Abstract
The human body accommodates a multitude of microorganisms that inhabit various anatomical sites, such as skin, mucosa, gastrointestinal tract, respiratory tract, urogenital tract and mammary glands, and are collectively defined as the microbiome, the composition and functions of which are crucial to health and survival (Moos 2016). Among the different organ systems, that coordinate the functions of the human body, central nervous system, comprising the brain and spinal cord, plays a primary role in controlling awareness, movements, sensations, thoughts, speech and memory by integrating sensory information and responding accordingly. Furthermore, the microbiome engages in complex interactions with the organ systems of the human body thereby regulating the functions of both the entities. Such relationships between the central nervous system (CNS) and the gut microbiome have been explored in detail and is termed as microbiome-gut-brain (MGB) axis, a complex bidirectional inter-communication that exists between the gut microbiome and the crucial areas of the CNS (Malan-Muller et al. 2018; Cryan 2019). Various neuroactive compounds such as neurotransmitters, metabolites, cytokines, and hormones are synthesised by the gut microbiota and the host as a result of this interaction. These neuromodulatory substances gain access to the brain by different pathways, thus affecting the local homeostasis. Dysregulation of the MGB axis has been implicated in the pathogenesis of various neuroinflammatory, neurodevelopmental and neurodegenerative diseases which is mediated by either a direct line of communication through vagus nerve or immune system activation or both (Cryan 2019). In turn, gut microbiota composition is influenced by the brain in response to stress and endocrine factors (Tremlett et al. 2017).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The human body accommodates a multitude of microorganisms that inhabit various anatomical sites, such as skin, mucosa, gastrointestinal tract, respiratory tract, urogenital tract and mammary glands, and are collectively defined as the microbiome, the composition and functions of which are crucial to health and survival (Moos et al. 2016). Among the different organ systems that coordinate the functions of the human body, central nervous system comprising the brain and spinal cord, plays a primary role in controlling awareness, movements, sensations, thoughts, speech and memory by integrating sensory information and responding accordingly. Furthermore, the microbiome engages in complex interactions with the organ systems of the human body thereby regulating the functions of both the entities. Such relationships between the central nervous system (CNS) and the gut microbiome have been explored in detail and is termed as microbiome-gut-brain (MGB) axis, a complex bidirectional inter-communication that exists between the gut microbiome and the crucial areas of the CNS (Malan-Muller et al. 2018; Cryan 2019). Various neuroactive compounds such as neurotransmitters, metabolites, cytokines, and hormones are synthesised by the gut microbiota and the host as a result of this interaction. These neuromodulatory substances gain access to the brain by different pathways, thus affecting the local homeostasis. Dysregulation of the MGB axis has been implicated in the pathogenesis of various neuroinflammatory, neurodevelopmental and neurodegenerative diseases which is mediated by either a direct line of communication through vagus nerve or immune system activation or both (Cryan 2019). In turn, gut microbiota composition is influenced by the brain in response to stress and endocrine factors (Tremlett et al. 2017).
The specific aetiology and trigger for many of the neurodevelopmental and neurodegenerative diseases remain elusive to the scientific community. An intricate interplay of genetic and environmental factors is attributed to their pathogenesis (Tremlett et al. 2017). There is accumulating evidence suggesting a definitive role of human gut microbiome in the pathogenesis of Parkinson’s disease (PD), multiple sclerosis (MS) and autism spectrum disorders (ASD) whereas animal and human studies are ongoing to elucidate the role of microbiome in Alzheimer disease (AD), stroke, traumatic brain injury, amyotrophic lateral sclerosis and glioma (Cryan 2019). Prebiotics, probiotics, diet modifications, aerobic exercises and microbial transplantation are the few interventions employed for targeting gut dysbiosis in neurological disorders, subject to critical screening and evaluation (Tremlett et al. 2017; Kang et al. 2017; Gubert et al. 2018; Yang et al. 2019).
2 Microbiome-Gut-Brain Axis
The bidirectional interactions in the MGB axis are perpetuated through direct and indirect pathways. These pathways are mediated by the following components, (i) endocrine (hypothalamic-pituitary-adrenal [HPA] axis); (ii) immune-reactive molecules (cytokines and chemokines); (iii) metabolic pathways; (iv) limbic system; and (v) neural system (afferent and efferent pathways) (Malan-Muller et al. 2018). The communication is also dependent on the permeability of the blood-brain barrier (BBB) and the intestinal epithelial barrier (IEB) (Lyte and Cryan 2014).
2.1 Gut Microbiota
The microbiota colonising the human gastrointestinal tract is a collection of bacteria, archaea and eukaryotes which have co-evolved in a mutual relationship over the years. An estimated 1014 organisms inhabit the gastrointestinal tract which supersedes the number of human cells in the body (Cani 2018). Recent genomic studies suggest that the ratio of bacterial to human cells is 1.3:1 (Gill et al. 2006; Sender et al. 2016). The inherited human genome is non-modifiable, while the microbiome composition is highly dynamic and diverse at various stages of life, with the changes being driven by extraneous factors like diet, physical activity and stress. Earlier studies had classified the gut microbiota into 3 enterotypes—Bacteriodes (Enterotype 1), Prevotella (Enterotype 2) and Ruminococcus (Enterotype 3) which is currently considered obsolete (MetaHIT Consortium et al. 2011). The gut microbiota is primarily composed of 2 major phyla, Bacteroidetes (largely composed of Bacteroides and Prevotella species) and Firmicutes (comprised of Clostridium, Lactobacillus and Ruminococcus species). Bacteria in the human gut belong to either of these phyla, although a few gut pathogens from phylum Proteobacteria are also present (Gill et al. 2006).
2.2 Bi-directional Pathways between Gut and Brain
The bidirectional interaction pathways existing between the CNS and the gut microbiome have also been termed and referred to as microbial endocrinology (Lyte and Cryan 2014). Many neuroactive compounds (gamma-aminobutyric acid [GABA], acetylcholine, serotonin, dopamine and norepinephrine) are produced by both the host and the microbiota. Food consumed by humans contains pre-existing functional neuroactive components as well as substrates required to produce these compounds. Gut microbiota can directly produce neuroactive compounds from these substrates (Wall et al. 2014; Strandwitz 2018).
