Abstract
Laccase was identified very early but the potential of the enzyme has cached the eyes of the researchers globally for two decades. The multifarious applications of laccase have enabled its application in various industrial and environmental sectors. The enzyme has been used for the delignification of lignocellulosic biomass, paper, and pulp industries. The manufacture of fibreboard via the chemical treatment releases formaldehyde and pollutes the environment thereby harming flora, fauna, and humans residing in the nearby areas. Thus, the heed for developing non-polluting technologies gained attention amongst the scientific community and laccase was one of the most apt alternatives for the synthesis of the fibreboard via biological treatment methods. As biological treatment methods are used the synthesis process is eco-friendly, non-polluting, and sustainable as well. Thus, the chapter would elaborate the structure of laccase, the general mode of action of laccase, its role in the synthesis of composite and its mechanism of action on plant fiber. Further to gain better insight other reported applications of laccases are also discussed along with its limitations and future prospect.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Around 2.45 billion years ago, oxygen (O2) concentration in the biosphere increased which gradually oxidized water-soluble iron (Fe) II to water-insoluble Fe III. Due to Fe III’s insolubility, iron was not readily available to the living systems for their metabolic processes. Under this evolutionary pressure, living systems such as aerobic organisms were forced to find naturally available iron-like metals with high redox potentials. As a response, they started utilizing copper (Cu II/Cu I) and manganese (Mn III/Mn II) which had a similar function as iron (Fe II/Fe III) (Andrews et al. 2003). Copper-containing proteins are mostly are extracellular (Crichton and Pierre 2001). They help with O2 transport and activation, as well as electron transfer during redox reactions. These proteins are multicopper oxidases (MCOs) and can oxidize huge range of substrates with help of O2 as an electron acceptor and function as electron transfer proteins (Janusz et al. 2020). One such interesting MCO is laccase. Laccase was first described by Yoshida (1883) that he found in exudates of Rhus vernicifera. Laccases along with peroxidases help in the development of plant cell walls. The presence of high levels of laccase-like MCO and its expression in vascular tissues of Liriodendron tulipifera indicated the requirement for the uptake of high-efficiency iron pumps in lignified tissues (Hoopes and Dean 2004). Laccases found in Anarcardiaceae resin ducts are thought to aid in defense against herbivores as well as a bacterial and fungal invasion (Mayer and Staples 2002). Most of the high redox potentials laccases are from fungi havng biotechnological and industrial significance (Nunes and Kunamneni 2018). It has been detected in several fungal strains and its production is most efficient in white-rot fungi (Shraddha et al. 2011). Fungal laccases only need oxygen and produce water as a byproduct. Because of their requirements and broad substrate specificity, they are regarded as green catalysts with biotechnological applications, including direct bio-electrocatalysis. Laccase and laccase-mediator system (LMS) have its applications in delignification (Virk et al. 2012), biocomposites (Nasir et al. 2014), biobleaching of pulp (Boruah et al. 2019), removal of aromatic pollutants (Khambhaty et al. 2015), treatment of industrial wastewater (Viswanath et al. 2014), biofuel cells and biosensors (Le Goff et al. 2015; Ribeiro et al. 2014) and degradation of diclofenac (DCF) and chloramphenicol (CAP) by laccase in presence mediators (Nguyen et al. 2014). Laccase has sparked tremendous interest for prospective biotechnological applications due to its catalytic characteristics (Abdel-Hamid et al. 2013). Laccase TEMPO oxidation treatment has been used used on cotton fibers for grafting octadecylamine grafting that enhanced the hydrophobic nature of the fiber (Ding et al. 2016). Bertrand et al. (2002) found the primary catalytic function of laccase in the lignification process. In the following year, laccase was applied in bioremediation processes. Pozdnyakova et al. (2006), demonstrated that laccase was used as a degradation tool to degrade polycyclic aromatic hydrocarbons (PAHs). Thus, with more knowledge and research, laccase was utilized in various industries such as food processing, textile industries, and wine stabilization. Table 1 gives an outline of the research varied out with laccase since 2000 to present 2021.
Thus, the present chapter would discuss the general mechanism of action of laccase, the role of laccase in the synthesis of fibreboard, and the mechanism for the synthesis of biocomposite. Further other reported applications of laccases its limitation and the future prospect have also been elaborated.
2 A Fascinating MCO-Laccase
Laccases (EC 1.10.3.2) bio-catalyze a electron (e−) oxidation of substrates and then passes four (e−) to the catalytic copper (Cu) atoms, that are oxidized without releasing partially reduced O2 called reactive oxygen species (ROS) (Janusz et al. 2020; Mehra et al. 2018).
2.1 Structure of Laccase
The 3D structure of MCOs is mainly constructed of β-sheets and turns. They contain a 10–20 kDa sized cupredoxin- like domain. MCOs are mainly of 3 types—2-domain, 3-domain, and 6-domain enzymes. Laccase consist of Greek key β barrel topology and it is ~500 amino acid residues structured in three successive domains. The first domain consists of 150 amino acids, second domain from 150 to 300 amino acids, and the third domain from 300 to 500 amino acids. The presence of disulfide bonds in-between the domains I and II and between I and III stabilizes the structure of laccase (Bertrand et al. 2002; Plácido and Capareda 2015). The structure of laccase has been studied using crystallography, isolating plant and animal laccase as crystals had been difficult to obtain due to the unavailability of proper purification protocols. Despite their broad taxonomic distribution and variety of substrates, it has been demonstrated that Cu in laccases exists in four different Cu catalytic forms per protein unit. These four catalytic Cu atoms are type 1 Cu (T1 Cu) and tri-nuclear Cu clusters (T2 Cu, T3α Cu, and T3β Cu) at the T2/T3 site across all multicopper oxidases. These four Cu ions are divided into three types of structures: Type 1 (paramagnetic ‘blue’ Cu), Type 2 (paramagnetic “normal/non-blue” Cu) and, Type 3 (diamagnetic spin coupled Cu-Cu pair). The majority of the proteins are represented in Table 2.
