Abstract
Lignocellulosic biomass can be a potential source of bioethanol by a microorganism such as yeast and bacteria. Hydrolysis of cellulose resulted in reducing sugars and fermentation of sugar produces bioethanol. Fermentable sugar can be obtained by pretreatment of lignocellulosic biomass which involves physic-chemical techniques along with biological pretreatment. Many fungal organisms such as white fungus and enzymes obtained from them have been reported to carry out the pretreatment process. Several models have been proposed to validate the hydrolysis of cellulose and hemicellulose. Tools of metabolic engineering and genetic engineering are used for the modification of microorganism so that they can utilize the different forms of carbon and perform the fermentation process at a wide range of pH and temperature. Process optimization and kinetic studies of microorganism can help in enhancing the productivity of bioethanol. Monod model and its modifications are used to describe the growth kinetics whereas Leudeking–Piret model for product formation kinetics. Different kinds of unit operations as a tool of downstream processing can be coupled with fermenter to prevent the product toxicity and increase the yield of the ethanol. Thus fuelling the future, the engineered microorganism can be explored for the production of next-generation lignocellulosic bioethanol.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
The global energy requirement is fulfilled by fuel which represents about 70% of the total energy demands (Gouveia and Oliveira 2009). The global energy runs on energy. The high cost of the fossil fuel and conservation of fossil fuel resources forced to produce biofuels via microbial fermentation of biomass (Wargacki et al. 2012). An economic growth and rising population compel for high energy demand. The need of energy will be drastically increased by almost 60% more than today in 2030 by the world of this 45% will be accounted for by India and China together (Patil et al. 2008). Thermochemical conversion and biochemical conversion are primarily used for the conversion of lignocellulosic biomass into simple sugars. In industries the biochemical conversion process produces ethanol. The first generation ethanol can be produced by fermentation of sugars or starch while second-generation ethanol is produced by lignocellulogic biomass which can be converted into sugars. Bioethanol is used in spark ignition engine alternative to petrol as blended fuel E85 (85% bioethanol and 15% gasoline) in most of the developed countries like Brazil, Indonesia, and USA (Jayed et al. 2011; Mussatto et al. 2010). Several developed and developing countries like Brazil, the United States (USA), Australia, Canada, Colombia Japan, India, China, and Europe are interested in economic development by their internal major biofuel markets. Such interests are developed by
-
(I)
increasing the oil prices,
-
(II)
concern about greenhouse gas (GHG) emissions measured by carbon footprint,
-
(III)
the requirements of the “Paris Agreement”.
These days biofuels are the favorable choice of fuel consumption due to generating an acceptable quantity of exhaust gases (Demirbas 2008).
Lignocellulosic biomass such as agricultural residue, forest residue, non-feed energy crops, and municipal solid waste (MSW) are used by lignocellulosic refineries (Chandel et al. 2018). The main constituents of lignocellulosic biomass are cellulose (32–54%), hemicelluloses (11–37%), and lignin (17–32%). Cellulose which is a polymer of glucose formed via β,1 → 4 glycosidic bond and hemicelluloses is made up of xylopyranose units linked through β,1 → 4 glycosidic bonds are chain polysaccharides. Lignin is heteropolymer arranged by cross-linked three dimension phenolic polymers formed from the oxidative combinatorial coupling of three monolignol monomers such as (p-coumaryl alcohol [C9H10O2], coniferyl alcohol [C10H12O3] and sinapyl alcohol [C11H14O4]) (Cao et al. 2017). Figure 14.1 shows lignocellulosic biomass components and their degradable products.
Lignocellulosic biomass components and their degradable products. Dashed line denotes the secondary degradation products (Zabed et al. 2017)
Lignocellulosic biomass pretreatment is used to remove cellulose, hemicellulose, and lignin which enhances cellulose hydrolysis to produce reducing sugars (Sun and Cheng 2002). The effective utilization of both cellulose and hemicellulose consisting of C6 and C5 carbon respectively is required for the production of biofuels and fine chemicals. Figure 14.2 shows the comparative analysis of ethanol production as 1st and 2nd generation biofuel.
Schematic representation of the biofuel production process (Bugg et al. 2011)
14.2 Kinetics of Solubilization
The mechanism of hydrolysis of cellulose by cellulose has been actively studied over the past 70 years. Bansal et al (2009) described the cellulose hydrolysis kinetic model. Figure 14.3 shows the steps in cellulose hydrolysis.
