Abstract
Laccase has the ability to act on a broad range of substrates, as a result of which multiple compounds have been used for laccase assay and numerous applications have been discovered over time. Thus, considering the above points, in the present study, white and blue laccase from Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus were used for laccase assay using various substrates which includes 2,2′ azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,6-dimethoxyphenol (2, 6 DMP), guaiacol (GCL), and syringaldazine (SYZ). The most effective substrate of the four substrates was ABTS for white and blue laccase. The white and blue laccase were further used for the synthesis of bio-material via fungal-assisted treatment of wheat bran (WB) and sugarcane bagasse (SB) followed by micro-wave pre-treatment and the addition of starch and glycerol to the treated agro-residue in the ratio of 5:1:1. The thickness swelling and water absorption percentage was minimum for wheat bran synthesized bio-material using white laccase in comparison to the other synthesized bio-material.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
10.1 Introduction
Laccase is widely distributed in nature and is found in plants, fungi, insects [1], and bacteria [2]. Laccase, due to its substrate specificity, can act on a broad range of substrates, as a result of which various substrates have been used for laccase assay, and the most widely used substrates include ABTS, 2,6 DMP, GCL, and SYZ. The other aspect of the enzyme involves the reuse of agro-residues which are generated in huge amounts throughout the globe. Only a small fraction of these residues is used, and the rest are either dumped in wasteland or are burned, thereby contributing to environmental pollution. They have immense applications, but the lignin content in these agro-residues acts as a barrier for its effective utilization [3]. However, laccase has been used for the delignification of agro-residues [4] which can further help in the utilization of these agro-residues in lignocellulosic biorefinery and synthesis of bio-material. Thus, laccase substrate affinity and its application in various sectors have attracted considerable attention from environmental, industrial, and biotechnological prospect [5].
In the present study, the focus was on two aspects: first is on laccase assay using various substrates, which include 2,2′ azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,6-dimethoxyphenol (2,6 DMP), guaiacol (GCL), and syringaldazine (SYZ) from newly isolated white laccase-producing strain Myrothecium verrucaria ITCC-8447 and blue laccas-producing strain Pleurotus ostreatus as the reference strain. This was followed by fungal-assisted synthesis of bio-material using wheat bran (WB) and sugarcane bagasse (SB) with white and blue laccase-producing strain Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus. The fungal pre-treated substrate was subjected to optimized microwave treatment in our laboratory. The bio-material was synthesized using starch and glycerol. The volume, thickness swelling, and water absorption percentage of the synthesized bio-material were determined. These synthesized bio-materials can help in effectively reutilizing agro-residue which would otherwise be rendered useless, thereby contributing toward management and recycling of agricultural waste.
10.2 Materials and Methods
10.2.1 Materials
The chemicals used were of analytical grades and purchased from Sigma, Hi-Media, and Merck, India.
10.2.2 Culture Condition and Inoculum Preparation
The fungal strain Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus were used in this study. The newly isolated strain Myrothecium verrucaria ITCC-8447 was isolated from Rajasthan, India, and maintained on malt extract agar plates, whereas Pleurotus ostreatus was a gift culture from the Institute of Forstbotanik, Gottingen, Germany, used as the reference culture and maintained on potato dextrose agar plates. The medium composition used for laccase assay was as follows for Myrothecium verrucaria ITCC-8447 (g/L): glucose 5, peptone 5, yeast extract 2, KH2PO4 1, MgSO4 0.5, ZnSO4 0.01, MnSO4 0.001, CaCl2 0.01, FeSO4 0.01, and pH 7.0 ± 0.5 [6] with slight modifications, and for Pleurotus ostreatus, Bushnell Haas Media (BHM) supplemented with (g/L) C6H12O6 pH 5 ± 0.5 [7] was used for laccase assay. For the inoculum preparation, 7-day-old culture was used, and two 6 mm cubes were inoculated in 50 mL of media using 250 mL Erlenmeyer flask and incubated at 28 ± 2 °C under static condition. The crude enzyme was withdrawn every 48 h for laccase assay, and the experiment was carried out for a period of 12 days. The crude extract from the 10th day was centrifuged at 10,000 rpm for 5 min used for further study.
