Abstract
Autism spectrum disorder (ASD) is a severe neurodevelopmental or neuropsychiatric disorder with elusive etiology and obscure pathophysiology. Cognitive inabilities, impaired communication, repetitive behavior pattern, and restricted social interaction and communication lead to a debilitating situation in autism. The pattern of co-occurrence of medical comorbidities is most intriguing in autism, compared to any other neurodevelopmental disorders. They have an elevated comorbidity burden among which most frequently are seizures, psychiatric illness, and gastrointestinal disorders. The gut microbiota is believed to play a pivotal role in human health and disease through involvement in physiological homoeostasis, immunological development, glutathione metabolism, amino acid metabolism, etc., which in a reasonable way explain the role of gut-brain axis in autism. Branded as a neurodevelopmental disorder with psychiatric impairment and often misclassified as a mental disorder, many experts in the field think that a therapeutic solution to autism is unlikely to emerge. As the pathophysiology is still elusive, taking into account of the various symptoms that are concurrent in autism is important. Gastrointestinal problems that are seen associated with most of the autism cases suggest that it is not just a psychiatric disorder as many claim but have a physiological base, and alleviating the gastrointestinal problems could help alleviating the symptoms by bringing out the much needed overall improvement in the affected victims. A gut disorder akin to Crohn’s disease is, sometimes, reported in autistic children, an extremely painful gastrointestinal disease which is named as autistic enterocolitis. This disturbed situation hypothesized to be initiated by dysbiosis or microbial imbalance could in turn perturb the coordination of microbiota-gut-brain axis which is important in human mental health as goes the popular dictum: “fix your gut, fix your brain.”
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
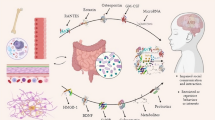
Life will not be possible as we know it today without microbes. Hitherto unknown and unpredicted, the role of gut microbiome in human health and disease is being explored eloquently with the help of next-generation techniques and allied improvements. Coined by Joshua Lederberg, the term microbiome signifies the natural community of microorganisms that factually share our body space and can be categorized as commensal, symbiotic, or pathogenic and have been all but ignored as determinants of health and disease [1]. Gone are the times when the role of microbes in human health was just focused on identifying and treating pathogens in patients, usually with antibiotics. Having colonized by trillions of microorganisms [2], these microbial communities outnumber the total number of human cells by a factor of 10 and contain 150 times as many genes as those encoded by the human genome [3], suggesting us to consider human gut microbiota as an organ or superorganism that coevolved with the human host to achieve a beneficial symbiotic relationship [4]. The term “superorganism” would refer to a collection of individuals which behave as a single unit with enhanced function [4]. The human host is immensely benefitted by the gut microbiota, attested by the fact that there is a plethora of interactions happening between them throughout the development [4] and its disruption could lead to manifold negative consequences [5]. The major role of these microbiota can be defined in terms of a metabolic one. A marvelous metabolic capacity equivalent to that of the liver is envisioned by the combined metabolic capacity of the microbiota, justifying their portrayal as an additional human organ [6] and revealing the exemplary host-microbiota mutualism in the gut. Their supreme role in the maintenance of host health includes facilitating digestion of otherwise indigestible dietary fibers into readily absorbable short-chain fatty acids (SCFAs), synthesis of vitamins and other beneficial metabolites, immune system regulation, detoxification of harmful substances, and enhanced resistance against colonization by pathogenic microorganisms [5]. The defense against pathogen colonization is achieved by nutrient competition and production of antimicrobial activities [7]. Their role in fortification of intestinal epithelial barrier and their ability to induce secretory IgA to limit penetration of bacteria into tissues are not to be overlooked. Researches on germ-free mice reveal that absence of microbes causes significant immune defects at structure levels like decreased Peyer’s patches, lamina propria, and isolated lymphoid follicles. At cellular levels, defects like decreased intestinal CD8+ T cells and CD4+ T helper 17 cells and reduced B-cell production of secretory IgA have been reported [8] (Fig. 13.1).