2.2 General Mode of Action
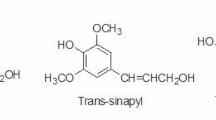
Catalytic participation of laccase’s in coupling reactions is dependent on C–C, C–N, and C–O molecule linkages. Laccase cleaves phenolic components in three ways: Cα–Cβ cleavage, Cα oxidation, and aryl–alkyl cleavage. In laccase-catalyzed oxidation, reaction the initial e− acceptor is T1 Cu that is situated in the cavity near the enzyme surface. The reduction of T1 Cu is a rate-limiting step and the internal electron then moves from T1 to T2 to T3 Cu. Meanwhile, at T2 and T3 Cu sites, O2 is reduced to H2O. Laccase converts phenolic compounds to phenoxyl radicals, which are then polymerized by radical rearrangement or coupling. However, based on the stability of the phenoxyl radicals, redox reversibility with oxidation of a targeted substrate is observed. By acting as mediators, radical-based coupling/redox recycling of phenolic substrates broadens the spectrum of laccase substrates (Patel et al. 2019; Agrawal et al. 2018; Kunamneni et al. 2007).
3 Laccase in the Synthesis of Biocomposite
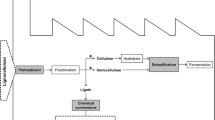
The bio/wood composite is made by the use of two components the i.e., the wood fiber and the adhesive. In the case of synthetic adhesive, formaldehyde and phenol formaldehyde are generally used. However, due to its toxic and harsh effects, the shift has occurred towards the biological synthesis of bio/wood composites (González-García et al. 2011; Moubarik et al. 2010). Also, the Government of Korea stated that the emission level above 4.0 mg/m2.h for the total volatile organic compound (TVOC) is prohibited (JIS A 1901, small chamber method) (ASTM-D6007-96 1996; Kim et al. 2007). The lignin component of the plants is the second most abundantly available polymer after cellulose. As lignin has structural similarity to the phenol-formaldehyde it has been regarded as a potential substitute for the already available synthetic adhesive (Zhou et al. 2011; Kumar et al. 2009). However, despite the two advantages i.e., high availability and a potential substitute for synthetic adhesive it has restricted use as most (80–85%) lignin available are either burned or discarded (Vishtal and Kraslawski 2011; Mai et al. 2000; Pizzi 2003). Thus, the use of lignin can be a game-changer for the industry of biocomposites and as it is a renewable resource the fear of its scarcity in the future would not be an issue (Agrawal and Verma 2020d).
4 Mechanism of Action of Laccase on Plant Fiber
The fiber modification has been an integral part of the synthesis of biocomposite and various physical-chemical, methods have been applied and reported in literature e.g., alkaline, microwave, high temperature, and steam treatments (Verma et al. 2005, 2009, 2011; Verma and Mai 2010). However, due to high cost, energy requirement, and less environmental sustainability, the past decades have been diverted towards the biological and enzyme-mediated treatment of fibers. Laccase is ubiquitous and multi-dimensional protein and has been used for the removal of lignin (Agrawal et al. 2019; Agrawal and Verma 2020a). The biological treatment methods are milder, specific, and more sustainable and cause minimal/no damage to the biological structure of the fiber (Kunamneni et al. 2008). Laccase enzyme is large and cannot penetrate the cells of the fiber it only results in surface modification (van de Pas et al. 2011). It acts on phenolic polymers of lignin with the resulting in reduction of O2 to H2O (Witayakran and Ragauskas 2009). It is due to these properties that laccase is an intensively studied oxidoreductase having numerous applications and recently in biocomposite synthesis (Agrawal et al. 2019; Agrawal and Verma 2020a, e). Also, laccase-mediated oxidation of lignin, free radicals of phenol and polyphenols are formed. As these free radicals are highly reactive it results in depolymerization, co-polymerization, and grafting (Saastamoinen et al. 2012). Further, the structure of lignin exhibits similarity to phenol-formaldehyde and can thus be a potential adhesive for the synthesis of biocomposite (Zhou et al. 2011; Kumar et al. 2009). Despite the advantage of lignin, the major drawback is its transformation to insoluble lignin and thus requires additional cross-linking e.g., maleic anhydride (Syukri et al. 2021). The laccase mediated treatment also has numerous advantages such as improvement in crystallinity index (Agrawal et al. 2019; Agrawal and Verma 2020a) removal of amorphous phenolic and non-phenolic components with no effect on the microfibril core that ultimately enhances the crystallinity of the cellulose of the fiber along with surface modification to form an effective biocomposite (Nasir et al. 2015) (Fig. 1).
5 Other Applications of Laccases
An overview of the various scientific and industrial applications has been represented in Fig. 2 and has been elaborated in the following section.
5.1 Paper and Pulp Industry
Wood is made of small wood fibers that are adhered to by lignin. To separate these wood fibers chemical and physical methods of pulping are used. In chemical pulping fibers are separated by dissolving and degrading lignin using chemical agents whereas in physical pulping fibers are physically ripped apart (Bilal et al. 2019a, b; Singh et al. (2015). Pulping is followed by sheeting which results in the production of paper. Chlorine-based chemicals are used for pulp bleaching; as a result, chlorinated aliphatic and aromatic compounds are formed. The compounds are said to be carcinogenic, mutagenic, and toxic. Extensive research has been undertaken in recent years to develop environmentally sustainable enzymatic bleaching technologies. Pulp bio-bleaching has been demonstrated using laccase-mediated systems, but the lack of proper and cheap mediators has hampered their practicality. Laccases can remove potentially toxic phenols produced during lignin degradation, allowing them to depolymerize lignin and delignify wood pulps. Laccase starts by interacting with small phenolic fragments of lignin, which then react to degrade with the lignin polymer. Moreover, the use of ligninolytic fungi to pretreat wood chips strengthens the pulp while lowering the energy required for mechanical pulping. It is also used to reduce the kappa number of pulp and improve the pulp’s papermaking properties. Thus, the use of laccases in bio-bleaching processes in the pulp and paper industry is an environmentally safe approach (Bilal et al. 2019a, b; Virk et al. 2012).
