymethyl xylans characterization
The CMX were characterized as follows: DS determination, thermogravimetric analysis and differential scanning calorimetry (TG/DSC), and absorption spectroscopy in the infrared region with Fourier transform (FT-IR). After the synthesis, the CMX were grinded in an IKA A11 basic mill, conditioned in a desiccator for 24 h and storage in hermetically closed bottles.
The CMX yield was calculated on the xylans dry weight basis, as described by Eq. 3.
where mCMX is the mass of dried CMX (g) and mX is the arabinoxylan mass (g).
Determination of degree of substitution (DS)
The average DS is defined as the average number of hydroxyl groups substituted in each d-xylose unit of the polymeric chain. The degree of substitution of CMX samples was determined according to standard ASTM CK-G06 (2005) with modifications: 4 g of the sample was weighted in a beaker and 75 mL of ethanol 99.5% were added, and the suspension was stirred for 5 min. After this, 5 mL of concentrated HNO3 were added and the media was heated to boiling. Then the media was cooled and stirred for 10 min, followed by successive washings with 150 mL of ethanol 80% (v/v) at 60 °C. After the washings the suspension was centrifuged at 3000 rpm for 5 min until complete acid removal from sample. The sample was then washed with 10 mL of methanol and dried in oven at 105 °C for 3 h.
For the DS determination, 1 g of dried sample was mixed with 100 mL of distilled water and 25 mL of NaOH 0.3 mol L−1. The solution was heated to boiling for 15 min. Then the solution was titrated with standard solution of HCl 0.3 mol L−1 at 60 °C, with phenolphthalein as indicator.
The DS was calculated according to Eqs. 4 and 5.
where A is the number of milliequivalents of acid per gram of sample, B is the volume of NaOH solution added (L), C is the molarity of NaOH, D is the HCl volume consumed in the titration (L), E is the molarity of HCl and F is the mass of the sample (g).
where 0.132 is the molecular mass of one unit of anhydroxylose; 0.058 is the raise on the molecular mass of the unit of anhydroxylose for each carboxymethyl group added after carboxymethylation.
All analyses were performed in duplicates.
Differential scanning calorimetry
TG/DSC analyses were carried out in a Shimadzu DSC-60 calorimeter. Initial temperature was 25 °C and final temperature was 400 °C, with a thermal scanning rate of 5 °C min−1. The sample was placed in a hermetic aluminum crucible under N2 atmosphere (100 mL min−1).
Fourier-transform infrared spectroscopy
The FT-IR spectra were obtained on a Jasco FT-IR 4100 spectrometer, with attenuated total reflectance accessories (ATR). The scanning of the spectra was performed in the wavenumber range from 4000 to 400 cm−1 and was obtained from an average of 256 scanning with 4 cm−1 of resolution.
Results and discussion
Chemical composition of corn fibers
The contents of monosaccharides, extractives, lignin, acetyl groups, uronic acids and ash of the corn fiber material are shown in Table 3.
The corn fibers presented a total sugar content of 66.7%. When compared to literature data, this content is lower than that observed in corn stover, for example (82.9%) (Silva et al. 2015), but higher than values found in Eucalyptus globulus (63.5%) (Garrote et al. 2007) and in rice straw (51.7%) (Vegas et al. 2008). The reason for the differences in sugar contents is related to the unusually high values of xylans and arabinans present in corn fiber. The corn fiber high arabinan content (Silva et al. 2015; Garrote et al. 2007; Vegas et al. 2008; Laine et al. 2015), associated with the presence of uronic acids and acetyl groups suggest that the corn fiber hemicelluloses could be classified as 4-O-methyl-glucuronoarabino-xylans acetate (GAX), similarly to those proposed, for example, for the corn stover hemicelluloses (Silva et al. 1998). Another unique characteristic of corn fibers is their high content of extractives (20.8%) caused probably by contamination during the processing of corn kernel. These may include starch, proteins and oils that are susceptible to extraction by the eluotropic solvent series procedure applied.
Extraction and characterization of arabinoxylans
The alkali extraction was carried out with six different concentrations of alkali, namely: 2, 4, 6, 10, 14 and 18% NaOH (w/v), and these treatments were called as X2, X4, X6, X10, X14, X18, respectively.
