Abstract
Currently, the majority of chemicals and energy are derived from fossil fuel-based resources. The continued depletion of these fossil resources and their attributed negative environmental impacts have boosted the search for green and renewable feedstock. In this context, lignocellulosic biomass has been identified as a sustainable alternative raw material to produce platform chemicals, biofuels and biomaterials. The production of added value products from this biomass generally involves first fractionation processes to separate its principal constituents: cellulose, hemicellulose and lignin. Fractionation of lignocellulosic material leads to release of cellulosic fibres and opens the cell wall structure by solubilization of lignin and hemicellulose. An ideal fractionation process should provide selective separation of each component of lignocellulosic material, easy access and isolation of the components after separation and recovery of each component in high yield. Pretreatment has been extensively studied, since it can be the most expensive process in biomass-to-fuel conversion. Therefore, this chapter aims to provide a complete perspective on the fractionation/pretreatment processes of lignocellulosic biomass to produce second-generation biofuels and recover high-added-value products. In particular, the most relevant marketable bio-based chemicals, namely, hydroxymethylfurfural (HMF), 2,5-furandicarboxylic acid (FDCA) and levulinic acid that can be obtained in a biorefinery scheme, are approached in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lignocellulosic biomass
- Pretreatment methods
- Green solvents
- Emerging methods
- Hydroxymethylfurfural
- 2,5-Furandicarboxylic acid
- Levulinic acid
1 Introduction
At present, there is a great loss of natural resources, and high consumption of energy and chemicals due to the unstoppable employment of fossil fuels as the main resource for today’s life (Takkellapati et al. 2018; Usmani et al. 2020). In this sense, in order to fulfil the unavoidable demand for energy and chemicals in the increasing world’s population, up to 80% of the energy and 90% of the chemicals are intimately dependent on this nonrenewable resource, leading not only to its depletion but also to a great and negative impact in the environment due to harmful greenhouse gas (GHG) emissions and the production of toxic materials (Takkellapati et al. 2018). This challenging situation drives to seek for natural, more efficient, sustainable and renewable resources as a way to substitute the fossil derivatives (Usmani et al. 2020).
In this scenario, biorefineries play a key role in enabling a more sustainable and environmentally friendly world by converting biomass into valuable products (Solarte-Toro et al. 2018), building the bases of the bioeconomy and achieving the objectives established in the agenda 2030 (Solarte-Toro and Cardona Alzate 2021). Thus, biomass is considered the cheapest and most abundant source that can be employed for that aim, having a great potential for the production of biofuels and high-added-value products (Cho et al. 2020; Yiin et al. 2021). Specifically, lignocellulosic biomass (LCB)—including wood, agricultural residues and energy crops—is a carbon-neutral material with large reserves, reaching productions of up to 200 billion tons per year (Chen et al. 2022; Ashokkumar et al. 2022), which may facilitate the transition to a greener resource utilization (Thoresen et al. 2020) and the mitigation of the carbon emissions and the global climatic issues derived (Usmani et al. 2020).
The LCB possesses a complex three-dimensional matrix structure, composed of a blend of cellulose fibres coated by hemicelluloses and lignin at different proportions depending on the species (Velvizhi et al. 2022). The valorization of LCB cues the production of biofuels and/or green platform chemicals not dependent on fossil resources, although their recalcitrant structure enables the straightforward obtainment of these useful end products. Hence, pretreatments are needed to promote the separation of the main constituents of LCB in separated streams, providing a more efficient valorization (Usmani et al. 2020). This process is a key step for the valorization of LCB since it may account for about 20% of the total cost in a lignocellulosic biorefinery (Fírvida et al. 2021; Saravanan et al. 2022). Although the fractionation processes can be performed using a wide variety of reagents (acid or alkali), the employment of green solvents has appeared as an interesting novel alternative in order to use available at large scale, cheap, recyclable, energy-efficient, low toxicity, biodegradable and, in general, stable solvents (Wang and Lee 2021).
In this sense, the selective fractionation of LCB would allow its conversion into green bio-based platform chemicals. With that aim, the transformation of simple sugars via chemical or biochemical pathways enables the obtainment of valuable building blocks such as those from the furanic-aliphatic family, i.e. hydroxymethylfurfural (HMF), 2,5-furandicarboxylic acid (FDCA) and levulinic acid (Rivas et al. 2021). These platform chemicals were highlighted by the US Department of Energy (DoE) as one of the most promising chemicals coming from biomass that may substitute a wide range of valuable products such as polymer materials, pharmaceutical derivatives, fuels or food products (Sajid et al. 2018; Davidson et al. 2021). Figure 5.1 shows a scheme with the different pretreatment methods described in this chapter and the value-added products discussed in it.
Hence, this book chapter aims to display a comprehensive overview of the fractionation processes and the high-added-value products that can be obtained within second-generation biorefinery schemes.
2 Composition and Sources of Lignocellulosic Biomass
Lignocellulosic biomass (LCB) is a renewable resource that includes a wide range of feedstock from forestry (hardwoods and softwoods), agricultural or industrial practices, energy crops, etc. (Cai et al. 2017; Rodionova et al. 2022). LCB is mainly constituted of two polymers with saccharide nature (cellulose and hemicelluloses) and one-third polymer with phenolic nature (lignin). Approximately 90% of dry matter in LCB consists of these three polymers (Nanda et al. 2014), whereas the rest comprises minor amounts of other components such as extractives and minerals.
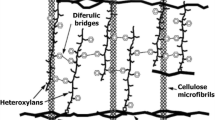
Distribution of cellulose, hemicelluloses and lignin is not uniform within the cell walls of LCB. The quantity and structure of these polymers are variable depending on the species, tissues and maturity of the plant cell wall (Barakat et al. 2013). Thus, the organization of these nonuniform three-dimensional matrices depends on the source of LCB in a highly variable composition as shown in Table 5.1. Related to this structural complex is the following: (a) cellulose has a crystalline fibrous conformation that acts as the main constituent of the plant cell wall and is responsible for the complicated and recalcitrant nature of LCB (Mankar et al. 2021); (b) hemicelluloses are binding agents between lignin and cellulose and add rigidity to the entire biomass complex (Isikgor and Becer 2015; Mankar et al. 2021); and (c) lignin behaves like a glue, filling and strongly binding the gap between hemicelluloses and cellulose. The function of lignin in the LCB is to provide impermeability and resistance against oxidative and microbial attack. Thus, LCB has evolved to resist degradation, thanks to the hydrophobicity of lignin, the recalcitrance of cellulose and the encapsulation of cellulose by the lignin-hemicelluloses complex (Isikgor and Becer 2015).
Cellulose is a linear polymer with around 100,000 average molecular weight and a molecular formula of (C6H10O5)n, constituted by d-anhydroglucopyranose units (glucose) bonded by β-(1 → 4) glycosidic links, which form the disaccharide cellobiose. Hydroxyl groups (OH) in the structure of d-anhydroglucopyranose, the primary (C6) and the secondary (C2 and C3), have different polarities that confer them the possibility to participate in intermolecular and intramolecular hydrogen bond interactions (Batista Meneses et al. 2022). In fact, cellulose contains both crystalline (organized) and amorphous (not well organized) regions alternating with each other as microfibrils; this crystalline region is resistant to degradation and the amorphous region is easy to degrade. Because of the fibrous nature and strong hydrogen bonding, cellulose is found to be insoluble in the majority of the solvents (Nanda et al. 2014; Kumar et al. 2016).
Hemicelluloses are branched heteropolymers, mainly constituted by different five and six carbon saccharide units, pentoses (xylose, arabinose) and hexoses (mannose, glucose, galactose) (Bhatia et al. 2020), interlinked via β-1,4 glycosidic linkages. Hemicelluloses also present substituents as acetyl and uronyl groups. Hemicelluloses, unlike cellulose, have a random and amorphous structure, which is composed of several heteropolymers including xylan, galactomannan, glucuronoxylan, arabinoxylan, glucomannan and xyloglucan. Hemicelluloses in woods differ in composition, since in hardwoods they are mainly constituted by xylan and in softwoods by glucomannans.
Lignin is a three-dimensional polymer made up by aromatic components, unlike the carbon backbones that constitute cellulose and hemicelluloses (Batista Meneses et al. 2022). This complex structure contains methoxyl, phenolic hydroxyl and some terminal aldehyde groups in the side chains. The three monolignols are p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol. The aromatic rings, which are abundantly present in lignin in the form of the basic phenylpropane units, are responsible for the antioxidant properties. The phenylpropane units are linked by β-O-4, β-5 and β–β′ bonds (Zhang and Naebe 2021). Lignin contains three types of phenylpropanoids, p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S), and their nature and quantity are variable according to species, tissues, maturity and spatial localization in the cell. As a general trend, the lignin content is lower in hardwoods than in softwoods. In contrast, hardwoods exhibit a higher amount of holocellulose and extractives than softwoods (Nanda et al. 2014). Related to the particularities of lignin from different LCB, in softwoods it mainly consists of guaiacyl units and minor percentage of p-hydroxyphenyl units (HG-lignin), while in hardwoods it is mainly made up by guaiacyl and syringyl units (GS-lignin) (Lourenço and Pereira 2018). Nonwoody LCB contains all the three units; thus, grass lignin belongs to HGS-lignin (Rodionova et al. 2022). It should be noted that H units in grasses are the lowest among the three lignin units, but their proportion is much higher than in hardwood and softwood.
Extractives are nonstructural components of LCB, which can be soluble in water or neutral organic solvents. They include biopolymers such as steroids, terpenoids, resin acids, lipid fats, waxes and phenolic constituents as stilbenes, flavonoids, tannins or lignans (Kumar et al. 2016). Elemental composition in LCB refers to major elements, such as K, Na, Mg, Ca or Si, and minor ones as S, Al, Fe, P or Mn; they can be found in less than 1% by weight in wood or shells, while in husks and straws, they reach up to 25% by weight (Nanda et al. 2014; Kumar et al. 2016).
3 Pretreatment Methods
Lignocellulosic pretreatment methods can be classified into traditional and emerging pretreatment technologies (Chen et al. 2022). Traditional methods can be divided into two types depending on whether or not they use chemical reagents. In addition, some traditional pretreatments can be described as fundamental methods, which are necessary as a previous stage to obtain biomass materials that meet the production requirements of the industry (Wang 2021).
3.1 Fundamental Pretreatment Methods
Fundamental pretreatment methods include washing, drying and mechanical pretreatment, which are necessary to exclude impurities and obtain shape uniformity (Wang 2021). Mechanical pretreatment consists of applying shear or compression forces to reduce the size of the raw material (Rezania et al. 2020). The commonly used mechanical pretreatments include grinding, milling and chipping. Chipping is performed during the collection of biomasses to get particles of 10–30 mm, while after milling or grinding, the size achieved is between 0.2 and 2 mm (Mankar et al. 2021). Milling and grinding decrease the biomass crystallinity and reduce polymerization degree improving the enzymatic digestibility (Dalena et al. 2018; Raynie et al. 2020).
Various types of milling instruments have been used so far, such as hammer milling, disk milling, ball milling and vibratory milling (Rezania et al. 2020). The more effective instrument reported is vibrating balls (Raynie et al. 2020). The main disadvantage of this method is that high-power consumption is usually associated with low efficiency. For this reason, the grinding process accounts for a significant part of the operating costs in the whole lignocellulosic biomass pretreatment process (Rezania et al. 2020).
3.2 Traditional Pretreatment Methods
3.2.1 Pretreatments with Chemical Reagents
Traditional chemical pretreatments include the use of different reagents such as acids, alkalis, oxidizing agents and organic solvents. As shown in Table 5.2, these methods are still being investigated as an alternative to the pretreatment of lignocellulosic biomass.