Cellobiohydrolase acting on a cellulosic substrate (Bansal et al. 2009)
The hydrolysis of cellulose involved the following critical steps:
-
1.
Cellulases get adsorbed on the substrate with the help of binding domain.
-
2.
The bonds susceptible to hydrolysis on the substrate surface are localized.
-
3.
The enzyme-substrate complex is formed.
-
4.
The β-glycosidic bonds present on the cellulose chain are hydrolyzed by the action of the enzyme and simultaneous forward sliding of the enzyme.
-
5.
Cellulases desorption from the substrate
-
6.
Cellobiose hydrolysis by the action of β-glucosidase for the formation of glucose.
Several kinetics models have been studies, which proposed the hydrolysis of cellulose and hemicelluloses (Shi et al. 2017a, b). dos Santos Rocha et al. (2017) summarized the models as follows:
Model 1: Cellulose hydrolysis (Saeman 1945).
The kinetics model of lignocellulosic material hydrolysis such as wood was initially proposed by Saeman (1945) at high temperature and in the presence of dilute acid. This model was designed for cellulose hydrolysis to glucose.
Model 2: Hemicellulose hydrolysis (Conner 1984).
Conner (1984) proposed a model to show the degradation of hemicellulose.

Model 3: Hemicellulose degradation into xylooligomers and monomers (Pronyk and Mazza 2010).
A model proposed by Pronyk and Mazza (2010) describes the formation of xylooligomers and sugars by the degradation of hemicelluloses.
14.2.1 Kinetics of Cellulosic Solubilization
The release of sugar from cellulosic biomass is one of the expensive operation (Shi et al. 2017a, b). The sequential steps in the degradation of cellulose are described in Fig. 14.4.
A first-order sequential reactions was proposed to describe the cellulose degradation, by the following equations:
where
- k1:
-
rate of solubilization for cellulosic fractions in monomers,
- k2:
-
rate of solubilization for cellulosic fractions in glucooligomers,
- k3:
-
rate of solubilization of glucooligomers to monomers,
- k4:
-
rate of transformation of glucose monomers degradation to hydroxymethylfurfural
- k5:
-
rate of solubilization of monomers to final degradable products,
- k6:
-
rate of solubilization of hydroxymethylfurfural to final degradable products.
14.2.2 Kinetics of Hemicellulosic Solubilization
The degradation of hemicellulosic fraction during hydrothermal pretreatment can be described in Fig. 14.5.
A first-order sequential reactions steps are proposed to describe the degradation of a hemicellulosic fraction by the following equations:
where
- k1:
-
rate of solubilization for hemicellulose into monomeric fractions,
- k2:
-
rate of solubilization for hemicellulose into xylooligomers,
- k3:
-
rate of solubilization of xylooligomers to monomers,
- k4:
-
rate of transformation of xylose monomers to furfural,
- k5:
-
rate of solubilization of xylose to final degradable products,
- k6:
-
rate of solubilization of furfural to final degradable products.
14.3 Pretreatment Methods
Several physical, chemical, physicochemical, and biological methods have been developed for the pretreatment of lignocellulosic biomass to get fermentable sugars which have been briefly summarized as follows (Larsen et al. 2018; Tian et al. 2018).
14.3.1 Milling
Milling (Mechanical grinding) which involves size reduction of biomass to increase the surface area is generally treated as the first step of the pretreatment process. Different milling methods such as ball milling (to reduce cellulose crystallinity), two-roll milling, hammer milling, vibro energy milling, colloid milling, and disk milling are used in bioethanol production processes which resultant in the particles size reduction to 0.2–2 mm. High energy requirement is one of the most important drawbacks of this process (Veluchamy et al. 2018)
14.3.2 Steam Explosion Pretreatment
Steam explosion is the most widely and commonly used physicochemical method of biomass pretreatment. Biomass is usually treated with high-pressure saturated steam at temperatures 160–240 °C, and pressures 0.7–4.8 MPa, which resulted into digestibility of the lignocellulosic biomass (Agbor et al. 2011; Chiaramonti 2012).
14.3.3 Liquid Hot Water Treatment (LHW)
Liquid hot water (LHW) which is used in hydrothermal pretreatment is used to reduce cell wall rigidity of lignocellulosic biomass. In addition, LHW pretreatment which maintains water in the liquid state at elevated temperatures (160–240 °C) is a green approach, does not need any chemicals (Zhuang et al. 2016).