5.2 Dye Degradation
Massive quantities of wastewaters are released by the textile industries which are contaminated by a large spectrum of chemicals for example azo dyes, which are the primary source of environmental pollution (Paździor et al. 2019). For the environment’s safety, treatment of industrial wastewater has become very important before its safe release into the environment (Salem et al. 2019). These effluents contain recalcitrant dyes (e.g., azo) that pollute the freshwater with their color and carcinogenic intermediates such as the aromatic amines. These chemical reagents are usually complex, synthetic and are unaffected to decolorization in presence of H2O, light, and different chemicals. They are also resistant to various existing dye degradation methods e.g., chemical treatments that are ineffective, and results in the production of intermediate compounds that are mutagenic or carcinogenic (Bilal et al. 2019a, b). As a result, the laccase-assisted dye bioremediation has gained interest due to their diverse potential for the degradation of various dyes via sustainable approach (Couto and Toca-Herrera 2006; Verma 2001; Verma and Madamwar 2002a, b). Since traditional treatment methods based on chemical or physical processes are extremely costly and involve massive quantities of resources, various techniques have recently been investigated as alternatives. Laccase due to its ability to catalyze reactions that can degrade a wide variety of pollutants. For textile wastewater treatment, many aerobic and anaerobic bioprocesses have been developed and extensive research has been done fungal laccases for the production of laccase to improve bioprocesses for the degradation of dyes (Verma and Madamwar 2005). The majority of current dye wastewater treatment processes are inefficient and costly. As a result of their ability to degrade dyes fungal laccase mediated remediation of dyes may provide an appealing solution for a sustainable future.
5.3 Bioremediation
Major issues globally today are polluted air, soil, and water that have disastrous consequences. Industrialization and the widespread use of pesticides in agriculture, contamination of the atmosphere is major problem. Industries have been subjected to stringent regulations to handle their waste effluents before their discharge. Numerous remediation strategies have been reported but only a few have been adopted by the industries. Recently, the ability of fungi to transmute diverse chemicals has sparked the interest of the scientific community (Bollag et al. 2003). Also, low cost, high efficiency, and environmental friendliness it has been considered as a feasible alternative to the pre-established chemical-physical methods (Balcázar-López et al. 2016). Further, enzymatic therapy is now being considered as an substitute strategy for the removal of xenobiotics (Balcázar-López et al. 2016). Laccases can remediate polluted soils via immobilization as they can oxidize toxic organic contaminants including chlorophenols, PAHs, etc. (Zhang et al. 2008; Niu et al. 2013). Farnet et al. (2000) investigated the ability of Marasmius quercophilus laccase to treatalkylphenols. Saparrat et al. (2010) investigated the detoxification of “alpeorujo,” which is a solid by-product from the olive oil extraction industry by Coriolopsis rigida laccase. Laccase has been reported for the removal of dichlorodiphenyltrichloroethane (Yuechun et al. 2010) and 2,4-dichlorophenol (Bhattacharya et al. 2009). The degradation of PAHs by Pleurotus ostreatus laccase has been reported by Pozdnyakova et al. (2006) and high tannin from wastewater by Coriolopsis gallica laccase (Yagüe et al. 2000).
5.4 Food Processing
Laccases have a lot of potential as food additives and manufacturing aids in the food industry (Osma et al. 2010). Laccase-based biocatalysts are energy-efficient and biodegradable, making them ideal for food industries and also to produce low-cost, nutritious foods (Brijwani et al. 2010). Laccases can reduce food processing costs while still being environmentally friendly and to fully realize its ability a detailed understanding of their mode of action is required. Laccase’s versatility in action and widespread presence in many fungi species attest to its ease of use in biotechnological processes. Despite the presence of turbidity, after treatment with laccase and active filtration color consistency improved in fruit juices. Also, the phenolic content of juices reduced after laccase treatment along with stability of color (Ribeiro et al. 2010). Dough enhancement additives are added in bread-making process to improve its taste, texture, volume, and freshness. Thus, laccase addition in dough had an oxidizing effect thereby increasing gluten structures strength in baked goods. Also it improved the crumb structure, softness, increased volume, stability, weight, and, reduced stickiness. It also has to be noted that where laccase decreased extensibility in both flour and gluten dough and increased its resistance. The laccase and proteolytic enzymes when added to oat flour increased loaf specific volume and reduced crumb stiffness, chewiness respectively, and eventually improved its texture. Also, Jurado et al. (2009) stated that the induction of laccase acts as a fermentation inhibitor and increased the output of ethanol from steam-exploded wheat straw and reduced phenolic compounds (Larsson et al. 1999). The polymerization of phenols and polyphenols and the natural co-oxidation reactions have resulted in unwanted fragrance and color changes (Ribeiro et al. 2010). Thus, laccase has been reported and used for the clarification of fruit juices (Narnoliya et al. 2019). Giovanelli and Ravasini (1993) investigated the use of laccase along with filtration for stabilizing apple juice. Phenols were removed more efficiently by laccase treatment over other treatments, such as activated coals (Brijwani et al. 2010). Ribeiro et al. (2010) stated that treatment by laccase significantly decreased the phenolic content of juices while increasing color stability. It has also been found to be more beneficial as compared to traditional treatments e.g., addition of ascorbic acid and sulfites along with the enhancement of its functionality as well as sensory properties. Laccase also contributes in beverage stabilization, role in overall food quality improvement, and use in the baking industry (Manhivi et al. 2018; Di Fusco et al. 2010). Further knowledge of laccase kinetic parameters would be beneficial for functional applications of the enzyme.