The raw corn fiber presented an ash content of 0.7%. This value was very low when compared to the ones presented in the extracted arabinoxylans (GAX), probably caused by the presence of inorganics from the starting material and residuals of NaNO3 and HNO3 from acidification process. The purity of GAX increased when NaOH concentration decreased from 18 to 6%, reaching values up to almost 24.0% (Table 4). This behavior is explained by, at least, three factors: first, the ash content is lower for the lower NaOH charges due to the lower consumption of inorganic reagents in the extraction and neutralization procedures; second, the degree of arabinoxylans degradation increases with increasing NaOH concentration and; third, the higher NaOH concentration enhances the extraction of glucans that goes along than arabinoxylans. The lower purity observed for the treatments at 2 and 4% NaOH concentration may be explained by low arabinoxylans extraction efficiency at these low levels of alkalinity.
Arabinoxylans yield
The optimum NaOH charge for arabinoxylans extraction was defined on the basis of extraction yield, arabinoxylans purity and viscosity as shown in Fig. 1. Samples X2, X4, X6, X10, X14 and X18 are ordered from 1 to 6, respectively. The calculated yields (Eq. 2) were relatively low and these values may be due to GAX losses during centrifugation, and also due to the presence of lignin residuals, which might retain some hemicelluloses, making them unavailable for extraction. Frequently, hemicelluloses are covalently bonded to lignin, through ester bond with acetyl groups and with p-hydroxicynamic acid, which inhibits their release from matrix to be extracted. In fact, a method to selectively extract all hemicelluloses from lignocellulosic materials at a high level of purity and yield, without considerable degradation, has not yet being developed (Pen et al. 2012). The best yield and arabinoxylans purity values were observed at the NaOH 6% extraction (sample 3). This extraction was performed in mild conditions which could preserve arabinoxylans, reaching high values of viscosity, when compared to samples 4–6 (NaOH 10 to 18%). By this way, we considered that the optimized extraction may be carried out at NaOH 6%.
Viscosity
The viscosity is directly correlated with the degree of polymerization of the arabinoxylans and purity. The results of viscosity of GAX in Table 5 show that the increase of NaOH charge, in the range of 6% to 18% (w/v) decreased the GAX viscosity, due probably to the degradation of hemicelluloses during the extraction. Considering the results showed in Table 4 the extraction of GAX may be preferably carried out at a NaOH content of 6%.
Carboxymethylation of arabinoxylans
Figure 2a–c present the relation between yield and mean degree of substitution at the samples 1–6 (heterogeneous activation), 7 to 11 (homogeneous activation in 2-propanol) and 12 to 16 (homogeneous activation without suspension media), respectively. After heterogeneous activation (Fig. 2a), the yield of CMX increased with increasing the ratio NaOH:SMCA for all samples 1–6, except sample 3.
Sample 3 was produced under the highest ratio NaOH:SMCA, which may not preserve properly the DS of the starting xylan polymer chain. Thus depolylimerization may give further lowering of yield by degradation processes. Additionally, the remaining sites for SMCA substitution on the xylan structure could not be effectively carboxymethylated, due to low content of SMCA available on media, when sample 3 is compared to samples 1 and 2 (Peng et al. 2011). On the other hand, the homogeneous activation experiments (Fig. 2b) showed the highest yield values of all experiments. The improved efficiency of the homogeneous activation may be due to increased reaction rate under homogeneous media, higher NaOH charges and higher reaction temperature. Samples obtained from the homogeneous activation without suspension medium (Fig. 2c) presented higher yield than those from heterogeneous activation and lower than those obtained from homogeneous activation in 2-propanol medium. The presence of organic media probably increased the number of effective collisions between NaOH and xylans, consequently elevating the rate of reaction and yield. As can be observed, the yield increased when the average DS increases, due to the raise of the molecular weight related with the addition of the carboxymethyl groups onto the arabinoxylans.
Characterization of carboxymethyl xylans
Degree of substitution
Tables 6 and 7 presents of DS obtained for, samples 1–6 (heterogeneous activation), samples 7–11 (homogeneous activation in 2-propanol) and samples 12–16 (homogeneous activation without suspension media), respectively.
Heterogeneous activation
According to Table 6, the increase of DS is due to the elevation on the NaOH:SCMA ratio in all samples, except sample 3 (DS = 0.15). The statistically highest DS values were found in sample 6 (DS = 0.32), at a molar ratio of AXU:SCMA:NaOH of 1:10:10. The DS found in the heterogeneous activation for this study were lower than those found elsewhere for birch xylans (Petzold et al. 2006) and xylans of beech (Konduri and Fatehi 2016).