14.3.4 Ammonia Fiber Expansion (AFEX) Pretreatment
Ammonia-based pretreatment method uses liquid ammonia in a batch reactor under pressure (1.72–2.06 MPa) and moderate temperature (60–120 °C) for several minutes (30–60 min) followed by rapid pressure release is used for lignocellulosic biomass pretreatment. AFEX treatment process resulted in cleavage of carbohydrate and lignin complex (Mood et al. 2013; Yang and Wyman 2008).
14.3.5 CO2 Explosion Pretreatment
Supercritical carbon dioxide (SC–CO2) explosion method uses inexpensive CO2 which acts as a green solvent at critical temperature (Tc) of 31 °C and critical pressure (Pc) of 7.4 MPa, is used for the pretreatment of wet lignocellulosic biomass (Brodeur et al. 2011).
14.3.6 Wet Oxidation Technology
Wet oxidation technology includes water and oxygen or air as a catalyst which is carried out at a temperature above 120 °C and pressures (0.5–2 MPa) for about 30 min. Formation of inhibitors such as furfural and hydroxymethylfurfural (HMF) is lower in the wet oxidation pretreatment (Talebnia et al. 2010).
14.3.7 Acid and Base Pretreatment
Concentrated and dilute acids such as sulphuric acid (H2SO4), hydrochloric acid (HCl), phosphoric acid (H3PO4), nitric acid (HNO3), etc., are used for the pretreatment of lignocellulosic biomass. The process of enzymatic hydrolysis can be improved with the pretreatment of acids to release fermentable sugars (Kumar et al. 2009). Some bases such as sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide [Ca(OH)2], ammonium hydroxide (NH4OH), etc., has been reported for the hydrolysis of biomass which is less harsh as compared to other pretreatment methods can be carried out at lower temperature and pressure. The effect of alkaline treatment depends on the content of lignin present in the biomass. It has been observed that alkaline pretreatment causes less sugar degradation as compared to the acid treatment (Hendriks and Zeeman 2009).
14.3.8 Ozonolysis Pretreatment
Ozonolysis pretreatment includes ozone gas as an effective oxidant in order to break down lignin and hemicelluloses complex and increase cellulose biodegradability and sugar yield (Chaturvedi and Verma 2013).
14.3.9 Organosolvation
Organosolvation process uses an organic acid such as oxalic, acetylsalicylic, and salicylic acids as catalysts or aqueous organic solvents such as methanol, ethanol, acetone, ethylene glycol, triethylene glycol, and tetrahydrofurfuryl alcohol mixture with inorganic acid catalysts (HCl or H2SO4) for lignin and hemicelluloses bond breakage during lignocellulosic biomass pretreatment (Zhu and Pan 2010; Kumar et al. 2009).
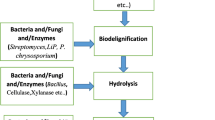
14.3.10 Biological Pretreatment
Biological pretreatment methods include either pure or crude enzyme for hydrolysis of different lignocellulosic biomass. Brown, white, and soft rot fungi have been reported for the degradation of lignin and hemicelluloses and very little cellulose. Several white-rot fungi such as Phanerochaete chrysosporium, Ceriporia lacerata, Cyathus stercolerus, Ceriporiopsis subvermispora, Pycnoporus cinnarbarinus and Pleurotus ostreaus has been reported for their lignin degradation efficiency (Alvira et al. 2010). The main advantages of biological treatment are low energy requirement and mild environment conditions (Taherzadeh and Karimi 2008; Sindhu et al. 2016). Table 14.1 shows the pros and cons of lignocellulosic biomass pretreatment methods.
14.4 Microbes for Bioethanol Production
Microorganism such as Saccharomyces cerevisiae, Schizosaccharomyces pombe, Zymomonas mobilis, Fusariumoxys porum, etc., plays a vital role during ethanol fermentation.
In ethanol fermentation, glucose can be utilized via oxidative metabolism (leads to cell growth) and fermentative metabolism (leads to ethanol fermentation) which are the two different energy producing pathways (Ji et al. 2016). Combined aerobic and anaerobic fed-batch operations are recommended to enhance the ethanol production. Table 14.2 shows the comparison among Zymomonas mobilis, Escherichia coli, and Saccharomyces cerevisiae.
Yeast is most commonly used for the ethanol fermentation due to the utilization of a different range of substrate (Mansouri et al. 2016). The rate of glycolysis is regulated by dissolved oxygen concentration.
