Abstract
Lignocellulosic or 2G ethanol has enticed a great deal of attention as one of the promising renewable energy fuels which will permit shift from finite fossil fuel to infinite biomass as a resource. The demand for ethanol in the market especially in blended petroleum-based products is increasing day by day as ethanol has higher octane number making it thermally efficient than traditional fuels. Additionally, fuel extracted primarily from biomass can be converted into all the three forms such as solid, liquid, and gaseous fuels. First-generation bioethanol or bioethanol from feedstocks has a fundamental disadvantage that it is mainly based on utilizing crops in the food-feed chain; therefore, interest in second-generation bioethanol from non-edible lignocellulose feedstock is ramping up as an economical and eco-friendly sustainable energy system. The copious availability of lignocellulosic biomass lowers the investment cost, and the obtained biofuel liberates less greenhouse gasses mitigating adverse environmental impacts. In this perspective, the present chapter aims to discuss lignocellulosic ethanol as a substitute to fossil-reliant resources. The chapter highlights the secondary and tertiary sources and optimum condition of fermentation to produce first-, second-, and third-generation bioethanol.
The first section of the chapter emphasizes on the different sources of celluloses and types of microbes which can reduce or ferment multiple substrates and their use in pretreatment as well as fermentation to obtain ethanol. It may be concluded that use of optimum conditions (temperatures, pH, and enzymes) and selecting the most efficient microorganisms give apparently high yield and concentration of bioethanol from lignocellulose biomass. The second section includes an overview of existing techniques used for pretreatment and fermentation. The study also discussed the current and future scenario of bioethanol production in the Indian context. Further efficient utilization of by-products and improved valorization which will directly reduce the final cost of ethanol are cohesively discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Global warming is one of the environmental issues which has emphatic and inevitable impacts on our planet as well as all the life forms existing on the planet. Multiple lines of evidence have strongly established that combustion of fossil fuels leading to emissions of greenhouse gas results in global warming. Fossil fuel consumption by the world’s transportation sector is nearly 60%, which eventually leads to depletion of natural resources and generation of massive pollution (Aditiya et al. 2016).
India is considered as third biggest oil importer in the world. It has consumed almost 3.3 million tons of fuel estimated in 2020–2022. The United States Department of Agriculture (USDA) stated that India’s average blending of ethanol in petrol rises up to 5.8% in 2019. It was also reported that the USA continues to be the biggest ethanol supplier and ethanol import in India from the country grows up to 750 million liters. Visualizing the above parameters of fuel consumption, we see that there’s an urgent need to acquire low-cost, clean, alternative, and renewable energy, which can be achieved by production of biofuels to replace fossil fuels (Gonçalves et al. 2016). This will not only help in reducing environmental impact by fossil fuel usage but also contribute to energy self-sufficiency.
Bioenergy with lesser greenhouse gas emission assures phasing out the fossil-derived energy making bioenergy a substantial contributory of clean and efficient energy simultaneously avoiding depletion of fossil fuels. Bioenergy obtained traditionally by combustion of biomass and its modern substitutes such as biogas, biofuels, biorefineries, etc. allows to gain energy security and economic growth in addition to stabilizing the environment (Fischer and Schrattenholzer 2001; Menon and Rao 2012; Popp et al. 2014; Sadhukhan et al. 2018; Jeswani et al. 2020; Muh et al. 2020; Kaniapan et al. 2021; Manikandan et al. 2022).
Different types of lignocellulosic biomass available can be categorized into three categories as primary sources (sugarcane, sugar beet, fruits) produced as crop, secondary sources (starchy crops, straw, bagasse, and rice husks) obtained from residue of crops, and tertiary sources (municipal wastes, wood trimmings, sludge from sewage treatment). Laboratory and industrial ethanol is generally produced by acid-catalyzed reaction of ethylene or obtained by fermentation where microorganisms such as yeast and bacteria are used to metabolize the sugars producing ethanol and CO2 (Kang et al. 2014). Producing bioethanol mainly involves fermentation of simple sugar and starch which are mainly derived from polysaccharides, and the ethanol generated is generally first-generation bioethanol. These substrates need to be fermented in a highly efficient manner which is quite expensive and non-sustainable in nature as these fuel sources are used as an important part of global food supply (Uçkun Kiran and Liu 2015; Battista et al. 2016). Production of bioethanol from lignocellulose biomass (second-generation biofuel) is sustainable and cost-effective which accounts for potential renewable energy sources (Jørgensen et al. 2007; Kang et al. 2014; Cutzu and Bardi 2017).
Lignocellulosic biomass generated from agricultural, industry, forestry, or municipal waste acts as a precursor for the production of lignocellulosic or 2G bioethanol. In recent years, it is found that environmental pollution is increasing due to the improper disposal of agro-industrial waste. This waste includes agricultural by-products such as straws, leaves, and husk as well as waste produced from food processing industries such as fruit peels, oil cakes, plant residues, etc. (Sadh et al. 2018). The bioethanol obtained from renewable resources such as crops, starch, and sugar helps in the reduction of greenhouse gas emission and when blended with road transport fuels leads to reducing air pollution. Bioethanol produced shows 94% less greenhouse gas emissions when compared to gasoline. It was also reported that for producing 1000 L bioethanol from lignocellulosic feedstocks, 2.6 mg of CO2 emission is saved (Aditiya et al. 2016; Mohapatra et al. 2017). The energy content of bioethanol is approximately 40% lower than gasoline, and oxygen content is 35% higher that enable a cleaner combustion and the emission of less toxic substances (Ahmed et al. 2016).
Bioethanol serves as a promising alternative as well as renewable and sustainable liquid biofuel, successful in dealing with environment quality and today’s global energy crisis (Aditiya et al. 2016).
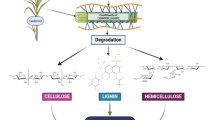
Worldwide bioethanol is mainly used as transportation fuel. Bioethanol has advantages like renewability, it is less toxic than other fuels, it has particulate free burning nature, and lower VOCs and NOx pollutants are emitted by its emission (Cutzu and Bardi 2017). Steps such as pretreatment, fermentation, and distillation are needed to turn lignocellulosic biomass into ethanol (Fig. 11.1). Various methods used for pretreatment and fermentation should be appropriate and effective to achieve high bioethanol yield.
2 Source of Cellulose
2.1 Structure
An organic compound “cellulose” with chemical formula (C6H10O5)n is a polysaccharide that consists of thousands of d-glucose units linked by β(1 → 4) linkage. The long chains of anhydro-d-glucopyranose units (AGU) are present where each molecule has three -OH groups per AGU, with the exception of the terminal ends. One end is the reducing end with d-glucose unit, and the other end is a non-reducing end with C4-OH group. Cellulose contributes to the structure of cell walls of plants, many algae, and oocytes. Molecular structure of cellulose has significant features like hydrophilic nature having contact angle of 20–30°, degradability range, and chemical variability as it has a high reactive -OH group. Due to these properties, cellulose is stated as an abundant polymer in nature. The content of cellulose varies in different substrates, e.g., wood contains 40–50%, dried hemp consists of approximately 57%, and cotton fibers consist of 90% cellulose. Some bacterial species are also able to secrete cellulose, and they produce one of the purest forms of cellulose. A strain of Clostridium bacteria TU-103, found in waste produced by zebra, is able to convert almost any form of cellulose to butanol which is a biofuel (Kulkarni Vishakha et al. 2012; Gupta et al. 2019; Seddiqi et al. 2021; Butnariu and Flavius 2022; Nisa et al. 2022).
Few additional properties of cellulose are that it is insoluble in water and other organic solvent, odorless, chiral, and biodegradable. Pulse tests given by Krumm et al. (2016) showed that the melting temperature was 467 °C. The carbon, hydrogen, and oxygen contents in cellulose are 44.44%, 6.17%, and 49.39%, respectively (Chen 2014; Krumm et al. 2016). Studying cellulose (Fig. 11.2) is important because it has various uses, out of which biofuel production from cellulosic biomass is taking advancement rapidly, as it is a homopolymer of glucose.
Another polysaccharide which comprises 20% biomass of plants is hemicellulose. Unlike cellulose, they are made up of units of sugars like xylose, mannose, arabinose, galactose, and glucose. The chains of hemicellulose are shorter 500–3000 sugar units, and they are branched. Hemicellulose also plays an important role in biofuel production along with cellulose (Chen 2014).
2.2 Types of Cellulose
The material that produces cellulose is called cellulosic material. According to the sources, cellulose can be categorized into plant cellulose, bacterial cellulose, algal cellulose, wood cellulose, and non-wood cellulose, and all these sources of lignocellulose biomass which have potential to be used as a substrate for ethanol production are shown in Fig. 11.3.
2.2.1 Plant Cellulose
Cellulose is the main part of the cell wall of plants. Most of the higher plants’ cellulose has a crystalline structure with a very long length but a narrow width of only a couple of nanometers (Šturcová et al. 2004). Plant cellulose is a mesh-like, tough material giving structural support to make cell wall. Cellulose is categorized into four different types of polymorphs, that is, celluloses I, II, III, and IV. The types of cellulose that are produced by plants are of categories I and II, and they are called as native cellulose that is found in two different crystalline forms. Type I cellulose is less stable thermodynamically than type II. Type II cellulose is mainly found in marine algae which is formed when type I cellulose is reacted with sodium hydroxide. When types I and II are treated with liquid ammonia, it produces type III crystalline form, which if further heated produces type IV cellulose (Lavanya et al. 2011).
2.2.2 Cellulose from Wood
Wood is one of the major sources of cellulose. Both softwood and hardwood contain cellulose and hemicellulose. Softwood contains 40–45% of cellulose, while hardwood contains 45–47% of cellulose (Kang et al. 2014). Examples of wood cellulose are aspen (Populus tremuloides) and cottonwood (Populus trichocarpa); both contain 56.5% and 52.0% of cellulose, respectively (Wayman 1958).
2.2.3 Cellulose from Non-wood
Non-wood biomass includes plants with weak steam; it also includes corncob, husk, nutshells of fruits, and agricultural and garden waste. Based on the output, bamboos are considered as the most abundant non-wood sources. Non-woody plants are gaining popularity as alternative sources of cellulose due to low lignin content, high cellulose yield, and relatively short rotation period leading to low production costs (Pennells et al. 2019). The valorization of non-wood agricultural residues is also beneficial with respect to reducing the burden of disposal of waste.
2.2.4 Bacterial Cellulose
Bacterial cellulose is gaining importance recently as a source of producing biofuel. Microorganisms usually make microbial cellulose, which is a source of pure cellulose. Due to its high purity and unique physical and chemical properties, it has many uses in many industries, such as the food and biomedical industries. It can also be used to make biobased polymers and nanocomposites (Lahiri et al. 2021).
Bacterial cellulose is naturally produced; many studies focus on enhancing the cellulose growth from culture in laboratory. These approaches have led to tailorable microbial cellulose, and desired properties can be achieved. Bacterial cellulose has almost the same formula like plant but has different properties like high purity and greater tensile strength and hydrophilicity and is more volatile than plant cellulose as it can be produced in lab. It can be grown in any shape because it has high movability at the time of formation. Gluconacetobacter xylinus, a Gram-negative bacterium, can synthesize cellulose (conventionally known as Acetobacter xylinum). Agrobacterium, Acetobacter, Rhizobium, Azotobacter, Sarcina, Alcaligenes, and Pseudomonas can also produce bacterial cellulose (Dien et al. 2003; Kumar et al. 2019; Lahiri et al. 2021).