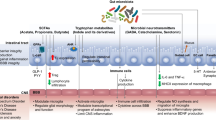
Neuroactive compounds produced in the gut are known to influence the host behavioural functions via two pathways. These compounds are either taken up from the gut into the portal circulation and then to systemic circulation, ultimately reaching the brain or they directly interact with the G protein-coupled receptors (GPCR-43)/neurokinin (NK)1R or NK2R receptors expressed on the enteric nervous system (ENS) which innervates the gastrointestinal tract (Aresti Sanz and El Aidy 2019). The brain functions influenced by these pathways can result in alteration of behaviour or cognition and also determine the food preferences and appetite. The brain also regulates the composition of the microbiota through the specific release of certain neurochemicals like substance P, calcitonin gene-related peptide, neuropeptide Y, somatostatin and vasoactive intestinal polypeptide into the gut lumen. This leads to the bidirectional vicious axis between the CNS and the gut microbiome (Fig. 7.1) (Holzer and Farzi 2014; Lyte and Cryan 2014).
Microbiome-gut-brain (MGB) axis. BBB blood-brain barrier, CCK cholecystokinin, CD4 cluster differentiation-4, CGRP calcitonin gene-related peptide, CNS central nervous system, GLP-1 glucagon-like peptide-1, GPCR43/41 G protein-coupled receptor 43/41, HDAC histone deacetylase, HPA hypothalamic-pituitary-adrenal axis, IEL intra-epithelial lymphocyte, IL interleukin, LAG-3 lymphocyte activation gene-3, LPS lipopolysaccharide, MCT monocarboxylate transporter, PYY peptide YY, SCFA short-chain fatty acid, SMCT sodium-coupled monocarboxylate transporter, Treg regulatory T cell, VIP vasoactive intestinal peptide
2.3 Role of Neurotransmitters in MGB Axis Homeostasis
Numerous neurotransmitters secreted by a wide spectrum of microbial species have been identified in the human gut which can impact the CNS. These neurotransmitters can potentially enter the brain from gut through portal circulation, but is limited by the intact BBB. Neurotransmitters like serotonin, dopamine, GABA and norepinephrine cannot typically breach the BBB. Yet, their precursors have the capability to cross the BBB and subsequently get converted to active neurotransmitters. For example, tryptophan is the serotonin precursor and tryptophan concentration in the blood plasma has been shown to correlate with brain serotonin levels (Strandwitz 2018). The alternate hypothesis is that the microorganism-derived neurotransmitters affect the brain through the vagus nerve and its afferent neurons. Vagus nerve signalling plays a crucial role in mediating stress, mood and satiety (Browning et al. 2017).
2.4 Role of Gut Neuroactive Metabolites in MGB Axis
Among the various bacterial metabolic by-products, short-chain fatty acids (SCFAs) have been identified as the key mediators of the brain-gut interaction. SCFAs are composed of up to 6 carbon atoms and are produced by the anaerobic fermentation of dietary fibres and resistant starches by the large intestine microbiota (Pascale et al. 2018). The predominant SCFAs produced are acetate (60%), propionate (20%) and butyrate (20%). They are absorbed by colonocytes via H+ or sodium-dependent monocarboxylate transporters into the portal circulation and are metabolised in the liver (Vijay and Morris 2014). Butyrate is primarily consumed as a preferred fuel source by colonocytes. A minor fraction reaches the systemic circulation and crosses the BBB thereby impacting CNS functions (Silva et al. 2020).
SCFAs play a key role in maintaining the gut-brain axis which includes preserving the integrity of colonocytes and IEB, preventing the translocation of bacteria or their toxic products, maintaining colonic mucus production, regulation of gut mucosal immunity and systemic inflammation via inhibition of histone deacetylation and T regulatory cells (Tregs) differentiation and regulation of appetite, sleep and whole-body homeostasis (Arpaia et al. 2013; Smith et al. 2013). Histone deacetylase (HDAC) inhibitors like butyrate and other SCFAs are emerging as promising therapeutic options due to their effects on transcription activation and gene regulation in the CNS (Dietz and Casaccia 2010).
2.5 Role of Gut Microbiota in Modulation of Microglia and Astrocyte Biology
Early microbiome studies to determine the influence of gut microbiota on CNS modulation was performed on germ-free (GF) or gnotobiotic animals. These gnotobiotic animals have been a powerful tool to determine causation and to study the effect of a particular bacteria or a dietary intervention on the MGB axis. A few animal studies have shown the role of gut microbiota on biological changes in microglia and astrocyte (Erny et al. 2015; Fung et al. 2017). Microglia constitute 10–15% of all cells in the CNS and as scavenger cells of brain, remove apoptotic nerve cells, kill invasive pathogens and trim redundant synaptic connections (Nayak et al. 2014). Microglial maturation and function are affected by the gut microbiota. Compared to conventionally colonised (specific pathogen-free, SPF) controls, GF mice have microglia with abnormal morphology, altered gene expression and impaired functional response to stimulation. The microglial changes were observed to revert to normal morphology once the diet for GF mice was supplemented with SCFAs (Erny et al. 2015). Similar changes in astrocyte morphology and functions due to the gut microbiota have been reported. Astrocytes are the major glial cells in the CNS that provide physical and metabolic support to the neurons. They take part in synaptogenesis and maintain the integrity of BBB by supporting the lining endothelial cells. Animal studies have shown that microbial metabolites bind to the aryl hydrocarbon receptor (AHR) in astrocytes, reducing symptoms of experimental autoimmune encephalitis (EAE) by inhibiting type I interferon signalling. This effect was reversed when mice were treated with antibiotics. Microbiota-dependent metabolism of tryptophan into AHR ligands engages AHR on astrocytes, thus leading to an increase in astrocyte expression of the inhibitor protein SOCS2 and consequently inhibiting activation of the transcription factor NF-κB and thereby limiting CNS inflammation (Rothhammer et al. 2016).
2.6 Role of Immune System in MGB Axis
Inflammation in the gut has been shown to mirror systemic and CNS inflammation. Peripheral gut insults like antibiotic use and unbalanced diet disrupt the IEB leading to translocation of bacteria and bacterial products like lipopolysaccharide (LPS) into the bloodstream creating a systemic inflammatory response by cytokine release. Dysregulated cytokine release can impact the permeability of BBB, thereby allowing entry of preformed neurotransmitters and pro-inflammatory cells and molecules to initiate a CNS inflammatory milieu (Pan et al. 2011).
