Abstract
This study investigated the physical properties (rheological and thermogravimetric analysis) of cornstarch-tannin adhesives and the mechanical properties (dry tensile strength and 3-point bending strength) of plywood made using cornstarch-tannin adhesives. This adhesive was evaluated for its utility in interior plywood manufacture. The optimum cure temperature and cure time of cornstarch-tannin adhesives were 170°C and 4 min, respectively. Plywood bonded with formaldehyde-free cornstarch-tannin adhesive exhibited excellent mechanical properties comparable to commercially available phenol-formaldehyde plywood adhesives. It was found that cornstarch-tannin panels which do not contain formaldehyde and with an emission equal to that of heated but unbound wood can be obtained by the use of hexamethylenetetramine (hexamine) as hardener.
The work has indicated that an environmentally friendly wood adhesive can be prepared from a natural renewable resource (cornstarch and wattle tannin) for bonding interior-type plywood.
Zusammenfassung
In dieser Studie werden die physikalischen (rheologische und thermogravimetrische Analyse) sowie die mechanischen Eigenschaften (Trockenzugfestigkeit und 3-Punkt-Biegefestigkeit) von Sperrholz, das mit einem Maisstärke-Tannin-Klebstoff hergestellt wurde, untersucht. Dabei wurde der Klebstoff bezüglich seiner Brauchbarkeit für im Innenbereich verwendetes Sperrholz untersucht. Die optimale Aushärtungstemperatur dieses Klebstoffes lag bei 170°C und die Aushärtungszeit bei 4 min. Sperrholz, das mit einem formaldehydfreien Maisstärke-Tannin-Klebstoff verklebt wurde, wies ausgezeichnete mechanische Eigenschaften auf, die mit denjenigen von handelsüblichen Phenol-Formaldehyd-Sperrholzklebstoffen vergleichbar sind. Es hat sich gezeigt, dass Maisstärke-Tannin-Platten, die kein Formaldehyd enthalten und deren Emission der von erhitztem, nicht verklebtem Holz entspricht, mit Hexamethylentetramin (Hexamin) als Härter hergestellt werden können.
Die Arbeit hat gezeigt, dass aus einem natürlichen nachwachsenden Rohstoff (Maisstärke und Akazientannin) ein umweltfreundlicher Holzklebstoff für die Verklebung von Sperrholz für den Innenbereich hergestellt werden kann.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The wood composites industry is one of the largest manufacturers in Europe. Wood adhesives are essential components in wood composites. At present, formaldehyde-based adhesives such as phenol-formaldehyde (PF) and urea-formaldehyde (UF) resins are predominantly used. In 1998, according to the European Panel Federation (EPF), the quantity of the adhesives used in Western Europe was estimated at approximately 3.4 million tons (Mansouri et al. 2006, Mansouri and Pizzi 2007). These adhesives are synthetically produced from non-renewable resources such as petroleum and natural gas. With dwindling petroleum resources and unstable fossil fuel prices, a great deal of uncertainty regarding the future cost and availability of synthetic adhesives exists (Imam et al. 2001). Several efforts have been made to reduce or replace formaldehyde contents in adhesive formulations (Mozaffar et al. 2004, Nihat and Nilgül 2002, Thompson 1991, Pizzi 1977, Yoosup et al. 2008) or to develop adhesives from natural materials (Pizzi 2006, Pizzi et al. 1995, Pichelin et al. 2006, Aldo Ballerini and Pizzi 2005, Yuan and Kaichang 2007, Trosa and Pizzi 2001, Li et al. 2004).
Condensed tannin adhesive is an excellent example of a formaldehyde-free adhesive from renewable resources. Certain condensed tannins such as “quebracho” and “wattle” are produced commercially from woods and barks and they are used as a raw material for the production of wood adhesives since the 1970s (Pizzi 1994, 2000). The chemical structure of wattle tannin is shown in Fig. 1. The free C2’, C5’ and C6 sites on the A-ring can react with hexamine as a hardener because of their strong nucleophilicity to form the adhesive (Pichelin et al. 1999, 2006). 13C-NMR has confirmed (Pichelin et al. 1999, Kamoun et al. 2003) that in presence of chemical species with very reactive nucleophilic sites, such as condensed flavonoid tannins, hexamine is not at all a formaldehyde-yielding compound. The very reactive imines and iminoaminomethylene intermediates initially formed in the decomposition do react with the phenolic species present without ever passing through the formation of formaldehyde (Pizzi and Tekely 1995, 1996, Pizzi et al. 1996, Kamoun et al. 2003).