3.2.1.1 Acid Pretreatment
Acidic hydrolysis is a traditional pretreatment that has been used to solubilize hemicellulose, precipitate lignin and make cellulose more accessible for enzymatic hydrolysis. Acid reagents can destroy the glycosidic bonds and break all polysaccharide-lignin linkages achieving the recovery of most monomeric sugars (Raynie et al. 2020; Rezania et al. 2020). The liquid phase rich in soluble sugars is usually submitted to a detoxification treatment before the subsequent fermentation. The commonly used reagents include organic acids such as acetic acid and inorganic acids like sulphuric and phosphoric acid (Rezania et al. 2020; Chen et al. 2022) The key parameters in acid pretreatment are temperature, residence time, acid concentration and solid loading. Dilute acid pretreatment reduces acid consumption but requires higher temperatures to achieve a reasonable yield of glucose from cellulose (Rezania et al. 2020). LCB pretreatment is usually applied within the acid concentration of 0–5% (w/w) under 120–215 °C for 1–120 min. The overall fermentable sugar and solid recovery can reach 60–75% and 3565%, respectively (Chen et al. 2022). These variations in the recovery of sugars may be related to the different operating conditions and the type of lignocellulosic materials. Gonzales et al. (2016) evaluated the pretreatment of different biomasses (empty palm fruit bunch, rice husk and pine tree wood pellets) with 5% (v/v) dilute sulphuric acid at 121 °C for 30–90 min, and the sugar recovery yield ranged between 39.7 and 60.7%. Moreover, the increase of the reaction time from 30 to 90 min provoked a three- to fourfold increase in the concentration of furfural and 5-hydroxymethylfurfural (HMF). The dilute acid pretreatment has been extensively optimized using experimental designs to find the optimal conditions that lead to a high solubilization of sugars (Gonzales et al. 2016). For example, Martínez-Patiño et al. (2017) reported solubilization of hemicellulosic sugars of 71% under 2.4% H2SO4, 84 min and 130 °C, when the acid pretreatment was applied to olive tree biomass (Martínez-Patiño et al. 2017).
The main advantage of acidic hydrolysis is the high delignification efficiency (>50%) and removal of hemicellulose (Raynie et al. 2020). On the other hand, the equipment corrosion, the high cost of acid recovery and the formation of inhibitors such as furfural and HMF are some of the drawbacks of this process. Using high concentrated acids can also damage the lignin structure. It is worth knowing that acid pretreatment processes have improved in economic and environmental aspects (Raynie et al. 2020; Rezania et al. 2020; Chen et al. 2022).
3.2.1.2 Alkaline Pretreatment
Alkaline hydrolysis involves solvation and saponification reactions achieving the cleave of the ester linkages between the hemicellulose with the lignin and cellulose. This method reduces cellulose crystallinity and dissolves lignin and part of the hemicellulose, increasing the accessibility of enzymes (Raynie et al. 2020). The commonly used reagents are NaOH, KOH, NH3-H2O, Ca(OH)2, etc. (Chen et al. 2022). Ca(OH)2 is a cheap and safe chemical that can be easily recovered and recycled by washing the biomass with water which can then be saturated with carbon dioxide to form a calcium carbonate precipitate. Therefore, it is preferred to select Ca(OH)2 for lignocellulose pretreatment in the industry (Wang 2021).
Alkaline pretreatment enables the extraction of lignin selectively without losing reducing sugar and carbohydrates and removing acetyl groups in hemicellulose structure (Raynie et al. 2020), enhancing porosity and surface area of biomass and therefore improving enzymatic hydrolysis (Rezania et al. 2020). Besides, it can be applied for a wider temperature range achieving versatile performance even at low temperatures (Chen et al. 2022). The main disadvantage of this method is the longer reaction times compared to other pretreatments (Rezania et al. 2020). Alkaline hydrolysis can also reduce delignification efficiency due to the condensation and redistribution of lignin and make cellulose denser and thermodynamically more stable than its native structure (Raynie et al. 2020).
LCB pretreatment is typically applied within alkali concentration of 2–7% and under 100–200 °C for a short contact time (10–90 min), or a concentration range of 0–2% at 50–100 °C for several hours (Chen et al. 2022). For example, when chestnut shells are treated with 7.2% NaOH at 80 °C for 30 min, a delignification of 92.6% was obtained (Morales et al. 2018). In another study, the alkali treatment of barley straw under optimal conditions (2% NaOH, 10 min and 105 °C) led to maximum lignin and hemicellulose removal of 84.8% and 79.5%, respectively (Haque et al. 2012). Gullón et al. (2017) studied a sequential fractionation of vine pruning based on the first stage of autohydrolysis at 201 °C, to recover hemicellulosic sugars, followed by a delignification of the autohydrolysed solid using 2% NaOH, 124 °C and 105 min. These conditions allowed the removal of 67.7% of the lignin, obtaining a solid with 69.4% of glucan (Gullón et al. 2017).
3.2.1.3 Oxidative Pretreatment
Oxidative pretreatment involves different chemical reactions such as electrophilic reaction, site chain dislocation and aliphatic-organic bond cleavages. Some common reagents are solutions of per-acids, e.g. hydrogen peroxide, and ozone (Raynie et al. 2020).
The decomposition of hydrogen peroxide into OH− and O2− contributes to the cleavage of alkyl propylene ether bonds and aromatic nuclei destroying lignin structure without releasing inhibitory by-products. Hydrogen peroxide under alkali conditions is unstable and is decomposed in the presence of transition metals (Chen et al. 2022). Overall, lignin removal rises with increasing H2O2 charge and reaction time (Yuan et al. 2018). For example, when treating wheat straw (previously extracted with NaOH) with H2O2 loads of 20 and 40 mg H2O2/g biomass for 12 h, removal of the residual lignin of about 35.2% and 58.1% was achieved, respectively (Yuan et al. 2018). However, in this same work, the authors reported that when using a high H2O2 charge (80 mg H2O2/g biomass), the increased reaction time from 6 h to 15 h only led to an additional 1.8% lignin removal.
Ozone can selectively remove lignin and decompose hemicellulose by breaking down the hydrogen bonds (Raynie et al. 2020; Chen et al. 2022). Due to ozonolysis being performed at room temperature, this method does not modify cellulose structure and any toxic by-product is released during the process. The major disadvantage of this method is the high ozone requirements, which makes it more expensive than other pretreatments (Raynie et al. 2020). Several authors have stated that ozone dose, treatment time and pH are key parameters that affect lignin removal (Kumar et al. 2020; Osuna-Laveaga et al. 2020; Chen et al. 2022). Thus, Kumar et al. (2020) applied 8.87 g ozone/g to treat wheat straw and observed that when the treatment time is extended from 2 to 6 h, the glucose yield increased five times (Kumar et al. 2020). Osuna-Laveaga et al. (2020) evaluated the effect of ozone dose on enzymatic hydrolysis of sugarcane bagasse and reported the highest glucose yields for the highest ozone dose (45.09% using 100 mg O3/g biomass vs 22.81% using 50 mg O3/g biomass) (Osuna-Laveaga et al. 2020).
3.2.1.4 Organosolv Pretreatment
The organic solvent pretreatment is employed to degrade lignin and hemicellulose and promote enzymatic hydrolysis of cellulose. Organic solvents can break the α-O-aryl bond and 4-O-methyl glucuronic acid ester bonds in lignocellulose (Chen et al. 2022). A wide range of organic solvents has been used for the pretreatment of LCB such as methanol, ethanol, tetrahydrofuran, acetone, ethylene glycol, formaldehyde, dioxane and amines with or without catalyst. Some organic acids and bases are also employed as reagents (Mankar et al. 2021; Chen et al. 2022). The main advantages of this pretreatment are the high efficiency in the fractionation of lignocellulose biomass in cellulose, hemicellulose and lignin with high purity and the easy recovery and reuse of the solvents (Rezania et al. 2020). In some cases, the organic solvents are too expensive, so it is necessary to recover as much as possible, making the process energy-intensive and costly. Besides, the high flammability and volatility of the solvents lead the process to be carried out under controlled conditions (Mankar et al. 2021).
Typically, organosolv pretreatment is performed in the range of 150–220 °C, and below 60 °C may result in lower lignin removal efficiency (Chen et al. 2022).
For instance, Paulownia wood treated with 50% ethanol, at 200 °C for 1 h, resulted in a lignin removal of 65%, and when the treatment time was extended to 4 h, only an increase of 6% in lignin removal was achieved (del Río et al. 2020). Chu et al. (2021) evaluated the effect of the addition of an acid catalyst (H2SO4) in the ethanol organosolv pretreatment of poplar sawdust. The authors found that when acid concentration was raised from 10 to 20 mM, lignin removal was enhanced from 22.23% to 59.87% (Chu et al. 2021). The combination of different organic solvents can improve the lignin removal efficiency. For example, the mixture of n-propylamine (10 mmol/g dry biomass) and 60% ethanol used to treat corn stover (at 140 °C for 40 min) increased lignin removal by 82% compared to single ethanol treatment (Tang et al. 2017).
3.2.1.5 Ammonia Fibre Expansion (AFEX)
The ammonia fibre expansion (AFEX) pretreatment method is similar to that of the steam explosion pretreatment (Rezania et al. 2020). The process involves anhydrous ammonia (1:1) at mild temperatures of 60–170 °C and high pressures between 15 and 30 bar for a short time (5–60 min) (Mankar et al. 2021).
Due to ammonia and oxyhydrogen ions released from liquid ammonia at high pressure, the ester and ether bonds between lignin and hemicellulose are broken (Chen et al. 2022). Besides, hemicellulose is degraded into oligomeric sugar and is deacetylated, while cellulose crystallinity is reduced by the rearrangement of the hydrogen bonding within its fibres (Rezania et al. 2020; Mankar et al. 2021).
The AFEX method achieves higher accessibility of enzymes for hydrolysis with negligible production of inhibitors, mild temperatures, short residence times and high retention of cellulose/hemicellulose content. The main drawbacks are the high capital cost of the equipment to withstand the high pressure involved in the process, the energy requirements for ammonia recycling and the high cost of this reagent (Rezania et al. 2020; Mankar et al. 2021). In addition, it has been reported that the method is less effective for hardwoods and softwoods with high lignin contents (e.g. 25–30%) compared with the high efficiencies obtained with agricultural residues and herbaceous crops (Zhao et al. 2017; Li et al. 2022).
Kamm et al. (2017) used aqueous ammonia (25% w/v) instead of liquid ammonia to test its influence on sugar concentration and its enzymatic yield. They concluded that conversions of more than 90% can be reached in both cases. Despite modified AFEX pretreatment needing higher temperature, inhibitors were neither produced (Kamm et al. 2017). Zhao et al. (2017) proposed hydrogen peroxide presoaking prior to ammonia fibre expansion (H-AFEX) to treat Miscanthus. The authors reported that the addition of 0.5% H2O2 (g/g dry biomass) led to an increase in glucose yield of about 10% (Zhao et al. 2017). Other examples of AFEX pretreatment are listed in Table 5.3.
3.2.2 Pretreatments Without Chemical Reagents
Traditional methods that avoid chemical reagents include those pretreatments that only require water as reaction media, such as hydrothermal, steam explosion and biological pretreatments. Some recent studies are listed in Table 5.3, which also includes examples of AFEX pretreatment to compare it with the steam explosion.
3.2.2.1 Hydrothermal Pretreatment
Hydrothermal pretreatment (also known as liquid hot water, autohydrolysis and hydrothermolysis) involves water with a solid loading rate of 2–30% w/w at temperatures of 160–240 °C and pressures between 6.2 and 33 bar, respectively, during 0–50 min (Rezania et al. 2020; del Río et al. 2022). The high temperatures produce the autoionization of water into hydronium ions (H3O+) that interact with the oxygen of the glycosidic bonds and allow the release of hemicellulosic compounds, such as acetyl groups in the form of acetic acid. In turn, this organic acid acts as a mild catalyst that promotes the solubilization of other hemicellulose-derived compounds (Raynie et al. 2020; del Río et al. 2022).
The high selectivity for hemicellulose solubilization as oligosaccharides, which are high value-added products, can make lignocellulosic biorefineries more competitive (del Río et al. 2022). In addition, this method does not use corrosive, expensive and toxic chemical reagents (Bhatia et al. 2020).
Several research works have highlighted the suitability of autohydrolysis as the first stage of a biorefinery, since it enables the selective solubilization of the hemicellulosic fraction in the liquid phase, remaining the cellulose and lignin almost unaltered in the solid fraction (del Río et al. 2020) In this context, Gullón et al. (2017) studied the non-isothermal autohydrolysis treatment of vine shoots to obtain xylooligosaccharides (XOS). When the operation was performed at 200 °C, 83.1% of the xylan was converted into XOS (corresponding to 12.2 g/L). However, the solid obtained under these conditions presented a low enzymatic susceptibility (obtaining 49.5% of total glucan hydrolysis after 96 h) (Gullón et al. 2017). In another study, the hydrothermal processing at 205 °C of Paulownia wood allowed obtaining a solution with a high concentration of XOS (21.33 g/L) and a solid with good enzymatic susceptibility (with a glucose yield close to 70%) (del Río et al. 2020).