Several studies suggest that local and systemic immune activation has critical effects on neural plasticity and behavioural effects, thereby confirming the contribution of immune system in the MGB axis homeostasis (Blander et al. 2017). Recognition of microbe-associated molecular patterns (MAMPs) in microbes and their metabolic by-products (SCFAs) can activate distinct immunological pathways for maintaining the CNS homeostasis (Thursby and Juge 2017). The mode of delivery (caesarean section) and antibiotic use have been linked with immune activation phenomenon in the newborn due to gut dysbiosis (Moos et al. 2016). These early gut dysbiotic changes have been positively correlated with defective neuronal plasticity and behavioural development, classically observed in ASD (Dzidic et al. 2018). The gut colonisers, Lactobacillus and Bifidobacterium, produce the neurotransmitter, acetylcholine, modulating the vagal nerve signalling, thereby attenuating the release of harmful cytokines (IL-1β, IL-6 and TNF-α) (El Aidy et al. 2014). A potential mechanism by which immune activation affects physiology and behaviour is via actions on the serotonergic system. As observed in mice studies by Lowry et al., serotonergic neurons in the dorsal raphe regulate mood and behaviour in response to the immune stimuli. Vagal afferent nerve fibres are also involved in transferring signals from peripheral immune activation to the CNS (Lowry et al. 2007). In addition, microbial infections can trigger antibody-mediated CNS autoimmunity and consequent neurodegeneration (Wu and Wu 2012). Altogether, peripheral immune response regulation by the gut microbiota and exogenous microbial challenges are critical in moulding CNS function and behaviour.
2.7 Composition and Correlation of Dysbiosis with Brain Behaviour
Increase in the members of the phylum Firmicutes or decrease in the members of phylum Bacteroidetes or their subclasses (altered Bacteroidetes/Firmicutes ratio) has been consistently observed in individuals with CNS disorders. In addition to the ratio, relative abundance or deficiency of certain genera in gut microbiota have been strongly linked with CNS disorders (Gill et al. 2006). Colonic bacterial spp. Faecalibacterium prausnitzii (Clostridial cluster IV), Coprococcus, Roseburia (Clostridial cluster XIV), Eubacterium and Anaerostipes have been identified to produce high concentrations of butyrate, an HDAC inhibitor. Butyrate-producing Faecalibacterium and Coprococcus were consistently associated with high mental quality-of-life (QOL) indicators (Cheung et al. 2019). The genera Akkermansia, Oscillospira and Lactococcus were found to be relatively abundant in individuals with higher sociability, less anxiety or depression (Johnson 2020). A relative abundance of Desulfovibrio and Sutterella was associated with less sociable behaviour and autism (Wang et al. 2011; De Angelis et al. 2013).
3 Impact of MGB Axis in Neurological Disorders
The dysregulation of gut microbiome composition is attributed to the pathomechanism of several neurological disorders (Fig. 7.2). In the next section, we will discuss the role of gut microbiome in a few neurological conditions based on the current evidence obtained from animal and human studies.
3.1 Multiple Sclerosis
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disease of the CNS characterised by the presence of widespread ‘plaques’ in the brain, spinal cord and optic nerve. MS plaques are pathologically heterogeneous revealing combinations of demyelination, inflammation, gliosis, axonal loss and remyelination (Reich et al. 2018). The commonest clinical phenotype of the disease at onset is the relapsing-remitting MS (RRMS) which occurs in more than 85%, while the remaining have progressive onset MS. The therapeutic approach is centred around immunomodulatory disease-modifying therapies (DMTs) which reduce the relapses but have unsatisfactory effect on the disease progression and long-term disability (Rae-Grant et al. 2018).
Immune dysregulation and pro-inflammatory immune shift are the hallmarks of MS, and active research is ongoing to identify the trigger for this. The pathogenesis is thought to be multifactorial with environmental influence prevailing over genetic factors. Evidence for the role of gut dysbiosis in MS susceptibility, pathogenesis, and disease course is compelling. Studies in animal models and persons with MS show alteration of gut microbiota which contribute to the change in immune homeostasis. CNS inflammation simulating MS can be induced in experimental animals by priming and activating the immune system with injection of myelin peptides and this is referred to as the EAE model. EAE can also be spontaneously generated in transgenic mice which express myelin-specific immune receptors (Glatigny and Bettelli 2018). Gut microbiota is essential for EAE induction as was evidenced by low frequency and reduced severity of EAE in GF mice. These mice had concomitant decreased pro-inflammatory TH17 differentiation, low levels of pro-inflammatory cytokines and enhanced Tregs. EAE could be re-established in them by allowing gut colonisation with segmented filamentous bacteria (Ivanov et al. 2009). Transfer of gut microbiota from MS patients could also induce EAE. In a study of 34 sets of monozygotic twins who were discordant for MS, transfer of gut microbiota from an affected twin induced spontaneous EAE more frequently in GF mice compared to the healthy twin (Berer et al. 2017). Conversely, administration of normal gut commensal bacteria, particularly those belonging to phyla Bacteroidetes and Firmicutes, was shown to reduce the incidence of EAE and the inflammatory response in susceptible models (Mowry and Glenn 2018).
In human studies, no difference was noted in the overall diversity and abundance of bacteria in MS and non-MS guts (Mowry and Glenn 2018; Mirza et al. 2020). The composition and relative richness of bacterial groups at sub-phylum level in MS were different from controls across multiple studies (Mirza et al. 2020). Members of phylum Bacteroidetes (Bacteroidaceae, Bacteroides and Prevotella) and phylum Firmicutes (Fecalibacterium) were reduced in MS gut (Cantarel et al. 2015; Miyake et al. 2015; Chen et al. 2016). These bacteria have key role in the generation of SCFAs which are anti-inflammatory. Studies also showed an increased abundance of methanogenic bacterial genera, Methanobrevibacter and Akkermensia (Tremlett et al. 2016; Jangi et al. 2016). They have pro-inflammatory effects related to respectively recruiting and activating immune cells and altering genes involved in immune responses. However, methanogenic bacteria may be increased secondary to constipation in MS and hence their abundance may result from MS than cause it. A recent study noted that the profile of gut microbiota in treatment-naïve MS varies across different ethnic groups with relative abundance of Clostridia being a common observation in all the groups (Ventura et al. 2019). Exposure to DMT can result in significant alterations in the beta diversity of gut microbiota (Tremlett et al. 2016; Mirza et al. 2020).
Studies in paediatric MS are of particular interest as children have fewer exposure to environmental stimuli and hence could shed light on the pathogenic role of the gut microbiota. Phylum Actinobacteria and genus Desulfovibrio were more abundant in MS gut. (Tremlett et al. 2016). These bacteria are also increased in other autoimmune inflammatory diseases including inflammatory bowel disease (Tremlett and Waubant 2018). A three-fold higher risk of an early relapse was noted among paediatric MS with Fusobacteria being absent in stool samples (Tremlett et al. 2016).