10.2.3 Enzyme Assay and Protein Concentration Determination
Laccase activity was determined quantitatively by measuring the absorption change of various substrates, which included ABTS at 420 nm, ε420 = 36,000 M−1 cm−1 [8]; 2,6 DMP at 469 nm, ε469 = 14,800 M−1 cm−1 [9]; GCL at 465 nm, ε465 = 12,100 M−1 cm−1 [10]; and SYZ at 525 nm, ε525 = 65,000 M−1 cm−1 [11] for 5 min at 30 °C in UV-visible spectrophotometer (Make: Dynamica; Model: Halo DB-30). The concentration of the substrate was varied and was in the following range: ABTS – 0.5–1.5 mM; 2, 6 DMP, 1–5 mM; GCL, 20–30 mM; and SYZ, 10–30 mM, followed by the determination of the optimal concentration of substrates for laccase assay. The laccase activity was calculated as per Holme and Peck, 1996 [12]. One activity unit (U) was defined as the amount of enzyme required to oxidize 1 mmole ABTS per minute at 420 m, 2,6 DMP at 469 nm, GCL at 465 nm, and SYZ 525 nm. The protein estimation was done by Lowry’s method, 1951 [13], with BSA as the standard.
10.2.4 Synthesis of Bio-material Using White and Blue Laccase
The fungal-assisted synthesis of bio-material was done by inoculating the agro-residue WB and SB with Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus under SSF for 15 days at 28 ± 2 °C. The agro-residue was then soaked in 5 mM ammonium molybdate, followed by microwave-assisted pre-treatment using microwave reaction system SOLV, Multiwave Pro (Make: Anton Parr, Austria), at 150 °C, 6 lb. pressure for 45 min, as per Verma et al. [14], with slight modifications which were established in our laboratory. It was followed by the addition of starch and glycerol to the treated agro-residue in the ratio of 5:1:1. The bio-material was then synthesized at 140 °C for 30 min. The thickness swelling (%) and water absorption (%) were measured as per Jeefferie et al. [15]:
where Wi is the initial weight of bio-material before water absorption and Wf is the final weight of bio-material after water absorption.
where Ti is the initial thickness of bio-material before water absorption and Tf is the final thickness of bio-material after water absorption.
10.3 Results and Discussions
10.3.1 Laccase Activity Using Various Substrates
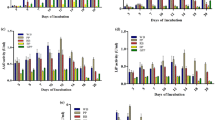
Laccase activity was evaluated with different substrates for a period of 12 days using Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus (Fig. 10.1a, b). The white laccase activity for Myrothecium verrucaria ITCC-8447 was detected 153.3 UL−1 by ABTS, whereas no activity was observed for 2,6 DMP and GCL on the fourth day. However, on the tenth day, white laccase activity using 2,6 DMP and GCL was 2.4 UL−1 and 4.3 UL−1 which was negligible as compared to ABTS 95.8 UL−1. In the reference strain Pleurotus ostreatus; significant blue laccase activity was detected on the fourth day with 2,6 DMP and ABTS of 34.9 UL−1 and 16.7 UL1, respectively, and it was 11.4 UL−1 for GCL, whereas on the tenth day, the laccase activity was 155.7 UL−1, 223.1 UL−1, and 87.4 UL−1 for 2,6 DMP, ABTS, and GCL. The observed difference in laccase activity may be due to the specificity of the crude extract to different substrates.
10.3.2 Laccase Assay Using Various Substrates
The most effective substrate of the four substrates was ABTS for both white and blue laccase from Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus (Table 10.1). Multiple substrates were used for laccase assay in the work done by Eichlerová (2012) [7], and laccase activity using SYZ was only obtained with purified enzyme; similarly in the present study, both white and blue laccase did not exhibit oxidation with SYZ. The optimal concentration of various substarte was determined, and it was observed that, for white laccase from Myrothecium verrucaria ITCC-8447, it was ABTS (0.5 mM), 2,6 DMP (2 mM), and GCL (30 mM), and for blue laccase from Pleurotus ostreatus, it was ABTS (1.5 mM), 2,6 DMP (2 mM), and GCL (30 mM). Similarly, the protein concentration for white and blue laccase was 1.82 mg/mL and 0.29 mg/mL, respectively (Table 10.1).
10.3.3 Synthesis of Bio-material Using White and Blue Laccase
The bio-material synthesized from WB and SB using white and blue laccase from Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus had a volume of 15.3 mm3 and 10.5 mm3 (Fig. 10.2a, b) and 25.7 mm3 and 54.9 mm3 (Fig. 10.2c, d), respectively. The percentage of thickness swelling observed in case of both WB and SB was negligible when synthesized using white laccase; however, in blue laccase-synthesized bio-material, it was 37.2 and 3.0 (Fig. 10.2e). These results are in accordance with Jeefferie et al. [15] which states that the thickness swelling is directly proportional to the size of fiber, i.e., smaller the size of the fiber less is the thickness swelling and vice versa [15]. The percentage of water absorption for white laccase-synthesized bio-material was 46.7 and 100 for WB and SB, respectively, whereas for blue laccase-synthesized bio-material, the percentage of water absorption was 100 and 76 (Fig. 10.2f). The water absorption of the bio-material significantly affects the structural integrity of bio-materials and results due to the saturation of the fibers in the presence of water; it also gives an insight into the water diffusivity of the bio-material [15].