5.5 Personal Care Applications
Laccase-generated products contain antimicrobial, detoxifying, or personal care active ingredients and has been used to synthesize anesthetics, anti-inflammatory medicines, etc. (Upadhyay et al. 2016). Couto and Toca-Herrera (2006) stated that the dyeing formula’s hydrogen substitution method based on laccase can resolve the inconvenience of chemical dyes by replacing the hydrogen with oxide. In recent years, skin lightening has been also used for cosmetics and dermatological preparations containing staining proteins. Laccase can be used as fragrant agents in personal care items such as toothpaste, mouthwash, detergent, and soap.
5.6 Pharmaceutical Pollutants
Active pharmaceutical ingredients have been detected in wastewater, and no effective method for the removal of are currently in use at large scale. Also these pollutants when released in water severely damages the aquatic environment or drinking water sources (Sui et al. 2010). This perilous condition necessitated the creation of a system for effectively removing pharmaceutical-based pollutants from wastewater. Researchers have confirmed bioremediation and removal of various pharmaceutically active ingredients using laccase (Rana et al. 2017; Xu et al. 2015). Lonappan et al. (2018) confirmed DCF biodegradation by immobilized laccase and enzyme’s binding improved when biochars were pretreated with citric acid. Remarkably, mature pig biochar immobilized laccase demonstrated a notable ability to fully extract DCF (500 μg L−1) in 2 h. Naghdi et al. (2017) investigated the removal of carbamazepine by immobilized laccase. After three cycles of reusability, the immobilized biocatalytic device retained 70% of its original operation and removed 83% of the carbamazepine from the spiked water. In a study by Taheran et al. (2017) used polyacrylonitrile-biochar composite that was home-prepared for laccase immobilization to degrade chlortetracycline from aqueous solution medium. Furthermore, the composite nanofibrous membrane-immobilized laccase demonstrated notable chlortetracycline removal efficacy (Taheran et al. 2017).
5.7 Biosensor
Oxidation of various organic pollutants, present in wastewater, especially phenolic compounds is catalyzed by laccases. It has a significant effect on the production of biosensors for both environmental and clinically relevant metabolites and it does not need any cofactors for e− transfer reactions. Due to laccase’s wide substrate range in biosensor technology, a large range of phenolics and azides can be detected (Rodríguez-Delgado et al. 2015; Sezgintürk et al. 2005). Laccase coupled multi-walled carbon nano tubes-based biosensors are used to calculate the polyphenol index in wines. A bio-sensor based on laccases coupled with multi-walled carbons nano-tubes measures the index of polyphenols in wine. This biosensor gives a clear and fast amperometric response to gallic acid (Di Fusco et al. 2010). The ultrasensitive amperometric detection of nanomolar catecholamine neurotransmitters (dopamine, epinephrine, and norepinephrine) is achieved by co-immobilization-based enzyme electrodes and laccase on glassy carbon electrodes. The enzyme’s selectivity to different phenolic compounds has been altered by the hybrid material of Nafion/sol-gel silicate used to immobilize laccase (Abdullah et al. 2007).
5.8 Miscellaneous Pollutants
As the population is increasing, agriculture production is being improved. This has led to heavy industrialization and excessive use of pesticides, which has caused a dreadful environmental condition. This has polluted the soil, water and, air with toxic chemicals which can create havoc on human health and climate. Due to these factors, it has become a major concern for the world. Potentially hazardous substances such as fungicides, herbicides, pesticides pharmaceutical compounds, phenolic compounds, PPCPs, and recalcitrant synthetic compounds can be biodegraded by laccase. Bisphenol-A, which has a carcinogenic effect, can be degraded by glutaraldehyde cross-linked chitosan beads. Laccase can degrade a wide range of substances, including polyvinyl chloride (Sumathi et al. 2016), xenobiotics e.g., polynuclear aromatic hydrocarbons (Dias et al. 2003; Cañas et al. 2007), polychlorinated biphenyls (Keum and Li 2004), etc. Laccase catalysis is used to regulate contaminants in the environment where fungal laccases can efficiently degrade and mineralize a variety of environmental contaminants, including BPA (Uchida et al. 2001), chlorophenol (Gaitan et al. 2011), nonylphenol (Tsutsumi et al. 2001), and chlorinated hydroxyl biphenyl (Schultz et al. 2001). It has also been used for the removal of 2,4,6-trinitrotoluene (TNT) (Cheong et al. 2006) and catechol (Tušek et al. 2017).
6 Limitations and Future Aspects
The main limitations of the application aspect using laccase are deactivation factors such as inhibitors, elevated pH, temperature, and non-reusability of free laccase. These drawbacks can be mitigated using new systems such as laccase-mediator or immobilized-laccase catalyzed systems. The lack of capacity to produce large quantities of active enzymes prevents its utility on a large scale. However, these issues can be addressed by recombinant organisms or screening for naturally hypersecretory strains. Thus, strain proficient of producing high titre of a suitable enzyme should be selected followed by optimization of the conditions for laccase production. Recent biotechnological advances, particularly in protein engineering and directed evolution, have enabled essential tools for the efficient development of better enzymes with improved properties with better applicability. Also, the production of new enzymes has been tailored to completely new areas of application where enzymes had not previously been used. Although laccase is still produced in limited quantities, their prospective ability is immense; many of these remain to be revealed. Enzyme immobilization could be used to overcome these limitations while also boosting biodegradation efficiency and enzyme reuse. Since the discovery of laccase, its use has expanded in a variety of sectors and has gained significant interest in the synthesis of biocomposites.