2.2.5 Algal Cellulose
Apart from the above, seaweeds have emerged as a source of cellulose and a major contributor in third-generation bioethanol production. Cellulose microfibrils are present in the cell wall of green, red, and brown seaweeds to give skeletal support to the microalgae. The members of Ulvales, Cladophorales, Ulotrichales, and Bryopsidales contain significant amount of cellulose which is predominantly an α-allomorph type with negligible or no lignin content. Algal cellulose is mainly comprised of two different crystalline structures Ια and Ιβ. These both celluloses are structured by parallel glucan chains in a flat-ribbon conformation, with alternating glucosyl units locked in opposite orientations by intramolecular hydrogen bonds. These intramolecular hydrogen bonding hold the chains together in flat sheets. Algal cellulose Ια is a triclinic unit cell. It has one chain with conformation- and hydrogen-bonding-different glycosyl residues (Nishiyama et al. 2003; Šturcová et al. 2004).
2.2.6 Animal Cellulose
An uncommon source of animal cellulose is tunicates. It is the only animal species that produces cellulose as skeletal frame in the tunic tissues. Tunicate-derived cellulose is composed purely of cellulose I allomorph making it superior to cellulose obtained from plants in terms of aspect ratio, surface area, crystallinity, and mechanical properties (Dunlop et al. 2020).
Cellulosic materials from different sources like plant cellulose, bacterial cellulose, algal cellulose, wood cellulose, and non-wood cellulose are popular substrates for bioethanol production. Mostly agricultural residues and sugary crops are considered as good sources of cellulose, but it can be extracted from sources such as sugarcane, corn, rice straw, and wheat straw (Lavanya et al. 2011). A list of sources with their composition of cellulose, hemicellulose, and lignin components is summarized in Table 11.1. For instance, cellulose content in agricultural waste such as wheat and corn straws ranges from 30% to 40% of biomass with least amount of lignin content making it a preferred substrate for bioethanol production. Similarly, cardoon plant also has high cellulose content but is accompanied with high hemicellulose and lignin content which means it needs to undergo additional pretreatment for optimum bioethanol production; however, high hemicellulose and lignin fraction make it valuable in paper and pulp production. In the case of waste generated in the food industry, the cellulose content ranges between 12% and 39%. As evident from the table, about 40–50% of bagasse is cellulose, but it is essential to reduce the lignin proportion to facilitate sugar hydrolysis and fermentation. In wood, it is reported that the cellulose content of hardwood and softwood is comparable with each other; however, higher lignin in softwoods makes it resistant to hydrolysis in comparison to hardwoods. A comparison of some seaweed-based cellulose is also described in Table 11.1. The cellulose contents in different seaweed species range from 35% to 56% with an average being 42.26%. It is also reported that cellulose prepared from seaweed has higher crystallinity index in comparison to other lignocellulose biomass. The chemical composition of lignocellulose biomass represented in the table can assist in deciding the energy-efficient pretreatment, hydrolysis, and fermentation parameters for obtaining maximum bioethanol yield.
3 Pretreatment
Pretreatment is a necessary step before forwarding the substrate for the fermentation process. Pretreatment accounts for more than 20% of ethanol production costs and is a costly capex procedure. It also affects biomass conversion steps (Pant et al. 2022). Any pretreatment method aims to break down cellulose, hemicellulose, and lignin into smaller fragments for enzymatic hydrolysis and other biorefining processes, increasing product yield (Sharma et al. 2019). This is mainly required because the original form is very complex that cellulase enzymes are not able to hydrolyze it into fermentable sugars. So, the pretreatment is a very important step before proceeding further for better yield (Oyegoke et al. 2022). The molecular structure of lignins determines the strong resistance of plant biomass to breakdown processes, which limits its use as a raw material in bioconversion processes such as cellulose bioethanol production. Lignins protect biomass from hydrolysis by forming a physical barrier between polysaccharides and hydrolytic enzymes (lignin-polysaccharide complexes) and inhibiting the activity of binding enzymes (Mikulski and Kłosowski 2022). The ideal pretreatment should promote sugar production or the ability to produce sugars after enzymatic digestion, reduce carbohydrate degradation or loss, prevent by-products that impede hydrolysis and fermentation, be cost-effective, and be environmentally friendly (Li et al. 2022).
The pretreatment is mainly used for size reduction of substrate for better extraction of hemicellulose, cellulose, starch, etc. before hydrolysis to get substantial amount of reducing sugars. This is a much better approach for respective enzymes to hydrolyze the pretreated substrate into fermentable sugars as the original form of sugars is difficult to hydrolyze because of its complex structure. Also, it helps in bringing down the degree of crystallization which is present in the cellulose fibers (Mosier et al. 2005; Sanchez and Cardona 2008; Sharma et al. 2019; Ummalyma et al. 2019; Zhao et al. 2022). The pretreatment for lignocellulosic material is considered to be successful; it is important to select the substrate accordingly. The selected material for fermentation should have good fermentation capability, low solid waste production, high ability to digest the pretreated residue, minimum heat and power requirements, the efficacy should be maintained in case of low moisture, decreasing the size of substrate should not be much required, minimal quantity of toxic generation, able to retain good sugar concentration, able to recover the amount of lignin and operating conditions and cost should be maintained (Alvira et al. 2010; Maurya et al. 2015). The pretreatment changes the properties of the substrate in different ways. Physical, chemical, physicochemical, and biological methods can be used for pretreatment, depending on the forces and amount of energy used in each process (Fig. 11.4). Few studies reported that combination of these processes results in a better yield (Banerjee et al. 2009; Mohapatra et al. 2017; Oyegoke et al. 2022). However, each pretreatment technique has its own benefits and drawbacks (Sharma et al. 2019).
3.1 Physical Pretreatment
The physical pretreatment mainly focuses on reducing the size of substrate and crystallinity in cellulose matrix. The physical pretreatment includes mechanical and extrusion treatment methods. Physical pretreatment is a pre-step to co-processing, optimizing equipment and conditions before scale-up, and minimizing costs (Li et al. 2022).
3.1.1 Mechanical
Mechanical pretreatment reduces particle size (10–30 mm after chipping, 0.2–2 mm after milling or grinding) to increase biomass surface area. It also decreases the crystalline nature of lignocellulose feedstock and reduces the extent of polymerization of hemicellulose, cellulose, etc. Various milling, grinding, and chipping methods contribute in reducing the physical size of the particle and to improve the lignocellulosic material digestibility. However, this method consumes a very high amount of energy and not efficient economically (Alvira et al. 2010; Maurya et al. 2015).
3.1.2 Extrusion/Pyrolysis Treatment
The extrusion method was utilized to produce gaseous products and residual char. The pretreatment is basically practiced at high temperatures, i.e., above 300 °C, followed by blending and shearing. This makes the chemical and physical modification in cellulose structure. Screw speed and barrel temperature may interrupt the lignocellulosic structure, causing fiber defibrillation, fibrillation, and shortening and enhanced carbohydrate arability to enzyme attack (Maurya et al. 2015; Rastogi and Shrivastava 2017).
3.1.3 Popping Pretreatment
Popping pretreatment employs heat and pressure to enhance cell wall enzyme accessibility (Nguyen et al. 2017). This technique is similar to water-impregnated steam explosion, which combines the mechanical forces of a rapid explosion with hydrolysis in high-temperature water and acetic acid generated from acetyl groups in the biomass. As biomass is generated by the pretreatment, there is a “pop-out.” In contrast to this process, the equipment used for popping pretreatment is a fairly simple system made up of a direct burner, a rotary reactor, and no steam generator. This method has a lot of advantages over other ways of doing things. For example, it has a much smaller effect on the environment and a higher saccharification efficiency than commonly used methods with similar properties. They are not suitable for a scale-up procedure, because of significant capital costs and pressure (Choi et al. 2013; Wi et al. 2013; Jang et al. 2021).
3.2 Physicochemical Pretreatments
The use of physicochemical processes is preferable over the use of chemical methods since they are more environmentally friendly. Despite this, the process becomes more involved, which may result in an increased energy requirement (Li et al. 2022).
3.2.1 Liquid Hot Water
The liquid hot water method, also known as hydrothermal pretreatment, incorporates both physical and chemical pretreatment techniques. In this, the lignocellulosic substrate is added in liquid at high temperature in range from 160 to 240 °C, pressure above 5 MPa, and time that varies for about an hour or more. It generally does not require any sort of rapid decompression, and no additional chemical or catalysts need to be added. Hot water can remove up to 80% of hemicellulose from herbaceous feedstocks and improve enzymatic digestibility. The advantages of this method are that it has good pH maintenance which reduces unnecessary degradation of polymers, it has high ability to recover pentose sugar, less inhibitors are formed, and no corrosion-resistant materials and chemicals are needed. This method requires a lot of energy and water, so it’s not commercially viable (Alvira et al. 2010; Yu et al. 2010).
3.2.2 Steam Explosion
Steam explosion, or autohydrolysis, is the most common pretreatment for lignocellulosic biomass. In this method, chopped biomass is subjected to high-temperature (160–260 °C) and high-pressure saturated steam (20 min) followed by a sudden release of pressure, which causes autohydrolysis of hemicellulose acetyl groups. This results in the segregation of individual fibers which destroy the structural cell wall. Due to steam explosion, the ability of enzymes to do hydrolysis is improved, there is lesser impact on nature, fewer chemicals are required that are not hazardous, and yield of sugar is increased, making it an acceptable method by industries. However, the process has few drawbacks: it is not preferred for softwoods, the hemicellulose content and lignin content are not degraded properly, there is generation of inhibitory materials, and this process is expensive as it requires more equipment for the addition of acid into chamber (Martín et al. 2002; Pan et al. 2005).
3.2.3 Ammonia Fiber Expansion
Ammonia fiber explosion is one of the physical and chemical pretreatments for biomass that are based on ammonia. In this, the lignocellulosic biomass is treated with liquid ammonia for the period of 30–60 min and at relatively moderate temperature (90–100 °C) and then sudden pressure release. After ammonia pretreatment at high pressure and temperature, lignin structure changes, increasing water holding capacity and causing cellulose swelling and phase change, improving the digestibility and reactivity of residual carbohydrates. The other ammonia-based pretreatment methods include soaking aqueous ammonia and ammonia recycle percolation. It has advantages as it enhances the area of reaction for enzymes causing increased digestibility. In this method, less amount of toxic compounds and inhibitors is formed. It is not preferred as this method does not work effectively with biomass containing a high amount of lignin; the amount of hemicellulose and ammonia is reduced largely which then affects the amount of sugar produced (Maurya et al. 2015; Rastogi and Shrivastava 2017).
3.2.4 Wet Oxidation Pretreatment
Wet air oxidation is the sub-critical oxidation of organics or oxidizable inorganics at high temperatures (125–320 °C) and pressures (0.5–2 MPa). Wet air oxidation is a promising alternative pretreatment method for fractionating lignocellulose into a solubilized hemicellulose fraction and a solid cellulose-rich fraction with minimal inhibitor formation, allowing improved enzymatic hydrolysis of the pretreated material for subsequent ethanol fermentation. Wet air oxidation opens cellulose’s crystalline structure, solubilizes hemicellulose, and degrades lignin into CO2, H2O, and carboxylic acids. Deacetylating hemicellulose produces carboxylic acids. At temperatures above 170 °C, adding oxygen makes the reaction exothermic, reducing energy use. Wet oxidation generates acids from hydrolysis and oxidative reactions. It has been shown to be an effective technique for solubilizing hemicelluloses and lignin, as well as increasing cellulose digestibility. It is the most extensively utilized method for producing ethanol, followed by simultaneous saccharification and fermentation. The only thermal energy necessary for WAO is the difference in enthalpy between the incoming and outgoing streams; consequently, minimal fuel is required. Although the initial investment for wet air oxidation is greater than that of other pretreatment techniques, the operational costs are essentially limited to the energy used to compress the air (Alvira et al. 2010; Banerjee et al. 2009).