The theoretical ethanol yield over glucose is 0.15 g/g and growth yield over glucose is 0.12 g/g. Optimum temperature and pH values for yeast are 30 °C to 35 °C and 4–6 respectively. Production of ethanol from C5 carbon such as xylose is described as follows (Tri and Kamei 2018).
Recently, thermophilic microorganism is in practice for ethanol production at elevated temperature (Shuler and Kargi 2002).
The cellulose and hemicelluloses fraction of lignocellulosic feedstocks can be converted to ethanol either by
-
(i)
simultaneous saccharification and fermentation (SSF)
-
(ii)
separate enzymatic hydrolysis and fermentation (SSF) process and
-
(iii)
consolidated bioprocessing (CBP)
Binod et al. (2010) describe the various ethanol processes as shown in Fig. 14.6.
Various methods of bioethanol production from lignocellulosic feedstocks (Nigam and Singh 2011)
Microbial consortium which may consist of a strain such as Trichoderma reesei, for enzyme production to hydrolyse lignocellulosic biomass and Saccharomyces cerevisiae, and Scheffersomyces stipitis, to utilize hexose and pentose sugars respectively could be used to perform consolidated bioprocessing (CBP) rather than a single microbe to increase the ethanol product yield (Rastogi, and Shrivastava 2017). Figure 14.7 shows the various metabolically engineered strains for ethanol production from pentose sugars.
Metabolically engineered strains for ethanol production from pentose sugars. Abbreviation rec recombinant (Hahn-Hägerdal et al. 2006)
Microorganisms like Saccharomyces cerevisiae, Candida shehatae, Zymomonas mobilis, Pichia stiplis, Pachysolen tannophilus, Escherichia coli, Kluveromyces marxianus, Thermophilic bacteria, Thermoanaero bacterium saccharolyticum, Thermoanaerobacter ethanolicus and Clostridium thermocellum have been reviewed for the production of bioethanol. The advantages and drawbacks of organisms used in lignocellulosic refinery have been depicted in Table 14.3.
14.5 Kinetics Models in Bioethanol Fermentation
Microbial growth kinetics is described by a logistic equation which is a common unstructured growth model. It deals with inhibition of growth which occurs in a batch process (Sewsunker-Sukai and Kana 2018).
Specific growth rate µ is given by Monod model
where
- X:
-
the biomass concentration (g/l),
- Xm:
-
the maximum biomass concentration which is identical to carrying capacity (g/l),
- μm:
-
the maximum growth rate (h−1),
- t:
-
the time (h).
The integration of the Eq. (14.15) with the boundary condition at t = 0, X = X0 gives logistic curve.
Product formation kinetic is described by the following equation:
where YP/S is yield coefficient.
In a batch process, substrate consumption kinetic is described by the following equation (Doran 1995):
where YX/S is yield coefficient and m is maintenance coefficient.
Monod model is generally used to describe the growth of the cells. Excess substrate concentration often leads to poor product formation (the ‘Crabtree effect’). Monod equation that includes a substrate and product inhibition is described as follows (Kashid and Ghosalkar 2018).
where
- P:
-
ethanol concentration (g/l),
- S:
-
substrate concentration (g/l),
- µ:
-
specific growth rate (h−1),
- µmax:
-
the maximum specific growth rate (h−1),
- Ks:
-
saturation constant (g/l),
- KI:
-
inhibition parameter for sugar,
- Pmax:
-
inhibition parameter for ethanol,
- Kp:
-
a constant representing the inhibitory effect due to product,
- n:
-
exponents governing ethanol inhibition of growth.
where Yp/s is the yield coefficient for ethanol on the substrate used for ethanol formation,
The value of substrate concentration at which the specific growth rate is maximum is given by the following equation (Rao 2010):
Substrate inhibition can overcome by fed-batch operation (Lin and Tanaka 2006).
where
- F:
-
feed rate (m3/h),
- V:
-
liquid volume (m3),
- x:
-
cell concentration (g/l),
- D:
-
dilution rate (h−1),
- µ:
-
the specific growth rate (h−1).
It is a differential equation for the rate of change of cell and substrate concentration in a fed-batch reactor. Where
- µ:
-
specific growth rate (h−1),
- qp:
-
the specific rate of product formation (h−1),
- SF:
-
feed concentration of glucose (g/l),
- YX/S:
-
true biomass yield from the substrate (g/g),
- Yp/s:
-
true product yield from the substrate (g/g),
- ms:
-
maintenance coefficient (g g−1h−1).