3.2 Neuromyelitis Optica Spectrum Disorder
Neuromyelitis optica spectrum disorder (NMSOD) is a severe multiphasic demyelinating disease driven by autoantibodies against aquaporin-4 water channels (AQP4). The B-cell responses in NMOSD are strongly influenced by T-cell-mediated cytotoxicity. Gut microbiota studies in NMOSD patients revealed over-abundance of Clostridium perfringens (Cree et al. 2016). This is of particular interest in the pathogenesis of the disease as AQP4 displays high degree of homology to an adenosine triphosphate-binding cassette transporter permease of C. perfringens and can show cross-reaction (Varrin-Doyer et al. 2012). A recent study from Indian NMOSD demonstrated increase in Clostridium boltea which also shares sequence similarity with AQP4 (Pandit et al. 2021).
3.3 Parkinson’s Disease
Parkinson’s disease (PD), a progressive neurodegenerative disease, is characterised by degeneration of the dopaminergic neurons projecting from the substantia nigra. PD is an alpha-synucleinopathy and its pathological hallmark is the presence of alpha-synuclein (α-synuclein) immunoreactive inclusions called Lewy bodies within neurons. Mutations of the gene for α-synuclein can cause familial forms of PD (Kalia and Lang 2016). Though the dominant clinical presentations of PD are motor disability, a range of non-motor signs have been described in PD with olfactory, sleep, gastrointestinal, autonomic, neuropsychiatric and sensory dysfunction (Poewe 2008). These non-motor symptoms often precede the neurological manifestations and have severe impact on the QOL (Pfeiffer 2016).
Gastrointestinal symptoms are frequent in PD among which constipation and delayed intestinal motility are the most common and distressing (Poewe 2008). Gut dysbiosis influences the gastrointestinal manifestations of PD as well as has role in the pathogenesis and severity of the disease. Mice transplanted with faecal microbiota from PD patients developed the classical motor symptoms of PD and neuroinflammation (Sampson et al. 2016). Neuroinflammation is being increasingly recognised as being integral to PD pathogenesis (Hirsch and Hunot 2009). The main mechanisms proposed for the influence of gut on PD include activation of the ENS through interactions with gut microbiota, activation of the local immune system and increase in gut permeability (Mulak 2015).
The contribution of gut in the pathogenesis was suggested by the detection of α-synuclein in the ENS in early PD (Hilton et al. 2014). Misfolding of α-synuclein could be induced by damage to enteric neurons and enteric glial cells leading to the accumulation of the inclusion bodies (Mulak 2015; Yang et al. 2019). Gut dysbiosis or an alternate environmental factor initiates the neuronal damage. Vagus nerve can transmit the α-synuclein pathology from the ENS to the dorsal vagal nucleus (Holmqvist et al. 2014). This hypothesis is supported by evidence from truncal vagotomy which prevented the spread of α-synuclein from gut to brain and the subsequent neurodegeneration in mouse models (Kim et al. 2019). Changes in gut flora and small intestinal bacterial overgrowth in PD contribute to inflammation and leaky gut which are hypothesised as substrates for the subsequent neurodegeneration (Tan et al. 2014; Yang et al. 2019).
In general, colonic biopsies and faecal analysis have revealed non-uniform data on gut microbiota in PD compared to controls. An increased diversity of bacterial species in PD gut was recorded in one study (Keshavarzian et al. 2015). Two studies showed a reduced abundance of Prevotellaceae species which reduced SCFAs implicated in PD pathogenesis. Other microbials noted to be decreased are Lachnospiraceae, Firmicutes, Clostridium coccoides, Bacteroides fragilis, Faecalibacterium prausnitzii, Enterococcaceae and Bacteroidetes, whereas Verrucomicrobiaceae species were increased in PD guts (Cryan et al. 2019). Enterobacteriaceae was associated with increased severity of postural instability and gait dysfunction in PD (Scheperjans et al. 2015).
Absorption and metabolism of levo-dopa which is the primary treatment for PD is influenced by the gut microbiota. Tyrosine decarboxylase from gut microbiota in mice reduced the plasma concentration of L-dopa (van Kessel et al. 2019). Conversely, PD medications have been linked to change in gut microbial status. Bacillaceae species was relatively abundant in patients who received levo-dopa compared to alternate therapy (Heintz-Buschart et al. 2018). A combination of monoamine oxidase inhibitors and amantadine also resulted in an increased microbial richness in PD (Bedarf et al. 2017).
3.4 Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common neurodegenerative disease which manifests clinically as progressive loss of episodic memory and other neurocognitive functions. The typical pathological substrates in the brain are extracellular deposition of amyloid plaques and dystrophic neurites containing hyperphosphorylated tau protein (DeTure and Dickson 2019). More recent evidence has linked neuroinflammation and systemic inflammation with AD pathogenesis (Heneka et al. 2015). Genetic and environmental factors play key roles in AD pathogenesis, the best understood being increasing age and the presence of the high-risk ε4 allele of apolipoprotein E gene (Xu and Wang 2016). Studies in animal models and gut microbiota in AD patients suggest a strong influence of the latter in AD pathogenesis. GF mice had reduced neuroinflammation and lower amyloid burden in brain. Antibiotics were shown to alter the neuropathology in transgenic mice by reducing accumulation of amyloid plaques (Jiang et al. 2017; Bostanciklioğlu 2019).
In human studies, alpha (within sample) and beta (between sample) diversities were reduced in AD compared to controls. Bacterial flora was different in AD compared to healthy controls with reduced abundance of Firmicutes in AD. Pro-inflammatory Escherichia and Shigella were increased with increase in inflammatory markers NLRP3, CXCL2, IL-6, and IL-1β in amyloid positive and cognitively impaired patients compared to cognitively preserved patients. AD gut also demonstrated reduced anti-inflammatory strain Bifidobacterium breve strain A1 and Escherichia rectale (Vogt et al. 2017; Cattaneo et al. 2017).
3.5 Autism Spectrum Disorder
Autism spectrum disorder (ASD) manifests in early childhood and is defined by the clinical features of stereotyped behaviours and rituals and deficits in communication and social interaction. The cause of ASD is largely attributed to the genetic and environmental factors and their interplay (Lord et al. 2018). Neuro-inflammation and gut microbiota has also been implicated as a cause for ASD in the recent studies (Li et al. 2017). Maternal factors including vaginal infections, periodontitis, prescription drug usage (sodium valproate), diet and obesity, mode of delivery (caesarean section vs vaginal delivery) and postnatal factors such as antibiotics and breastfeeding, host genetics and environment shape the gut microbiota in early life (Tamburini et al. 2016). Various human and animal studies have shown that these factors can alter the composition of gut microbiota and their metabolic products thus predisposing a child for autism (Li et al. 2017).