3.2.5 Oxidative Pretreatment
During oxidative pretreatment, H2O2 or peracetic acid (C2H4O3) is added to water-suspended biomass as H2O2 is a popular oxidizer. Studies show that 1–2% H2O2 at 25–30 °C can dissolve half the lignin and most of the hemicellulose. To increase cellulose accessibility, this pretreatment process eliminates hemicellulose and lignin from biomass. During pretreatment, electrophilic substitution, side chain displacement, alkyl/aryl ether linkage breakage, and oxidative aromatic nucleus cleavage may occur. The main disadvantage is that this process is costly due to the high cost of H2O2, and high-efficiency reaction vessels that can withstand such conditions are required (Maurya et al. 2015).
3.2.6 CO2 Explosion
Supercritical carbon dioxide, which has traditionally been used as an extraction solvent, is now being investigated for non-extractive applications due to its many benefits. The method is based on using CO2 as a supercritical fluid, which is a gaseous fluid that is compressed to a density resembling liquid at temperatures higher than its critical point. Lignin can be effectively removed under supercritical pretreatment conditions, improving substrate digestibility. Co-solvents like ethanol, when added, can enhance CO2 delignification at high pressure. Carbonic acid is produced when CO2 and water react, facilitating the breakdown of polymers. Because CO2 molecules are the same size as water and ammonia, they can fit through the lignocellulose’s tiny pores. High pressure facilitates this mechanism. After the CO2 pressure is released explosively, the structure of cellulose and hemicellulose is disrupted, and the substrate’s available surface area to enzyme attack rises. Supercritical CO2 is widely used as a non-extraction solvent due to its environmental acceptability, non-toxicity, low cost, easy recovery, and non-flammability. CO2 forms carbonic acid in water, accelerating hydrolysis. CO2 explosion is more cost-effective than ammonia expansion and produces fewer inhibitors than steam explosion. High pressure is required (Kim and Hong 2001; Alvira et al. 2010; Maurya et al. 2015).
3.2.7 Microwave Pretreatment
Microwave irradiation is a commonly utilized method due to its great heating efficiency and simple operation. Since microwave-based pretreatment frequently involves both thermal and non-thermal effects, it might be considered a physicochemical process. Microwave pretreatment improves starch digestibility, which can increase enzyme accessibility. Submerging the biomass in diluted chemical reagents and exposing the resulting slurry to microwave radiation for 5–20 min of pretreatment. This alternative energy generates hot nuclei by interacting with the polar molecules of the solvent, which is usually water. It has the potential to change the ultrastructure of cellulose by decomposing lignin and hemicelluloses and increasing the enzymatic sensitivity of lignocellulosic materials. Thus, the reactions may be carried out more rapidly and with improved yields and selectivity. The heating is done by the rotation of the dipoles, in which the polar molecules try to line up in the rapidly changing electromagnetic field caused by the microwaves, and by ionic conduction, which is the instantaneous superheating of the ionic substance caused by friction between the ionic molecules caused by the motion that creates the electric field. The main benefit of this method is that the reactions happen quickly and the mixture is heated evenly. The pretreatment of biomass with the help of microwaves could be a good way to save time, energy, and the formation of inhibitors. It could be thought of as one of the most promising ways to change the native structure of cellulose by breaking down lignin and hemicelluloses, making it more sensitive to enzyme hydrolysis. The sugar production from the substrate could be increased by combining microwave technology with the addition of additives (Maurya et al. 2015; Harahap et al. 2022; Mikulski and Kłosowski 2022; Oyegoke et al. 2022).
3.2.8 Ultrasound Pretreatment
Ultrasound has been used to get hemicelluloses, cellulose, and lignin out of lignocellulosic biomass, but less research has been done on how easily lignocellulosic materials can be broken down by water. Even though there hasn’t been much research on ultrasonic pretreatment of lignocellulose, some researchers have shown that ultrasonic pretreatment makes saccharification of cellulose work better. Higher enzymatic hydrolysis yields after ultrasound pretreatment might be because the introduction of ultrasound field into the enzyme processing solution causes cavitation effects that greatly speed up the movement of enzyme macromolecules toward the substrate surface. Also, the mechanical impacts caused by the collapse of cavitation bubbles make it easier for enzymes to work on solid substrates. Cavitation works best at 50 °C, which is the best temperature for many enzymes (Sun and Tomkinson 2002; Alvira et al. 2010).
3.3 Chemical Pretreatment
Chemical pretreatment increases biomass hydrolysis. Acids, alkalis, organic solvents, and ionic liquids break down lignocellulosic biomass. Due to its simplicity and efficiency, chemical pretreatment is widely employed in industry. Chemical pretreatment can produce inhibitors and environmental pollution (Li et al. 2022).
3.3.1 Concentrated or Diluted Acid
Acid pretreatment is most widely used method because of its high efficiency. Acid pretreatment solubilizes hemicellulose and lignin and makes cellulose more enzyme-accessible. Acid pretreatment can be done with concentrated or diluted acid, but concentrated acid produces inhibitory chemicals. Acids remove hemicellulose or lignin from cellulose to increase glucose recovery. Concentrated acids are toxic, corrosive, and hazardous, necessitating corrosion-resistant equipment. However, when these chemicals are used at high temperatures, large amounts of breakdown products are formed. They can also corrode equipment, pollute residues, and harm the environment, among other things. Higher concentrations of these compounds reduce reaction times and eliminate the need for enzymes, but low concentrations require higher temperatures and pressures to obtain optimal hydrolysis efficiencies. One of the drawbacks of using concentrated chemicals is the need to neutralize the samples after they have been treated, which raises the overall expense of the process (Mosier et al. 2005; Maurya et al. 2015; Velazquez-Lucio et al. 2018).
At an industrial scale, diluted acid pretreatment is the best choice. There are two ways to do dilute acid pretreatment: at a high temperature (>180 °C) for a short time and at a lower temperature (>120 °C) for a longer time (30–90 min). Acid that has been diluted seems to be a better way to treat lignocellulosic biomass before it is used in industry. This has been studied for a wide range of lignocellulosic biomass. Organic acids like fumaric and maleic acids are emerging as potential cellulose hydrolysis enhancers for ethanol synthesis (Alvira et al. 2010; Maurya et al. 2015; Rastogi and Shrivastava 2017; Velazquez-Lucio et al. 2018).
With dilute sulfuric acid, which is also the most extensively used acid, high hydrolysis yields have been reported. H2SO4, H3PO4, NH3, C2H4O3, C2H2O4, HCOOH, CH3COOH, and C4H4O4 have all been investigated (Maurya et al. 2015).
3.3.2 Alkali Treatment
Alkaline chemistries are better at pretreating agricultural wastes and herbaceous crops. Alkaline pretreatment removes lignin from biomass by breaking the ester bonds that hold lignin and xylan together. This leaves more cellulose and hemicellulose in the fractions, which is similar to how soda or kraft pulping works (McIntosh and Vancov 2010).
The alkaline treatment entails the application of alkali, often sodium, potassium, calcium, or ammonium hydroxides, to biomass under ambient conditions. Pretreatment with alkali can be done in a wide range of periods, from seconds to days, at room temperature. Because of the swelling caused by the NaOH, the cellulose’s internal surface area expands, while the degree of polymerization and crystallinity decreases. This causes the lignin structure to break. It has been observed that lowering the lignin concentration of hardwood from 24–55% to 20% with NaOH increases digestibility from 14% to 55%. The primary benefit of this method is the effective elimination of lignin from biomass. If you want anything that works, NaOH is your best bet. When compared to other methods, it was found to be superior in expanding usable interior space. However, softwoods with a lignin level higher than 26% showed no reaction to dilute NaOH. Alkaline pretreatment [NaOH/Ca(OH)2], which helps get rid of lignin, can be made more effective by adding air or oxygen. However, the substantial processing expenses incurred after the initial purchase of lime or other hydroxides make the entire process prohibitively expensive. Washing the calcium and sodium salts used in the process uses a lot of water. Furthermore, they are challenging to eliminate (Mosier et al. 2005; Alvira et al. 2010; Maurya et al. 2015).
This procedure removes acetyl and uronic acid from hemicelluloses, increasing enzyme accessibility. Hydrolysis of ester bonds between xylan and hemicellulose residues also occurs. This technique improves cellulose digestibility and lignin solubilization while solubilizing little cellulose and hemicellulose as compared to acid pretreatment. It has been demonstrated that alkaline pretreatment of biomass prior to fermentation can result in greater saccharification yields. Pretreatment with an alkaline solution at high temperatures breaks down hemicelluloses into sugar monomers, reduces cellulose crystallinity, and increases biomass porosity, resulting in faster enzymatic hydrolysis. Sodium hydroxide is primarily used in the alkaline-based technique. Alkaline pretreatment reduces sugar deterioration and is cost-effective because many caustic salts can be recovered and/or regenerated. This approach is distinguished by the induction of solvation and saponification reactions, which result in the formation of pores in the cell wall. This allows intracellular chemicals to escape and reduces starch polymers, cellulose, and starch crystallinity (Maurya et al. 2015; Wang et al. 2016; Mohapatra et al. 2017; Velazquez-Lucio et al. 2018; Yang et al. 2019).
3.3.3 Organosolv
Organosolv extracts lignin from lignocellulosic biomass using organic or aqueous solvents with inorganic acid catalysts. Acetone, methanol, ethanol, ethylene glycol, triethylene glycol, and tetrahydrofurfuryl alcohol are being used. Some organic or aqueous organic solvents can be used as catalysts at higher temperatures with or without organic acids. In some studies, hemicellulose bonds are broken using acid catalysts (HCl, H2SO4, oxalic, or salicylic). In a two-stage fractionation, it has been proposed to combine the organosolv process with prior acid hydrolysis to separate the fiber’s hemicellulose and lignin. It is possible to achieve high lignin removal (70%) and minimal cellulose loss (less than 2%). The addition of acid typically results in a high yield of xylose. By raising the process temperature (above 185 °C), this acid addition can be avoided for a satisfactory delignification. An important application of the organosolv process is the extraction of high-quality lignin, a product with added value. Due to the effective removal of lignin, this process has demonstrated high levels of enzymatic hydrolysis of treated biomass (around 90%). The main benefit of the organosolv process, when compared to other chemical pretreatments, is the recovery of relatively pure lignin as a by-product (Mosier et al. 2005; Maurya et al. 2015; Mohapatra et al. 2017; Alvira et al. 2010; Joy and Krishnan 2022).
Solvent and catalyst costs are the process’s main drawback. Solvents’ high commercial price is another industrial consideration. Solvent recovery reduces operational costs. Organic solvents are flammable and can cause fires and explosions if used improperly. Solvents may inhibit enzymatic hydrolysis and fermentative microorganisms which raises costs. Organic solvents also stop enzymatic hydrolysis from occurring, so they have to be taken away for enzymatic hydrolysis to work. Solvents need to be taken out of the system using the right extraction and separation methods, such as evaporation and condensation. Solvents should also be recycled to cut down on operational costs. So, getting rid of organic solvents also comes with an extra cost. For economic reasons, ethanol and methanol, which have low molecular weights and low boiling points, are the best solvents (Mosier et al. 2005; Alvira et al. 2010; Maurya et al. 2015; Mohapatra et al. 2017; Rastogi and Shrivastava 2017).