Substituting µ = D, Monod equation is changed
Rearrangement of Eq. (14.32) gives an expression of substrate concentration as a function of the dilution rate.
Reciprocal plot (1/D vs. 1/S) is used to find out the value of Ks and μmax by interpreting the slope and intercept (Srimachai et al. 2015).
In chemostat culture with µ = D, a plot of \(\frac{1}{{Y_{X/S}^{{^{obs} }} }}\) verses \(\frac{1}{D}\) gives a straight line with slope \(m_{s}\) and intercept \(\frac{1}{{Y_{X/S}^{true} }}\)
where
- \(\frac{1}{{Y_{X/S}^{{^{obs} }} }}\) :
-
the observed biomass yield from the substrate,
- \(\frac{1}{{Y_{X/S}^{true} }}\) :
-
the true biomass yield from the substrate,
- \(m_{s}\) :
-
maintenance coefficient.
The formation of ethanol by microbes can be represented by Leudeking and Piret model (Mansouri et al. 2016).
Ethanol production rate in batch mode is represented by the following equation:
where
- qp:
-
specific product formation rate,
- μ:
-
specific growth rate,
- α:
-
growth-associated product formation coefficient,
- β:
-
nongrowth-associated product formation coefficient,
- P:
-
bioethanol as product concentration,
- X:
-
cell biomass concentration.
Immobilization of yeast within porous or polymeric matrices results in high cell concentrations in the reactor and therefore, high ethanol productivities. Immobilized cells reactors may be in the form of packed columns or fluidized beds. The immobilization kinetic has been given in the equation (Ariyajaroenwong et al. 2016).
where,
- De:
-
effective diffusivity of the substrate,
- µ max :
-
the specific growth rate of the organism (h−1),
- K S :
-
the saturation constant (kg/m−3)
- S :
-
the concentration of the limiting substrate (kg/m−3)
- r:
-
the distance measured radially from the center.
Figure 14.8, shows the method of immobilization of yeast cells. The action of microbes on lignocellulosic feedstocks and optimization parameters for growth conditions is listed in Table 14.4.
The methods of immobilization of yeast cells in a calcium alginate beads and b agar agar cubes (Behera et al. 2010)
14.6 Technologies Used for Development of Strains
14.6.1 CRISPR-Cas9 Genome Editing Technology
Saccharomyces cerevisiae genome can be edited by the CRISPR-Cas9 technology for the utilization of xylose for lignocellulosic ethanol production. This technology has made the genome editing easier in diploid organisms and enable the engineering of 5-10 pathways in yeast genome simultaneously (Jansen et al. 2017; Wang 2015; Löbs, et al 2017). Figure 14.9 shows CRISPR-Cas9-mediated genome editing.

(Source Löbs et al. 2017)
CRISPR-Cas9-mediated genome editing [HR Homologous recombination; NHEJ Nonhomologous end-joining]
14.6.2 Protein Engineering
Protein engineering has improved the pentose uptake kinetics in yeast by the modification of amino acid sequences in proteins (Ko and Lee 2018). Figure 14.10, shows the role of protein engineering for fuel production.
Protein engineering for fuel production (Ko and Lee 2018)
14.6.3 Metabolic Engineering
Tools of system biology as metabolic engineering have improved the production of ethanol in nonconventional yeast by the modification of the pathways as shown in Fig. 14.11 (Löbs et al. 2017).
Metabolic engineering of yeast for biofuels production (Jin and Cate 2017)
14.6.4 Evolutionary Engineering
Evolutionary engineering is used to improve the traits of the organisms. It uses adaptive laboratory evolution for relevant industrial traits selection (Mans et al. 2018). Through adaptive laboratory evolution, yeast strain has been improved which can be grown on pentose sugar to enhance the yield of ethanol (Fig. 14.12).
Evolutionary engineering for strain improvement (Mans et al. 2018)
14.7 Downstream Processing of Ethanol from Fermentation Broth
Conventional distillation is commonly used for ethanol purification. Vacuum fermentation with cell recycling is used for volatile ethanol extraction which enhances the overall process productivity of ethanol (Cardona and Sánchez 2007). Ethanol can be recovered from fermentation broth through gas stripping. Pervaporation which is membrane-based technology is used for ethanol removal and keeping the ethanol concentration below the inhibitory level of the microorganism when coupled with fermentation (Chovau et al. 2011). Extractive fermentation is another promising technique for ethanol recovery. Figure 16.13, shows different modes of ethanol recovery from the fermentation broth.