Despite the advantages, some remaining challenges still must be overcome, such as the high energy and water consumption, the low concentration of sugars, the fermentation inhibitors and the lack of literature that collects studies for the co-production of biofuels and value-added compounds within a multiproduct biorefinery approach (Rezania et al. 2020; del Río et al. 2022).
3.2.2.2 Steam Explosion Pretreatment
Steam explosion (SE) is an effective, environmental-friendly and industrially scalable pretreatment method that involves two stages: autohydrolysis and instantaneous decompression stage. As described before, hemicellulose is hydrolysed by acetic acid derived from acetyl groups and other acids during the hydrothermal stage. At the same time, cellulose crystallinity is reduced, and lignin depolymerized. The sudden release of pressure in the second stage destroys the structure of fibrous materials, breaking the glycosidic and hydrogen bonds, thereby modifying the biomass both physically and chemically (Yu et al. 2022a).
Steam explosion can be considered an economical approach, due to the process efficiency and the lack of chemicals required (Yu et al. 2022a). Owing to these advantages, it is one of the most used methods to remove hemicellulose and lignin with efficacy. The incomplete lignin removal and the generation of the inhibitory compound are some drawbacks of this method (Rezania et al. 2020; Chen et al. 2022).
SE pretreatment is applied with hot steam at 180–240 °C and high pressure of 1–3.5 MPa, followed by an explosive decompression to atmospheric pressure. Other key parameters are residence time and the selection of catalysts (Yu et al. 2022a). Recently, Álvarez et al. 2021 applied SE (180 °C for 30 min) to barley straw to obtain XOS, fermentable sugars and lignin (Álvarez et al. 2021). Romero-García et al. (2022) compared autohydrolysis and SE of olive tree pruning biomass, and the results showed that both treatments performed similarly, although the former yielded the highest overall sugar recovery, 92%, at lower operation temperature (180 °C) versus 80.4% for SE at 220 °C (Romero-García et al. 2022).
3.2.2.3 Biological Pretreatment
Biological pretreatment presents various advantages compared with physical and chemical pretreatments such as (1) low operational cost, (2) no need of chemical reagents and (3) lower energy requirements. In addition, another important benefit of biological pretreatment is that compounds that affect subsequent hydrolysis and fermentation are not generated (Sindhu et al. 2016; Chen et al. 2022). However, long incubation times and the loss of carbohydrates are the main disadvantages of this pretreatment (Martínez-Patiño et al. 2018). Biological pretreatment is performed using bacteria, fungi or enzymes to help degrade lignin from LCB (Chen et al. 2022). Fungi such as white-rot fungi, brown-rot fungi and soft-rot fungi are the most effective microorganisms to break down lignin due to the secretion of several oxidative enzymes, namely, lignin peroxidase (LiP), manganese peroxidase (MnP), laccase (Lac) and versatile peroxidase (VP) (Martínez-Patiño et al. 2018). For example, the treatment of poplar wood using white-rot basidiomycete Peniophora incarnata under room temperature for 7 days led to a 70% lignin removal. Enzymatic hydrolysis showed that the maximum yield of glucose reached 33.4% that was improved sevenfold relative to the untreated group (Ma et al. 2021). Martínez-Patiño et al. (2018) screened seven white-rot fungi to treat olive tree biomass. In this study, the authors studied the changes in biomass composition, secretion of ligninolytic enzymes and enzymatic hydrolysis efficiency after 15, 30 and 45 days of solid-state fermentations. The results indicated that the treatment with Irpex lacteus for 45 days improved enzyme susceptibility compared with the non-inoculated sample (31% vs 13.5%) (Martínez-Patiño et al. 2018). Xu et al. (2018) applied Bacillus subtilis to treat corn stalk for 24 h which resulted in a lignin degradation of 23% and cellulose crystallinity decrease of 4.1% (Xu et al. 2018).
3.3 Emerging Methods
In the last decades, greener solvents such as supercritical fluids, ionic liquids and deep eutectic solvents gained interest because they allowed performing under milder conditions, which reduces the release of degradation products and energy consumption, using less toxic and more environmental-friendly chemicals (Raynie et al. 2020; Bhatia et al. 2020). Other emerging methods such as non-thermal plasma (NTP), co-solvent enhanced lignocellulosic fractionation (CELF), microwave and ultrasound pretreatment have been developed recently to decrease the required quantity of harsh chemicals and processing time. Microwave and ultrasound pretreatments are the most extensively studied of these nonconventional methods (Sidana and Yadav 2022).
3.3.1 Microwave-Assisted Heating Pretreatment
Microwave heating pretreatment involves the use of non-ionizing electromagnetic radiations typically in the frequency range of 300 MHz–300 GHz with a wavelength of 1 m to 1 mm. LCB and water absorb microwave radiations which produce the alignment of the molecule dipoles. The realignment of polar molecules generates heat, and its propagation is carried out through two mechanisms: ionic conduction and bipolar rotation (Aguilar-Reynosa et al. 2017; Siddique et al. 2022).
The localized heating produced by microwave irradiation disrupts chemical bonds in the lignocellulosic biomass, depolymerizes lignin and releases the hemicellulose fraction (Sidana and Yadav 2022). LCB usually has a low dielectric constant, which reduces the efficiency of pretreatment. However, the microwave absorption capacity can be enhanced by the presence of moisture and inorganic components (Sidana and Yadav 2022).
The main advantage of the method is the reduction of the reaction times to ten times less in comparison with other heating systems, thus decreasing the energy consumption. Due to the short reaction times, the amount of side products is also reduced. Besides, the heat loss is reduced with the use of non-conductor vessels that allows the passage of microwaves without being heated. The main drawback is the formation of hot spots due to the nonhomogeneous properties of the LCB. Besides, some materials have low-energy absorption due to their dielectric properties, and in some cases, these properties can change with temperature (Aguilar-Reynosa et al. 2017).
Microwave-assisted heating is usually performed at 70–230 °C for 5–120 min with microwaves of 2450 MHz and power ranging from 250 to 1000 W (Sidana and Yadav 2022).
Due to the advantages of microwave heating, it has been used to obtain oligosaccharides through hydrothermal treatment from several LCBs. For example, Mihiretu et al. (2017) applied this technology to extract xylan from aspenwood and sugarcane trash with maximal yields of 66% and 50%, respectively (Mihiretu et al. 2017). Using microwave-assisted hydrothermal treatment at 200 °C, Luo et al. (2017) reported a high removal of hemicellulose (more than 95%) from bamboo (Luo et al. 2017). Dávila et al. (2021) solubilized hemicelluloses from vine shoots, under microwave irradiation to evaluate the impact of temperature and time on the production of oligosaccharides. In this same work, the authors compared the extraction of oligosaccharides using autohydrolysis assisted by microwave and by conventional heating. The authors found that operating under conditions that maximize the production of oligosaccharides, the microwave process enabled reducing both extraction time and energy consumption by 2.6 and 3.5 times, respectively, compared to the conventional treatment (Dávila et al. 2021).
3.3.2 Ultrasonic-Assisted Heating Pretreatment
Ultrasounds are acoustic waves with frequencies over the hearing range (>20 kHz). Their propagation through low-pressure areas in the medium produces minute gas or vapour bubbles that gradually increase in size until implosion, giving rise to a phenomenon called acoustic cavitation. The implosion of the cavitation bubbles releases a large amount of energy, creating local hot spots with temperatures of 2000–5000 K and pressures up to 1800 atm (Mankar et al. 2021; Sidana and Yadav 2022).
Ultrasonic pretreatment causes physical and chemical changes in the lignocellulosic biomass through thermal effects and shearing forcers developed over implosion, but also due to the oxidative radicals produced for the decomposition of the water molecules. The rupture of hydrogen bonds reduces the cellulose crystallinity. Besides, the ultrasonic method breaks α-O-4 and β-O-4 ether linkages in lignin and increases hemicelluloses solubility (Mankar et al. 2021; Sidana and Yadav 2022).
One of the main advantages, as in the case of microwave-assisted pretreatment, is the reduction of hydrolysis time compared with the conventional hydrothermal method. This advantage, along with low instrumental requirements, high efficiency and reliable repeatability, can make this process more cost-effective (de Carvalho Silvello et al. 2019). Nonetheless, Bundhoo and Mohee (2018) concluded that sonication is energetically inefficient based on the lab-scale studies reviewed. Besides, more studies on pretreatment condition optimization, scale-up and economic and environmental sustainability analysis are needed (Bundhoo and Mohee 2018; Sidana and Yadav 2022).
The pretreatment efficiency does not increase with frequencies greater than 100 kHz. Typically, the sonication method is conducted at 20–80 kHz for 20–150 min (Chen et al. 2022). Other relevant parameters are biomass composition, particle size and reaction configuration geometry (Mankar et al. 2021). Ultrasonic irradiation is usually combined with chemical (acid, alkali, ionic liquid, deep eutectic solvents) or physical methods, achieving higher efficiencies (Sidana and Yadav 2022).
Several works have evaluated the optimization of ultrasonic pretreatment of various biomass feedstock. In this line, Onu Olughu et al. (2021) examined the effect of the operational variables (acoustic power, solid-solvent ratio, hammer mill screen size and sonication time) on the delignification yield. The results revealed that the sonication time was the factor that had the greatest impact on delignification followed by acoustic power. Under optimized extraction conditions (50 min, 1/25 g/mL, 180 W, 3.2 mm), UAE led to a delignification yield of 20.11% (Onu Olughu et al. 2021).
The potential of ultrasonic treatment has also been used to improve the performance in enzymatic saccharification of LCB. For example, de Carvalho Silvello et al. (2019) demonstrated that the application of ultrasonic waves during the enzymatic hydrolysis of sugarcane bagasse led to an 89.37% higher concentration of reducing sugars in comparison to the unsonicated biomass (de Carvalho Silvello et al. 2019).
Table 5.4 gathers selected recent studies of microwave- and ultrasonic-assisted pretreatment of lignocellulosic biomass.
3.3.3 Green Solvents
3.3.3.1 Supercritical Fluids (SCFs)
Supercritical fluids (SCFs) are substances at temperatures and pressures above their vapour-liquid critical point, defined as the endpoint of the liquid-vapour equilibrium curve. The substances exhibit an intermediate behaviour between liquid and gases, presenting high diffusivity and density, and low viscosity at these conditions. These properties allow these nontoxic and relatively low-cost solvents to penetrate the solid material easily due to faster mass transfer (Raynie et al. 2020; Bhatia et al. 2020; Mankar et al. 2021).
Supercritical carbon dioxide (SC-CO2) is the most widely used supercritical fluid due to its lower critical temperature and pressure (31.1 °C, 73.6 bar) compared with other compounds such as water (374.2 °C, 221.2 bar), ammonia (132.3 °C, 112.8 bar) and methanol (240 °C, 79.6 bar) (Bhatia et al. 2020; Chen et al. 2022). SC-CO2 reacts with water present in biomass, forming a carbonic acid which works as a catalyst and increases the hydrolysis rate of the lignocellulosic biomass. A higher moisture content improves the reaction rate (Raynie et al. 2020; Mankar et al. 2021). Besides, high pressure and temperature enhance the interaction between the supercritical fluid and lignocellulose because the pore size and the surface area get expanded (Badgujar et al. 2021; Chen et al. 2022).
SC-CO2 is considered a clean and green solvent. It is nonflammable, nontoxic and non-reactive in nature. The pretreatment is performed under mild conditions, usually below 30 MPa and 200 °C; thus, it does not cause the disintegration of biomass components. It is not necessary a detoxification step before fermentation because SC-CO2 can be easily separated via depressurization. Furthermore, carbon dioxide liberated during fermentations can be used for pretreatment (Raynie et al. 2020; Badgujar et al. 2021). It has been reported that SC-CO2 pretreatment at 20–30 MPa for 6–72 h could improve sugar production by 45.4–101.45% (Chen et al. 2022).
The main drawback of supercritical fluid pretreatment is the lack of process evaluation at an industrial scale which needs further investigation. Supercritical fluids have been well studied at a large scale for biomass-derived compound extraction, but there are very few research articles on the scale-up of biomass pretreatment method (Badgujar et al. 2021).