3.3.4 Ozonolysis
In the last few decades, ozonolysis pretreatment has been shown to be effective by breaking down the lignin polymer and making the hemicellulose in lignocellulosic biomass slightly more soluble. During the process, the substrate is kept in a reaction vessel, and the ozone gas is passed through it. There could be packed beds, fixed beds, or stirred semi-batch reactors inside the vessel. Both the amount of water and the type of biomass are very important. It can be used to break up the structure of things like wheat straw, bagasse, pine, peanut, cotton straw, rye straw, and poplar sawdust. The next step, enzymatic hydrolysis, works better when there is less lignin in the wood. Compounds with conjugated double bonds and high electron densities in their functional groups react quickly with ozone. So, since lignin has a lot of C=C bonds, it is most likely to be oxidized when lignocellulosic materials are exposed to ozone. When ozone attacks lignin, it releases water-soluble, low molecular weight compounds, mostly organic acids like formic and acetic acid, which can cause the pH to drop from 6.5 to 2. Ozonolysis has been used to treat agricultural wastes like wheat straw and rye straw to increase the yield of enzymatic hydrolysis in both cases. Most of the time, ozonolysis is done at room temperature and pressure, and it doesn’t leave behind any toxic residues that could affect the hydrolysis and fermentation that come next. More research is needed to make ethanol from ozone-treated lignocellulosic materials. The process can be too expensive because of high ozone requirement (Garcia-Cubero et al. 2009; Alvira et al. 2010; Maurya et al. 2015; Rastogi and Shrivastava 2017).
3.3.5 Ionic Liquid Pretreatment
Ionic liquids are a new type of solvent that is effective, is safe for the environment, and can be used to turn lignocellulosic feedstocks into fuels and chemicals. This is because ionic liquids can break down and separate biomass efficiently. Ionic liquids have gotten a lot of attention as possible biomass pretreatment agents because they have “green” properties like not being volatile or flammable, being chemically and thermally stable, having a wide electro-chemical range, having high ionic conductivity, being able to be recycled, and being able to dissolve a wide range of things. Ionic liquids are a group of melted salts with unique physical and chemical properties. They have shown a lot of promise. This pretreatment process uses ionic liquids with a biomass-to-ionic liquid weight-to-weight ratio of 1:10 and temperatures between 100 and 150 °C. Antisolvents like water, methanol, and ethanol use soluble biomass to regenerate, which is then broken down by enzymes to make fermentable sugars. Ionic liquids behave similarly to salt, which consists of large organic cations and small inorganic anions. At low temperatures, they are liquids (room temperature). Because ionic liquids contain anions such as chloride, formate, acetate, and alkyl phosphonate, they can form hydrogen bonds with cellulose at high temperatures. Ionic liquids have a lot of potential for pretreating lignocellulosic biomass and making substrates that can break down more than 90% of cellulose (Maurya et al. 2015; Nargotra et al. 2018; Das et al. 2021a, b).
However, ionic liquids can be used as pretreatment agents; they are more expensive and aren’t as good at getting rid of lignin and hemicelluloses as other methods. This limits how widely they can be used. Even though the dissolved material was able to settle out, there are still problems with the cost, recycling, and biocompatibility of ionic liquids for further processing. Some choline-based aprotic ionic liquids have been studied and shown to be compatible with both enzymes and microbes. This enables the development of a one-pot reaction system, in which all process steps are performed in a single vessel without separation. Some protic ionic liquids have been called low cost, and this is mostly because they are easier to make. Ionic liquids like 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) have a special way of breaking down complex polysaccharides and getting rid of lignin at room temperature (Nargotra et al. 2018; Das et al. 2021a, b).
Ionic liquids that are still in the biomass could stop hydrolytic enzymes from working and stop fermentation from happening further down the line. It could change how much sugar and biofuel are made in the end. Flash distillation can be used to get ionic liquids back from antisolvents after they have been regenerated, so they can be used again. For ionic liquids to be used on a large scale, energy-efficient ways to recycle them must be found. Before using them in biomass pretreatment, it is also important to think about how toxic they are to enzymes and fermenting microorganisms (Maurya et al. 2015).
3.4 Biological Pretreatment
One crucial approach for digesting lignocellulosic biomass is biological processing. Given that it is carried out naturally without the use of chemicals, biological pretreatment is regarded as a “green” technology. Numerous researchers have used different microbes, and this zero-pollution strategy has drawn a lot of interest since it improves the pace of fermentation and enzymatic saccharification without requiring a substantial financial input. Low energy consumption and minimal to no waste stream output are the key advantages. It may be used for on-farm wet storage to produce biofuels from lignocellulosic biomass at a low cost. The biggest advantages of biological pretreatment over other methods are that there is no chemical recovery after pretreatment, the cost of treatment further down the line is low, and it is easy to use, uses less energy, is good for the environment, and makes less toxic materials like furfural and hydroxymethylfurfural. For biological pretreatment, microorganisms that release a variety of cell wall-degrading enzymes have been utilized successfully. This method primarily employs fungus and bacteria, or their enzymes, and is highly dependent on a variety of factors, including the culture medium, culture duration, strain type, generated enzymes, and degradation processes. These microbes include ruminant bacteria, wood rot fungus, and symbiotic bacteria that can coexist with some invertebrate animals (e.g., termites, earthworms). Through the use of ligninolytic enzymes, wood rot fungi such as white rot, brown rot, and soft rot can partially break down or alter lignin. Cellulolytic and hemicellulolytic systems, which are primarily in charge of hydrolyzing the biomass ingested by their hosts, can be produced by symbiotic bacteria in animal rumens or the digestive tracts of termites (Li et al. 2022; Narayanaswamy et al. 2013; Wan and Li 2013).
Cellulolytic and hemicellulolytic bacteria are typically used to hydrolyze cellulose and hemicellulose into monomeric sugars in biological pretreatment. When the fermentation process and the breakdown of lignocellulosic biomass are initiated simultaneously, bioproducts such as different enzymes, lactate, acetate, organic acids, etc. are produced as well as biofuels such as ethanol, hydrogen, methane, furfural, etc. Several elements impact a biological pretreatment’s success. This includes things about the substrate like biomass composition, inoculum concentration, aeration rate, moisture content, incubation time, incubation temperature, pH, and microbe species. For successful lignin and hemicellulose removal from biomass, an appropriate microbial consortium must be used. Optimal incubation temperature, pH, and time vary by biological consortium. Aeration helps oxygenate, remove CO2, maintain humidity, dissipate heat, and distribute metabolic volatiles (Sharma et al. 2019; Ummalyma et al. 2019; Sindhu et al. 2016).
It is common practice to use a solid-state fermentation technique to carry out biological pretreatment. Biological pretreatment is more time-consuming than thermochemical pretreatments because microorganisms, especially fungi, need more time to completely colonize and degrade biomass feedstocks. Because of this, it is less likely that a processing plant will use this technique for on-site pretreatment of lignocellulosic biomass. However, bacteria’s need for carbohydrates for their own growth and metabolism is a problem that must be addressed when using microbes in pretreatment. Consequently, lignin-degrading microorganisms are chosen for biological pretreatment. However, as compared to the status quo of pretreatment technologies, biological pretreatment has the potential to significantly lessen negative environmental effects and energy consumption. Furthermore, pretreated biomass residues obtained by fungal pretreatment applied as an on-farm wet storage pretreatment would have significantly greater cellulose digestibility than pretreated biomass residues obtained using ensilages that utilize lactic acid bacteria for fermentation (Wan and Li 2013; Li et al. 2022).
3.4.1 Bacterial Pretreatment
Numerous microorganisms produce diverse biomass-degrading enzymes utilized in biological pretreatment. The selection of the best bacteria strains for the pretreatment of lignocellulosic biomass, followed by enzymatic hydrolysis and fermentation, is an important part of making biofuel. Compared to lignin, cellulose and hemicellulose are relatively simpler to break down (Sharma et al. 2019). Bacteria degrade lignin less effectively than fungi, but they’re more adaptable, fast-reproducing, and useful. Bacillus spp., Rhodococcus spp., and Pseudomonas spp. are the predominant bacteria capable of degrading lignin from termite gut, calf stomach, soil, and compost. Bacterial pretreatment of lignocellulosic biomass has great potential for future industrial applications, but there are many unresolved issues, such as low lignin degradation efficiency and unclear metabolic network pathways and enzyme regulatory mechanisms (Li et al. 2022). Despite the fact that the breakdown of lignin by microorganisms has been studied extensively in fungi and less so in bacteria, scientists are very interested in bacterial lignin degradation due to recently discovered bacterial peroxidases, laccases, and β-etherases that can be used to degrade lignin (Sharma et al. 2019).
3.4.2 Fungal Pretreatment
Fungi are widely recognized microorganisms due to the cooperative influence of their enzymes on the decomposition of lignocellulosic waste. Fungi are abundant in nature, and many of them produce useful enzymes that break down plant matter such cellulolytic, hemicellulolytic, and ligninolytic. Because filamentous fungi are abundant and can be isolated from numerous sources, including soil, living plants, and lignocellulosic waste, they are widely utilized. In contrast, lignin’s complex delignification route is proving to be a significant roadblock in the way of studying and choosing the most effective fungal strain. By destroying the cell wall structure of lignocellulosic biomass, white-rot fungi are the best microorganisms for the pretreatment of the majority of lignocellulosic materials, according to the findings of these studies. As the hyphae of white-rot fungi grow and erode the cell wall structure of lignocellulosic biomass, they parasitize the wood system’s cell cavities. To gain access to lignocellulose, the white-rot fungus secretes laccase, lignin peroxidase, manganese peroxidase, and other peroxidases. Diverse white-rot fungi can degrade 30–70% of lignin from various lignocellulosic biomasses in 7–80 days (Sharma et al. 2019; Alvira et al. 2010; Maurya et al. 2015; Li et al. 2022).
Several white-rot fungi, such as Ceriporia lacerata, Phanerochaete chrysosporium, Pleurotus ostreatus, Cyathus stercoreus, Pycnoporus cinnabarinus, and Ceriporiopsis subvermispora, produce lignin peroxidases, which are enzymes that break down lignin. Few white-rot fungi like Trametes versicolor and Ceriporiopsis subvermispora can break down both lignin and whole cellulose (cellulose and hemicellulose). This means that cellulose recovery is low. To get closer to a biological pretreatment of lignocellulose that is cost-effective and improve the hydrolysis so that ethanol yields can be increased, more basidiomycetes fungi that can delignify plant material quickly and efficiently need to be studied and tested (Alvira et al. 2010; Maurya et al. 2015; Sindhu et al. 2016; Li et al. 2022).
To get closer to a biological pretreatment of lignocellulose that is cost-effective and improve the hydrolysis so that ethanol yields can be increased, more basidiomycetes fungi that can delignify plant material quickly and efficiently need to be studied and tested (Alvira et al. 2010; Maurya et al. 2015). Due to process bottlenecks, such as low sugar yields, poor feedstock capacity, lengthy fungal pretreatment time, and sterilization requirements, the pretreatment of fungi on a biorefinery scale does not appear to be economically viable at the current state of technology; significant process improvements are still required to meet product cost targets. White-rot fungi have the ability to degrade a variety of materials, making it particularly challenging to screen strains when utilizing various lignocellulosic biomasses. In addition, selecting a fungal strain that can efficiently degrade lignin and recover cellulose remains challenging, and its commercial application has not advanced (Sharma et al. 2019; Li et al. 2022).