Different modes of ethanol recovery from the fermentation broth. a Vacuum fermentation with cell recycling. b Fermentation coupled with gas stripping. c Fermentation coupled with pervaporation. d Extractive fermentation (Cardona and Sánchez 2007)
Furthermore fuelling the future, the engineered microorganism can be used for next-generation bioethanol production depending upon lignocellulosic biomass utility by bacteria and fungi (Liao et al. 2016). A portion of hemicellulose can be hydrolyzed through the pretreatment method such as acid pretreatment. The main industrial ethanol producer such as conventional yeast (Saccharomyces cerevisiae) and Zymomonas mobilis cannot utilize xylose (major pentose sugar) as a source of carbon. In an attempt to circumvent this problem, a group of yeast and bacteria have been engineered to utilize xylose with varying degree of success (Fig. 14.14).
Overview of biofuel production from lignocellulosic biomass (Liao et al. 2016)
14.8 Conclusions and Future Prospect
Bioethanol production from lignocellulosic feedstocks by means of microbes is an alternative to renewable energy. But the development of an economically viable process and optimization of pretreatment methods are still required for lignocellulosic feedstocks to enhance the yield of ethanol. Bioethanol production has some major obstacles such as pretreatment process, enzymatic hydrolysis, fermentation, and distillation which are required to overcome by means of efficient technology. Production of fermentable sugars in high concentration by hydrolysis process is yet to be achieved as biomass processing is a major challenging task. Fermentation process requires both pentose and hexose sugars in presence of engineered microbial strains. However much work is still required to bring ethanol production by engineered microorganisms to an industrial level. Distillation is an energy-consuming process, an alternative green process such as pervaporation should be commercialized on industrial scale. Thus, in near future different types of biomass can be effectively utilized and optimized for bioethanol production with the improvement of technologies.
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685
Alvira P, Tomás-Pejó E, Ballesteros MJ, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13):4851–4861
Ariyajaroenwong P, Laopaiboon P, Salakkam A, Srinophakun P, Laopaiboon L (2016) Kinetic models for batch and continuous ethanol fermentation from sweet sorghum juice by yeast immobilized on sweet sorghum stalks. J Taiwan Inst Chem Eng 66:210–216
Bansal P, Hall M, Realff MJ, Lee JH, Bommarius AS (2009) Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol Adv 27(6):833–848
Barbosa CD, Lacerda IC, de Souza Oliveira E (2018) Potential evaluation of Saccharomyces cerevisiae strains from alcoholic fermentation of mango pulp. Afr J Biotechnol 17(28):880–884
Behera S, Kar S, Mohanty RC, Ray RC (2010) Comparative study of bio-ethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomyces cerevisiae cells immobilized in agar agar and Ca-alginate matrices. Appl Energy 87(1):96–100
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101(13):4767–4774
Brandenburg J, Poppele I, Blomqvist J, Puke M, Pickova J, Sandgren M, Rapoport A, Vedernikovs N, Passoth V (2018) Bioethanol and lipid production from the enzymatic hydrolysate of wheat straw after furfural extraction. Appl Microbiol Biotechnol 102(14):6269–6277
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S (2011) Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res 2011
Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R (2011) Pathways for degradation of lignin in bacteria and fungi. Nat Product Rep 28(12):1883–1896
Cao X, Sun S, Sun R (2017) Application of biochar-based catalysts in biomass upgrading: a review. RSC Adv 7(77):48793–48805
Cardona CA, Sánchez ÓJ (2007) Fuel ethanol production: process design trends and integration opportunities. Bioresour Technol 98(12):2415–2457
Chandel AK, Garlapati VK, Singh AK, Antunes FA, da Silva SS (2018) The path forward for lignocellulose biorefineries: bottlenecks, Solutions, and perspective on commercialization. Bioresour Technol 264:370–381
Chaturvedi V, Verma P (2013) An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 3(5):415–431
Chiaramonti D, Prussi M, Ferrero S, Oriani L, Ottonello P, Torre P, Cherchi F (2012) Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 46:25–35