Homogeneous activation
The DS of samples obtained from homogeneous activation (Table 7) increased when NaOH:SCMA ratio increases in 2-propanol media and without suspension media. CMX produced at a 2-propanol media reached higher DS than those from activation without suspension media. The organic media is able to disperse better aqueous NaOH along with the carbohydrate structure, in order to enable hydroxide availability to react. This is explained by the low dielectric constant of 2-propanol, which may immobilize the sodium hydroxide near the xylans surface prior to reaction (Pushpamalar et al. 2006; Bhattacharyya et al. 1995). The use of solvents with lower dielectric constant than water may be an advantage when long chains of CMX are desired in the final products. Surfactants and similar compounds may be obtained by carboxymethylation with low dielectric suspension media. In this case, the challenge is related with the attendance to environmental friendly products, like bioethanol, for example. On the other hand, when products with low DS are required, aqueous media may be used.
Samples 11 (DS = 1.24) and 16 (DS = 0.98) presented higher DS values than those found in literature for one route carboxymethylation (Petzold et al. 2006; Konduri and Fatehi 2016). The use of 2-propanol as a suspension media promoted better DS values probably due to the inhibition of undesirable side reactions which produce sodium salts of glycolic and diclycolic acids. According to Alekhina et al. (2014), these side-products are recalcitrant to purification processes. The homogeneous activation method is more efficient than the heterogeneous one, giving higher reactivity to hemicelluloses, due to the increased accessibility to xylan hydroxyl groups.
Differential scanning calorimetry
Figure 3 present the DSC curve for arabinoxylans. The glass transition temperatures (Tg) and fusion enthalpy (ΔH) of CMX obtained by heterogeneous activation, homogeneous activation in 2-propanol and homogeneous activation without 2-propanol are presented in Figs. 4 and 5a, b, respectively.
The arabinoxylans presented a glass transition temperature (Tg) of 202.74 °C and a fusion enthalpy (ΔH) of − 104.86 mJ. Some authors report that after approximately 200 °C the decomposition of the polymer chains and substituents bond cleavages take place as main thermal events (Alekhina et al. 2014; Konduri and Fatehi 2016). The presence of substituents at CMX may cause, higher levels of thermal stability, when compared to starting material (arabinoxylan) (Konduri and Fatehi 2016), although this correlation is not well defined in this work.
A small variation of Tg is observed in all samples when compared to the GAX sample (reference). After heterogeneous activation (Fig. 4) sample 1:1:1 (AXU:SMCA:NaOH) presented the lower Tg value (176.54 °C) while sample 1:10:10 had the highest Tg value (208.93 °C). Sample 1:1:2 presented the highest amount of ΔH (in modulus), which may be explained by the optimum addition of sodium ions among the chains of the xylans, giving a high DS, as can be seen in Table 6. This ion addition probably did not affect considerably the original structure of the polymer chains. Thus, the carboxymethyl groups further added in the place of the ions probably had created new inter-chain interactions, increasing ΔH. CMX from homogeneous activation in 2-propanol presented no good correlation among DS and Tg or ΔH values. On the other hand, the average Tg from this process (188.2 °C) was higher than that observed for samples obtained by homogeneous activation without 2-propanol (184.5 °C). These results indicate that the use of 2-propanol improved the thermal resistance of the CMX produced. The ΔH at the glass transition phase ranged from − 265.70 mJ to − 446.92 mJ, presenting higher energy levels of thermal decomposition than that required for the arabinoxylan (− 104.86 mJ), probably due to branched chains obtained by the methyl groups added to the original backbone (Konduri and Fatehi 2016). A progressive increase of ΔH was observed at CMX obtained by homogeneous activation without 2-propanol. The increase of SMCA concentration used in samples—ranging from 1:1:4.1 to 1:4:4.1 (AXU:SMCA:NaOH)—probably favored effective substitutions (DS increase), enabling the formation of more hydrogen bonds than those found in the original arabinoxylan. The hydrogen bond increase caused by carboxymethylation at higher SMCA concentrations resulted at higher ΔH (in modulus). The exception of this trend is observed on sample 1:4:4.1, probably due to a steric hindrance from the carboxymethyl groups, which may enable distortions, thus lowering polymer chain superposition, and finally reducing the number of hydrogen bonds between chains.