Putrino et al. (2020) evaluated SC-CO2 pretreatment in different contact time conditions (3 and 5 h) and the addition of various polarity modifiers (NaOH, NaHSO4 and ethanol) to enhance enzymatic hydrolysis of cellulose present in coconut fibres. The authors concluded that the method caused changes in the morphology of the raw material, increasing porosity, and reducing the phenolic compound content. However, there was no significant increase in sugar yield after enzymatic hydrolysis because there is hardly any reduction of the lignin content (Putrino et al. 2020).
Takada et al. (2021) studied the topochemistry of the delignification of the Fagus crenata wood using supercritical methanol treatment (270 °C, 27 MPa). After 30 min, more than 70% of the lignin was eluted. The authors observed that the lignin in the secondary wall was easily decomposed and removed, while the middle lamella lignin was initially resistant to the pretreatment method (Takada et al. 2021).
Several studies have evaluated the combination of supercritical fluids with co-solvents and other pretreatment methods. In this line, Silveira et al. (2015) achieve a 41.9% delignification, high carbohydrate recoveries and high enzymatic digestibility by combining SC-CO2 with the ionic liquid 1-butyl-3-methylimidazolium acetate and ethanol as co-solvents to pretreat sugarcane bagasse (Silveira et al. 2015).
3.3.3.2 Ionic Liquids (ILs)
Ionic liquids (ILs) are molten salts with a melting point lower than 100 °C, usually composed of organic cations, and organic or inorganic anions (Usmani et al. 2020; Chen et al. 2022). ILs can be classified depending on the cation, being the most used for lignocellulosic biomass pretreatment imidazolium-based ([(C3N2)Xn]+), but also ammonium-based ([NX4]+), pyrrolidinium-based ([(C4N)Xn]+), pyridinium-based ([C5N)Xn]+), phosphonium-based ([PX4]+), sulphonium-based ([SX3]+) and choline-based have been used widely for this purpose. The most commonly used anions are chloride and acetate (Rezania et al. 2020; Usmani et al. 2020; Chen et al. 2022).
Several semi-empirical and empirical parameters have been used to predict and simulate the solubility of biomass and other biomolecules in ionic liquids. COSMO-RS and the Kamlet-Taft parameters describe polarity, while Hansen parameters quantify the solubility (Usmani et al. 2020). The cellulose dissolving capability can be attributed to the affinity of the anion to hydrogen bonds, which promotes the formation of an electron donor-electron acceptor complex between cellulose and the ionic liquid. On the other hand, the lignin solubility in ILs is attached to the interactions between the aromatic components of lignin and the cations (Usmani et al. 2020; Chen et al. 2022).
ILs are considered a green alternative to volatile organic solvents because they are easy to obtain, do not form hazardous chemicals, selectively remove lignin and hemicelluloses and can be operated in continuous mode with high biomass input (Bhatia et al. 2020; Chen et al. 2022). ILs also have intrinsic and interesting properties such as nonflammability, wide electrochemical windows, broad liquid regions, low vapour pressure, high viscosity, low conductivity and high thermal and chemical stability. The tuneable nature of ILs allows for the design of a solvent with specific properties to perform the required extraction selectivity and capacity (Usmani et al. 2020).
The main drawbacks are related to the recovery and reuse of ILs which is crucial due to the high cost of the solvents (Bhatia et al. 2020). Desirable properties in a solvent such as low vapour pressure, high viscosity and high dissolution capacity for polar molecules become a challenge in its recovery. Distillation is not, in general, an adequate method to remove impurities from ILs, and many polar compounds such as water, inorganic salts or acids tend to accumulate in the solvent. The solutions become viscous and difficult to handle post cellulose extraction, making it challenging to recover hemicellulose and lignin. Besides, some ILs are toxic to microorganisms and enzymes (Usmani et al. 2020).
In the last decade, the solvent and catalytic capacities of ILs for biomass treatment have also attracted much interest. In this context, Asim et al. (2021) using an ionic liquid-based on [PyH] HSO4·(H2SO4)3 under mild conditions (60 °C, 2 h) reported a high delignification (79%) and lignin recovery (77%) from wheat straw (Asim et al. 2021). In this same line, Portela-Grandío et al. (2021) proposed an organosolv process catalysed with 1-butyl-3-methylimidazolium hydrosulphate for the fractionation of invasive species such as Acacia dealbata wood. The authors found that under optimized conditions (190 °C, 60% ethanol, 60 min of reaction time and 0.6 g 1-butyl-3-methylimidazolium hydrosulphate/g wood), this treatment led to high solubilization of lignin and hemicelluloses and cellulose recovery (87.5%, 88.7% and 88.3%, respectively) (Portela-Grandío et al. 2021).
3.3.3.3 Deep Eutectic Solvents (DESs)
Deep eutectic solvents (DESs) are liquid eutectic mixtures composed of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) at specific molar ratios, with freezing points lower than those of the individual components. They have favourable features similar to ionic liquids, such as low vapour pressure, high thermal stability and tunable physicochemical properties. Moreover, compared with traditional solvents and ionic liquids, DESs are less toxic, more biodegradable, more compatible with enzymes and microorganisms and more cost-effective in the synthesis process than ILs (Chen et al. 2020, 2022; Wang and Lee 2021).
Chlorine-chloride (ChCl) is the most used HBA for biomass pretreatment for its low cost (65 US$/kg) and safe and healthy nature, and it can be derived from biomass (Xu et al. 2020b; Wang and Lee 2021). Other quaternary ammonium salts such as betaine, benzyltrimethylammonium chloride (BTMAC) and triethyl benzyl ammonium chloride (TEBAC) are also used as HBAs in DES (Chen et al. 2020).
DESs can be classified into four groups according to the functional group of HBDs carboxyl acid-based DESs, amine-/amide-based DESs, polyalcohol-/carbohydrate-based DESs and phenolic compound-based DESs (Zhou et al. 2022). Lactic acid (LA), formic acid (FA) and oxalic acid (OA) are some biomass-derived carboxylic acids that have been used as HBDs in DES synthesis. The acid strength, amount of carboxyl groups and molar ratio are closely related to lignin extraction efficiency. The presence of active protons promotes proton-catalysed cleavage of various bonds in biomass (Chen et al. 2020; Zhou et al. 2022).
Amide-based (e.g. urea), amine-based (e.g. monoethanolamine) and imidazole-based compounds are used to synthesize amine-/amide-based DESs. This kind of DESs exhibits alkaline behaviour, which facilitates the selective lignin extraction through deprotonation of phenolic hydroxyl groups in lignin. Polyalcohol-/carbohydrate-based DESs contain chemicals with hydroxyl groups such as polyalcohols (e.g. glycerol (Gly) and ethylene glycol (EG)) and carbohydrates (e.g. glucose) and usually exhibit neutral or near-neutral pH conditions. Polyalcohol-based DESs have been widely used for lignocellulosic pretreatment, being ChCl/Gly the most popular as Gly is a low-cost by-product generated in the biodiesel industry, and high delignification (60–90%) has been reported by several studies. On the other hand, carbohydrate-based DESs generally have a low capability of lignin extraction, being more used as solvents in chemical reactions rather than for biomass pretreatment. More recently, lignin-derived compounds, such as vanillin, p-coumaric acid (PCA), catechol, p-hydroxybenzyl alcohol (PBA), p-hydroxybenzoic acid (PB) or p-hydroxybenzaldehyde (PHA), have been used in phenolic-based DESs synthesis, which can aid in the development of a closed-loop biorefinery (Chen et al. 2020; Zhou et al. 2022).
As in the case of ILs (see Sect. 5.3.3.3.2), the Kamlet-Taft parameters are used as a quantitative tool to describe the solvatochromic properties of DESs, thus reducing the efforts to select the appropriate DES for treatment (Wang and Lee 2021; Zhou et al. 2022). DES-based pretreatment can be enhanced manifold by applying microwaves and ultrasound, and also catalysts and co-solvents such as water, acids and metal salts have been tested to boost the performance of lignin extraction. Even though DES solvents are expected to become one of the most popular biomass pretreatments in the future, a deeper understanding of the interactions of DESs with the different biomass fractions is still missing (Raynie et al. 2020; Mankar et al. 2021; Zhou et al. 2022). Besides, further studies are needed to develop effective DESs recycling technologies and understand the properties of different DES lignin to broaden their applications (Chen et al. 2020).
Zhao et al. (2018) studied a series of three ethanolamine-based DESs and three amide- or glycerol-based DESs to treat wheat straw. The authors evaluated the influence of different parameters on the delignification performance such as properties of DESs (pH and viscosity), pretreatment temperature (50, 70, 90, 110, 130 °C) and pretreatment time (1, 6, 9, 12, 24 h). Among all tested DESs, the choline chloride/monoethanolamine (ChCl/MEA (1:6)) system using a liquid/solid ratio of 20:1 during 9 h and 70 °C showed the highest lignin removal (71.4%) while preserving 93.7% of the cellulose. In addition, this solid exhibited good enzymatic digestibility with cellulose and xylan conversion of 89.8% and 62%, respectively (Zhao et al. 2018).
Another work conducted by Mankar et al. (2022) evaluated the ability of four ChCl-carboxylic acid-based eutectic mixtures to be applied combined with microwave-assisted technology for the extraction of lignin from coconut coir. The authors also compared the effect of different heating types (microwave and conventional heating) on the lignin removal efficiency. Under optimum conditions (150 °C for 20 min using ChCl/LA (1:4)), the lignin yield was six times higher using microwave irradiation compared to conventional heating (82% vs 13.5%) (Mankar et al. 2022).
Table 5.5 shows recent studies on supercritical fluids (SCFs), ILs and DESs pretreatment of lignocellulosic pretreatment.
3.3.4 Other Emerging Methods
Several advanced treatment methods used in food industries for improving the nutritional quality and shelf-life of ready-to-eat food products have been gaining interest as biomass pretreatment techniques over the last decade. These nonconventional methods have been proved successful at a laboratory scale, but they are in an early research state. Nonthermal plasma (NTP), hydrodynamic cavitation, high hydrostatic pressure homogenization, electron beam irradiation and gamma irradiation are some of the pretreatments discussed by Sidana and Yadav (2022).
Co-solvent enhanced lignocellulosic fractionation (CELF) technique is another novel method where a mixture of tetrahydrofuran (THF) and water is used as a monophasic solvent to simultaneously extract lignin from biomass and transform glucan-rich solid residue into various fuel precursors, 5-hydroxymethyl furfural, furfural and levulinic acids in high yields (Bhatia et al. 2020; Mankar et al. 2021).
4 Lignocellulosic Biomass to Value-Added Biochemicals
In recent years, the investigation of the furanic-aliphatic family has substantially increased, such as versatile building blocks derived from renewable resources. For the development of bio-based platform chemicals, simple sugars present in the lignocellulosic materials can be converted by chemical or biochemical processes into value-added building blocks, which contributes to reaching the desired bio-based economy. In this context, the most relevant marketable bio-based chemicals, namely, hydroxymethylfurfural (HMF), 2,5-furandicarboxylic acid (FDCA) and levulinic acid that can be obtained in a biorefinery scheme, are approached in this chapter.
4.1 Hydroxymethylfurfural (HMF)
Hydroxymethylfurfural or 5-hydroxymethylfurfural (HMF) containing a hydroxymethyl group (an aldehyde group and a furan ring) is one of these platform chemicals. This versatile chemical structure allows its transformation into higher-value derivative compounds (such as levulinic acid, 2,5-dimethylfuran, ɣ-valerolactone, 5-hydroxymethyl-2-furan carboxylic acid, 2,5-diformylfuran, 5-formylfuran carboxylic acid and 2,5-furandicarboxylic acid), with applications in different sectors such as plastic, pharmaceutical, fuels, fragrance and textile industries. For this reason, HMF was identified by the US Department of Energy as one of the most promising bio-derived molecules from lignocellulosic biomass. Accordingly, the global market of HMF is expected to reach $61 million by 2024 (Market Study Report 2019).