There are a number of benefits to using biological pretreatment over other pretreatment methods. The primary problems with this method are that it takes a long time and the sugars could be taken by the microorganisms for their own growth. The economics of a process can be improved by using it with the combined pretreatment process, combining a biological process with physical and chemical approaches to shorten the pretreatment time (Ummalyma et al. 2019).
3.5 Combined Pretreatment
The physical, chemical, and biological pretreatments of lignocellulosic biomass for biofuel applications have been thoroughly examined. Numerous variables affect the degradability of biomass, including lignin concentration, cellulose crystallinity, and lignin-cellulose bonds. Consequently, a single pretreatment procedure does not produce the desired outcomes due to its intrinsic limitations and limited working modes. In addition, a single pretreatment technique is impractical in terms of cost and efficiency. Combination pretreatment was reported to be more successful than chemical and biological pretreatment alone, according to studies. Combinational pretreatment is an effective method for the development of sophisticated biomass pretreatment systems (Wang et al. 2016; Das et al. 2021a, b).
4 Biomass Fermentation
Numerous microbes are capable of converting biomass into ethanol. Bioethanol is currently regarded as the most important biofuel for the future. It is manufactured through chemical synthesis or fermentation and is subject to major industrial development around the world (Ahmed et al. 2016).
When lignocellulosic biomass is chosen for ethanol fermentation, it needs to be hydrolyzed to glucose before fermentation. Producing ethanol requires microorganism in various ways. Microorganisms are capable of the conversion of sugars to bioethanol mainly in two ways: firstly, by glycolysis, in which two pyruvate molecules are formed by one monosaccharide, by releasing two NAD+, and secondly, by fermentation under anaerobic conditions, NAD+ will be regenerated by transfer of two electrons from NADH to acetaldehyde (which is an intermediate formed during decarboxylation of pyruvate) forming ethanol (Maicas et al. 2002; Balat 2011; Munjal et al. 2012).
The most successful ethanol production is acquired by microbes such as yeast Saccharomyces cerevisiae. In bioethanol production, other microorganisms such as Escherichia coli, Zymomonas mobilis, Thermoanaerobacter ethanolicus, Pichia stipitis, Klebsiella oxytoca, Candida shehatae, Mucor indicus, etc. were also studied. But a good replacement for S. cerevisiae has not yet been found. The most efficient bacterial nanocellulose producers belong to Komagataeibacter genus, which synthesize high amounts and food-grade cellulose (Florea et al. 2016; Bušić et al. 2018).
Although all the enlisted organisms are capable of ethanol production, the wild-type strains produce much less amount of ethanol and thus are inadequate to fulfill the requirement as a viable alternative for bioethanol production. To overcome the restrictions in the utilization of microbes to their full potential, all the above microbes are tailored to improvise hyper-cellulolytic activity, fermentation capacity, uptake of substrate, resistance to stressor, and valorization of multiple biomasses. Several methods such as random and site-directed mutagenesis, protoplast fusion, gene editing, genome editing, metabolic engineering, cell surface engineering, etc. have made it possible to inculcate necessary traits in the microbes allowing us to solve the challenges associated with them and ramp up the ethanol production (Lugani et al. 2020).
Fermentation is a crucial step in the bioethanol production process because bioethanol is made by converting carbohydrates (starch and cellulose) into ethanol with the aid of microorganisms. After the substrate has been pretreated, the lignin is removed so that it can be fermented easily. Combination processing methods, such as simultaneously saccharifying and fermenting, simultaneously saccharifying and co-fermenting, and consolidated bioprocessing, can be used to add value and possibly save money. Using an integrated biorefinery method, the waste from making bioethanol can be used to make biochemicals, fertilizer, heat, and energy, among other things (Velazquez-Lucio et al. 2018; Dhungana et al. 2022). During the fermentation process, yeast cells absorb a portion of the sugar and turn the remainder into glycerol, acetaldehydes, and lactic acid (Braide et al. 2016).
Lignocellulosic biomass is an abundant and readily available ethanol source as it can be used locally without competing with food (Guerrero et al. 2018). The best way to turn lignocellulosic materials into bioethanol is decided by how much it costs, how it affects the environment, and how well it uses energy. There are celluloses, hemicelluloses, lignin, and soluble polar and non-polar polysaccharides in lignocellulosic materials. Even though producing ethanol from lignocellulosic materials is hard, it can replace making bioethanol from food products (Braide et al. 2016). Starchy crops (corn, wheat, barley), sugar crops (sugarcane, sugar beet, sorghum, fruits), and cellulose crops (stems, leaves, trunks, branches, husks) are all acceptable options for alcoholic fermentations (Cutzu and Bardi 2017). Through metabolism, microorganisms are grown in these materials to turn sugars and starches into ethanol (Tran et al. 2019). Yeasts, especially those from the Saccharomyces species, are the most common microorganisms used in the alcoholic fermentation process. Industrial bioethanol production is best when it has a high ethanol yield; a high ethanol tolerance; a high ethanol productivity (>5.0 g/L/h); the ability to grow in simple, cheap, and undiluted media; and the ability to grow in the presence of inhibitors, at a low pH, or at a high temperature (Cutzu and Bardi 2017).
Recent studies have explored using microorganisms to convert biomass into ethanol. Braide et al. (2016) achieved maximum percentage ethanol yield of 6.72% (sugarcane bagasse), 6.23% (sugarcane bark), 6.17% (cornstalk), 4.17% (corncob), and 3.45% (cornhusk) at 72 h of fermentation and at pH 3.60, 3.82, 4.00, 3.64, and 3.65, respectively (Braide et al. 2016). García-Torreiro et al. (2016) used a sequential SSF system to produce ethanol, conducting the saccharification process first for the first 24 h before inoculating P. tannophilus to begin the fermentation. The white-rot fungus Irpex lacteus was used to pretreat the materials. Final ethanol yields for wheat straw, corn stover, barley straw, and corncob were 79 ± 14, 102 ± 8, 91 ± 2, and 106 ± 10 (mg ethanol/g dry substrate), respectively, after 94 h of SSF. Final ethanol concentrations after 94 h of SSF were 12.5 ± 0.8, 13.5 ± 1.0, 10.8 ± 0.2, and 11.5 ± 1.1 g/L (García-Torreiro et al. 2016). Also, by cultivating the cellulase-producing Streptomyces sp. T3-1, a technique developed by Hsu et al. (2011), can significantly increase the hydrolysis efficiency of corncob-based cellulosic material. After 2 days, the fermentation process was complete, with the glucose having been nearly completely depleted and ethanol having reached a maximum concentration of 24.6 g/L (Hsu et al. 2011). Also, Selvakumar et al. (2022) chemically pretreated corncobs using H2SO4 and CH3COOH in varied ratios. Saccharomyces cerevisiae hydrolysates were fermented at 30 °C and 200 rpm for 4 days. This study suggests binary acid pretreatment for lignocellulosic biomass (Selvakumar et al. 2022). Densifying lignocellulosic biomass with alkaline chemicals, corn stover yielded 21.4 g of ethanol per 100 g of corn stover, which was increased using a standard steam autoclave. Without washing or detoxifying the pretreated biomass, 70.6 g/L of ethanol was produced (Chen et al. 2021). Cabral et al. (2016) prepared coconut fibers with alkali pretreatment, hydrolyzed them enzymatically, and then fermented them with Saccharomyces cerevisiae. Despite significant cellulose loss (4.42% for the fiber and 17.9% for the original content), alkaline (5% NaOH) pretreatment solubilized lignin (80%), making coconut fibers a viable raw material for 2G ethanol production trials. Eighty-seven percent of the sugars were converted through enzymatic hydrolysis, and 81% of the hydrolysate substrate was used in ethanolic fermentation, yielding a 59.6% efficiency for sugar to ethanol conversion (Cabral et al. 2016). Choi et al. (2015) established a unique method for producing bioethanol from single-source citrus peel waste (orange, mandarin, grapefruit, lemon, or lime) or citrus peel waste combined with other fruit waste (banana peel, apple pomace, and pear waste). According to the study, yeast fermentation produced higher ethanol concentrations (14.4–29.5 g/L) and yields (90.2–93.1%) than immobilized cell reactor fermentation alone (Choi et al. 2015). In addition, Guerrero et al. (2018) tested fermentation with 0.25 g/L S. cerevisiae cells and no mineral salt addition. PSSF (8 h) and SSF fermentation configurations yielded 4.8% and 4% (v/v) ethanol solutions from banana rachis and pseudo-stem, respectively, indicating 87% and 74% of maximal ethanol production (Guerrero et al. 2018). Moreover, by using banana pseudo-stem as a source for the bioethanol production by S. cerevisiae NCIM 3570, Ingale et al. (2014) investigated crucial aspects of the pretreatment of fungi with A. ellipticus and A. fumigatus for the saccharification of cellulosic substrate for the manufacture of ethanol. The fermentation of 4.1 g/L cellulosic hydrolysate resulted in a maximum yield of 84% and productivity of 0.024 g/h, yielding 17.1 g/L of ethanol after 72 h (Ingale et al. 2014).
Sherpa et al. (2022) researched sugarcane top bioethanol. Separate fermentation produces 3.76% (v/v) ethanol in 48 h, while SSF produces 5.69% (v/v) ethanol in 30.67 h. To boost fermentation efficiency, a partially consolidated bioprocessing method was developed. This process combines pretreatment and saccharification with laccase and cellulase enzyme mixes, followed by co-fermentation with S. cerevisiae and xylose-fermenting yeast AKBR 212. This method produced 7.57% ethanol in 24.30 h (Sherpa et al. 2022). The effects of popping pretreatment on saccharification and fermentation for individual and mixed biomass were investigated by Nguyen et al. in 2017 (coffee husk, cassava stem, and coconut coir) versus simultaneous saccharification and fermentation vs. separate hydrolysis and fermentation. Better than simultaneous fermentation and hydrolysis was simultaneous saccharification. Popping pretreatment enhanced both individual and mixed biomass saccharification efficiency (Nguyen et al. 2017). Wang et al. (2016) investigated the impact of pretreatment with diluted sulfuric acid, ultrasound-assisted alkali, and high pressure-assisted alkali on cotton stalk bioethanol production. Cotton stalk produced the highest reducing sugar yields (271.70 mg/dry biomass) and ethanol yields (45.53%) when high pressure was used to assist the alkali pretreatment. High pressure-assisted alkali pretreatment was proven effective for cotton stalk ethanol generation (Wang et al. 2016). The impact of hydrotrope as a pretreatment method on rice straw for bioethanol production is examined by Devendra and Pandey (2016). Rice straw was delignified using sodium cumene sulfonate and sodium xylene sulfonate as hydrotropes. While sodium cumene sulfonate hydrolysate could be fermented (with Saccharomyces cerevisiae) to produce 0.74% weight percent (w/v) of ethanol with a conversion efficiency of 73.5%, sodium xylene sulfonate hydrolysate could only produce 0.4% w/v ethanol with a conversion efficiency of 79.6% when standard glucose was used as the control (Devendra and Pandey 2016). With this, Yadav et al. (2011) attempted a study to produce bioethanol from rice straw by co-culturing of S. cerevisiae and P. stipitis in a way that allows both hexose and pentose sugars to be turned into ethanol at the same time. The concentration of ethanol was discovered to be 12 g/L by co-culturing OVB 11 (Saccharomyces cerevisiae) and Pichia stipitis NCIM 3498. The yield, volumetric ethanol productivity, and fermentation efficiency were each found to be 0.33 g/L h, 0.4 g/g, and 95%, respectively (Yadav et al. 2011). Moreover, Battista et al. (2016) recovered hydrogen and bioethanol from olive mill wastewater and olive pomace mixture using Saccharomyces cerevisiae anaerobic fermentation. Pretreatments (ultrasonic pretreatment, basic pretreatment, and calcium carbonate addition). Basic pretreatment reduced polyphenol content in the reaction medium and led to cellulose hydrolysis, which increased ethanol concentration from 2.50 g/L to over 10.00 g/L, increasing the process 2.33-fold (Battista et al. 2016).