Cornstarch is abundant, inexpensive, and renewable, it is widely used in numerous industrial applications such as the paper, textile, food, pharmaceutical, cosmetics and adhesives industry (Richardson and Gorton 2003). Cornstarch is a mixture of two highly polymeric and isotactic molecules: amylose (linear chain molecule composed of α-D-glycopyranose units which are linked by α-D-(1–4) bonds) and amylopectine (branched molecule composed of α-D-glycopyranose held together by 1,4-linkage except at the branch points which are 1,6-bonds). (Van Steene and Masschelein-Kleiner 1980). More recently, the development of a starch-based wood adhesive for interior applications has been described by Imam et al. (1999). Starch yields adhesives with excellent affinity for polar materials such as cellulose. In this regard, starch based adhesives wet the polar surface of cellulose, penetrate crevices and pores and thus, form strong adhesive bonds. The bonding is the result of both mechanical interlocking and Van der Waals forces (Imam et al. 1999).
In this study, the physical properties (rheological and thermogravimetric analysis) of adhesives and the mechanical properties (dry tensile strength, modulus of rupture “MOR” and modulus of elasticity “MOE”) of plywood manufactured using formaldehyde-free cornstarch-tannin adhesives were investigated.
2 Materials and experimental methods
2.1 Materials
Unmodified commercial grade cornstarch (extra pure) was obtained from ACROS ORGANICS; the moisture content was in the range of 10 to 12%. A sodium hydroxide (M w \( = \) 40.00 g/mol) was purchased from VWR prolabo. Commercial flavonoid wattle tannin (Wattle OP) was provided by SILVATEAM. The hardener, hexamine 99%, was supplied by Aldrich.
2.2 Preparation of cornstarch-tannin adhesives
To prepare 500 g of adhesive, a cornstarch water solution was prepared at 65% (p/v) concentration, by dissolving 130 g of cornstarch in 200 ml of deionised water and stirring at room temperature, to which 13 g of wattle tannin are added. Hardener content used was 5% hexamine by weight on tannin extract solids content. The hexamine was dissolved in water to yield a 30% concentration solution in water before being added to the cornstarch-tannin solution. The solution was mixed and 100 ml of sodium hydroxide (33%) was added. The resulting adhesives were mixed for 45 min at room temperature and then used to bond plywood.
2.3 Plywood preparation and testing
5 ply laboratory plywood panels of dimension 200 × 200 ×10 mm3 were prepared from 2 mm thick maritime pine veneers with a moisture content of 4% at a glue mix spread of 225 g/m2 single glueline. In the case of cornstarch-tannin adhesives, hot pressing was carried out at 12 bar pressure and 170°C for 8 min press time (optimal conditions). While plywood bonded with commercial phenol-formaldehyde resin was assembled and hot pressed at 12 bar pressure and 125°C for 6 min press time.
Fifteen samples were cut from conditioned plywood and the following properties were determined in accordance with appropriate European standards: dry tension strength (EN 314, 1993), static bending (modulus of rupture – MOR) and modulus of elasticity-MOE (EN 310, 1993), and the results obtained are shown in the tables.
2.4 Rheological characterization
The adhesives were characterized with an rotational rheometer (ARES) in the parallel plates geometry, which was used for all the measurements; plates of diameter 25 mm and a gap of 1.5 mm. Silicone oil was used to prevent water evaporation. All measurements were repeated at least three times.
2.5 Thermogravimetric analysis (TGA)
Ten milligrams of each cured sample was placed in a previously tarred stainless steel pan (PerkinElmer Life And Analytical Sciences, Inc., Boston, MA) inside a thermogravimetric Analyzer, model TGA Q50. Samples were heated from room temperature (~20°C) up to 300°C at the rate of 5°C/min. in air atmosphere. Thermograms of the sample weight as a function of temperature and its first derivative were considered for the analysis. All measurements were repeated three times.