HMF is produced by acid dehydration of hexoses or their corresponding polysaccharides obtained from lignocellulosic biomass. Its efficient large-scale production requires deep understanding of dehydration mechanisms using the adequate catalyst and the optimization of the process. Among several processes used for their production, the use of liquid ionic and biphasic systems has been extensively explored (Naz et al. 2021; Yousatit et al. 2022) due to the interesting yields reported (Sousa et al. 2015). More recently, emerging green solvents such as DESs for the sustainable production of HMF have been also considered, taking into account their inherent advantages as solvents (Zuo et al. 2021). Generally, the main strategies used for the HMF production are (1) the heterogonous acid catalysis using catalysts such as zeolites and polymeric resins and (2) homogenous catalysis catalysed by the ionic liquid and organic or mineral acids. HMF production involves a wide range of conditions that can be evaluated (such as extractive solvent, reactive phase and/or water/organic media). The scientific community has devoted huge efforts to develop more efficient and sustainable processes for HMF production. Among several strategies, the use of microwave technology as an alternative to conventional heating systems allows employing lower reaction times and obtaining higher yields. The use of biphasic systems employing soluble solvents (such as dimethyl sulfoxide (DMSO), acetone or poly(ethylene) glycol (PEG)) reduces undesired hydration reactions of HMF compared to aqueous media. In addition, the catalytic process can be combined with an enzymatic reaction in order to improve HMF yields. For instance, immobilized glucose isomerase enzymes can be used for the isomerization of glucose into fructose, being subsequently dehydrated in HMF (Alipour 2016). On the other hand, high-pressure CO2 has been also evaluated as a low-cost alternative to acid catalysts. One of the main challenges for the advance in the HMF production includes the direct transformation from lignocellulosic biomass within a one-pot approach (Zhao et al. 2011; Xu et al. 2020a).
4.2 2,5-Furandicarboxylic Acid (FDCA)
Particularly, 2,5-furandicarboxylic acid (FDCA), obtained by complete oxidation of HMF, has also been identified as one of the top 12 high-potential bio-based products to be obtained from biomass (Bozell and Petersen 2010), and its worldwide market is expected to reach $850 million by 2025 (Acumen Research and Consulting 2022). Its major industrial use is as a substitute for terephthalic acid (TPA), which can be used to synthesize several polyesters such as polyethylene furanoate (PEF). PEF is obtained by the polymerization of FDCA with ethylene glycol to replace the petroleum-derived polyethylene terephthalate plastic (PET) (Gubbels et al. 2013; Motagamwala et al. 2018). The European Union has funded a consortium of 11 companies, known as “PEFerence”, in order to develop an innovative production process for FDCA and PEF (https://peference.eu/). FDCA may also be employed as a building block in the production of medicines, plasticizers, thermosets, coatings and polyamides (Hu et al. 2018).
Large-scale production of FDCA requires two key steps: (1) dehydration of hexoses (such as fructose, glucose and C6 polysaccharides/cellulose) to obtain HMF and (2) further catalytic oxidation to produce FDCA (by chemical catalysis, electrocatalysis or enzymatic catalysis). Most studies employ chemical catalysis using a methodology based on a Pt/C catalyst for efficient oxidation. High FDCA yields have been also reported using several types of nanoparticles as catalysts in oxidation conditions (>90%) (Siankevich et al. 2014). Direct conversion of fructose into FDCA was also obtained using a triphasic system achieving a global yield of 78% (Yi et al. 2015). Nevertheless, this typical chemical route implies the generation of by-products, impurities, wastes and the need for downstream processing, which compromises the ecological footprint. In this sense, biocatalysis using enzymes for bioconversion of HMF into FDCA shows several advantages as an alternative route, namely, the mild reaction conditions and the high selectivity of the enzymes (Domínguez de María and Guajardo 2017).
The enzymatic oxidation of HMF includes three consecutive oxidation steps. Some authors have proposed the combination of enzymes with chemocatalysis (Krystof et al. 2013; Qin et al. 2015). Moreover, this biocatalytic reaction can be carried out using whole cells, which entails several advantages such as no need of cofactor regeneration and no tedious purification of enzymes. The first whole-cell biocatalyst reported for FDCA production using HMF as substrate was using a Pseudomonas putida S12 strain, which was modified to express the hmfH gene from Cupriavidus basilensis (encoding an HMF/furfural oxidoreductase) (Koopman et al. 2010).
Alternatively, furfural was also employed as a more sustainable and economical substrate for FDCA production. Furfural can be industrially obtained by acid-catalysed thermohydrolysis of the hemicellulose process. The biocatalytic conversion was also reported using a recombinant Escherichia coli expressing two enzymes (oxidase and carboxylase) to convert furfural into 2-furoic acid and subsequently furoic acid into FDCA, respectively (Kawanabe et al. 2021). The increase of industrial patent application shows the interest in this approach for FDCA production (de Bont et al. 2018). Nevertheless, higher productivities should be obtained to validate this strategy at an industrial level.
4.3 Levulinic Acid (LVA)
Levulinic acid (LVA), also known as 4-oxopentanoic acid, is a low molecular weight carboxylic acid also included in the top 12 bio-based platform chemicals due to a wide range of applications, namely, personal care, adsorbents, lubricants, drug delivery and as a precursor for the production of biofuels, chemicals and polymers. The global market size for LVA is projected to achieve US $5.02 billion by 2028 (Grand View Research 2021). LVA is produced from HMF by acid degradation of cellulose in an aqueous medium and subsequent rehydration of HMF in an acidic medium. In this reaction, formic acid is also formed in equimolar quantities, which is also beneficial since formic acid can be used as plasticizers, rubbers, formaldehyde, textiles and drugs. Furfural has been also employed for LVA conversion via hydrogenation to furfuryl alcohol and subsequent ethanolysis (Dutta and Bhat 2021). LVA production by desirable one-pot conversion has been reported using simple sugars such as glucose and polysaccharides such as cellulose, as well as directly from biomass (Mukherjee et al. 2015; Morone et al. 2015). Reaction conditions for LVA production are harsher than for cellulose or HMF conversion. Generally, low LVA yields are related to side reactions that are derived in the formation of dark and coloured solid denominated humins.
Hemicellulose containing pentoses and hexoses (as oligomer and/or monomer) obtained by hydrothermal treatment of Pinus pinaster was used for LVA production by homogenous catalysis with sulphuric acid, yielding 66% of LVA of the stoichiometric value (Rivas et al. 2013). As an alternative to mineral acids, heterogeneous catalysts have also been evaluated for LVA production (Sajid et al. 2021). Similar to HMF conversion, the selectivity and yield of LVA are strongly dependent on acid strength. Temperatures in the range of 80–200 °C for 0.5–24 h have been evaluated for glucose conversion to LVA, obtaining yields varying 20–79% using catalysts such as HCl, FeCl3 or Amberlite IR-120 (Sajid et al. 2021). For LVA production from xylose, temperatures in the range of 120–200 °C for 0.5–6 h were employed using solvents such as water-acetone, water or water-methyl ethyl ketone catalysed with Cu-NbP or H2SO4 (Sajid et al. 2021).
Despite the high value of LVA and the possibility of its production from renewable resources such as lignocellulosic biomass, the research focused on the development of more stable catalysts using non-metals with synthetic polymers is still necessary. In this sense, the pretreatment that allows a suitable fractionation of lignocellulosic biomass is mandatory for its utilization.
5 Conclusion
The complexity and recalcitrance of LCB, composed of a blend of cellulose fibres coated by hemicelluloses and lignin at different proportions depending on the species, do not allow a straightforward valorization to produce biofuels and/or green platform chemicals not dependent on fossil resources. Hence, pretreatments are needed to promote the separation of the main constituents of LCB in separated streams, providing a more efficient valorization. Traditional pretreatments, including the use of different chemicals such as acids, alkalis, oxidizing agents and organic solvents, or even those pretreatments avoiding the use of reagents, such as steam explosion, autohydrolysis or biological treatments, have shown certain limitations, hampering full-fledged commercial adaptation of such traditional processes. In the last years, alternative green and sustainable pretreatment technologies have been developed, aiming to overcome the bottlenecks of traditional technologies. Microwave- and ultrasonication-assisted technologies have shown high effectivity on a variety of lignocellulosic biomass. On the other hand, green solvents, e.g. supercritical fluid, ionic liquid and deep eutectic solvent (DES)-based pretreatment techniques, have already shown great potential in the pretreatment of different LCB.
The transformation of biomass feedstock into 5-HMF, FDCA and LA is of particular interest. These compounds, included in the DOE Platform Chemical List, show strong potential as platform chemicals may serve as a primary building block of the biorefinery and can provide direct substitutes for existing petrochemicals. Although there have been remarkable advances in the last years, these processes are still emerging for fulfilling industrial needs. Some remaining challenges still have to be overcome in bio-based products from biorefinery carbohydrates.
Abbreviations
- AFEX:
-
Ammonia fibre expansion
- BTMAC:
-
Benzyltrimethylammonium
- CELF:
-
Co-solvent enhanced lignocellulosic fractionation
- ChCl:
-
Choline chloride
- COSMO-RS:
-
Conductor-like screening model for real solvents
- DES:
-
Deep eutectic solvent
- DMSO:
-
Dimethyl sulfoxide
- EG:
-
Ethylene glycol
- FA:
-
Formic acid
- FDCA:
-
Furandicarboxylic acid
- GHG:
-
Greenhouse gases
- Gly:
-
Glycerol
- HBA:
-
Hydrogen bond acceptors
- HBD:
-
Hydrogen bond donors
- HMF:
-
Hydroxymethylfurfural
- IL:
-
Ionic liquid
- LA:
-
Lactic acid
- Lac:
-
Laccase
- LCB:
-
Lignocellulosic biomass
- LiP:
-
Lignin peroxidase
- LVA:
-
Levulinic acid
- MEA:
-
Monoethanolamine
- MnP:
-
Manganese peroxidase
- NTP:
-
Nonthermal plasma
- OA:
-
Oxalic acid
- PB:
-
p-Hydroxybenzoic acid
- PBA:
-
p-Hydroxybenzyl alcohol
- PCA:
-
p-Coumaric acid
- PEG:
-
Poly(ethylene) glycol
- PET:
-
Polyethylene terephthalate
- PHA:
-
p-Hydroxybenzaldehyde
- SCFs:
-
Supercritical fluids
- SE:
-
Steam explosion
- TEBAC:
-
Triethyl benzyl ammonium
- XOS:
-
Xylooligosaccharides
References
Acumen Research and Consulting (2022) 2,5-Furandicarboxylic acid (FDCA) market surpass $850 million by 2025 | CAGR 11%. https://www.acumenresearchandconsulting.com/press-releases/2-5-furandicarboxylic-acid-fdca-market. Accessed 23 Feb 2022
Aguilar-Reynosa A, Romaní A, Ma. Rodríguez-Jasso R et al (2017) Microwave heating processing as alternative of pretreatment in second-generation biorefinery: an overview. Energy Convers Manag 136:50–65. https://doi.org/10.1016/j.enconman.2017.01.004