Fourier-transform infrared spectroscopy
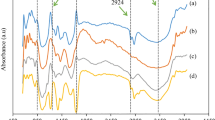
The FT-IR spectra can characterize the main functional groups present in the arabinoxylan structure, enabling a qualitative chemical description of the polymer (Magaton et al. 2008; Marques 2014; Jayapal et al. 2013; Samanta et al. 2012). Figure 6 presents the FT-IR spectra of sample 5 of CMX and the of the original corn fiber xylan. As can be seen, a typical band at 1030 cm−1 is observed due to glucosidic C–O–C bonds and to C–O and C–C stretching from xylose units (Magaton et al. 2008; Marques 2014; Eduardo da Silva et al. 2012). At the region of absorption associated to the deformation of C–H from anomeric hydrogens (950–700 cm−1), a band at 886 cm−1 with low intensity is observed indicating the presence of β-anomers, due to β-glucosidic bonds between xylose units of the xylan chain (Marques 2014; Jayapal et al. 2013; Samanta et al. 2012; Eduardo da Silva et al. 2012). The band observed around 1643 cm−1 is referred to water absorption vibrations (Magaton et al. 2008; Marques 2014; Jayapal et al. 2013). Bands at 2859 cm−1 and 2925 cm−1 are related to the C–H aliphatic stretching modes (Magaton et al. 2008; Marques 2014; Jayapal et al. 2013; Eduardo da Silva et al. 2012). The O–H vibrations are revealed by a band observed at 3334 cm−1 due to water molecules presence as an effect of xylans hygroscopy (Marques 2014; Jayapal et al. 2013; Samanta et al. 2012; Eduardo da Silva et al. 2012). The absence of a band around 1720 cm−1 indicates that no oxidation of the hydroxyl groups of hemicelluloses occurred during the xylan isolation procedure. At 1738 cm−1 (C=O stretching modes) no absorption band is observed, revealing that the acetyl groups were hydrolyzed during the extraction of hemicelluloses (Magaton et al. 2008; Eduardo da Silva et al. 2012). A band observed at 1363 cm−1 refers to sodium nitrate byproduct derived from the alkali extract neutralization with nitric acid.
In the CMX spectrum, a band at 1584 cm−1 is observed, due to the COO− stretching, revealing that the arabinoxylans were carboxymethylated (Peng et al. 2015; Mondal et al. 2015; Ren et al. 2008; Velkova et al. 2015). The band observed at 2914 cm−1 is refers to the C–H stretching and bending vibrational modes from methylene groups (Konduri and Fatehi 2016). Bands observed at 1308 cm−1 and 1411 cm−1 are due to the C=O stretching modes of carboxylic groups and of CH2 from methylene (Peng et al. 2015; Mondal et al. 2015; Konduri and Fatehi 2016; Ren et al. 2008; Velkova et al. 2015).
These results confirm the effective carboxymethylation of arabinoxylans.
Conclusions
The corn fibers represent a potential renewable source of hemicelluloses for the development of high quality value-added products. Their competitiveness is also related to their low cost, as an abundant agroindustry residue. Xylose and arabinose chains, containing acetyl and uronic acids groups, mainly compose the hemicelluloses from corn fibers (GAX). The optimal NaOH concentration for arabinoxylan extraction at acceptable yield is about 6% (w/v). The GAX carboxymethylation, carried out in mild conditions, was more effective when 2-propanol was applied in the homogeneous activation, giving a hemicellulosic derivatives from corn fibers, with high degree of substitution and good thermal resistance.