2.6 Formaldehyde emission by desiccator method
The formaldehyde emissions from the plywood were determined according to the European Standard (ISO/CD 12460-4 2007) using a glass desiccator. The 24-h desiccator method uses a common glass desiccator with a volume of 10 L. Eight test pieces, with dimensions of 150 × 50 × 10 mm3, which were cut from the plywood, were positioned in the desiccator. The formaldehyde released from the test pieces at 23 ± 2°C and 50 ± 10% relative humidity, during 24 h is absorbed in a Petri dish filled with 30 ml of distilled water and determined photometrically. Three replicates were used for each adhesive.
3 Results and discussions
First of all, the physical properties of cornstarch-tannin adhesives were characterized. Various characterizations like stability of cornstarch-tannin adhesives, classification of adhesives, optimisation of reaction time and reaction temperature were studied. Secondl, the mechanical properties of plywood prepared with cornstarch-tannin adhesives were studied.
3.1 Physical properties of cornstarch-tannin adhesives
Dynamic oscillatory measurements (time sweep) were carried out in order to examine the stability of cornstarch-tannin adhesives. In the complex modulus, the elasticity can be described by the storage shear modulus G’, and the viscous property can be described by the loss shear modulus G’’. Figure 2 shows the variation of the elastic modulus G’ and viscous modulus G’’ with time at 25°C, 1% strain and 1 rad/s of formaldehyde-free cornstarch-tannin adhesives. It can be seen that dynamic moduli (G’ and G’’) increase progressively with time up to 4 h. Beyond this they remain constant showing excellent structural stability of the adhesive. On the other hand, this figure shows a predominant elastic character of the adhesive (G’ > G’’).
The dependence of G’ and G’’ on frequency (frequency sweep) can be used to characterize or classify a dispersion. The four most common and traditional classifications are that of a dilute solution, an entanglement network system (or a concentrated solution), a weak gel and a strong gel. A dilute solution shows loss moduli (G’’) larger than shear moduli (G’) over the entire frequency range, yet the moduli approach each other at higher frequencies (Clark and Ross-Murphy 1987, Steffe 1996). An entanglement network system shows G’’ and G’ curves intersecting at the middle of the frequency range, indicating a clear tendency for more solid-like behaviour at higher frequencies (Ross-Murphy 1984). Weak gels have G’ higher than G’’ with moduli almost parallel to each other. Strong gels also have G’ higher than G’’. However, G’ has a slope of 0, and G’’ displays a minimum at intermediate frequencies (Clark and Ross-Murphy 1987).
Figure 3 displays the G’ and G’’ plotted against frequency for cornstarch-tannin adhesives after stabilization at 25°C. As can be seen from Fig. 3, the dynamic moduli (G’ and G’’) increase with frequency. G’ is seen to be greater than G’’ over the entire frequency range studied. Furthermore, G’ and G’’ are almost parallel to each other. According to the classification mentioned above, the cornstarch-tannin adhesives belong to weak gels.
TGA can check the thermal decomposition and thermal stability of adhesives. Figure 4 shows the thermogravimetric TGA curve and its derivative (DTG) of the cornstarch-tannin adhesive in air atmosphere at a heating rate of 5°C/min. The DTG curve shows that there are two obvious mass losses at 175°C and 225°C. The decomposition of the cornstarch is produced at about 175°C, whereas the decomposition of the wattle tannin starts at 225°C. The results obtained with thermogravimetric analysis confirm that 170°C is the optimal polymerization temperature. Pyrolysis of starches at 175°C in a stream of air has been described to give CO2, CO, water, acetaldehyde, furan and 2-methyl furan (Bryce and Greenwood 1963). For cornstarch, levoglucosan is usually the main constituent of the decomposed products, besides complex gases and liberated water (Greenwood 1967).
Figure 5 illustrates the time dependence of storage modulus (G’) and loss modulus (G’’) of the cornstarch-tannin adhesive at optimal polymerization temperature (170°C), 1% strain and 1 rad/s. It can be observed that the elastic modulus (G’) and viscous modulus (G’’) increase progressively with increasing time up to 4 min. Beyond this a sharp decrease in G’ and G’’ is observed indicating that the optimum cure time was 4 min.