Chovau S, Gaykawad S, Straathof AJ, Van der Bruggen B (2011) Influence of fermentation by-products on the purification of ethanol from water using pervaporation. Bioresour Technol 102(2):1669–1674
Conner AH (1984) Kinetic modeling of hardwood prehydrolysis. Part I. Xylan removal by water prehydrolysis. Wood Fiber Sci 16(2):268–277
Demiray E, Karatay SE, Dönmez G (2018) Evaluation of pomegranate peel in ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Energy 159:988–994
Demirbas A (2008) Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers Manag 49(1):125–130
Doran PM (1995) Bioprocess engineering principles. Elsevier
dos Santos Rocha MS, Pratto B, de Sousa Junior R, Almeida RM, da Cruz AJ (2017) A kinetic model for hydrothermal pretreatment of sugarcane straw. Bioresour Technol 228:176–185
Ferreira J, Santos VA, Cruz CH (2018) Ethanol production by co-culture of Zymomonas mobilis and Pachysolen tannophilus using banana peels hydrolysate as substrate. Acta Scientiarum Technol 40:e35169
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36(2):269–274
Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G (2006) Bio-ethanol—the fuel of tomorrow from the residues of today. Trends in Biotech 24(12):549–556
Hendriks AT, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Tech 100(1):10–18
Jansen ML, Bracher JM, Papapetridis I, Verhoeven MD, de Bruijn H, de Waal PP, van Maris AJ, Klaassen P, Pronk JT (2017) Saccharomyces cerevisiae strains for second-generation ethanol production: from academic exploration to industrial implementation. FEMS Yeast Res 17(5):1–20
Jayed MH, Masjuki HH, Kalam MA, Mahlia TM, Husnawan M, Liaquat AM (2011) Prospects of dedicated biodiesel engine vehicles in Malaysia and Indonesia. Renew Sustain Energy Rev 15(1):220–235
Ji M, Miao Y, Chen JY, You Y, Liu F, Xu L (2016) Growth characteristics of freeze-tolerant baker’s yeast Saccharomyces cerevisiae AFY in aerobic batch culture. SpringerPlus 5(1):503
Jin YS, Cate JH (2017) Metabolic engineering of yeast for lignocellulosic biofuel production. Curr Opin Chem Biol 41:99–106
Kashid M, Ghosalkar A (2018) Evaluation of fermentation kinetics of xylose to ethanol fermentation in the presence of acetic acid by Pichia stipitis: modeling and experimental data comparison
Ko JK, Lee SM (2018) Advances in cellulosic conversion to fuels: engineering yeasts for cellulosic bioethanol and biodiesel production. Curr Opin Biotech 50:72–80
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729
Larsen J, Poulsen NN, Jeppesen MD, Mogensen KK (2018) Methods of processing lignocellulosic biomass using single-stage autohydrolysis pretreatment and enzymatic hydrolysis. U.S. Patent 9,920,345, issued March 20, 2018
Liao JC, Mi L, Pontrelli S, Luo S (2016) Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat Rev Microbiol 14(5):288
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38(4):449–467
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69(6):627–642
Löbs AK, Schwartz C, Wheeldon I (2017) Genome and metabolic engineering in non-conventional yeasts: current advances and applications. Synth Syst Biotechnol 2(3):198–207
Mans R, Daran JMG, Pronk JT (2018) Under pressure: evolutionary engineering of yeast strains for improved performance in fuels and chemicals production. Curr Opin Biotechnol 50:47–56
Mansouri A, Rihani R, Laoufi AN, Özkan M (2016) Production of bioethanol from a mixture of agricultural feedstocks: Biofuels characterization. Fuel 185:612–621
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5(5):597–609
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93
Mussatto SI, Dragone G, Guimarães PM, Silva JP, Carneiro LM, Roberto IC, Vicente A, Domingues L, Teixeira JA (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28(6):817–830
Mustofa A (2018) Bioethanol production from banana stem by using simultaneous saccharification and fermentation (SSF). In: IOP conference series: materials science and engineering, vol 358, no 1, p 012004. IOP Publishing
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37(1):52–68
Nwuche CO, Murata Y, Nweze JE, Ndubuisi IA, Ohmae H, Saito M, Ogbonna JC (2018) Bioethanol production under multiple stress condition by a new acid and temperature tolerant Saccharomyces cerevisiae strain LC 269108 isolated from rotten fruits. Process Biochem 67:105–112
Patil V, Tran KQ, Giselrød HR (2008) Towards sustainable production of biofuels from microalgae. Int J Mol Sci 9(7):1188–1195