Alipour S (2016) High yield 5-(hydroxymethyl)furfural production from biomass sugars under facile reaction conditions: a hybrid enzyme- and chemo-catalytic technology. Green Chem 18:4990–4998. https://doi.org/10.1039/C6GC00749J
Almeida RO, Moreira A, Moreira D et al (2022) High-performance delignification of invasive tree species wood with ionic liquid and deep eutectic solvent for the production of cellulose-based polyelectrolytes. RSC Adv 12:3979–3989. https://doi.org/10.1039/D1RA08410K
Álvarez C, González A, Ballesteros I, Negro MJ (2021) Production of xylooligosaccharides, bioethanol, and lignin from structural components of barley straw pretreated with a steam explosion. Bioresour Technol 342:125953. https://doi.org/10.1016/j.biortech.2021.125953
Ashokkumar V, Venkatkarthick R, Jayashree S et al (2022) Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—a critical review. Bioresour Technol 344:126195. https://doi.org/10.1016/j.biortech.2021.126195
Asim AM, Uroos M, Naz S, Muhammad N (2021) Pyridinium protic ionic liquids: effective solvents for delignification of wheat straw. J Mol Liq 325. https://doi.org/10.1016/j.molliq.2020.115013
Badgujar KC, Dange R, Bhanage BM (2021) Recent advances of use of the supercritical carbon dioxide for the biomass pre-treatment and extraction: a mini-review. J Indian Chem Soc 98:100018. https://doi.org/10.1016/j.jics.2021.100018
Bai Y, Zhang XF, Wang Z et al (2022) Deep eutectic solvent with bifunctional Brønsted-Lewis acids for highly efficient lignocellulose fractionation. Bioresour Technol 347:126723. https://doi.org/10.1016/j.biortech.2022.126723
Banerji A, Balakrishnan M, Kishore VVN (2013) Low severity dilute-acid hydrolysis of sweet sorghum bagasse. Appl Energy 104:197–206. https://doi.org/10.1016/j.apenergy.2012.11.012
Barakat A, de Vries H, Rouau X (2013) Dry fractionation process as an important step in current and future lignocellulose biorefineries: a review. Bioresour Technol 134:362–373. https://doi.org/10.1016/j.biortech.2013.01.169
Batista Meneses D, Montes de Oca-Vásquez G, Vega-Baudrit JR et al (2022) Pretreatment methods of lignocellulosic wastes into value-added products: recent advances and possibilities. Biomass Convers Biorefin 12:547–564. https://doi.org/10.1007/s13399-020-00722-0
Besserer A, Obame SN, Safou-Tchima R et al (2022) Biorefining of Aucoumea klaineana wood: Impact of steam explosion on the composition and ultrastructure the cell wall. Ind Crop Prod 177:114432. https://doi.org/10.1016/j.indcrop.2021.114432
Bhatia SK, Jagtap SS, Bedekar AA et al (2020) Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Bioresour Technol 300:122724. https://doi.org/10.1016/j.biortech.2019.122724
Bhatia R, Lad JB, Bosch M et al (2021) Production of oligosaccharides and biofuels from Miscanthus using combinatorial steam explosion and ionic liquid pretreatment. Bioresour Technol 323:124625. https://doi.org/10.1016/j.biortech.2020.124625
Borrega M, Nieminen K, Sixta H (2011) Degradation kinetics of the main carbohydrates in birch wood during hot water extraction in a batch reactor at elevated temperatures. Bioresour Technol 102:10724–10732. https://doi.org/10.1016/j.biortech.2011.09.027
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 12:539. https://doi.org/10.1039/b922014c
Bundhoo ZMA, Mohee R (2018) Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: a review. Ultrason Sonochem 40:298–313. https://doi.org/10.1016/j.ultsonch.2017.07.025
Cai J, He Y, Yu X et al (2017) Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sustain Energy Rev 76:309–322. https://doi.org/10.1016/j.rser.2017.03.072
Caparrós S, Garrote G, Ariza J et al (2007) Xylooligosaccharides production from Arundo donax. J Agric Food Chem 55:5536–5543. https://doi.org/10.1021/jf063159p
Chen Z, Wan C (2018) Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour Technol 250:532–537. https://doi.org/10.1016/j.biortech.2017.11.066
Chen Z, Ragauskas A, Wan C (2020) Lignin extraction and upgrading using deep eutectic solvents. Ind Crop Prod 147. https://doi.org/10.1016/j.indcrop.2020.112241
Chen C, Zhao L, Sun ZF et al (2022) Advances in pretreatment of lignocellulosic biomass for bioenergy production: challenges and perspectives. Bioresour Technol 343:126123. https://doi.org/10.1016/j.biortech.2021.126123
Cho EJ, Trinh LTP, Song Y et al (2020) Bioconversion of biomass waste into high value chemicals. Bioresour Technol 298:122386. https://doi.org/10.1016/j.biortech.2019.122386
Chourasia VR, Pandey A, Pant KK, Henry RJ (2021) Improving enzymatic digestibility of sugarcane bagasse from different varieties of sugarcane using deep eutectic solvent pretreatment. Bioresour Technol 337. https://doi.org/10.1016/j.biortech.2021.125480
Chu Q, Tong W, Chen J et al (2021) Organosolv pretreatment assisted by carbocation scavenger to mitigate surface barrier effect of lignin for improving biomass saccharification and utilization. Biotechnol Biofuels 14:136. https://doi.org/10.1186/s13068-021-01988-w
Dai L, Gu Y, Xu J et al (2022) Toward green production of xylooligosaccharides and glucose from sorghum straw biowaste by sequential acidic and enzymatic hydrolysis. Ind Crop Prod 179:114662. https://doi.org/10.1016/j.indcrop.2022.114662
Dalena F, Senatore A, Iulianelli A, et al (2018) Ethanol from biomass: future and perspectives. In: Ethanol: science and engineering. Elsevier Inc., Amsterdam
Davidson MG, Elgie S, Parsons S, Young TJ (2021) Production of HMF, FDCA and their derived products: a review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem 23:3154–3171. https://doi.org/10.1039/D1GC00721A
Dávila I, Gullón P, Labidi J (2021) Influence of the heating mechanism during the aqueous processing of vine shoots for the obtaining of hemicellulosic oligosaccharides. Waste Manag 120:146–155. https://doi.org/10.1016/j.wasman.2020.11.014
Daza Serna LV, Orrego Alzate CE, Cardona Alzate CA (2016) Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour Technol 199:113–120. https://doi.org/10.1016/j.biortech.2015.09.078
de Bont JAM, Ruijssenaars HJ, Werij J (2018) FDCA-decarboxylating monooxygenase-deficient host cells for producing FDCA
de Carvalho Silvello MA, Martínez J, Goldbeck R (2019) Increase of reducing sugars release by enzymatic hydrolysis of sugarcane bagasse intensified by ultrasonic treatment. Biomass Bioenergy 122:481–489. https://doi.org/10.1016/j.biombioe.2019.01.032
del Río PG, Domínguez VD, Domínguez E et al (2020) Comparative study of biorefinery processes for the valorization of fast-growing Paulownia wood. Bioresour Technol 314:123722. https://doi.org/10.1016/j.biortech.2020.123722
del Río PG, Gullón B, Romaní A, Garrote G (2021) Fast-growing Paulownia wood fractionation by microwave-assisted hydrothermal treatment: a kinetic assessment. Bioresour Technol 338:125535. https://doi.org/10.1016/j.biortech.2021.125535
del Río PG, Gullón B, Wu J et al (2022) Current breakthroughs in the hardwood biorefineries: hydrothermal processing for the co-production of xylooligosaccharides and bioethanol. Bioresour Technol 343:126100. https://doi.org/10.1016/j.biortech.2021.126100
Din NAS, Lim SJ, Maskat MY, Zaini NAM (2021) Bioconversion of coconut husk fibre through biorefinery process of alkaline pretreatment and enzymatic hydrolysis. Biomass Convers Biorefin 11:815–826. https://doi.org/10.1007/s13399-020-00895-8
Domínguez de María P, Guajardo N (2017) Biocatalytic valorization of furans: opportunities for inherently unstable substrates. ChemSusChem 10:4123–4134. https://doi.org/10.1002/cssc.201701583
Dutta S, Bhat NS (2021) Recent advances in the value addition of biomass-derived levulinic acid: a review focusing on its chemical reactivity patterns. ChemCatChem 13:3202–3222. https://doi.org/10.1002/cctc.202100032
el Hage R, Chrusciel L, Desharnais L, Brosse N (2010) Effect of autohydrolysis of Miscanthus x giganteus on lignin structure and organosolv delignification. Bioresour Technol 101:9321–9329. https://doi.org/10.1016/j.biortech.2010.06.143
Fírvida I, del Río PG, Gullón P et al (2021) Alternative lime pretreatment of corn stover for second-generation bioethanol production. Agronomy 11:155. https://doi.org/10.3390/agronomy11010155
Fu D, Mazza G, Tamaki Y (2010) Lignin extraction from straw by ionic liquids and enzymatic hydrolysis of the cellulosic residues. J Agric Food Chem 58:2915–2922. https://doi.org/10.1021/jf903616y
Gomes DG, Michelin M, Romaní A et al (2021) Co-production of biofuels and value-added compounds from industrial Eucalyptus globulus bark residues using hydrothermal treatment. Fuel 285:119265. https://doi.org/10.1016/j.fuel.2020.119265
Gonzales RR, Sivagurunathan P, Kim S-H (2016) Effect of severity on dilute acid pretreatment of lignocellulosic biomass and the following hydrogen fermentation. Int J Hydrog Energy 41:21678–21684. https://doi.org/10.1016/j.ijhydene.2016.06.198
Grand View Research (2021) Lactic acid market share | Industry report, 2021–2028. https://www.grandviewresearch.com/industry-analysis/lactic-acid-and-poly-lactic-acid-market. Accessed 14 Mar 2022
Gubbels E, Jasinska-Walc L, Koning CE (2013) Synthesis and characterization of novel renewable polyesters based on 2,5-furandicarboxylic acid and 2,3-butanediol. J Polym Sci A Polym Chem 51:890–898. https://doi.org/10.1002/pola.26446
Guerrero AB, Ballesteros I, Ballesteros M (2017) Optimal conditions of acid-catalysed steam explosion pretreatment of banana lignocellulosic biomass for fermentable sugar production. J Chem Technol Biotechnol 92:2351–2359. https://doi.org/10.1002/jctb.5239
Gullón B, Eibes G, Dávila I et al (2017) Valorization of vine shoots based on the autohydrolysis fractionation optimized by a kinetic approach. Ind Eng Chem Res 56:14164–14171. https://doi.org/10.1021/acs.iecr.7b02833
Haque MA, Barman DN, Kang TH et al (2012) Effect of dilute alkali on structural features and enzymatic hydrolysis of barley straw (Hordeum vulgare) at boiling temperature with low residence time. J Microbiol Biotechnol 22:1681–1691. https://doi.org/10.4014/jmb.1206.06058
Hassan E-SRE, Mutelet F (2022) Evaluation of miscanthus pretreatment effect by choline chloride based deep eutectic solvents on bioethanol production. Bioresour Technol 345:126460. https://doi.org/10.1016/j.biortech.2021.126460
Hassan SS, Ravindran R, Jaiswal S et al (2020) An evaluation of sonication pretreatment for enhancing saccharification of brewers’ spent grain. Waste Manag 105:240–247. https://doi.org/10.1016/j.wasman.2020.02.012
Hernández-Mendoza AG, Saldaña-Trinidad S, Martínez-Hernández S et al (2021) Optimization of alkaline pretreatment and enzymatic hydrolysis of cocoa pod husk (Theobroma cacao L.) for ethanol production. Biomass Bioenergy 154:106268. https://doi.org/10.1016/j.biombioe.2021.106268
Hu L, He A, Liu X et al (2018) Biocatalytic transformation of 5-hydroxymethylfurfural into high-value derivatives: recent advances and future aspects. ACS Sustain Chem Eng 6:15915–15935. https://doi.org/10.1021/acssuschemeng.8b04356
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/C5PY00263J
Kamireddy SR, Kozliak EI, Tucker M, Ji Y (2014) Determining the kinetics of sunflower hulls using dilute acid pretreatment in the production of xylose and furfural. Green Process Synth 3:69–75. https://doi.org/10.1515/gps-2013-0095
Kamm B, Leiß S, Schönicke P, Bierbaum M (2017) Biorefining of lignocellulosic feedstock by a modified ammonia fiber expansion pretreatment and enzymatic hydrolysis for production of fermentable sugars. ChemSusChem 10:48–52. https://doi.org/10.1002/cssc.201601511
Kawanabe K, Aono R, Kino K (2021) 2,5-Furandicarboxylic acid production from furfural by sequential biocatalytic reactions. J Biosci Bioeng 132:18–24. https://doi.org/10.1016/j.jbiosc.2021.03.001