Time evolution of storage modulus (G’) and loss modulus (G’’) of cornstarch-tannin adhesive at 170°C, 1 rad/s and 1%. (G’: Δ; G’’: □; Tan_delta: ◊)
Zeitlicher Verlauf des Speichermoduls (G’) und des Verlustmoduls (G’’) des Maisstärke-Tannin-Klebstoffes bei 170°C, 1 rad/s und 1%. (G’: Δ; G’’: □; Tan_delta: ◊)
Figure 6 shows the time dependence of G’ and G’’ for cornstarch-tannin adhesive at 85°C, 1% strain and 1 rad/s. The results obtained by this technique show two stages. In the course of the first stage (between 0 and 1500 s), both dynamic moduli (G’ and G’’) increase progressively with increasing time which may be due to the removal of water. This stage corresponded to the water evaporation. In the second stage (between 1500 and 3000 s), G’ and G’’ increase shortly with time corresponding to the activation of polymerization. The foam formation induces the decrease of the complex modulus at the end of the experiment. Results show the double effects of the temperature (water evaporation and activation of polymerization).
3.2 Mechanical properties of plywood
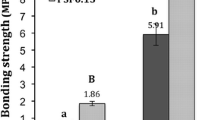
In Table 1, the results of laboratory plywood prepared when using cornstarch-tannin as an adhesive are reported. The results indicate that formaldehyde-free cornstarch-tannin adhesive is capable of giving dry modulus of rupture, modulus of elasticity and tensile strength results which are comparable to those obtained with synthetic commercial phenol-formaldehyde resin. The dry tensile strength tests revealed that in most cases, the rupture is not adhesive but interfacial and more precisely in the wood side. Of particular interest in Table 1 are the formaldehyde emission tests performed on the panels according to the European Standard (ISO/CD 12460-4 2007). The formaldehyde emission results are, however, considerably lower than those observed for the commercial phenol-formaldehyde resin. Emission values of 0.2 mg formaldehyde/m2/h in Table 1 are only due to the formaldehyde generated just by heating of the wood, and are not due to the resin. In the presence of fast-reacting species, hexamine is not at all a formaldehyde-yielding compound (Pichelin et al. 2006). The very reactive amino-immine intermediates initially formed in the decomposition do react with the phenolic or aminoplastic species present without ever passing through the formation of formaldehyde (Pizzi and Tekely 1995, 1996, Pizzi et al. 1996, Kamoun et al. 2003).
4 Conclusion
The cornstarch-tannin adhesives prepared without using formaldehyde which was substituted by a nonvolatile, nontoxic, aldehyde, (hexamine) yield good mechanical properties for the plywood to pass comfortably relevant international standard specifications. At laboratory level, wattle tannin hardened with hexamine has shown to be a formaldehyde-free system. This useful effect is based on the double mechanism of slow hexamine decomposition to reactive imino-amino methylene bases and their immediately subsequent very rapid reaction with the tannin.
Research is in progress to further improve cornstarch-tannin adhesive formulations by employing other nonvolatile, non-toxic and aldehyde hardeners (glyoxal or dioxal).
References
Aldo Ballerini AD, Pizzi A (2005) Non-toxic, zero emission tannin-glyoxal adhesives for wood panels. Holz Roh- Werkst 63:477–478
Bryce DJ, Greenwood CT (1963) Aspects of the thermal degradation of starch. Starch/Stärke 15(5):166
Clark AH, Ross-Murphy SB (1987) Structural and mechanical properties of biopolymer gels. Adv Polym Sci 83:57–192
EN 310 (1993) Wood-based panels. Determination of modulus of elasticity in bending and of bending strength. The European committee for standardization
EN 314-1/-2 (1993) Plywood–bond quality, Part 1. Test methods. European Committee for Standardization, Brussels
Greenwood CT (1967) The thermal degradation of starch. Adv Carbohyd Chem Bi 22:483–515
Imam SH, Lijun M, Liang C, Greene RV (1999) Wood adhesive from crosslinked poly(vinyl alcohol) and partially gelatinized starch: preparation and properties. Starch/Stärke 51(6):225–229