Many clinical and physiological changes seen in ASD including diarrhoea, constipation, gaseous distension, gastroesophageal reflux disease, intolerance to certain food, abnormal metabolites and neuro-inflammation are attributed to the less diverse gut microbiota in the ASD children (Pulikkan et al. 2019). Aberrant behaviours of irritability, hyperactivity, social withdrawal and stereotypy were found to be more in ASD children with gastrointestinal problems (Chaidez et al. 2014; Moradi et al. 2021). Histopathology from ASD subjects with gastrointestinal symptoms was inconsistent with some showing lymphoid nodular hyperplasia, follicular lymphoid hyperplasia, ulcerative colitis and others with either non-specific inflammation or normal findings (Kang et al. 2014). From pyrosequencing study of faecal microflora, higher counts of microbial organisms belonging to Bacteroidetes and Proteobacterium phyla in children with ASD were detected as compared to neurotypical children in whom Firmicutes and Actinobacterium were predominant (Finegold et al. 2010). Similar findings were also observed in a recent study in ASD children between age group 2–4 years which showed higher colonisation by Bacteroidetes, Proteobacteria and butyrate producing Faecalibacterium prausnitzii, whereas a significant reduction of Bifidobacterium longum was observed (Coretti et al. 2018). Contradictory to the above findings, an increase in the ratio of Firmicutes/Bacteroidetes in ASD was reported in some studies (Strati et al. 2017; Moradi et al. 2021). In ASD subjects with constipation, high levels of Escherichia, Shigella and Clostridium cluster XVIII were found (Strati et al. 2017).
As mentioned in the initial part of this chapter, gut microbiota and their metabolites play a central role in brain behaviour modulated via immune, neural and endocrine pathways originating from leaky intestines. In ASD, metabolites from the transformed gut microbiome metabolism lead to increased production of SCFAs including acetate, propionate and butyrate (Li et al. 2017). A decrease in the gut colonisation by E. coli affects the catabolism of 3,3 phenylpropionate. SCFAs activate inflammasomes, innate immune system receptors and sensors thereby modulating downstream inflammatory pathways involving Tregs and TH17 cells contributing to aberrant behaviour in ASD. Levels of pro-inflammatory markers such as TNFα, IL-10, TGFβ, NT and SORT-1 were found to be significantly higher in ASD patients as compared to controls thus confirming that the immune pathways were altered in them (Carissimi et al. 2019). Desulfovibrio sp. and B. fragilis, a predominant gut coloniser in ASD, produce LPS which is an important antigenic component triggering immunological response (Finegold et al. 2010). Thus, intestinal dysbiosis has effects on immunological, biochemical and neuroendocrine system which ultimately influences the brain and behaviour.
3.6 Glioma
Table 7.1 provides a summary of the pattern in gut dysbiosis observed in major neurodegenerative diseases as per reliable research studies conducted in the recent past.
Tumours of CNS cause substantial morbidity and mortality, and the most common histologic type is glioma (Patel et al. 2019). The gut microbiota was recently implicated in the pathogenesis of glioma since the microbiota exert pleiotropic effects in tumour progression, growth, survival, transformation and response to therapeutic agents. A recent study of tumours involving breast, lung, brain, ovary, pancreas, melanoma and bone revealed presence of intracellular bacteria in both cancer and immune cells (Nejman et al. 2020). In the mouse glioma models, reduction in Bacteroidetes and Firmicutes were seen with an abundance of Verrucomicrobia especially of Akkermansia genus after tumour growth was observed. The metabolites such as dihydroxy phenylacetic acid (DOPAC), 5-hydroxy indole acetic acid (5-HIAA), adenosine, histamine, acetate, propionate, butyrate, norepinephrine, GABA, tryptophan, valerate and aspartic acid levels were found to be reduced in them (Dono et al. 2020). The circulating immune cells such as T and B cells and macrophages cross the BBB and play a role in gliomagenesis. Tumour survival and growth is promoted by an immunosuppressed microenvironment created by immune cells and cytokine profile in which Tregs play a crucial role (Mehrian-Shai et al. 2019).
4 Therapeutic Interventions
The gut microbiome composition can be manipulated by the application of probiotics, prebiotics, diet modifications and faecal microbial transplantation. Relying on such alternative interventions to augment the gut microbiome, post proper evaluation and trials will reduce the huge burden on complex drugs and associated side effects, and promote the QOL of patients suffering from neurological disorders.
Probiotics are lactic acid-producing bacteria (Lactococci, Lactobacilli, Bifidobacteria and Saccharomycetes) that augment the IEB and may prevent intestinal inflammatory diseases. Prebiotics are non-digestible oligosaccharides that encourage the growth of beneficial bacteria. A few studies have reported beneficial effects of probiotics/prebiotics in neuropsychiatric symptoms such as anxiety and depression (Li et al. 2017).
Two studies in MS compared outcomes of probiotic preparations with Lactobacillus species versus placebo and noted improvement in markers of inflammation; however, intestinal bacterial loads were not assessed in them (Kouchaki et al. 2017; Tamtaji et al. 2017). Another study showed an anti-inflammatory shift of the flora with an increased abundance of Lactobacillus, and decreased abundance of Akkermansia, Dorea and Blautia with probiotic therapy (Tankou et al. 2018). Probiotics/prebiotics can potentially alter the gut dysbiosis in PD and have been tried as therapeutic strategies. Increased complete bowel movements were reported in one study in PD patients with probiotics/prebiotics compared to placebo (Barichella et al. 2016). Treatment with probiotics (Lactobacillus and Bifidobacterium species) produced an increase in mini-mental status examination (MMSE) scores in a small cohort of patients with AD (Akbari et al. 2016). Larger studies looking into changes in the gut flora and cognitive scores with therapy are needed to assess therapeutic effects further. In a recent systemic review of eight clinical trials that involved the use of pre/probiotic supplementation in children with ASD, prebiotics combined with a gluten and casein-free diet was found to improve certain gastrointestinal symptoms along with significant reduction in anti-sociability scores. But probiotics have limited evidence in alleviating the gastrointestinal or neurobehavioral symptoms in ASD children (Ng et al. 2019).
There is currently no standardised pre/probiotics regimen with regard to type and concentration of different strains, duration of treatments and monitoring parameters in the management of neurological disorders.