Synthesis of bio-material via fungal-assisted treatment of wheat bran (WB) and sugarcane bagasse (SB) using white laccase (a–b) and blue laccase (c–d) followed by microwave pre-treatment and addition of starch and glycerol. (e) Thickness swelling (%) of blue laccase-synthesized bio-material (f) water absorption (%) of the white and blue laccase-synthesized bio-material
10.4 Conclusion
The present study states that the most effective substrate was ABTS for white and blue laccase-producing strain Myrothecium verrucaria ITCC-8447 and Pleurotus ostreatus. The fungal-assisted treatment using white and blue laccase followed by pre-treatment was employed for the synthesis of bio-material. The WB-synthesized bio-material via white laccase exhibited minimum water absorption percentage in comparison to the other synthesized bio-material. Thus, the delignification capability of the agro-residues and its role in bio-material synthesis using pre-treatment and binding agents can be effectively utilized for the reutilization of waste agro-residues in synthesis of bio-material, thereby fitting in the recent concept of “waste recycling.”
References
Hattori M, Konishi H, Tamura Y et al (2005) Laccase-type phenoloxidase in salivary glands and watery saliva of the green rice leafhopper, Nephotettix cincticeps. J Insect Physiol 51(12):1359–1365
Surwase SV, Patil SA, Srinivas S et al (2016) Interaction of small molecules with fungal laccase: a surface plasmon resonance based study. Enzym Microb Technol 82:110–114
Martinez AT, Ruiz-Duenas FJ, Martinez MJ, del Rio JC, Gutierrez A (2009) Enzymatic delignification of plant cell wall: from nature to mill. Curr Opin Biotechnol 20:348–357
Karp SG, Faraco V, Amore A, Letti LAJ, Thomaz Soccol V, Soccol CR (2015) Statistical optimization of laccase production and delignification of sugarcane bagasse by Pleurotus ostreatus in solid-state fermentation. Biomed Res Int 2015:1–8
Afreen S, Anwer R, Singh RK et al (2016) Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J Biol Sci 25:1446–1453
Olga VKS, Elena VS, Valeria PG, Olga VM, Natalia VL, Aida ND, Alexander IJ, Alexander M (1998) Purification and characterization of the constitutive form of laccase from basidiomycete Coriolus hirsutus and effect of inducers on laccase synthesis. Biotechnol Appl Biochem 28:47–54
Eichlerová I, Šnajdr J, Baldrian P (2012) Laccase activity in soils: considerations for the measurement of enzyme activity. Chemosphere 88(10):1154–1160
Bourbonnais R, Paice M, Reid I et al (1995) Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2, 2′-azinobis (3-ethylbenzthiazoline-6-sulfonate) in Kraft lignin depolymerization. Appl Environ Microbiol 61(5):1876–1880
Wu J, Kim KS, Lee JH et al (2010) Cloning, expression in Escherichia coli, and enzymatic properties of laccase from Aeromonas hydrophila WL-11. J Environ Sci 22(4):635–640
Jolivalt C, Madzak C, Brault A et al (2005) Expression of laccase IIIb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl Microbiol Biotechnol 66(4):450–456
Rajagopalu D, Show PL, Tan YS et al (2016) Recovery of laccase from processed Hericium erinaceus (Bull.: Fr) Pers. fruiting bodies in aqueous two-phase system. J Biosci Bioeng 122(3):301–306
Holme DJ, Peck H (1996) Resolución de Problemas de Bioquímica Analítica, first ed. Acribia, Zaragoza 160:114–126
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Verma P, Watanabe T, Honda Y, Watanabe T (2011) Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated by H2O2. Bioresour Technol 102(4):3941–3945
Jeefferie AR, Fariha ON, Warikh AR, Yuhazri MY, Sihombing H, Junid R (2011) Preliminary study of the physical and the mechanical properties of tapioca starch/sugarcane fiber cellulose composite. J Eng Appl Sci 6(4):7–15
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Agrawal, K., Verma, P. (2020). Laccase-Mediated Synthesis of Bio-material Using Agro-residues. In: Sadhukhan, P., Premi, S. (eds) Biotechnological Applications in Human Health. Springer, Singapore. https://doi.org/10.1007/978-981-15-3453-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-3453-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3452-2
Online ISBN: 978-981-15-3453-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)