References
Alekhina M, Mikkonen KS, Alen R, Tenkanen M, Sixta H (2014) Carboxymethylation of alkali extracted xylan for preparation of bio-based packaging films. Carbohydr Polym 100:89–96. https://doi.org/10.1016/j.carbpol.2013.03.048
ASTM (2005) Analytic method for determining degree of substitution in the product, CK-G06, 5
Bhattacharyya D, Singhal RS, Kulkarni PR (1995) A comparative account of conditions for synthesis of sodium carboxymethyl starch from corn and amaranth starch. Carbohydr Polym 27:247–253. https://doi.org/10.1016/0144-8617(95)00083-6
Deutschmann R, Dekker RFH (2012) From plant biomass to bio-based chemicals: latest developments in xylan research. Biotechnol Adv 30:1627–1640. https://doi.org/10.1016/j.biotechadv.2012.07.001
Eduardo da Silva A, Marcelino HR, Gomes MCS, Oliveira EE, Nagashima TJ, Egito EST (2012) Xylan, a promising hemicellulose for pharmaceutical use. InTech, pp 61–84. http://www.intechopen.com/books/products-and-applications-ofbiopolymers. Accessed 16 Feb 2017
Erbringerová A (2006) Structural diversity and application potential of hemicelluloses. Macromol Symp 232:1–12. https://doi.org/10.1002/masy.200551401
Garrote G, Kabel MA, Schols HA, Falque E, Domínguez H, Parajó JC (2007) Effects of Eucalyptus globulus autohydrolysis conditions on the reaction products. J Agric Food Chem 55:9006–9013. https://doi.org/10.1021/jf0719510
Goldschimid O (1971) Lignins: occurrence, formation, structure and reactions. J Polym Sci C Polym Lett 10:228–230. https://doi.org/10.1002/pol.1972.110100315
Gomes KR, Chimphango AFA, Gorgenst JF (2015) Modifying solubility of polymeric xylan extracted from Eucalyptus grandis and sugarcane bagasse by suitable side chain removing enzymes. Carbohydr Polym 131:177–185. https://doi.org/10.1016/j.carbpol.2015.05.029
Heinze T, Pfeiffer K (1999) Studies on the synthesis and characterization of carboxymethylcellulose. Die Angew Makromol Chem 266:37–45. https://doi.org/10.1002/(SICI)1522-9505(19990501)266:1%3c37:AID-APMC37%3e3.0.CO;2-Z
Heinze T, Liebert T, Heinze U, Schwikal K (2004) Starch derivatives of high degree of functionalization 9: carboxymethyl starches. Cellulose 11:239–245. https://doi.org/10.1023/B:CELL.0000025386.68486.a4
Hilpmann G, Becher N, Pahner FA, Kusema B, Mäki-Arvela P, Lange R, Murzin DY, Salmi T (2016) Acid hydrolysis of xylan. Catal Today 259:376–380. https://doi.org/10.1016/j.cattod.2015.04.044
Jayapal N, Samanta AK, Kolte AP, Semani S, Suresh KP, Sampath KT (2013) Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Ind Crops Prod 42:14–24. https://doi.org/10.1016/j.indcrop.2012.05.019
Konduri MKR, Fatehi P (2016) Synthesis and characterization of carboxymethylated xylan and its application as a dispersant. Carbohydr Polym 146:26–35. https://doi.org/10.1016/j.carbpol.2016.03.036
Laine C, Kemppainen K, Kuutti L, Varhimo A, Asikainen S, Grönroos A, Määthänen M, Buchert J, Harlin A (2015) Extraction of xylan from wood pulp and brewer’s spent grain. Ind Crops Prod 70:231–237. https://doi.org/10.1016/j.indcrop.2015.03.009
Magaton AS, Veloso DP, Colodette JL (2008) Caracterização das O-acetil-(4-O-metilglicurono)xilanas isoladas da madeira de Eucalyptus urograndis. Quim Nova 31:1085–1088. https://doi.org/10.1590/S0100-40422008000500027
Marques AIF (2014) Isolamento de xilanas por precipitação com antisolventes. Dissertation, Técnico Lisboa, p 141
Mondal IH, Yeasmin S, Rahman S (2015) Preparation of food grade carboxymethyl cellulose from corn husk agro-waste. Int J Biol Macromol 79:144–150. https://doi.org/10.1016/j.ijbiomac.2015.04.061
Nascimento GE, Hamm LA, Baggio CH, Werner MFP, Lacomini M, Cordeiro LMC (2013) Structure of a galactoarabinoglucuronoxylan from tamarillo (Solanum betaceum), a tropical exotic fruit, and its biological activity. Food Chem 141:510–516. https://doi.org/10.1016/j.foodchem.2013.03.023
Paes MCD (2007) Manipulação da composição química do milho: impacto na indústria e na saúde humana Embrapa digital, pp 1–6. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/50216/1/Manipulacao-composicao.pdf. Accessed 26 Feb 2017
Peng XW, Ren JL, Peng F, Sun RC (2011) Rapid carboxymethylation of xylan-rich hemicelluloses by microwave irradiation. Adv Mat Res 236–238:292–296