7 Conclusion
Laccase has tremendous potential in the application of biocomposite using plant fibers and the research must now be directed toward less focused aspects of the enzyme to broaden the enzyme’s applications. One of the major limitations of using laccase is the high cost of downstream processes such as laccase purification that raises the overall cost of production, preventing it from being commercialized. As a result, research should concentrate on the development of more efficient and cost-effective methods for large-scale production and commercialization of laccase-based applications. It would facilitate the development of a “greener” approach for a “clean” environment by contributing towards chemical-free treatment in industries, development of a chemical-free biocomposite.
References
Abdel-Hamid AM, Solbiati JO, Cann IK (2013) Insights into lignin degradation and its potential industrial applications. Adv Appl Microbiol 82:1–28
Abdullah J, Ahmad M, Lee YH, Karuppiah N, Sidek H (2007) An optical biosensor based on immobilization of laccase and MBTH in stacked films for the detection of catechol. Sensors 7:2238–2250
Agrawal K, Verma P (2019a) Biodegradation of synthetic dye Alizarin Cyanine Green by yellow laccase producing strain Stropharia sp. ITCC-8422. Biocatal Agric Biotechnol 21:101291
Agrawal K, Verma P (2019b) Column bioreactor of immobilized Stropharia sp. ITCC 8422 on natural biomass support of L. cylindrica for biodegradation of anthraquinone violet R. Bioresour Technol Rep 8:100345
Agrawal K, Verma P (2020a) Production optimization of yellow laccase from Stropharia sp. ITCC 8422 and enzyme-mediated depolymerization and hydrolysis of lignocellulosic biomass for biorefinery application. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00869-w
Agrawal K, Verma P (2020b) Myco-valorization approach using entrapped Myrothecium verrucaria ITCC-8447 on synthetic and natural support via column bioreactor for the detoxification and degradation of anthraquinone dyes. Int Biodeterior Biodegradation 153:105052
Agrawal K, Verma P (2020c) Potential removal of hazardous wastes using white laccase purified by ATPS–PEG–salt system: an operational study. Environ Technol Innov 17:100556
Agrawal K, Verma P (2020d) Production optimization of yellow laccase from Stropharia sp. ITCC 8422 and enzyme-mediated depolymerization and hydrolysis of lignocellulosic biomass for biorefinery application. Biomass Convers Biorefin 9:1–20
Agrawal K, Verma P (2020e) Laccase-mediated synthesis of bio-material using agro-residues. In: Biotechnological applications in human health. Springer, Singapore, p 87
Agrawal K, Chaturvedi V, Verma P (2018) Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess 5:1–12
Agrawal K, Bhardwaj N, Kumar B, Chaturvedi V, Verma P (2019) Process optimization, purification and characterization of alkaline stable white laccase from Myrothecium verrucaria ITCC-8447 and its application in delignification of agroresidues. Int J Biol Macromol 125:1042–1055
Amari A, Alzahrani FM, Alsaiari NS, Katubi KM, Rebah FB, Tahoon MA (2021) Magnetic metal organic framework immobilized laccase for wastewater decolorization. Processes 9(5):774
Andrews SC, Robinson AK, Rodríguez-Quiñones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237
Ardhaoui M, Bhatt S, Zheng M, Dowling D, Jolivalt C, Khonsari FA (2013) Biosensor based on laccase immobilized on plasma polymerized allylamine/carbon electrode. Mater Sci Eng C 33:3197–3205
Arias ME, Arenas M, Rodríguez J, Soliveri J, Ball AS, Hernández M (2003) Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl Environ Microbiol 69:1953–1958
Ashrafi SD, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA (2013) The enzymatic decolorization and detoxification of synthetic dyes by the laccase from a soil-isolated ascomycete, Paraconiothyrium variabile. Int Biodeterior Biodegrad 85:173–181
ASTM-D6007-96 (1996) Standard test method for determining formaldehyde concentrations in air from wood products using a small scale chamber
Balcázar-López E, Méndez-Lorenzo LH, Batista-García RA, Esquivel-Naranjo U, Ayala M, Kumar VV, Savary O, Cabana H, Herrera-Estrella A, Folch-Mallol JL (2016) Xenobiotic compounds degradation by heterologous expression of a Trametes sanguineus laccase in Trichoderma atroviride. PLoS One 11:e0147997
Barrios-Estrada C, de Jesús R-AM, Parra AL, Belleville MP, Sanchez-Marcano J, Iqbal HM, Parra-Saldívar R (2018) Potentialities of active membranes with immobilized laccase for Bisphenol A degradation. Int J Biol Macromol 108:837–844
Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C (2002) Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry 41:7325–7333
Bhattacharya SS, Karmakar S, Banerjee R (2009) Optimization of laccase mediated biodegradation of 2,4-dichlorophenol using genetic algorithm. Water Res 43:3503–3510
Bilal M, Jing Z, Zhao Y, Iqbal HM (2019a) Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal Agric Biotechnol 19:101174
Bilal M, Rasheed T, Nabeel F, Iqbal HMN, Zhao Y (2019b) Hazardous contaminants in the environment and their laccase-assisted degradation—a review. J Environ Manag 234:253–264
Bollag JM, Chu HL, Rao MA, Gianfreda L (2003) Enzymatic oxidative transformation of chlorophenol mixtures. J Environ Qual 32:63–69
Boruah P, Sarmah P, Das PK, Goswami T (2019) Exploring the lignolytic potential of a new laccase producing strain Kocuria sp. PBS-1 and its application in bamboo pulp bleaching. Int Biodeterior Biodegradation 143:104726