Kelbert M, Romaní A, Coelho E et al (2015) Lignocellulosic bioethanol production with revalorization of low-cost agroindustrial by-products as nutritional supplements. Ind Crop Prod 64:16–24. https://doi.org/10.1016/j.indcrop.2014.10.056
Kim KH, Dutta T, Sun J et al (2018) Biomass pretreatment using deep eutectic solvents from lignin derived phenols. Green Chem 20:809–815. https://doi.org/10.1039/c7gc03029k
Kim TH, Kwak H, Kim TH, Oh KK (2021) Reaction characteristics of organosolv-fractionation process for selective extraction of carbohydrates and lignin from rice husks. Energies (Basel) 14:686. https://doi.org/10.3390/en14030686
Koopman F, Wierckx N, de Winde JH, Ruijssenaars HJ (2010) Efficient whole-cell biotransformation of 5-(hydroxymethyl)furfural into FDCA, 2,5-furandicarboxylic acid. Bioresour Technol 101:6291–6296. https://doi.org/10.1016/j.biortech.2010.03.050
Krystof M, Pérez-Sánchez M, Domínguez de María P (2013) Lipase-mediated selective oxidation of furfural and 5-hydroxymethylfurfural. ChemSusChem 6:826–830. https://doi.org/10.1002/cssc.201200954
Kumar A, Gautam A, Dutt D (2016) Biotechnological transformation of lignocellulosic biomass into industrial products: an overview. Adv Biosci Biotechnol 07:149–168. https://doi.org/10.4236/abb.2016.73014
Kumar B, Bhardwaj N, Agrawal K et al (2020) Current perspective on pretreatment technologies using lignocellulosic biomass: an emerging biorefinery concept. Fuel Process Technol 199:106244. https://doi.org/10.1016/j.fuproc.2019.106244
Li X, Shi Y, Kong W et al (2022) Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—a review. Energy Rep 8:696–709. https://doi.org/10.1016/j.egyr.2021.12.015
López-Linares JC, García-Cubero MT, Lucas S et al (2019) Microwave assisted hydrothermal as greener pretreatment of brewer’s spent grains for biobutanol production. Chem Eng J 368:1045–1055. https://doi.org/10.1016/j.cej.2019.03.032
Lourenço A, Pereira H (2018) Compositional variability of lignin in biomass. In: Lignin trends and applications. InTech, London
Luo Y, Fan J, Budarin VL et al (2017) Microwave-assisted hydrothermal selective dissolution and utilisation of hemicellulose in Phyllostachys heterocycla cv. pubescens. Green Chem 19:4889–4899. https://doi.org/10.1039/C7GC02300F
Ma J, Yue H, Li H et al (2021) Selective delignification of poplar wood with a newly isolated white-rot basidiomycete Peniophora incarnata T-7 by submerged fermentation to enhance saccharification. Biotechnol Biofuels 14:135. https://doi.org/10.1186/s13068-021-01986-y
Mankar AR, Pandey A, Modak A, Pant KK (2021) Pretreatment of lignocellulosic biomass: a review on recent advances. Bioresour Technol 334:125235. https://doi.org/10.1016/j.biortech.2021.125235
Mankar AR, Pandey A, Pant KK (2022) Microwave-assisted extraction of lignin from coconut coir using deep eutectic solvents and its valorization to aromatics. Bioresour Technol 345:126528. https://doi.org/10.1016/j.biortech.2021.126528
Market Study Report (2019) Global 5-hydroxymethylfurfural (5-HMF) (CAS 67-47-0) market 2019 by manufacturers, regions, type and application, forecast to 2024—market study report. https://www.marketstudyreport.com/reports/global-5-hydroxymethylfurfural-5-hmf-cas-67-47-0-market-2019-by-manufacturers-regions-type-and-application-forecast-to-2024. Accessed 23 Feb 2022
Martínez-Patiño JC, Ruiz E, Romero I et al (2017) Combined acid/alkaline-peroxide pretreatment of olive tree biomass for bioethanol production. Bioresour Technol 239:326–335. https://doi.org/10.1016/j.biortech.2017.04.102
Martínez-Patiño JC, Lu-Chau TA, Gullón B et al (2018) Application of a combined fungal and diluted acid pretreatment on olive tree biomass. Ind Crop Prod 121:10–17. https://doi.org/10.1016/j.indcrop.2018.04.078
Meléndez-Hernández PA, Hernández-Beltrán JU, Hernández-Guzmán A et al (2021) Comparative of alkaline hydrogen peroxide pretreatment using NaOH and Ca(OH)2 and their effects on enzymatic hydrolysis and fermentation steps. Biomass Convers Biorefin 11:1897–1907. https://doi.org/10.1007/s13399-019-00574-3
Michelin M, Teixeira JA (2016) Liquid hot water pretreatment of multi feedstocks and enzymatic hydrolysis of solids obtained thereof. Bioresour Technol 216:862–869. https://doi.org/10.1016/j.biortech.2016.06.018
Mihiretu GT, Brodin M, Chimphango AF et al (2017) Single-step microwave-assisted hot water extraction of hemicelluloses from selected lignocellulosic materials—a biorefinery approach. Bioresour Technol 241:669–680. https://doi.org/10.1016/j.biortech.2017.05.159
Mittal A, Chatterjee SG, Scott GM, Amidon TE (2009) Modeling xylan solubilization during autohydrolysis of sugar maple and aspen wood chips: reaction kinetics and mass transfer. Chem Eng Sci 64:3031–3041. https://doi.org/10.1016/j.ces.2009.03.011
Mokomele T, da Costa SL, Balan V et al (2019) Incorporating anaerobic co-digestion of steam exploded or ammonia fiber expansion pretreated sugarcane residues with manure into a sugarcane-based bioenergy-livestock nexus. Bioresour Technol 272:326–336. https://doi.org/10.1016/j.biortech.2018.10.049
Moniz P, Pereira H, Duarte LC, Carvalheiro F (2014) Hydrothermal production and gel filtration purification of xylo-oligosaccharides from rice straw. Ind Crop Prod 62:460–465. https://doi.org/10.1016/j.indcrop.2014.09.020
Morales A, Gullón B, Dávila I et al (2018) Optimization of alkaline pretreatment for the co-production of biopolymer lignin and bioethanol from chestnut shells following a biorefinery approach. Ind Crop Prod 124:582–592. https://doi.org/10.1016/j.indcrop.2018.08.032
Morales M, Arvesen A, Cherubini F (2021) Integrated process simulation for bioethanol production: effects of varying lignocellulosic feedstocks on technical performance. Bioresour Technol 328:124833. https://doi.org/10.1016/j.biortech.2021.124833
Morone A, Apte M, Pandey RA (2015) Levulinic acid production from renewable waste resources: bottlenecks, potential remedies, advancements and applications. Renew Sustain Energy Rev 51:548–565. https://doi.org/10.1016/j.rser.2015.06.032
Mota TR, Oliveira DM, Morais GR et al (2019) Hydrogen peroxide-acetic acid pretreatment increases the saccharification and enzyme adsorption on lignocellulose. Ind Crop Prod 140:111657. https://doi.org/10.1016/j.indcrop.2019.111657
Motagamwala AH, Won W, Sener C et al (2018) Toward biomass-derived renewable plastics: production of 2,5-furandicarboxylic acid from fructose. Sci Adv 4. https://doi.org/10.1126/sciadv.aap9722
Mukherjee A, Dumont M-J, Raghavan V (2015) Review: sustainable production of hydroxymethylfurfural and levulinic acid: challenges and opportunities. Biomass Bioenergy 72:143–183. https://doi.org/10.1016/j.biombioe.2014.11.007
Nanda S, Mohammad J, Reddy SN et al (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers Biorefin 4:157–191. https://doi.org/10.1007/s13399-013-0097-z
Naz S, Uroos M, Muhammad N (2021) Effect of molecular structure of cation and anions of ionic liquids and co-solvents on selectivity of 5-hydroxymethylfurfural from sugars, cellulose and real biomass. J Mol Liq 334:116523. https://doi.org/10.1016/j.molliq.2021.116523
Nitsos CK, Choli-Papadopoulou T, Matis KA, Triantafyllidis KS (2016) Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain Chem Eng 4:4529–4544. https://doi.org/10.1021/acssuschemeng.6b00535
Oh Y, Park S, Jung D et al (2020) Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int J Biol Macromol 165:187–197. https://doi.org/10.1016/j.ijbiomac.2020.09.145
Onu Olughu O, Tabil LG, Dumonceaux T (2021) Ultrasonic delignification and microstructural characterization of switchgrass. Energies (Basel) 14:263. https://doi.org/10.3390/en14020263
Osuna-Laveaga DR, García-Depraect O, Vallejo-Rodríguez R et al (2020) Integrated ozonation-enzymatic hydrolysis pretreatment of sugarcane bagasse: enhancement of sugars released to expended ozone ratio. Processes 8:1274. https://doi.org/10.3390/pr8101274
Pérez-Armada L, Rivas S, González B, Moure A (2019) Extraction of phenolic compounds from hazelnut shells by green processes. J Food Eng 255:1–8. https://doi.org/10.1016/j.jfoodeng.2019.03.008
Pielhop T, Larrazábal GO, Studer MH et al (2015) Lignin repolymerisation in spruce autohydrolysis pretreatment increases cellulase deactivation. Green Chem 17:3521–3532. https://doi.org/10.1039/C4GC02381A
Portela-Grandío A, Peleteiro S, Yáñez R, Romaní A (2021) Integral valorization of Acacia dealbata wood in organic medium catalyzed by an acidic ionic liquid. Bioresour Technol 342:126013. https://doi.org/10.1016/j.biortech.2021.126013
Putrino FM, Tedesco M, Bodini RB, de Oliveira AL (2020) Study of supercritical carbon dioxide pretreatment processes on green coconut fiber to enhance enzymatic hydrolysis of cellulose. Bioresour Technol 309:123387. https://doi.org/10.1016/j.biortech.2020.123387
Qin Y-Z, Li Y-M, Zong M-H et al (2015) Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents. Green Chem 17:3718–3722. https://doi.org/10.1039/C5GC00788G
Ramesh R, Nair A, Jayavel A et al (2020) Choline chloride-based deep eutectic solvents for efficient delignification of Bambusa bambos in bio-refinery applications. Chem Pap 74:4533–4545. https://doi.org/10.1007/s11696-020-01259-2
Raynie DE, Roy R, Rahman MS (2020) Recent advances of greener pretreatment technologies of lignocellulose. Curr Res Green Sustain Chem 3:100035. https://doi.org/10.1016/j.crgsc.2020.100035
Rezania S, Oryani B, Cho J et al (2020) Different pretreatment technologies of lignocellulosic biomass for bioethanol production: an overview. Energy 199:117457. https://doi.org/10.1016/j.energy.2020.117457
Rivas S, González-Muñoz MJ, Vila C et al (2013) Manufacture of levulinic acid from pine wood hemicelluloses: a kinetic assessment. Ind Eng Chem Res 52:3951–3957. https://doi.org/10.1021/ie3018725
Rivas S, González-Muñoz MJ, Santos V, Parajó JC (2014) Acidic processing of hemicellulosic saccharides from pine wood: product distribution and kinetic modeling. Bioresour Technol 162:192–199. https://doi.org/10.1016/j.biortech.2014.03.150
Rivas S, Santos V, Parajó JC (2017) Aqueous fractionation of hardwood: selective glucuronoxylan solubilisation and purification of the reaction products. J Chem Technol Biotechnol 92:367–374. https://doi.org/10.1002/jctb.5014
Rivas S, Vila C, Alonso JL et al (2019) Biorefinery processes for the valorization of Miscanthus polysaccharides: from constituent sugars to platform chemicals. Ind Crop Prod 134:309–317. https://doi.org/10.1016/j.indcrop.2019.04.005
Rivas S, Rigual V, Domínguez JC et al (2020) A biorefinery strategy for the manufacture and characterization of oligosaccharides and antioxidants from poplar hemicelluloses. Food Bioprod Process 123:398–408. https://doi.org/10.1016/j.fbp.2020.07.018
Rivas S, López L, Vila C, Parajó JC (2021) Organosolv processing of vine shoots: fractionation and conversion of hemicellulosic sugars into platform chemicals by microwave irradiation. Bioresour Technol 342:125967. https://doi.org/10.1016/j.biortech.2021.125967
Rochón E, Cabrera MN, Scutari V et al (2022) Co-production of bioethanol and xylosaccharides from steam-exploded eucalyptus sawdust using high solid loads in enzymatic hydrolysis: effect of alkaline impregnation. Ind Crop Prod 175:114253. https://doi.org/10.1016/j.indcrop.2021.114253
Rodionova MV, Bozieva AM, Zharmukhamedov SK et al (2022) A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int J Hydrog Energy 47:1481–1498. https://doi.org/10.1016/j.ijhydene.2021.10.122
Rojas-Sossa JP, Zhong Y, Valenti F et al (2019) Effects of ammonia fiber expansion (AFEX) treated corn stover on anaerobic microbes and corresponding digestion performance. Biomass Bioenergy 127:105263. https://doi.org/10.1016/j.biombioe.2019.105263