Pen F, Peng P, Xu F, Sun RC (2012) Fractional purification and bioconversion of hemicelluloses. Biotechnol Adv 30:879–903. https://doi.org/10.1016/j.biotechadv.2012.01.018
Peng P, Meizhi Z, Diao E, Yuefang G (2015) Synthesis and characterization of carboxymethyl xylan-g-poly(propylene oxide) and its application in films. Carbohydr Polym 133:117–125. https://doi.org/10.1016/j.carbpol.2015.07.009
Petzold K, Schwikal K, Heinze T (2006) Carboxymethyl xylan—synthesis and detailed structure characterization. Carbohydr Polym 64:292–298. https://doi.org/10.1016/j.carbpol.2005.11.037
Pushpamalar V, Langford SJ, Ahmad M, Lim YY (2006) Optimization of reactions conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr Polym 64:312–318. https://doi.org/10.1016/j.carbpol.2005.12.003
Ren JL, Sun RC, Peng F (2008) Carboxymethylation of hemicelluloses isolated from sugarcane bagasse. Polym Degrad Stab 93:786–793. https://doi.org/10.1016/j.polymdegradstab.2008.01.011
Samanta AK, Senani S, Kolte AP, Sridhar M, Sampath KT, Jayapal N, Devi A (2012) Production and in vitro evaluation of xylooligosaccharides generated from corn cobs. Food Bioprod Process 90:466–474. https://doi.org/10.1016/j.fbp.2011.11.001
Scott RW (1979) Colometric determination of hexuronic acids in plant materials. Anal Chem 51:936–941. https://doi.org/10.1021/ac50043a036
Silva SS, Carvalho RR, Fonseca JLC, Garcia RB (1998) Extração e Caracterização de Xilanas de Sabugos de Milho. Quim Nova 8:25–33. https://doi.org/10.1590/S0104-14281998000200005
Silva R, Haraguchi SK, Muniz EC, Rubira AF (2009) Aplicações de fibras lignocelulósicas na química de polímeros e em compósitos. Quim Nova 32:661–671
Silva JC, Oliveira RC, Neto AS, Pimentel VC, Santos AA (2015) Extraction, addition and characterization of hemicelluloses from corn cobs to development of paper properties. Proc Mater Sci 8:793–801. https://doi.org/10.1016/j.mspro.2015.04.137
Sindimilho (2005) Sindicato da Industria de Milho, Soja e seus derivados no Estado de São Paulo; Milho e suas riquezas, História. www.fiesp.com.br/sindmilho/curiosidades. Accessed 28 Feb 2016
Sólar R, Kacik F, Mlecer I (1987) Simple method for determination of O-acetyl groups in wood and related materials. Nord Pulp Pap Res J 2:139–141. https://doi.org/10.3183/NPPRJ-1987-02-04-p139-141
Technical Association of the Pulps and Paper Industry (TAPPI) (1994) TAPPI standard T230 om-94, Viscosity of pulp (capillary viscometer method), Atlanta
Technical Association of the Pulps and Paper Industry (TAPPI) (2007) TAPPI standard T264 cm-07, Acid-insoluble lignin in wood and pulp, Atlanta
Technical Association of the Pulps and Paper Industry (TAPPI) (2011) TAPPI standard T222 om-11, Acid-insoluble lignin in wood and pulp, Atlanta
Technical Association of the Pulps and Paper Industry (TAPPI) (2012) TAPPI standard T211 om-12, Ash in wood, pulp, paper and paperboard: combustion at 525°C, Atlanta
Vegas R, Kabel M, Schols HA, Alonso JL, Parajó JC (2008) Hydrothermal processing of rice husks: effects of severity on product distribution. J Chem Technol Biotechnol 83:965–972. https://doi.org/10.1002/jctb.1896
Velkova N, Doliska A, Zemljic LF, Vesel A, Saake B, Strnad S (2015) Influence of carboxymethylation on the surface physical–chemical properties of glucuronoxylan and arabinoxylan films. Polym Eng Sci 55:2706–2713. https://doi.org/10.1002/pen.24059
Viana LG, Cruz PS (2016) Reaproveitamento de resíduos agroindustriais. http://cobesa.com.br/2016/download/cobesa-2016/IVCOBESA-133.pdf. Accessed 13 June 2017
Wallis A, Wearne R, Wrigth P (1996) Chemical analysis of polysaccharides in plantation eucalyptus wood and pulps. Appita J 49:258–262
Acknowledgments
Financial support provided by the Coordination for the Improvement of Higher Education Personnel is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented at the 19th International Symposium on Wood, Fiber and Pulping Chemistry.
Rights and permissions
About this article
Cite this article
de Mattos, N.R., Colodette, J.L. & de Oliveira, C.R. Alkaline extraction and carboxymethylation of xylans from corn fiber. Cellulose 26, 2177–2189 (2019). https://doi.org/10.1007/s10570-018-02236-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-02236-5