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzyme Res 2010:149748
Camarero S, Ibarra D, Martinez AT, Romero J, Gutiérrez A, José C (2007) Paper pulp delignification using laccase and natural mediators. Enzym Microb Technol 40:1264–1271
Cañas AI, Alcalde M, Plou F, Martínez MJ, Martínez ÁT, Camarero S (2007) Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ Sci Technol 41:2964–2971
Cheong S, Yeo S, Song HG, Choi HT (2006) Determination of laccase gene expression during degradation of 2, 4, 6-trinitrotoluene and its catabolic intermediates in Trametes versicolor. Microbiol Res 161:316–320
Couto SR, Toca-Herrera JL (2006) Laccases in the textile industry. Biotechnol Mol Biol Rev 1:115–120
Crichton RR, Pierre J (2001) Old iron, young copper: from Mars to Venus. Biometals 14:99–112
Di Fusco M, Tortolini C, Deriu D, Mazzei F (2010) Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 81:235–240
Dias AA, Bezerra RM, Lemos PM, Pereira AN (2003) In vivo and laccase-catalysed decolourization of xenobiotic azo dyes by a basidiomycetous fungus: characterization of its ligninolytic system. World J Microbiol Biotechnol 19:969–975
Ding H, Wu Y, Zou B, Lou Q, Zhang W, Zhong J, Lu L, Dai G (2016) Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J Hazard Mater 307:350–358
El-Batal AI, ElKenawy NM, Yassin AS, Amin MA (2015) Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol Rep 5:31–39
Farnet AM, Criquet S, Tagger S, Gil G, Le Petit J (2000) Purification, partial characterization, and reactivity with aromatic compounds of two laccases from Marasmius quercophilus strain 17. Can J Microbiol 46:189–194
Gaitan IJ, Medina SC, González JC, Rodríguez A, Espejo ÁJ, Osma JF, Sarria V, Alméciga-Díaz CJ, Sánchez OF (2011) Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens. Bioresour Technol 102:3632–3635
Giovanelli G, Ravasini G (1993) Apple juice stabilization by combined enzyme—membrane filtration process. LWT-Food Sci Technol 26(1):1–7
González-García S, Feijoo G, Heathcote C, Kandelbauer A, Moreira MT (2011) Environmental assessment of green hardboard production coupled with a laccase activated system. J Clean Prod 19:445–453
Gutierrez-Sanchez C, Pita M, Vaz-Dominguez C, Shleev S, De Lacey AL (2012) Gold nanoparticles as electronic bridges for laccase-based biocathodes. J Am Chem Soc 134(41):17212–17220
Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J (2002) Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol 9:601–605
Hoopes JT, Dean FD (2004) Ferroxidase activity in a laccase-like multicopper oxidase from Liriodendron tulipifera. Plant Physiol Biochem 42:27–33
Hou H, Zhou J, Wang J, Du C, Yan B (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39:1415–1419
Janusz G, Pawlik A, Świderska-Burek U, Polak J, Sulej J, Jarosz-Wilkołazka A, Paszczyński A (2020) Laccase properties, physiological functions, and evolution. Int J Mol Sci 21:1–25
Jurado M, Prieto A, Martínez-Alcalá Á, Martínez ÁT, Martínez MJ (2009) Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour Technol 100(24):6378–6384
Keum YS, Li QX (2004) Fungal laccase-catalyzed degradation of hydroxy polychlorinated biphenyls. Chemosphere 56:23–30
Khambhaty Y, Ananth S, Sreeram KJ, Rao JR, Nair BU (2015) Dual utility of a novel, copper enhanced laccase from Trichoderma aureoviridae. Int J Biol Macromol 81:69–75
Kim S, Kim JA, Kim HJ (2007) Application of field and laboratory emission cell (FLEC) to determine formaldehyde and VOCs emissions from wood-based composites. J Korean Wood Sci Technol 35:24–37
Kumar S, Mohanty AK, Erickson L, Misra M (2009) Lignin and its applications with polymers. J Biobaased Mater Bioenergy 3:1–24
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase—a versatile enzyme for biotechnological applications. Communicating current research and educational topics and trends in applied microbiology 1:233–245
Kunamneni A, Camarero S, García-Burgos C, Plo FJ, Ballesteros A, Alcalde M (2008) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories 7:1–17
Larsson S, Reimann A, Nilvebrant NO, Jönsson LJ (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77:91–103
Le Goff A, Holzinger M, Cosnier S (2015) Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cell Mol Life Sci 72(5):941–952
Leite OD, Fatibello-Filho O, Barbosa ADM (2003) Determination of catecholamines in pharmaceutical formulations using a biosensor modified with a crude extract of fungi laccase (Pleurotus ostreatus). J Braz Chem Soc 14:297–303
Lettera V, Pezzella C, Cicatiello P, Piscitelli A, Giacobelli VG, Galano E, Amoresano A, Sannia G (2016) Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem 196:1272–1278
Lonappan L, Liu Y, Rouissi T, Pourcel F, Brar SK, Verma M, Surampalli RY (2018) Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem Eng J 351:985–994
Mai C, Majcherczyk A, Hüttermann A (2000) Chemo-enzymatic synthesis and characterization of graft copolymers from lignin and acrylic compounds. Enzym Microb Technol 27:167–175
Manhivi VE, Amonsou EO, Kudanga T (2018) Laccase-mediated crosslinking of gluten-free amadumbe flour improves rheological properties. Food Chem 264:157–163
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Mehra R, Muschiol J, Meyer AS, Kepp KP (2018) A structural-chemical explanation of fungal laccase activity. Sci Rep 8:1–16
Mogharabi M, Faramarzi MA (2014) Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv Synth Catal 356(5):897–927