Imam SH, Sherald HG, Lijun M, Liang C (2001) Environmentally friendly wood adhesive from a renewable plant polymer: characteristics and optimization. Polym Degrad Stabil 73:529–533
ISO/CD 12460-4 (2007) Wood-based panels – Determination of formaldehyde release – desiccator method. The European committee for standardization
Kamoun C, Pizzi A, Zanetti M (2003) Upgrading of MUF resins by buffering additives – Part 1: Hexamine sulphate effect and its limits. J Appl Polym Sci 90(1):203–214
Li K, Geng X, Simonsen J, Karchesy J (2004) Novel wood adhesives from condensed tannins and polyethylenimine. Int J Adhes Adhes 24:327–333
Mansouri HR, Pizzi A, Leban JM (2006) Improved water resistance of UF adhesives for plywood by small pMDI additions. Holz Roh- Werkst 64(3):218–220
Mansouri HR, Pizzi A (2007) Recycled micronized polyurethane powders as active extenders of UF and PF wood panel adhesives. Holz Roh- Werkst 65(4):293–299
Mozaffar AK, Sayed Marghoob A, Ved Prakash M (2004) Development and characterization of a wood adhesive using bagasse lignin. Int J Adhes Adhes 24:485–493
Nihat SC, Nilgül O (2002) Use of organosolv lignin in phenol-formaldehyde resins for particleboard production II. Particleboard production and properties. Int J Adhes Adhes 22:481–486
Pichelin F, Kamoun C, Pizzi A (1999) Hexamine hardener behaviour – effects on wood glueing, tannin and other wood adhesives. Holz Roh- Werkst 57(5):305–317
Pichelin F, Nakatani M, Pizzi A, Wieland S, Despres A, Rigolet S (2006) Structural beams from thick wood panels bonded industrially with formaldehyde-free tannin adhesives. Forest Prod J 56(5):31–36
Pizzi A (1977) Hot-setting tannin-urea-formaldehyde exterior wood adhesives. Adhess Age 20(12):27–35
Pizzi A (1994) Advanced wood adhesives technology. Marcel Dekker, New York
Pizzi A, Meikleham N, Dombo B, Roll W (1995) Autocondensation-based, zero-emission, tannin adhesives for particleboard. Holz Roh- Werkst 53:201–204
Pizzi A, Tekely P (1995) Mechanism of polyphenolic tannin resin hardening by hexamethylenetetramine: CP-MAS 13C NMR. J Appl Polym Sci 56:1645–1650
Pizzi A, Tekely P (1996) Hardening mechanisms by hexamethylenetetramine of fast-reacting phenolic wood adhesives – a CPMAS 13C NMR study. Holzforschung 50:277–281
Pizzi A, Tekely P, Panamgama LA (1996) A different approach to low formaldehyde emission aminoplastic wood adhesives. Holzforschung 50:481–485
Pizzi A (2000) Tannery Row – the story of some natural and synthetic wood adhesives. Wood Sci Technol 34(4):277–316
Pizzi A (2006) Recent developments in eco-efficient bio-based adhesives for wood bonding: opportunities and issues. J Adhes Sci Technol 20(8):829–846
Richardson S, Gorton L (2003) Characterisation of the substituent distribution in starch and cellulose derivatives. Anal Chim Acta 497:27–65
Ross-Murphy SB (1984) Rheological methods. In: Chan HW-S (ed) Biophysical methods in food research. Blackwell, Palo Alto, CA
Steffe JF (1996) Rheological methods in food process engineering. Freeman Press, East Lansing, MI
Thompson GE (1991) Demethylated kraft lignin as a substitute for phenol in wood adhesives. MS thesis, Colorado State University
Trosa A, Pizzi A (2001) A no-aldehyde emission hardener for tannin-based wood adhesives for exterior panels. Holz Roh- Werkst 59:266–271
Van Steene G, Masschelein-Kleiner L (1980) Modified starch for conservation purposes. Stud Conserv 25(2):64–70
Yoosup P, Dohertyb WOS, Halleya PJ (2008) Developing lignin-based resin coatings and composites. Ind Crop Prod 27:163–167
Yuan L, Kaichang L (2007) Development and characterization of adhesives from soy protein for bonding wood. Int J Adhes Adhes 27:59–67
Acknowledgements
We gratefully acknowledge the financial support from the “Conseil Général des Landes” (Aquitaine, France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moubarik, A., Allal, A., Pizzi, A. et al. Characterization of a formaldehyde-free cornstarch-tannin wood adhesive for interior plywood. Eur. J. Wood Prod. 68, 427–433 (2010). https://doi.org/10.1007/s00107-009-0379-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-009-0379-0