Ishola et al. (2013) developed simultaneous saccharification, filtration, and fermentation (SSFF) to produce lignocellulosic ethanol. The fermenting organism and the enzymes can be utilized under ideal conditions with SSFF, and the fermenting cultures can be recycled for several cultivations. At 68 and 72 h of fermentation, SSFF and simultaneous saccharification and fermentation (SSF) produce roughly the same quantity of ethanol, 84.2% and 85.3% of the theoretical output, respectively (Ishola et al. 2013). Raja Sathendra et al. (2019) used a hydrothermal method and a chemical method to remove lignin from palm wood that had already been treated. Trichoderma reesei MTCC 4876 was used to break down palm wood that had already been treated. After that, Kluyveromyces marxianus MTCC 1389 used palm wood hydrolysate to make bioethanol. Artificial neural network with a 5-2-1 topology was also used to find the best values for process parameters. At artificial neural network’s best conditions of 45 °C temperature, 156 rpm agitation rate, pH 5, substrate concentration of 8% (v/v), and inoculum size of 3.2% (v/v), the experimental bioethanol yield was 22.90 g/L (Raja Sathendra et al. 2019). Kumar et al. (2022) evaluated humic acid on alkaline pretreatment of Kentucky bluegrass biomass to extract 70.1% lignin and biocomponents. 7.5% (w/v) pretreated biomass and 16 FPU/g cellulase produced 0.55 g/g reducing sugars. Yeast fermentation of biomass hydrolysate produced 76.6% (w/w) ethanol (Kumar et al. 2022).
In addition, Yang et al. (2019) use alkali-catalyzed hot liquid water pretreatment of bamboo to produce bioethanol using Saccharomyces cerevisiae. Maximum bioconversion was achieved using 0.5% NaOH aqueous at 170 °C, separate enzymatic hydrolysis, and fermentation, providing 4.8 g/L ethanol (Yang et al. 2019). In the presence of Saccharomyces cerevisiae, Ahmed et al. (2016) use a solar batch fermenter to produce bioethanol from common date palm waste (Ahmed et al. 2016). In order to improve pretreatment conditions and increase sugar recovery yield for both cotton straw and sunflower straw, Yildirim et al. (2021) used a central composite design of response surface methodology. By fermentation with S. cerevisiae, the maximum ethanol concentration, ethanol yield, and ethanol productivity for cotton straw were 7.21 g/L, 0.41 g/g, and 0.10 g/L h, respectively, at the end of the study. For sunflower straw, these values were 8.05 g/L, 0.40 g/g, and 0.11 g/L h (Yildirim et al. 2021).
Nazar et al. (2022) examined the ligninolytic activity of Bacillus ligniniphilus L1 laccase for bioethanol generation from rice straw. Bacterial laccase increased rice straw cellulase hydrolysis, yielding 15.8 mg/mL glucose, 21.8 mg/mL reducing sugar, and 22.3 mg/mL bioethanol (Nazar et al. 2022). Tiwari et al. (2022) used Klebsiella oxytoca ATCC 13182 to produce bioethanol from rice husk. After 48–72 h of incubation at pH 7, 36 °C, bioethanol production can reach 32.61 0.45 g/L. Acid and biological pretreatment (especially with Aspergillus niger) increased bioethanol production by 1.47 times (47.98 ± 1.25 g/L) (Tiwari et al. 2022). A camel rumen endo-1,4-xylanase was described and tested on agricultural wastes. Rajabi et al. (2022) examined the contribution of endo-1,4-xylanase on B. subtilis AP ethanol production in an SSF system. The enzyme increased ethanol production (p 0.001) to 7.3 g/L with a yield of 26.8% from wheat bran (Rajabi et al. 2022). Ziaei-Rad et al. (2021) use [TEA][HSO4] to pretreat wheat straw. Three-hour pretreatment biomass yielded 43.1 g/L and 84.34% of the theoretical maximum yield after 48 h of fermentation. 10.76% of the biomass was untreated (Ziaei-Rad et al. 2021).
From Table 11.2, it was showed that Saccharomyces cerevisiae is mostly utilized for bioethanol production from a wide range of agricultural residues. However, more research is required to increase the yield of bioethanol through the utilization of various non-explored agricultural biomasses, easy and low-cost pretreatment methods, and new and modified microorganisms.
5 Green Energy Generation in India
Even though renewable energy is strongly emphasized globally and numerous initiatives aim to replace fossil fuel-based energy system, several countries still heavily depend on fossil fuels. Despite the fact that most of the countries are committed to shift to renewable power, the surging energy requirement reduces the transition from fossil fuel to non-fossil fuel energy resources. Although the share of renewables has improved to 28.3% of the global electricity in 2021, it is not up to the mark in agriculture, building, industry, and transport sector (Chaturvedi 2022; REN21 2022).
Amid these events, world policy makers have set new models, adapted novel norms, and proposed ambitious programs which are boosting the shift to renewable-based energy systems. All these landmark policies and programs are steadily gaining impetus as reflected by the escalating share of renewable energy in total energy demand globally. Figure 11.5 is global maps representing some of the leading countries in biofuel production, the USA and Brazil being the world’s biggest biofuel producers (15,000 and 7500 million gallons, respectively). World map of biofuel productivity is changing radically due to integration between different energy sectors and availability of energy storage options. This has allowed countries such as Europe, Indonesia, Germany, India, and China to be clustered as top countries in biofuel production (Chaturvedi 2022; REN21 2022).
Global map showing top leading countries in biofuel production (billion gallons) (Statista 2022)
Despite being listed in the top countries in biofuel production, as depicted in Fig. 11.6, in India, significant share of energy is still extracted from fossil fuels, followed by hybrid energy systems; however, contribution of renewable energy in total final energy demand remains low.
Fossil fuel and non-fossil fuel generation capacity installed in India (CEA 2022)
To suffice the energy needs, India has adopted new business models to reduce draining out of fossil fuels and accelerated installation of power capacity and grid connect. India has launched green energy corridor project and renewable energy hybrid projects and introduced schemes such as PM KUSUM, Roof Top Solar (RTS) Programme, and Solar Parks to achieve the clean energy target. The country is working on enhancing domestic manufacturing of energy equipment, generating skilled energy labor, and giving attractive incentives to provide clean energy. The Government of India aims to develop at least one city in each state as solar city and has set a target of 175 GW of renewable energy capacity by the year 2022, out of which 114 GW is already achieved till June 2022. India is well on its way to achieve about 40% cumulative electric power installed capacity from non-fossil fuel-based energy resources by 2030 (Chaturvedi 2022; REN21 2022).
6 Future Prospects
The production of advanced bioethanol from lignocellulosic biomass has undergone extensive research and development over the past few decades, and as a result, this biofuel is now an essential part of meeting the expected future demand for bioethanol. Cellulosic ethanol still needs to be fully commercialized, though, so higher production yields and lower costs are still needed. To maximize the use of lignocellulosic biomass sources and increase the sustainability of the conversion process, innovative and sophisticated technologies are required at each stage of the process (Duque et al. 2021). Traditional ethanol feedstocks including molasses, sugarcane juice, corn, etc. have societal and economic obstacles (Kumar et al. 2009). Genome engineering and cell wall modification are currently cutting-edge techniques for feedstock modification. There is currently no industrially viable pretreatment method for the removal of lignin that is both affordable and effective enough to meet the demand for bioethanol globally (Lamichhane et al. 2021). One microorganism modified with genes or microbial consortia reduces costs, but another problem is microbe survival and co-utilization of pentose and hexose carbohydrates. Now, researchers are developing modified strains that can break down polysaccharides and utilize carbohydrates even when hazardous substances are present (Devi et al. 2022).
Pretreatment technology provides a practical way to separate the main lignocellulose constituents and reveal the cellulose that is present (Zhao et al. 2022). The best bioethanol can be made from feedstock if the feedstock is pretreated first. There are different kinds of pretreatment. They can be either physical or chemical, and their strength can vary. The best type of pretreatment is one that doesn’t cost much and makes a lot of bioethanol (Oyegoke et al. 2022).
It is still necessary to create more sophisticated pretreatment technologies that can regulate mechanisms, adjust to the special characteristics of various types of biomass, and keep costs low (Mosier et al. 2005). It is crucial to lose as little sugar as possible, increase the concentration of solids as much as possible, and keep the price of reactors and other equipment low in order to further reduce the cost of the pretreatment step in the conversion of biomass into ethanol (Alvira et al. 2010). Investigating technologies that enable pretreatment and saccharification to occur simultaneously in the same vessel will result in cost savings, energy reductions, and process simplification (Li et al. 2022).
The low conversion efficiency of the technique has prevented widespread commercialization even though the proof of concept has already been established. It is possible to use a native single bacterium, a genetically altered organism, or a consortium (Singhania et al. 2022). So, the economic, ecological, and environmental effects of making and using bioethanol are something that should be looked at when trying to find a balance between the quality and harmony of the natural environment, the quality of life, and economic growth (Piwowar and Dzikuć 2022).
7 Conclusion
In the recent past, increased civilization and industrialization has led to the attenuation of fossil fuels and natural substances, thereby causing global warming. In this situation, there is an urgent need to explore alternate fuels. There are various lignocellulose materials listed in this chapter which can be valorized into not only first-generation and second-generation bioethanol but also the third-generation bioethanol produced in fair amount using algae. The chapter has made an attempt to summarize different technologies involved in the production of bioethanol. Some of the steps discussed here include pretreatment and hydrolysis methods which allow to obtain better yield or contribute toward generating almost negligible waste. Further, vast diversity of microbes available with different characteristics can be used as per need and can also be combined with each other for better bioethanol production. There are yet several areas of research in this field which are being explored all around the world to find the most efficient and effective way of producing bioethanol contribution to global energy demand. Cleaner and greener production of bioenergy is important for sustainable development, and this substitution will surely be an important factor in increasing the health of our Mother Nature.