Moubarik A, Allal A, Pizzi A, Charrier F, Charrier B (2010) Characterization of a formaldehyde-free cornstarch-tannin wood adhesive for interior plywood. Eur J Wood Wood Prod 68:427–433
Moya R, Hernández M, García-Martín AB, Ball AS, Arias ME (2010) Contributions to a better comprehension of redox-mediated decoloration and detoxification of azo dyes by a laccase produced by Streptomyces cyaneus CECT 3335. Bioresour Technol 101:2224–2229
Naghdi M, Taheran M, Brar SK, Kermanshahi-pour A, Verma M, Surampalli RY (2017) Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci Total Environ 584:393–401
Narnoliya LK, Agarwal N, Patel SN, Singh SP (2019) Kinetic characterization of laccase from Bacillus atrophaeus, and its potential in juice clarification in free and immobilized forms. J Microbiol 57(10):900–909
Nasir M, Gupta A, Beg MDH, Chua GK, Asim M (2014) Laccase application in medium density fibreboard to prepare a bio-composite. RSC Adv 4:11520–11527
Nasir M, Hashim R, Sulaiman O, Nordin NA, Lamaming J, Asim M (2015) Laccase, an emerging tool to fabricate green composites: a review. Bioresources 10:6262–6284
Nguyen LN, Hai FI, Price WE, Leusch FDL, Roddick F, McAdam EJ, Magram SF, Nghiem LD (2014) Continuous biotransformation of bisphenol a and diclofenac by laccase in an enzymatic membrane reactor. Int Biodeterior Biodegradation 95:25–32
Niu J, Dai Y, Guo H, Xu J, Shen Z (2013) Adsorption and transformation of PAHs from water by a laccase-loading spider-type reactor. J Hazard Mater 248:254–260
Nunes CS, Kunamneni A (2018) Laccases-properties and applications. In: Nunes CS, Kumar V (eds) Enzymes in human and animal nutrition: principles and perspectives, Academic Press, London, pp 133–161
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Uses of laccases in the food industry. Enzyme Res 2010:918761
Patel N, Shahane S, Majumdar R, Mishra U (2019) Mode of action, properties, production, and application of laccase: a review. Recent Pat Biotechnol 13:19–32
Pazarlıoǧlu NK, Sariişik M, Telefoncu A (2005) Laccase: production by Trametes versicolor and application to denim washing. Process Biochem 40:1673–1678
Paździor K, Bilińska L, Ledakowicz S (2019) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J 376:120597
Pizzi A (2003) Natural phenolic adhesives II: lignin. In: Handbook of adhesive technology, 2nd edn. Marcel Dekk, New York
Plácido J, Capareda S (2015) Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Bioresour Bioprocess 2(1):1–2
Pozdnyakova NN, Rodakiewicz-Nowak J, Turkovskaya OV, Haber J (2006) Oxidative degradation of polyaromatic hydrocarbons catalyzed by blue laccase from Pleurotus ostreatus D1 in the presence of synthetic mediators. Enzym Microb Technol 39:1242–1249
Rana RS, Singh P, Kandari V, Singh R, Dobhal R, Gupta S (2017) A review on characterization and bioremediation of pharmaceutical industries’ wastewater: an Indian perspective. Appl Water Sci 7:1–12
Renzetti S, Courtin CM, Delcour JA, Arendt EK (2010) Oxidative and proteolytic enzyme preparations as promising improvers for oat bread formulations: rheological, biochemical and microstructural background. Food Chem 119(4):1465–1473
Ribeiro DS, Henrique SM, Oliveira LS, Macedo GA, Fleuri LF (2010) Enzymes in juice processing: a review. Int J Food Sci Technol 45:635–641
Ribeiro FWP, Barroso MF, Morais S, Viswanathan S, de Lima-Neto P, Correia AN, Oliveira MBPP, Delerue-Matos C (2014) Simple laccase-based biosensor for formetanate hydrochloride quantification in fruits. Bioelectrochemistry 95:7–14
Rocasalbas G, Francesko A, Touriño S, Fernández-Francos X, Guebitz GM, Tzanov T (2013) Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr Polym 92:989–996
Rodríguez-Delgado MM, Alemán-Nava GS, Rodríguez-Delgado JM, Dieck-Assad G, Martínez-Chapa SO, Barceló D, Parra R (2015) Laccase-based biosensors for detection of phenolic compounds. Trends Anal Chem 74:21–45
Saastamoinen P, Mattinen ML, Hippi U, Nousiainen P, Sipilä J, Lille M, Suurnäkki A, Pere J (2012) Laccase aided modification of nanofibrillated cellulose with dodecyl gallate. Bio Res 7(4):5749–5770
Salem SS, Mohamed AA, Gl-Gamal MS, Talat M, Fouda A (2019) Biological decolorization and degradation of azo dyes from textile wastewater effluent by Aspergillus niger. Egypt J Chem 62:1799–1813
Sanghi R, Dixit A, Verma P, Puri S (2009) Design of reaction conditions for the enhancement of microbial degradation of dyes in sequential cycles. J Environ Sci 21:1646–1651
Saparrat MCN, Jurado M, Díaz R, Romera IG, Martínez MJ (2010) Transformation of the water soluble fraction from “alpeorujo” by Coriolopsis rigida: the role of laccase in the process and its impact on Azospirillum brasiliense survival. Chemosphere 78:72–76
Schultz A, Jonas U, Hammer E, Schauer F (2001) Dehalogenation of chlorinated hydroxybiphenyls by fungal laccase. Appl Environ Microbiol 67:4377–4381
Sezgintürk MK, Göktuğ T, Dinçkaya E (2005) A biosensor based on catalase for determination of highly toxic chemical azide in fruit juices. Biosens Bioelectron 21:684–688
Shraddha SR, Sehgal S, Kamthania M, Kumar A (2011) Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzyme Res 2011:217861
Singh G, Kaur K, Puri S, Sharma P (2015) Critical factors affecting laccase-mediated biobleaching of pulp in the paper industry. Appl Microbiol Biotechnol 99:155–164
Soares GM, de Amorim MP, Costa-Ferreira M (2001) Use of laccase together with redox mediators to decolourize Remazol brilliant blue R. J Biotechnol 89:123–129