Romero-García JM, López-Linares JC, del Mar Contreras M et al (2022) Exploitation of olive tree pruning biomass through hydrothermal pretreatments. Ind Crop Prod 176:114425. https://doi.org/10.1016/j.indcrop.2021.114425
Sajid M, Zhao X, Liu D (2018) Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): recent progress focusing on the chemical-catalytic routes. Green Chem 20:5427–5453. https://doi.org/10.1039/C8GC02680G
Sajid M, Farooq U, Bary G et al (2021) Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: progress, challenges, and prospects. Green Chem 23:9198–9238. https://doi.org/10.1039/D1GC02919C
Saratale GD, Saratale RG, Varjani S et al (2020) Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind Crop Prod 150:112425. https://doi.org/10.1016/j.indcrop.2020.112425
Saravanan A, Senthil Kumar P, Jeevanantham S et al (2022) Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour Technol 344:126203. https://doi.org/10.1016/j.biortech.2021.126203
Siankevich S, Savoglidis G, Fei Z et al (2014) A novel platinum nanocatalyst for the oxidation of 5-hydroxymethylfurfural into 2,5-Furandicarboxylic acid under mild conditions. J Catal 315:67–74. https://doi.org/10.1016/j.jcat.2014.04.011
Sidana A, Yadav SK (2022) Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. J Clean Prod 335:130286. https://doi.org/10.1016/j.jclepro.2021.130286
Siddique IJ, Salema AA, Antunes E, Vinu R (2022) Technical challenges in scaling up the microwave technology for biomass processing. Renew Sustain Energy Rev 153:111767. https://doi.org/10.1016/j.rser.2021.111767
Silveira MHL, Vanelli BA, Corazza ML, Ramos LP (2015) Supercritical carbon dioxide combined with 1-butyl-3-methylimidazolium acetate and ethanol for the pretreatment and enzymatic hydrolysis of sugarcane bagasse. Bioresour Technol 192:389–396. https://doi.org/10.1016/j.biortech.2015.05.044
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass—an overview. Bioresour Technol 199:76–82. https://doi.org/10.1016/j.biortech.2015.08.030
Sohni S, Hashim R, Nidaullah H et al (2020) Enhancing the enzymatic digestibility of oil palm biomass using supercritical carbon dioxide-based pretreatment towards biorefinery application. Ind Crop Prod 157:112923. https://doi.org/10.1016/j.indcrop.2020.112923
Solarte-Toro JC, Cardona Alzate CA (2021) Biorefineries as the base for accomplishing the sustainable development goals (SDGs) and the transition to bioeconomy: technical aspects, challenges and perspectives. Bioresour Technol 340:125626. https://doi.org/10.1016/j.biortech.2021.125626
Solarte-Toro JC, Chacón-Pérez Y, Cardona-Alzate CA (2018) Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron J Biotechnol 33:52–62. https://doi.org/10.1016/j.ejbt.2018.03.005
Sousa AF, Vilela C, Fonseca AC et al (2015) Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency. Polym Chem 6:5961–5983. https://doi.org/10.1039/C5PY00686D
Ståhl M, Nieminen K, Sixta H (2018) Hydrothermolysis of pine wood. Biomass Bioenergy 109:100–113. https://doi.org/10.1016/j.biombioe.2017.12.006
Sun FF, Zhao X, Hong J et al (2016) Industrially relevant hydrolyzability and fermentability of sugarcane bagasse improved effectively by glycerol organosolv pretreatment. Biotechnol Biofuels 9:59. https://doi.org/10.1186/s13068-016-0472-7
Takada M, Minami E, Kawamoto H (2021) Topochemistry of the delignification of japanese beech Fagus crenata wood by supercritical methanol treatment. ACS Omega 6:20924–20930. https://doi.org/10.1021/acsomega.1c02345
Takkellapati S, Li T, Gonzalez MA (2018) An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Techn Environ Policy 20:1615–1630. https://doi.org/10.1007/s10098-018-1568-5
Tang C, Shan J, Chen Y et al (2017) Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin. Bioresour Technol 232:222–228. https://doi.org/10.1016/j.biortech.2017.02.041
Tang W, Wu X, Huang C et al (2021) Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour Technol 324:124651. https://doi.org/10.1016/j.biortech.2020.124651
Teramura H, Sasaki K, Oshima T et al (2018) Effective usage of sorghum bagasse: optimization of organosolv pretreatment using 25% 1-butanol and subsequent nanofiltration membrane separation. Bioresour Technol 252:157–164. https://doi.org/10.1016/j.biortech.2017.12.100
Thoresen PP, Matsakas L, Rova U, Christakopoulos P (2020) Recent advances in organosolv fractionation: towards biomass fractionation technology of the future. Bioresour Technol 306:123189. https://doi.org/10.1016/j.biortech.2020.123189
Usmani Z, Sharma M, Gupta P et al (2020) Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour Technol 304:123003. https://doi.org/10.1016/j.biortech.2020.123003
Utekar PG, Kininge MM, Gogate PR (2021) @@Intensification of delignification and enzymatic hydrolysis of orange peel waste using ultrasound for enhanced fermentable sugar production. Chem Eng Process Process Intensif 168:108556. https://doi.org/10.1016/j.cep.2021.108556
Vasaki M, Sithan M, Ravindran G et al (2022) Biodiesel production from lignocellulosic biomass using Yarrowia lipolytica. Energy Convers Manag X 13:100167. https://doi.org/10.1016/j.ecmx.2021.100167
Velvizhi G, Balakumar K, Shetti NP et al (2022) Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: paving a path towards circular economy. Bioresour Technol 343:126151. https://doi.org/10.1016/j.biortech.2021.126151
Wang X (2021) Pretreatment: toward effectiveness and sustainability. In: Advances in 2nd generation of bioethanol production. Woodhead Publishing/Elsevier, San Diego, p. 87-112
Wang W, Lee D-J (2021) Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: a review. Bioresour Technol 339:125587. https://doi.org/10.1016/j.biortech.2021.125587
Wang Y, Meng X, Jeong K et al (2020) Investigation of a lignin-based deep eutectic solvent using p -hydroxybenzoic acid for efficient woody biomass conversion. ACS Sustain Chem Eng 8:12542–12553. https://doi.org/10.1021/acssuschemeng.0c03533
Xu W, Fu S, Yang Z et al (2018) Improved methane production from corn straw by microaerobic pretreatment with a pure bacteria system. Bioresour Technol 259:18–23. https://doi.org/10.1016/j.biortech.2018.02.046
Xu C, Paone E, Rodríguez-Padrón D et al (2020a) Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem Soc Rev 49:4273–4306. https://doi.org/10.1039/D0CS00041H
Xu H, Peng J, Kong Y et al (2020b) Key process parameters for deep eutectic solvents pretreatment of lignocellulosic biomass materials: a review. Bioresour Technol 310:123416. https://doi.org/10.1016/j.biortech.2020.123416
Yi G, Teong SP, Zhang Y (2015) The direct conversion of sugars into 2,5-furandicarboxylic acid in a triphasic system. ChemSusChem 8:1151–1155. https://doi.org/10.1002/cssc.201500118
Yiin CL, Yap KL, Ku AZE et al (2021) Recent advances in green solvents for lignocellulosic biomass pretreatment: potential of choline chloride (ChCl) based solvents. Bioresour Technol 333:125195. https://doi.org/10.1016/j.biortech.2021.125195
Ying W, Fang X, Xu Y, Zhang J (2022) Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenergy 158:106377. https://doi.org/10.1016/j.biombioe.2022.106377
Yousatit S, Osuga R, Kondo JN et al (2022) Selective synthesis of 5-hydroxymethylfurfural over natural rubber–derived carbon/silica nanocomposites with acid–base bifunctionality. Fuel 311:122577. https://doi.org/10.1016/j.fuel.2021.122577
Yu Y, Wu J, Ren X et al (2022a) Steam explosion of lignocellulosic biomass for multiple advanced bioenergy processes: a review. Renew Sustain Energy Rev 154:111871. https://doi.org/10.1016/j.rser.2021.111871
Yu Z, Ma H, den Boer E et al (2022b) Effect of microwave/hydrothermal combined ionic liquid pretreatment on straw: rumen anaerobic fermentation and enzyme hydrolysis. Environ Res 205:112453. https://doi.org/10.1016/j.envres.2021.112453
Yuan Z, Wen Y (2017) Evaluation of an integrated process to fully utilize bamboo biomass during the production of bioethanol. Bioresour Technol 236:202–211. https://doi.org/10.1016/j.biortech.2017.03.179
Yuan Z, Wen Y, Li G (2018) Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour Technol 259:228–236. https://doi.org/10.1016/j.biortech.2018.03.044
Zhang Y, Naebe M (2021) Lignin: a review on structure, properties, and applications as a light-colored UV absorber. ACS Sustain Chem Eng 9:1427–1442. https://doi.org/10.1021/acssuschemeng.0c06998
Zhang H, Lyu G, Zhang A et al (2018) Effects of ferric chloride pretreatment and surfactants on the sugar production from sugarcane bagasse. Bioresour Technol 265:93–101. https://doi.org/10.1016/j.biortech.2018.05.111
Zhao S, Cheng M, Li J et al (2011) One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted–Lewis–surfactant-combined heteropolyacid catalyst. Chem Commun 47:2176. https://doi.org/10.1039/c0cc04444j
Zhao C, Shao Q, Ma Z et al (2016) Physical and chemical characterizations of corn stalk resulting from hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment. Ind Crop Prod 83:86–93. https://doi.org/10.1016/j.indcrop.2015.12.018
Zhao C, Qiao X, Cao Y, Shao Q (2017) Application of hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment of energy crops. Fuel 205:184–191. https://doi.org/10.1016/j.fuel.2017.05.073
Zhao Z, Chen X, Ali MF et al (2018) Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour Technol 263:325–333. https://doi.org/10.1016/j.biortech.2018.05.016
Zhou H, Liu Q, Zhong X et al (2021) Flow chemistry for a better fractionation of lignocellulosic biomass in products structure and yield. Ind Crop Prod 173:114124. https://doi.org/10.1016/j.indcrop.2021.114124
Zhou M, Fakayode OA, Ahmed Yagoub AE et al (2022) Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew Sustain Energy Rev 156:111986. https://doi.org/10.1016/j.rser.2021.111986
Ziaei-Rad Z, Fooladi J, Pazouki M, Gummadi SN (2021) Lignocellulosic biomass pre-treatment using low-cost ionic liquid for bioethanol production: an economically viable method for wheat straw fractionation. Biomass Bioenergy 151:106140. https://doi.org/10.1016/j.biombioe.2021.106140
Zuo M, Wang X, Wang Q et al (2021) Aqueous-natural deep eutectic solvent-enhanced 5-hydroxymethylfurfural production from glucose, starch, and food wastes. ChemSusChem. https://doi.org/10.1002/cssc.202101889
Acknowledgements
The authors acknowledge the financial support received from the Spanish “Ministry of Economy and Competitiveness” (Project “Cutting-edge strategies for a sustainable biorefinery based on valorization of invasive species”, reference PID2019-110031RB-I00) and from “Xunta de Galicia” (contract ED431C 2017/62-GRC to Competitive Reference Group BV1, Project ED431 F 2020/03 and Project ED431F 2020/06). These projects are partially funded by the FEDER Program of the European Union (“Unha maneira de facer Europa”). Dr Beatriz Gullón, Gemma Eibes and Aloia Romaní would like to express their gratitude to the Spanish Ministry of Economy and Competitiveness for her postdoctoral grant (Reference RYC2018-026177-I, RYC2018-024846-I and RYC2020-030690-I, respectively). Sandra Rivas thanks to MINECO for the research contract with reference IJC2018-037665-I. Álvaro Lobato-Rodríguez would like to express his gratitude to the Spanish Ministry of Science and Innovation for his FPI doctoral grant (PRE2020-093359).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lobato-Rodríguez, Á. et al. (2023). State-of-the-Art Technologies for Production of Biochemicals from Lignocellulosic Biomass. In: Pathak, P.D., Mandavgane, S.A. (eds) Biorefinery: A Sustainable Approach for the Production of Biomaterials, Biochemicals and Biofuels. Springer, Singapore. https://doi.org/10.1007/978-981-19-7481-6_5
Download citation
DOI: https://doi.org/10.1007/978-981-19-7481-6_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7480-9
Online ISBN: 978-981-19-7481-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)