References
Aditiya HB, Mahlia TMI, Chong WT, Nur H, Sebayang AH (2016) Second generation bioethanol production: a critical review. Renew Sustain Energy Rev 66:631–653
Ahmed B, Mabrouk K, Cherif K, Boudjemaa B (2016) Bioethanol production from date palm fruit waste fermentation using solar energy. Afr J Biotechnol 15(30):1621–1627. https://doi.org/10.5897/ajb2016.15368
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13):4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Annamalai N, Al Battashi H, Anu SN, Al Azkawi A, Al Bahry S, Sivakumar N (2020) Enhanced bioethanol production from waste paper through separate hydrolysis and fermentation. Waste Biomass Valor 11(1):121–131
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52(2):858–875
Banerjee S, Sen R, Pandey RA, Chakrabarti T, Satpute D, Giri BS, Mudliar S (2009) Evaluation of wet air oxidation as a pretreatment strategy for bioethanol production from rice husk and process optimization. Biomass Bioenergy 33(12):1680–1686. https://doi.org/10.1016/j.biombioe.2009.09.001
Battista F, Mancini G, Ruggeri B, Fino D (2016) Selection of the best pretreatment for hydrogen and bioethanol production from olive oil waste products. Renew Energy 88:401–407. https://doi.org/10.1016/j.renene.2015.11.055
Braide W, Kanu IA, Oranusi US, Adeleye SA (2016) Production of bioethanol from agricultural waste. J Fundam Appl Sci 8(2):372–386. https://doi.org/10.4314/jfas.v8i2.14
Bušić A, Marđetko N, Kundas S, Morzak G, Belskaya H, Ivančić Šantek M, Komes D, Novak S, Šantek B (2018) Bioethanol production from renewable raw materials and its separation and purification: a review. Food Technol Biotechnol 56(3):289–311
Butnariu M, Flavius AI (2022) General information about cellulose. Biotechnol Bioprocess 3(3):2766–2314
Cabral MMS, Abud A KdS, Silva CEF, Almeida RMRG (2016) Bioethanol production from coconut husk fiber. Ciência Rural 46(10):1872–1877. https://doi.org/10.1590/0103-8478cr20151331
CEA (2022) Central Electricity Authority. https://cea.nic.in/. Accessed 25 Aug 2022
Chaturvedi P (2022) Future of renewable energy in India. In: Sayigh A (ed) Sustainable energy development and innovation: selected papers from the World Renewable Energy Congress (WREC) 2020. Springer International Publishing, Cham, pp 625–628
Chen H (2014) Chemical composition and structure of natural lignocellulose. In: Chen H (ed) Biotechnology of lignocellulose: theory and practice. Springer, Dordrecht, Netherlands, pp 25–71
Chen X, Yuan X, Chen S, Yu J, Zhai R, Xu Z, Jin M (2021) Densifying lignocellulosic biomass with alkaline chemicals (DLC) pretreatment unlocks highly fermentable sugars for bioethanol production from corn stover. Green Chem 23(13):4828–4839. https://doi.org/10.1039/D1GC01362A
Choi IS, Kim J-H, Wi SG, Kim KH, Bae H-J (2013) Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl Energy 102:204–210
Choi IS, Lee YG, Khanal SK, Park BJ, Bae H-J (2015) A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl Energy 140:65–74. https://doi.org/10.1016/j.apenergy.2014.11.070
Cutzu R, Bardi L (2017) Production of bioethanol from agricultural wastes using residual thermal energy of a cogeneration plant in the distillation phase. Fermentation 3(2). https://doi.org/10.3390/fermentation3020024
Danso-Boateng E, Ross AB, Mariner T, Hammerton J, Fitzsimmons M (2022) Hydrochars produced by hydrothermal carbonisation of seaweed, coconut shell and oak: effect of processing temperature on physicochemical adsorbent characteristics. SN Appl Sci 4(8):203
Das L, Achinivu EC, Barcelos CA, Sundstrom E, Amer B, Baidoo EEK, Simmons BA, Sun N, Gladden JM (2021a) Deconstruction of woody biomass via protic and aprotic ionic liquid pretreatment for ethanol production. ACS Sustain Chem Eng 9(12):4422–4432. https://doi.org/10.1021/acssuschemeng.0c07925
Das N, Jena PK, Padhi D, Mohanty MK, Sahoo G (2021b) A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01294-3
Devendra LP, Pandey A (2016) Hydrotropic pretreatment on rice straw for bioethanol production. Renew Energy 98:2–8. https://doi.org/10.1016/j.renene.2016.02.032
Devi A, Bajar S, Kour H, Kothari R, Pant D, Singh A (2022) Lignocellulosic biomass valorization for bioethanol production: a circular bioeconomy approach. Bioenergy Res. https://doi.org/10.1007/s12155-022-10401-9
Dhungana P, Prajapati B, Maharjan S, Joshi J (2022) Current trends in lignocellulosic bioethanol production. Int J Appl Sci Biotechnol 10(1):1–11
Dien BS, Cotta MA, Jeffries TW (2003) Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol 63(3):258–266
Dunlop MJ, Clemons C, Reiner R, Sabo R, Agarwal UP, Bissessur R, Sojoudiasli H, Carreau PJ, Acharya B (2020) Towards the scalable isolation of cellulose nanocrystals from tunicates. Sci Rep 10(1):19090
Duque A, Álvarez C, Doménech P, Manzanares P, Moreno AD (2021) Advanced bioethanol production: from novel raw materials to integrated biorefineries. Processes 9(2):206–236
Fernandes MC, Ferro MD, Paulino AFC, Mendes JAS, Gravitis J, Evtuguin DV, Xavier A (2015) Enzymatic saccharification and bioethanol production from Cynara cardunculus pretreated by steam explosion. Bioresour Technol 186:309–315
Fischer G, Schrattenholzer L (2001) Global bioenergy potentials through 2050. Biomass Bioenergy 20(3):151–159
Florea M, Hagemann H, Santosa G, Abbott J, Micklem CN, Spencer-Milnes X, de Arroyo Garcia L, Paschou D, Lazenbatt C, Kong D, Chughtai H, Jensen K, Freemont PS, Kitney R, Reeve B, Ellis T (2016) Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc Natl Acad Sci U S A 113(24):E3431–E3440
Garcia-Cubero MA, Gonzalez-Benito G, Indacoechea I, Coca M, Bolado S (2009) Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour Technol 100(4):1608–1613. https://doi.org/10.1016/j.biortech.2008.09.012
García-Torreiro M, López-Abelairas M, Lu-Chau TA, Lema JM (2016) Fungal pretreatment of agricultural residues for bioethanol production. Ind Crop Prod 89:486–492. https://doi.org/10.1016/j.indcrop.2016.05.036
Gonçalves FA, Ruiz HA, Silvino dos Santos E, Teixeira JA, de Macedo GR (2016) Bioethanol production by Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis from delignified coconut fibre mature and lignin extraction according to biorefinery concept. Renew Energy 94:353–365
Guerrero AB, Ballesteros I, Ballesteros M (2018) The potential of agricultural banana waste for bioethanol production. Fuel 213:176–185. https://doi.org/10.1016/j.fuel.2017.10.105
Gupta PK, Raghunath SS, Prasanna DV, Venkat P, Shree V, Chithananthan C, Choudhary S, Surender K, Geetha KJC (2019) An update on overview of cellulose, its structure and applications. Cellulose 201(9)
Harahap AFP, Husnil YA, Ramadhana MYA, Sahlan M, Hermansyah H, Bambang Prasetya MG (2022) Effect of microwave pretreatment on some properties of bamboo (Gigantochloa apus) for bioethanol production. Int J Adv Sci Eng Inf Technol 12(1):365–371
Hsu C-L, Chang K-S, Lai M-Z, Chang T-C, Chang Y-H, Jang H-D (2011) Pretreatment and hydrolysis of cellulosic agricultural wastes with a cellulase-producing Streptomyces for bioethanol production. Biomass Bioenergy 35(5):1878–1884. https://doi.org/10.1016/j.biombioe.2011.01.031
Ingale S, Joshi SJ, Gupte A (2014) Production of bioethanol using agricultural waste: banana pseudo stem. Braz J Microbiol 45(3):885–892
Ioelovich M (2014) Waste paper as promising feedstock for production of biofuel. J Sci Res Rep 3(7):905–916
Ishola MM, Jahandideh A, Haidarian B, Brandberg T, Taherzadeh MJ (2013) Simultaneous saccharification, filtration and fermentation (SSFF): a novel method for bioethanol production from lignocellulosic biomass. Bioresour Technol 133:68–73. https://doi.org/10.1016/j.biortech.2013.01.130
Jang YW, Lee KH, Yoo HY (2021) Improved sugar recovery from orange peel by statistical optimization of thermo-alkaline pretreatment. Processes 9(3):409–422
Jeswani HK, Chilvers A, Azapagic A (2020) Environmental sustainability of biofuels: a review. Proc Math Phys Eng Sci 476(2243):20200351
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. BioFpr 1(2):119–134
Joy SP, Krishnan C (2022) Modified organosolv pretreatment for improved cellulosic ethanol production from sorghum biomass. Ind Crop Prod 177:114409. https://doi.org/10.1016/j.indcrop.2021.114409
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. ScientificWorldJournal 2014:298153
Kaniapan S, Hassan S, Ya H, Patma Nesan K, Azeem M (2021) The utilisation of palm oil and oil palm residues and the related challenges as a sustainable alternative in biofuel, bioenergy, and transportation sector: a review. Sustainability 13(6):3110
Khalil SRA, Abdelhafez AA, Amer EAM (2015) Evaluation of bioethanol production from juice and bagasse of some sweet sorghum varieties. Ann Agric Sci 60(2):317–324
Kim KH, Hong J (2001) Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour Technol 77:139–144
Krumm C, Pfaendtner J, Dauenhauer PJ (2016) Millisecond pulsed films unify the mechanisms of cellulose fragmentation. Chem Mater 28(9):3108–3114
Kulkarni Vishakha S, Butte Kishor D, Rathod Sudha S, Mumbai N (2012) Natural polymers—a comprehensive review. Int J Res Pharm Biomed Sci 3(4):1597–1613
Kumar S, Singh SP, Mishra IM, Adhikari DK (2009) Recent advances in production of bioethanol from lignocellulosic biomass. Chem Eng Technol 32(4):517–526
Kumar A, Singh J, Baskar C (2019) Lignocellulosic biomass for bioethanol production through microbes: strategies to improve process efficiency. In: Rastegari A, Yadav A, Gupta A (eds) Prospects of renewable bioprocessing in future energy systems, vol 10. Springer Nature, Cham, pp 357–386
Kumar R, Basak B, Pal P, Chakrabortty S, Park Y-K, Ali Khan M, Chung W, Chang S, Ahn Y, Jeon B-H (2022) Feasibility assessment of bioethanol production from humic acid-assisted alkaline pretreated Kentucky bluegrass (Poa pratensis L.) followed by downstream enrichment using direct contact membrane distillation. Bioresour Technol 360:127521. https://doi.org/10.1016/j.biortech.2022.127521
Lahiri D, Nag M, Dutta B, Dey A, Sarkar T, Pati S, Edinur HA, Abdul Kari Z, Mohd Noor NH, Ray RR (2021) Bacterial cellulose: production, characterization, and application as antimicrobial agent. Int J Mol Sci 22(23):12984
Lamichhane G, Acharya A, Poudel DK, Aryal B, Gyawali N, Niraula P, Phuyal SR, Budhathoki P, Bk G, Parajuli N (2021) Recent advances in bioethanol production from lignocellulosic biomass. Int J Green Energy 18(7):731–744. https://doi.org/10.1080/15435075.2021.1880910
Lavanya D, Kulkarni PK, Dixit M, Raavi PK, Krishna LNV (2011) Sources of cellulose and their applications – a review. Int J Drug Formul Res 2(6):19–38
Li X, Shi Y, Kong W, Wei J, Song W, Wanga S (2022) Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—a review. Energy Rep 8:696–709
Lugani Y, Rai R, Prabhu AA, Maan P, Hans M, Kumar V, Kumar S, Chandel AK, Sengar RS (2020) Recent advances in bioethanol production from lignocelluloses: a comprehensive review with a focus on enzyme engineering and designer biocatalysts. J Biofuel Res J 7(4):1267–1295
Ma’ruf A, Pramudono B, Aryanti N (2017) Lignin isolation process from rice husk by alkaline hydrogen peroxide: lignin and silica extracted. AIP Conference Proceedings
Maicas S, Ferrer S, Pardo I (2002) NAD(P)H regeneration is the key for heterolactic fermentation of hexoses in Oenococcus oeni. Microbiology 148(1):325–332