Srebotnik E, Hammel KE (2000) Degradation of nonphenolic lignin by the laccase/1-hydroxybenzotriazole system. J Biotechnol 81:179–188
Sumathi T, Viswanath B, Sri Lakshmi A, SaiGopal DVR (2016) Production of laccase by Cochliobolus sp isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem Res Int 2016:9519527
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res 44(2):417–426
Syukri MSM, Rahman RA, Mohamad Z, Illias RM, Mahmood NAN, Jaafar NR (2021) Optimization strategy for laccase immobilization on polyethylene terephthalate grafted with maleic anhydride electrospun nanofiber mat. Int J Biol Macromol 166:876–883
Taheran M, Naghdi M, Brar SK, Knystautas EJ, Verma M, Surampalli RY (2017) Degradation of chlortetracycline using immobilized laccase on Polyacrylonitrile-biochar composite nanofibrous membrane. Sci Total Environ 605:315–321
Tsutsumi Y, Haneda T, Nishida T (2001) Removal of estrogenic activities of bisphenol A and nonylphenol by oxidative enzymes from lignin-degrading basidiomycetes. Chemosphere 42:271–276
Tušek AJ, Šalić A, Zelić B (2017) Catechol removal from aqueous media using laccase immobilized in different macro-and microreactor systems. Appl Biochem Biotechnol 182:1575–1590
Uchida H, Fukuda T, Miyamoto H, Kawabata T, Suzuki M, Uwajima T (2001) Polymerization of bisphenol A by purified laccase from Trametes villosa. Biochem Biophys Res Commun 287:355–358
Upadhyay P, Shrivastava R, Agrawal PK (2016) Bioprospecting and biotechnological applications of fungal laccase. 3. Biotech 6:15
van de Pas D, Hickson A, Donaldson L, Lloyd-Jones G, Tamminen T, Fernyhough A, Mattinen ML (2011) Characterization of fractionated lignins polymerized by fungal laccases. Bioresources 6:1105–1121
Verma P (2001) White rot fungi mediated integrated approach for lignocellulosic waste decomposition and textile dye decolorization. http://hdl.handle.net/10603/77403
Verma P, Madamwar D (2002a) Production of ligninolytic enzymes for dye decolorization by cocultivation of white-rot fungi Pleurotus ostreatus and Phanerochaete chrysosporium under solid-state fermentation. Appl Biochem Biotechnol 102:109–118
Verma P, Madamwar D (2002) Comparative study on transformation of azo dyes by different white rot fungi. NISCAIR-CSIR, New Delhi, pp 393–396. http://hdl.handle.net/123456789/19893
Verma P, Madamwar D (2005) Decolorization of azo dyes using Basidiomycete strain PV 002. World J Microbiol Biotechnol 21:481–485
Verma P, Mai C (2010) Hydrolysis of cellulose and wood powder treated with DMDHEU by a hydrolase enzyme complex, Fenton’s reagent, and in a liquid culture of Trametes versicolor. Holzforschung 64(1):69–75. https://doi.org/10.1515/hf.2010.007
Verma P, Mai C, Krause A, Militz H (2005) Studies on the resistance of DMDHEU treated wood against white-rot and brown rot fungi. International Research Group on Wood Protection, Stockholm, Sweden Doc. IRG/WP/05-10566
Verma P, Junga U, Militz H, Mai C (2009) Protection mechanisms of DMDHEU treated wood against white and brown rot fungi. De Gruyter 3:371–378. https://doi.org/10.1515/HF.2009.051
Verma P, Watanabe T, Honda Y, Watanabe T (2011) Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated by H2O2. Bioresour Technol 102:3941–3945
Virk AP, Sharma P, Capalash N (2012) Use of laccase in pulp and paper industry. Biotechnol Prog 28:21–32
Vishtal AG, Kraslawski A (2011) Challenges in industrial applications of technical lignins. Bioresources 6:3547–3568
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res 2014:163242
Witayakran S, Ragauskas AJ (2009) Modification of high-lignin softwood kraft pulp with laccase and amino acids. Enzym Microb Technol 44:176–181
Xu R, Tang R, Zhou Q, Li F, Zhang B (2015) Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chem Eng J 262:88–95
Yagüe S, Terrón MC, González T, Zapico E, Bocchini P, Galletti GC, González AE (2000) Biotreatment of tannin-rich beer-factory wastewater with white-rot basidiomycete Coriolopsis gallica monitored by pyrolysis/gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 14:905–910
Yoshida H (1883) Chemistry of lacquer (Urushi). J Chem Soc Trans 43:472–486
Yuechun ZHAO, Xiaoyun YI, Minghua LI, Lu LIU, Weijuan MA (2010) Biodegradation kinetics of DDT in soil under different environmental conditions by laccase extract from white rot fungi. Chin J Chem Eng 18:486–492
Zhang M, Wu F, Wei Z, Xiao Y, Gong W (2006) Characterization and decolorization ability of a laccase from Panus rudis. Enzym Microb Technol 39:92–97
Zhang J, Liu X, Xu Z, Chen H, Yang Y (2008) Degradation of chlorophenols catalyzed by laccase. Int Biodeterior Biodegradation 61:351–356
Zhou X, Tang L, Zhang W, Lv C, Zheng F, Zhang R, Du G, Tang B, Liu X (2011) Enzymatic hydrolysis lignin derived from corn stover as an intrinstic binder for bio-composites manufacture: effect of fiber moisture content and pressing temperature on boards’ properties. Bioresources 6:253–264
Conflict of Interest
Authors have no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Agrawal, K., Vaishnavi, S., Verma, P. (2022). Laccase Mediated Green Composite Synthesis: A Name Synonymous with Each Other. In: Deshmukh, S.K., Deshpande, M.V., Sridhar, K.R. (eds) Fungal Biopolymers and Biocomposites. Springer, Singapore. https://doi.org/10.1007/978-981-19-1000-5_14
Download citation
DOI: https://doi.org/10.1007/978-981-19-1000-5_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0999-3
Online ISBN: 978-981-19-1000-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)