Potential therapeutic approaches sought for modification of microbiota in MS also include vitamin D3 supplementation and diet modifications. Vitamin D3 supplementation in a small sample of MS patients improved immune tolerance and reduced inflammation with anti-inflammatory shift of gut microbiota in the subgroup who were not on immunotherapy (Cantarel et al. 2015). Dietary modifications have also been shown to impact the inflammatory profile. A diet rich in simple sugars, saturated fat, salt and high calorie content has been associated with increased risk for autoimmune demyelinating diseases. This has been shown to increase inflammatory cells in the gut and induce gut dysbiosis (Thorburn et al. 2014). A shift to normal gut flora can be induced by a calorie-restricted diet rich in fruits, vegetables and fish although robust studies for the utility in MS are lacking (Mowry et al. 2018).
Faecal microbiota transplantation (FMT) is a procedure wherein the faecal microbiota from a healthy person is transferred to a patient with gut-microbiome dysbiosis. FMT in patients with PD improved constipation and non-gastrointestinal manifestations (Yang et al. 2019). It was also tried in ASD; however, the safety profile and efficacy of this intervention needs to be established (Li et al. 2017). The principle of microbiota transfer therapy (MTT) is based on FMT protocol with minor modifications. Oral vancomycin treatment is given for 14 days followed by fasting (12–24 h) and bowel cleansing. Standardised Human Gut Microbiota (SHGM) either orally or rectally is then administered to repopulate the gut microbiota for a period of 7–8 weeks. Gastrointestinal and behavioural symptoms in ASD subjects significantly improved with MTT (Kang et al. 2017). There is insufficient evidence to translate FMT and MTT to clinical practice at present.
5 Conclusion
The bi-directional pathways involving gut microbiome and human brain connect host behaviour with the gut function thereby subserving a major role in health and disease. The marked differences in the composition of gut microbiota across various studies suggest a strong role for ethnicity, dietary patterns and other environmental influences (Cryan et al. 2019). Nevertheless, the perturbations in the gut microbiota have been linked to disease risk and progression in various neurological disorders including neurodegenerative, neurodevelopmental and autoimmune diseases, tumours and stroke. Gut microbiome dysregulation may not be directly causative, but when combined with other risk factors can be responsible for various neurological disorders (Tremlett et al. 2017). Many of these studies are limited by small sample size and the wide variability in the methods for collection, storage and analysis of samples (Cryan et al. 2019). Therefore, in-depth, standardised and multicentric studies at a global level are highly warranted to substantiate the relationship between gut microbiota and the numerous neurological diseases in which they have been implicated. Such studies possess the potential to identify biomarkers for disease onset, activity and co-morbid conditions. Simultaneously, the role of physiotherapy and non-strenuous, aerobic exercises in alleviating the symptoms in neurological disorders by strengthening the gut-brain axis can be further explored to gauge its potency as a promising intervention strategy in the future.
References
Akbari E, Asemi Z, Daneshvar Kakhaki R et al (2016) Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci 8:256. https://doi.org/10.3389/fnagi.2016.00256
Aresti Sanz J, El Aidy S (2019) Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Psychopharmacology 236:1597–1609. https://doi.org/10.1007/s00213-019-05224-0
Arpaia N, Campbell C, Fan X et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455
Barichella M, Pacchetti C, Bolliri C et al (2016) Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87:1274–1280. https://doi.org/10.1212/WNL.0000000000003127
Bedarf JR, Hildebrand F, Coelho LP et al (2017) Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med 9:39. https://doi.org/10.1186/s13073-017-0428-y
Berer K, Gerdes LA, Cekanaviciute E et al (2017) Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A 114:10719–10724. https://doi.org/10.1073/pnas.1711233114
Blander JM, Longman RS, Iliev ID et al (2017) Regulation of inflammation by microbiota interactions with the host. Nat Immunol 18:851–860. https://doi.org/10.1038/ni.3780
Bostanciklioğlu M (2019) The role of gut microbiota in pathogenesis of Alzheimer’s disease. J Appl Microbiol 127:954–967. https://doi.org/10.1111/jam.14264
Browning KN, Verheijden S, Boeckxstaens GE (2017) The Vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 152:730–744. https://doi.org/10.1053/j.gastro.2016.10.046
Cani PD (2018) Human gut microbiome: hopes, threats and promises. Gut 67:1716–1725. https://doi.org/10.1136/gutjnl-2018-316723
Cantarel BL, Waubant E, Chehoud C et al (2015) Gut microbiota in multiple sclerosis: possible influence of Immunomodulators. J Investig Med 63:729–734. https://doi.org/10.1097/JIM.0000000000000192
Carissimi C, Laudadio I, Palone F et al (2019) Functional analysis of gut microbiota and immunoinflammation in children with autism spectrum disorders. Dig Liver Dis 51:1366–1374. https://doi.org/10.1016/j.dld.2019.06.006
Cattaneo A, Cattane N, Galluzzi S et al (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68. https://doi.org/10.1016/j.neurobiolaging.2016.08.019
Cheung SG, Goldenthal AR, Uhlemann A-C et al (2019) Systematic review of gut microbiota and major depression. Front Psychiatry 10:34. https://doi.org/10.3389/fpsyt.2019.00034
Chaidez V, Hansen RL, Hertz-Picciotto I (2014) Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord 44:1117–1127. https://doi.org/10.1007/s10803-013-1973-x
Chen J, Chia N, Kalari KR et al (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 6:28484. https://doi.org/10.1038/srep28484
Coretti L, Paparo L, Riccio MP et al (2018) Gut microbiota features in young children with autism Spectrum disorders. Front Microbiol 9:3146. https://doi.org/10.3389/fmicb.2018.03146
Cree BAC, Spencer CM, Varrin-Doyer M et al (2016) Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann Neurol 80:443–447. https://doi.org/10.1002/ana.24718
Cryan JF, O’Riordan KJ, Cowan CSM et al (2019) The microbiota-gut-brain Axis. Physiol Rev 99:1877–2013. https://doi.org/10.1152/physrev.00018.2018
De Angelis M, Piccolo M, Vannini L et al (2013) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8:e76993. https://doi.org/10.1371/journal.pone.0076993
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32. https://doi.org/10.1186/s13024-019-0333-5