Malik K, Salama E-S, El-Dalatony MM, Jalalah M, Harraz FA, Al-Assiri MS, Zheng Y, Sharma P, Li X (2021) Co-fermentation of immobilized yeasts boosted bioethanol production from pretreated cotton stalk lignocellulosic biomass: long-term investigation. Ind Crop Prod 159:113122. https://doi.org/10.1016/j.indcrop.2020.113122
Manikandan S, Subbaiya R, Biruntha M, Krishnan RY, Muthusamy G, Karmegam N (2022) Recent development patterns, utilization and prospective of biofuel production: emerging nanotechnological intervention for environmental sustainability – a review. Fuel 314:122757
Martín C, Galbe M, Nilvebrant N-O, Jönsson LJ (2002) Comparison of the fermentability of enzymatic hydrolyzates of sugarcane bagasse pretreated by steam explosion using different impregnating agents. Appl Biochem Biotechnol 98–100:699–716
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5(5):597–609. https://doi.org/10.1007/s13205-015-0279-4
McIntosh S, Vancov T (2010) Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment. Bioresour Technol 101(17):6718–6727. https://doi.org/10.1016/j.biortech.2010.03.116
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38(4):522–550
Mikulski D, Kłosowski G (2022) Delignification efficiency of various types of biomass using microwave-assisted hydrotropic pretreatment. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-08717-9
Mohapatra S, Mishra C, Behera SS, Thatoi H (2017) Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass – a review. Renew Sustain Energy Rev 78:1007–1032. https://doi.org/10.1016/j.rser.2017.05.026
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Muh E, Tabet F, Amara S (2020) Biomass conversion to fuels and value-added chemicals: a comprehensive review of the thermochemical processes. Curr Altern Energ 4:1–23
Munjal N, Mattam A, Pramanik D, Srivastava P, Yazdani SS (2012) Modulation of endogenous pathways enhances bioethanol yield and productivity in Escherichia coli. Microb Cell Factories 11(1):145
Narayanaswamy N, Dheeran P, Verma S, Kumar S (2013) Biological pretreatment of lignocellulosic biomass for enzymatic saccharification. In: Fang Z (ed) Pretreatment techniques for biofuels and biorefineries. Green energy and technology. Springer, Berlin, pp 3–34
Nargotra P, Sharma V, Gupta M, Kour S, Bajaj BK (2018) Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour Technol 267:560–568. https://doi.org/10.1016/j.biortech.2018.07.070
Nazar M, Xu L, Ullah MW, Moradian JM, Wang Y, Sethupathy S, Iqbal B, Nawaz MZ, Zhu D (2022) Biological delignification of rice straw using laccase from Bacillus ligniniphilus L1 for bioethanol production: a clean approach for agro-biomass utilization. J Clean Prod 360. https://doi.org/10.1016/j.jclepro.2022.132171
Nguyen QA, Yang J, Bae H-J (2017) Bioethanol production from individual and mixed agricultural biomass residues. Ind Crop Prod 95:718–725. https://doi.org/10.1016/j.indcrop.2016.11.040
Nisa LLA, Aritonang S, Manawan MT, Sudiro T (2022) Structure of cellulose and its use: a review. Int J Educ Social Sci Res 5(2):54–67
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose I(alpha) from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125(47):14300–14306
Oyegoke T, Tongshuwar GT, Oguche JE (2022) Biomass pretreatment as a key process in bioethanol productions: a review. J Eng Sci XXIX(1):130–141
Pan X, Xle D, Gilkes N, Gregg DJ, Saddler JN (2005) Strategies to enhance the enzymatic hydrolysis of pretreated softwood with high residual lignin content. Appl Biochem Biotechnol 121–124:1069–1079
Pant S, Ritika, Kuila A (2022) Chapter 8 - Pretreatment of lignocellulosic biomass for bioethanol production. In: Tuli D, Kasture S, Kuila A (eds) Advanced biofuel technologies. Elsevier, Amsterdam, pp 177–194
Pennells J, Godwin ID, Amiralian N, Martin DJ (2019) Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 27(2):575–593
Piwowar A, Dzikuć M (2022) Bioethanol production in Poland in the context of sustainable development-current status and future prospects. Energies 15(7):10.3390/en15072582
Popp J, Lakner Z, Harangi-Rákos M, Fári M (2014) The effect of bioenergy expansion: food, energy, and environment. Renew Sustain Energy Rev 32:559–578
Premjet S (2018) Potential of weed biomass for bioethanol production. In: Basso TP, Basso LC (eds) Fuel ethanol production from sugarcane. IntechOpen, London
Raja Sathendra E, Baskar G, Praveenkumar R, Gnansounou E (2019) Bioethanol production from palm wood using Trichoderma reesei and Kluyveromyces marxianus. Bioresour Technol 271:345–352. https://doi.org/10.1016/j.biortech.2018.09.134
Rajabi M, Nourisanami F, Ghadikolaei KK, Changizian M, Noghabi KA, Zahiri HS (2022) Metagenomic psychrohalophilic xylanase from camel rumen investigated for bioethanol production from wheat bran using Bacillus subtilis AP. Sci Rep 12(1):8152. https://doi.org/10.1038/s41598-022-11412-4
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sustain Energy Rev 80:330–340. https://doi.org/10.1016/j.rser.2017.05.225
REN21 (2022) Renewables 2021 global status report (ISBN 978-3-948393-04-5). REN21 Secretariat, Paris
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1
Sadhukhan J, Martinez-Hernandez E, Murphy RJ, Ng DKS, Hassim MH, Siew Ng K, Yoke Kin W, Jaye IFM, Leung Pah Hang MY, Andiappan V (2018) Role of bioenergy, biorefinery and bioeconomy in sustainable development: strategic pathways for Malaysia. Renew Sustain Energy Rev 81:1966–1987
Sanchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99(13):5270–5295
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37(1):19–27
Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, Bacabac RG, Klein-Nulend J (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28(4):1893–1931
Selvakumar P, Adane AA, Zelalem T, Hunegnaw BM, Karthik V, Kavitha S, Jayakumar M, Karmegam N, Govarthanan M, Kim W (2022) Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel 321:124060. https://doi.org/10.1016/j.fuel.2022.124060
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valor 10:235–251. https://doi.org/10.1007/s12649-017-0059-y
Sherpa KC, Kundu D, Banerjee S, Ghangrekar MM, Banerjee R (2022) An integrated biorefinery approach for bioethanol production from sugarcane tops. J Clean Prod 352:131451. https://doi.org/10.1016/j.jclepro.2022.131451
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass—an overview. Bioresour Technol 199:76–82. https://doi.org/10.1016/j.biortech.2015.08.030
Singhania RR, Patel AK, Singh A, Haldar D, Soam S, Chen C-W, Tsai M-L, Dong C-D (2022) Consolidated bioprocessing of lignocellulosic biomass: technological advances and challenges. Bioresour Technol 354. https://doi.org/10.1016/j.biortech.2022.127153
Statista (2022). https://www.statista.com/statistics/281606/ethanol-production-in-selected-countries/. Accessed 20 Aug 2022
Šturcová A, His I, Apperley DC, Sugiyama J, Jarvis MC (2004) Structural details of crystalline cellulose from higher plants. Biomacromolecules 5(4):1333–1339
Sun R, Tomkinson J (2002) Comparative study of lignins isolated by alkali and ultrasound-assisted alkali extractions from wheat straw. Ultrason Sonochem 9(2):85–93
Takano M, Hoshino K (2018) Bioethanol production from rice straw by simultaneous saccharification and fermentation with statistical optimized cellulase cocktail and fermenting fungus. Bioresour Bioprocess 5(1):16
Tiwari S, Beliya E, Vaswani M, Khawase K, Verma D, Gupta N, Paul JS, Jadhav SK (2022) Rice husk: a potent lignocellulosic biomass for second generation bioethanol production from Klebsiella oxytoca ATCC 13182. Waste Biomass Valor 13(5):2749–2767. https://doi.org/10.1007/s12649-022-01681-5
Tran TTA, Le TKP, Mai TP, Nguyen DQ (2019) Bioethanol production from lignocellulosic biomass. In: Yun Y (ed) Alcohol fuels - current technologies and future prospect. IntechOpen, London, pp 1–13
Uçkun Kiran E, Liu Y (2015) Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 159:463–469
Ummalyma SB, Supriya RD, Sindhu R, Binod P, Nair RB, Pandey A, Gnansounou E (2019) Biological pretreatment of lignocellulosic biomass-current trends and future perspectives. In: Basile A, Dalena F (eds) Second and third generation of feedstocks. Elsevier, Amsterdam, pp 197–212
Velazquez-Lucio J, Rodríguez-Jasso RM, Colla LM, Sáenz-Galindo A, Cervantes-Cisneros DE, Aguilar CN, Fernandes BD, Ruiz HA (2018) Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Res J 5(1):780–791. https://doi.org/10.18331/brj2018.5.1.5
Ververis C, Georghiou K, Danielidis D, Hatzinikolaou DG, Santas P, Santas R, Corleti V (2007) Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour Technol 98(2):296–301
Wan C, Li Y (2013) Solid-state biological pretreatment of lignocellulosic biomass. In: Gu T (ed) Green biomass pretreatment for biofuels production. Springer, Berlin
Wang M, Zhou D, Wang Y, Wei S, Yang W, Kuang M, Ma L, Fang D, Xu S, Du S-k (2016) Bioethanol production from cotton stalk: a comparative study of various pretreatments. Fuel 184:527–532. https://doi.org/10.1016/j.fuel.2016.07.061
Wayman M (1958) The manufacture of chemical cellulose from wood. Can J Chem Eng 36:271–276
Wi SG, Choi IS, Kim KH, Kim HM, Bae H-J (2013) Bioethanol production from rice straw by popping pretreatment. Biotechnol Biofuels 6(166):1–7
Yadav KS, Naseeruddin S, Prashanthi GS, Sateesh L, Rao LV (2011) Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol 102(11):6473–6478. https://doi.org/10.1016/j.biortech.2011.03.019
Yang H, Shi Z, Xu G, Qin Y, Deng J, Yang J (2019) Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour Technol 274:261–266. https://doi.org/10.1016/j.biortech.2018.11.088
Yildirim O, Ozkaya B, Altinbas M, Demir A (2021) Statistical optimization of dilute acid pretreatment of lignocellulosic biomass by response surface methodology to obtain fermentable sugars for bioethanol production. Int J Energy Res 45(6):8882–8899. https://doi.org/10.1002/er.6423
Yu G, Yano S, Inoue H, Inoue S, Endo T, Sawayama S (2010) Pretreatment of rice straw by a hot-compressed water process for enzymatic hydrolysis. Appl Biochem Biotechnol 160(2):539–551
Zhao L, Sun Z-F, Zhang C-C, Nan J, Ren N-Q, Lee D-J, Chen C (2022) Advances in pretreatment of lignocellulosic biomass for bioenergy production: challenges and perspectives. Bioresour Technol 343:126123. https://doi.org/10.1016/j.biortech.2021.126123
Ziaei-Rad Z, Fooladi J, Pazouki M, Gummadi SN (2021) Lignocellulosic biomass pre-treatment using low-cost ionic liquid for bioethanol production: an economically viable method for wheat straw fractionation. Biomass Bioenergy 151:106140. https://doi.org/10.1016/j.biombioe.2021.106140
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Patil, N., Shendkar, T.G., Pardhi, A., Suthar, S.P., Patil, G.S., Pathak, P.D. (2023). Bioethanol Production from Agricultural Biomass: Sources of Cellulose, Pretreatment Methods, and Future Prospects. In: Pathak, P.D., Mandavgane, S.A. (eds) Biorefinery: A Sustainable Approach for the Production of Biomaterials, Biochemicals and Biofuels. Springer, Singapore. https://doi.org/10.1007/978-981-19-7481-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-19-7481-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7480-9
Online ISBN: 978-981-19-7481-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)