Pronyk C, Mazza G (2010) Kinetic modeling of hemicellulose hydrolysis from triticale straw in a pressurized low polarity water flow-through reactor. Ind Eng Chem Res 49(14):6367–6375
Rao DG (2010) Introduction to biochemical engineering. Tata McGraw-Hill Education
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sustain Energ Rev 80:330–340
Saeman JF (1945) Kinetics of wood saccharification-hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind Eng Chem 37(1):43–52
Sewsynker-Sukai Y, Kana EG (2018) Simultaneous saccharification and bioethanol production from corn cobs: process optimization and kinetic studies. Bioresour Tech 262:32–41
Sharma D, Sud A, Bansal S, Mahajan R, Sharma BM, Chauhan RS, Goel G (2018) Endocellulase production by Cotylidia pannosa and its application in saccharification of wheat bran to bioethanol. BioEnergy Res 11(1):219–227
Shi J, Wu D, Zhang L, Simmons BA, Singh S, Yang B, Wyman CE (2017a) Dynamic changes of substrate reactivity and enzyme adsorption on partially hydrolyzed cellulose. Biotechnol Bioeng 114(3):503–515
Shi S, Guan W, Kang L, Lee YY (2017b) Reaction kinetic model of dilute acid-catalyzed hemicellulose hydrolysis of corn stover under high-solid conditions. Ind Eng Chem Res 56(39):10990–10997
Shuler ML, Kargi F (2002). Bioprocess engineering: basic concepts, 2nd edn. Upper Saddle
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass—an overview. Bioresour Tech 199:76–82
Srimachai T, Nuithitikul K, Sompong O, Kongjan P, Panpong K (2015) Optimization and kinetic modeling of ethanol production from oil palm frond juice in batch fermentation. Energy Procedia 79:111–118
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Tech 83(1):1–11
Tabata T, Yoshiba Y, Takashina T, Hieda K, Shimizu N (2017) Bioethanol production from steam-exploded rice husk by recombinant Escherichia coli KO11. World J Microbiol Biotechnol 33(3):1–7
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9(9):1621–1651
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101(13):4744–4753
Tian SQ, Zhao RY, Chen ZC (2018) Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew Sustain Energy Rev 91:483–489
Tri CL, Kamei I (2018) The improvement of sodium hydroxide pretreatment in bioethanol production from Japanese bamboo Phyllostachys edulis using the white rot fungus Phlebia sp. MG-60. Int Biodeterior Biodegrad 133:86–92
Veluchamy C, Kalamdhad AS, Gilroyed BH (2018) Advanced pretreatment strategies for bioenergy production from biomass and biowaste. In: Handbook of environmental materials management, pp 1–19
Wang TY (2015) Engineering yeast for cellulosic ethanol production. Austin Chem Eng 2(2):1018
Wang X, He Q, Yang Y, Wang J, Haning K, Hu Y, Wu B, He M, Zhang Y, Bao J, Contreras LM (2018) Advances and prospects in metabolic engineering of Zymomonas mobilis. Metab Eng
Wargacki AJ, Leonard E, Win MN, Regitsky DD, Santos CN, Kim PB, Cooper SR, Raisner RM, Herman A, Sivitz AB, Lakshmanaswamy A (2012) An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335(6066):308–313
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin: Innov Sustain Econ 2(1):26–40
Yang P, Zhang H, Cao L, Zheng Z, Mu D, Jiang S, Cheng J (2018) Combining sestc engineered A. niger with sestc engineered S. cerevisiae to produce rice straw ethanol via step-by-step and in situ saccharification and fermentation. 3 Biotech 8(1):12
Yuan Z, Wen Y, Kapu NS (2018) Ethanol production from bamboo using mild alkaline pre-extraction followed by alkaline hydrogen peroxide pretreatment. Bioresour Technol 247:242–249
Zabed H, Sahu JN, Suely A, Boyce AN, Faruq G (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sustain Energy Rev 71:475–501
Zhu JY, Pan XJ (2010) Woody biomass pretreatment for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour Technol 101(13):4992–5002
Zhuang X, Wang W, Yu Q, Qi W, Wang Q, Tan X, Zhou G, Yuan Z (2016) Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour Technol 199:68–75
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, A., Singh, J., Baskar, C. (2019). Lignocellulosic Biomass for Bioethanol Production Through Microbes: Strategies to Improve Process Efficiency. In: Rastegari, A., Yadav, A., Gupta, A. (eds) Prospects of Renewable Bioprocessing in Future Energy Systems. Biofuel and Biorefinery Technologies, vol 10. Springer, Cham. https://doi.org/10.1007/978-3-030-14463-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-14463-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14462-3
Online ISBN: 978-3-030-14463-0
eBook Packages: EnergyEnergy (R0)