Dietz KC, Casaccia P (2010) HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol Res 62:11–17. https://doi.org/10.1016/j.phrs.2010.01.011
Dono A, Patrizz A, McCormack RM et al (2020) Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncology 9:CNS57. https://doi.org/10.2217/cns-2020-0007
Dzidic M, Boix-Amorós A, Selma-Royo M et al (2018) Gut microbiota and mucosal immunity in the neonate. Med Sci 6:56. https://doi.org/10.3390/medsci6030056
El Aidy S, Dinan TG, Cryan JF (2014) Immune modulation of the brain-gut-microbe axis. Front Microbiol 5:146. https://doi.org/10.3389/fmicb.2014.00146
Erny D, Hrabě de Angelis AL, Jaitin D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977. https://doi.org/10.1038/nn.4030
Finegold SM, Dowd SE, Gontcharova V et al (2010) Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16:444–453. https://doi.org/10.1016/j.anaerobe.2010.06.008
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155. https://doi.org/10.1038/nn.4476
Gill SR, Pop M, DeBoy RT et al (2006) Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1359. https://doi.org/10.1126/science.1124234
Glatigny S, Bettelli E (2018) Experimental autoimmune encephalomyelitis (EAE) as animal models of multiple sclerosis (MS). Cold Spring Harb Perspect Med 8:a028977. https://doi.org/10.1101/cshperspect.a028977
Gubert C, Kong G, Renoir T et al (2018) Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2019.104621
Heintz-Buschart A, Pandey U, Wicke T et al (2018) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder: nose and gut microbiome in PD and iRBD. Mov Disord 33:88–98. https://doi.org/10.1002/mds.27105
Heneka MT, Carson MJ, Khoury JE et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Hill-Burns EM, Debelius JW, Morton JT et al (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749. https://doi.org/10.1002/mds.26942
Hilton D, Stephens M, Kirk L et al (2014) Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127:235–241. https://doi.org/10.1007/s00401-013-1214-6
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. https://doi.org/10.1016/S1474-4422(09)70062-6
Holmqvist S, Chutna O, Bousset L et al (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820. https://doi.org/10.1007/s00401-014-1343-6
Holzer P, Farzi A (2014) Neuropeptides and the microbiota-gut-brain Axis. In: Lyte M, Cryan JF (eds) Microbial endocrinology: the microbiota-gut-brain Axis in health and disease. Springer, New York, NY, pp 195–219
Ivanov II, Atarashi K, Manel N et al (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498. https://doi.org/10.1016/j.cell.2009.09.033
Jangi S, Gandhi R, Cox LM et al (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:12015. https://doi.org/10.1038/ncomms12015
Jiang C, Li G, Huang P et al (2017) The gut microbiota and Alzheimer’s disease. JAD 58:1–15. https://doi.org/10.3233/JAD-161141
Johnson KV-A (2020) Gut microbiome composition and diversity are related to human personality traits. Human Microbiome J 15:100069. https://doi.org/10.1016/j.humic.2019.100069
Kalia LV, Lang AE (2016) Evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol 12:65–66. https://doi.org/10.1038/nrneurol.2015.249
Kang D-W, Adams JB, Gregory AC et al (2017) Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. https://doi.org/10.1186/s40168-016-0225-7
Kang V, Wagner GC, Ming X (2014) Gastrointestinal dysfunction in children with autism spectrum disorders. Autism Res 7:501–506. https://doi.org/10.1002/aur.1386
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360. https://doi.org/10.1002/mds.26307
Kim S, Kwon S-H, Kam T-I et al (2019) Transneuronal propagation of pathologic α-Synuclein from the gut to the brain models Parkinson’s disease. Neuron 103:627–641.e7. https://doi.org/10.1016/j.neuron.2019.05.035
Kouchaki E, Tamtaji OR, Salami M et al (2017) Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 36:1245–1249. https://doi.org/10.1016/j.clnu.2016.08.015
Li C, Cui L, Yang Y et al (2019) Gut microbiota differs between Parkinson’s disease patients and healthy controls in Northeast China. Front Mol Neurosci 12:171. https://doi.org/10.3389/fnmol.2019.00171
Li Q, Han Y, Dy ABC, Hagerman RJ (2017) The gut microbiota and autism Spectrum disorders. Front Cell Neurosci 11:120. https://doi.org/10.3389/fncel.2017.00120
Lin CH, Chen CC, Chiang HL et al (2019) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson's disease. J Neuroinflammation 16(1):129. https://doi.org/10.1186/s12974-019-1528-y
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392:508–520. https://doi.org/10.1016/S0140-6736(18)31129-2
Lowry CA, Hollis JH, de Vries A et al (2007) Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience 146:756–772. https://doi.org/10.1016/j.neuroscience.2007.01.067
Lyte M, Cryan JF (eds) (2014) Microbial endocrinology: the microbiota-gut-brain Axis in health and disease. Springer, New York, NY
Ma B, Liang J, Dai M et al (2019) Altered gut microbiota in Chinese children with autism spectrum disorders. Front Cell Infect Microbiol 9:40. https://doi.org/10.3389/fcimb.2019.00040
Malan-Muller S, Valles-Colomer M, Raes J et al (2018) The gut microbiome and mental health: implications for anxiety–and trauma-related disorders. OMICS: J Integr Biol 22:90–107. https://doi.org/10.1089/omi.2017.0077
Mehrian-Shai R, Reichardt JKV, Harris CC, Toren A (2019) The is, paving the way to brain cancer. Trends in Cancer 5:200–207. https://doi.org/10.1016/j.trecan.2019.02.008
MetaHIT Consortium (additional members), Arumugam M, Raes J et al (2011) Enterotypes of the human gut microbiome. Nature 473:174–180. https://doi.org/10.1038/nature09944
Mirza A, Forbes JD, Zhu F et al (2020) The multiple sclerosis gut microbiota: a systematic review. Mult Scler Relat Disord 37:101427. https://doi.org/10.1016/j.msard.2019.101427
Miyake S, Kim S, Suda W et al (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 10:e0137429. https://doi.org/10.1371/journal.pone.0137429
Moos WH, Faller DV, Harpp DN et al (2016) Microbiota and neurological disorders: a gut feeling. BioResearch Open Access 5:137–145. https://doi.org/10.1089/biores.2016.0010
Moradi K, Ashraf-Ganjouei A, Tavolinejad H et al (2021) The interplay between gut microbiota and autism spectrum disorders: a focus on immunological pathways. Prog Neuro-Psychopharmacol Biol Psychiatry 106:110091. https://doi.org/10.1016/j.pnpbp.2020.110091
Mowry EM, Azevedo CJ, McCulloch CE et al (2018) Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology 91:e2256–e2264. https://doi.org/10.1212/WNL.0000000000006644
Mowry EM, Glenn JD (2018) The dynamics of the gut microbiome in multiple sclerosis in relation to disease. Neurol Clin 36:185–196. https://doi.org/10.1016/j.ncl.2017.08.008
Mulak A (2015) Brain-gut-microbiota axis in Parkinson’s disease. WJG 21:10609. https://doi.org/10.3748/wjg.v21.i37.10609
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. https://doi.org/10.1146/annurev-immunol-032713-120240
Nejman D, Livyatan I, Fuks G et al (2020) The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 368:973–980. https://doi.org/10.1126/science.aay9189
Ng QX, Loke W, Venkatanarayanan N et al (2019) A systematic review of the role of prebiotics and probiotics in autism Spectrum disorders. Medicina (Kaunas) 55:129. https://doi.org/10.3390/medicina55050129
Pan W, Stone KP, Hsuchou H et al (2011) Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des 17:3729–3740. https://doi.org/10.2174/138161211798220918
Pandit L, Cox LM, Malli C et al (2021) Clostridium bolteae is elevated in neuromyelitis optica spectrum disorder in India and shares sequence similarity with AQP4. Neurol Neuroimmunol Neuroinflamm 8:e907. https://doi.org/10.1212/NXI.0000000000000907
Pascale A, Marchesi N, Marelli C et al (2018) Microbiota and metabolic diseases. Endocrine 61:357–371. https://doi.org/10.1007/s12020-018-1605-5
Patel AP, Fisher JL, Nichols E et al (2019) Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 18:376–393. https://doi.org/10.1016/S1474-4422(18)30468-X
Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22:S119–S122. https://doi.org/10.1016/j.parkreldis.2015.09.004
Poewe W (2008) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15(Suppl 1):14–20. https://doi.org/10.1111/j.1468-1331.2008.02056.x
Pulikkan J, Maji A, Dhakan DB et al (2018) Gut microbial dysbiosis in Indian children with autism spectrum disorders. Microb Ecol 76(4):1102–1114. https://doi.org/10.1007/s00248-018-1176-2
Pulikkan J, Mazumder A, Grace T (2019) Role of the gut microbiome in autism Spectrum disorders. Adv Exp Med Biol 1118:253–269. https://doi.org/10.1007/978-3-030-05542-4_13
Rae-Grant A, Day GS, Marrie RA et al (2018) Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 90:777–788. https://doi.org/10.1212/WNL.0000000000005347
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378:169–180. https://doi.org/10.1056/NEJMra1401483
Rothhammer V, Mascanfroni ID, Bunse L et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. https://doi.org/10.1038/nm.4106
Sampson TR, Debelius JW, Thron T et al (2016) Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480.e12. https://doi.org/10.1016/j.cell.2016.11.018
Scheperjans F, Aho V, Pereira PAB et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. https://doi.org/10.1002/mds.26069
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533. https://doi.org/10.1371/journal.pbio.1002533
Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 11:25. https://doi.org/10.3389/fendo.2020.00025
Smith PM, Howitt MR, Panikov N et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. https://doi.org/10.1126/science.1241165
Strandwitz P (2018) Neurotransmitter modulation by the gut microbiota. Brain Res 1693:128–133. https://doi.org/10.1016/j.brainres.2018.03.015
Strati F, Cavalieri D, Albanese D et al (2017) New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5:24. https://doi.org/10.1186/s40168-017-0242-1
Tamburini S, Shen N, Wu HC, Clemente JC (2016) The microbiome in early life: implications for health outcomes. Nat Med 22:713–722. https://doi.org/10.1038/nm.4142
Tamtaji OR, Kouchaki E, Salami M et al (2017) The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr 36:660–665. https://doi.org/10.1080/07315724.2017.1347074
Tan AH, Mahadeva S, Thalha AM et al (2014) Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord 20:535–540. https://doi.org/10.1016/j.parkreldis.2014.02.019
Tankou SK, Regev K, Healy BC et al (2018) A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 83:1147–1161. https://doi.org/10.1002/ana.25244
Thorburn AN, Macia L, Mackay CR (2014) Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40:833–842. https://doi.org/10.1016/j.immuni.2014.05.014
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474:1823–1836. https://doi.org/10.1042/BCJ20160510
Tremlett H, Bauer KC, Appel-Cresswell S et al (2017) The gut microbiome in human neurological disease: a review: gut microbiome. Ann Neurol 81:369–382. https://doi.org/10.1002/ana.24901
Tremlett H, Fadrosh DW, Faruqi AA et al (2016) Gut microbiota in early pediatric multiple sclerosis: a case−control study. Eur J Neurol 23:1308–1321. https://doi.org/10.1111/ene.13026
Tremlett H, Waubant E (2018) The gut microbiota and pediatric multiple sclerosis: recent findings. Neurotherapeutics 15:102–108. https://doi.org/10.1007/s13311-017-0574-3
van Kessel SP, Frye AK, El-Gendy AO et al (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10:310. https://doi.org/10.1038/s41467-019-08294-y
Varrin-Doyer M, Spencer CM, Schulze-Topphoff U et al (2012) Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize clostridium ABC transporter. Ann Neurol 72:53–64. https://doi.org/10.1002/ana.23651
Ventura RE, Iizumi T, Battaglia T et al (2019) Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci Rep 9:16396. https://doi.org/10.1038/s41598-019-52894-z
Vogt NM, Kerby RL, Dill-McFarland KA et al (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7:13537. https://doi.org/10.1038/s41598-017-13601-y
Xu R, Wang Q (2016) Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol 10:63. https://doi.org/10.1186/s12918-016-0307-y
Yang D, Zhao D, Ali Shah SZ et al (2019) The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol 10:1155. https://doi.org/10.3389/fneur.2019.01155
Vijay N, Morris M (2014) Role of Monocarboxylate transporters in drug delivery to the brain. CPD 20:1487–1498
Wall R, Cryan JF, Ross RP et al (2014) Bacterial neuroactive compounds produced by Psychobiotics. In: Lyte M, Cryan JF (eds) Microbial endocrinology: the microbiota-gut-brain Axis in health and disease. Springer, New York, New York, NY, pp 221–239
Wang L, Christophersen CT, Sorich MJ et al (2011) Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 77:6718–6721. https://doi.org/10.1128/AEM.05212-11
Wu H-J, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3:4–14. https://doi.org/10.4161/gmic.19320
Zurita MF, Cárdenas PA, Sandoval ME et al (2020) Analysis of gut microbiome, nutrition and immune status in autism spectrum disorder: a case-control study in Ecuador. Gut Microbes 11(3):453–464. https://doi.org/10.1080/19490976.2019.1662260
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sundaram, S., Ponnambath, D.K., Nair, S.S. (2022). Microbiota-Gut-Brain Axis in Neurological Disorders. In: Thomas, S. (eds) Human Microbiome . Springer, Singapore. https://doi.org/10.1007/978-981-16-7672-7_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-7672-7_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7671-0
Online ISBN: 978-981-16-7672-7
eBook Packages: MedicineMedicine (R0)