The human gut microbiota as a superorganism. The largest microbiome is accredited as that seen in our gastrointestinal tract. It is influenced by several external factors, such as diet, inflammation stage, environment, and xeno-metabolome. Each microbiome refers an individual phenotype, able to describe symbiosis-, dysbiosis-, and disease-related gut conditions (Adapted from Putignani et al. (2014) Pediatr Res 76:2–10)
The microbiome at a specific niche is believed to cause local as well as systemic effects on host biology [7]. Chronic low-grade intestinal inflammation seen in irritable bowel syndrome (IBS ) [9] and intense intestinal autoimmunity observed in the inflammatory bowel disease (IBD ) [8] are examples of local effects of the microbiome on host biology. The systemic effect of microbiome on host biology is evident from the fact that the gut microbiome contributes to the etiology of experimental disease models affecting remote organ systems. Immune cells stimulated at the intestinal site, like microbe-sensing antigen processing cells (APCs ) and adaptive immune cells, are trafficked to distal tissue sites by systemic diffusion [7].
The bidirectional interaction between the gut-intestine tract and brain via the microbiome is crucial for maintaining equilibrium between health and disease. The normal microbiome promotes increased metabolic capacity, immune system maturation, and SCFA production, while dysbiosis of microbiome leads to increased inflammation and overabundance of Enterobacteriaceae . This leads to decreased intestinal mucus, immune cell differentiation, gut-associated lymphoid tissue and metabolic capacity, and SCFA production, causing an overall ill health in the host [4]. Recent studies indicate that microorganisms within and among the body habitats exhibit intricate relationships. They play a critical role in driving physical factors such as oxygen, moisture, and pH. They, also, play a role in the host immunological regulation microbial interactions. All these point out the fact that the microbiome plays more crucial role in health and disease than expected so far [10].
The crucial intervention by intestinal communities implicated in diseases like allergies [11], late-onset autism [12], inflammatory bowel disease [13], cancer [14], obesity, and type 2 diabetes [15] reinstates that our understanding of microbial ecology will have a direct bearing on our ability to manage and maintain human health in the future. The major move toward studying changes in composition of the intestinal microbiota in relation to diseases relies primarily on the phylogenetic characterization of the microbiota of diseased individuals in comparison with healthy candidates. Given the substantial interindividual and intraindividual variations in addition to age-related changes in the composition of the intestinal microbiota, it is a difficult task to establish the presence and relative abundance of specific microbial communities in relation with human health status. However, options are realistic to use specific changes in compositional and functional diversity of microbiota as biomarkers for health or specific diseases.
The gut microbiota is a chief contributor in maintaining normal physiology and energy production throughout life. Energy-dependent processes like body temperature regulation, reproduction, and tissue growth are partly influenced by gut microbial energy production [16] further giving evidences for the importance of gut microbiota. The gut microbiome dysbiosis is believed to have a negative impact on gastrointestinal tract-related disorders such as Crohn’s disease and ulcerative colitis, systemic diseases like metabolomic disorders, and central nervous system (CNS )-related disorders [17]. This review highlights the importance of the gut microbiome in autism which is a non-immune-mediated CNS disorder in which the role of gut microbiome has been implicated in its exacerbation.
13.2 Plausible Mechanisms Involved in Gut-Brain Axis Through Gut Microbiome Intervention: Bidirectional Communication
Gut-brain axis (GBA ) can be defined as a physiological framework in which the gut microbiota communicates with the CNS and vice versa through neural, endocrine, and immune pathways. The communication being bidirectional, the role of the brain on the microbial content of the gut and how the intestinal microbiota influences the brain and behavior solicit equal attention. Homeostases of several systems like gastrointestinal tract (GI) function, appetite, and weight control are a few major factors that are significantly affected by the GBA. The microbial habitat is determined by GI motility as well as epithelial functions. Since these factors can be regulated by the CNS, any changes induced by the CNS can result in alteration and perturbation of intestinal microbiota leading to several pathological situations [9].
The enteric microbiota can assert their influence on gut homeostasis by means of regulation of bowel motility, modulation of intestine pain, immune responses, and nutrient processing [18]. Mounting evidences indicate that gut microbiome does affect the development and regulation of hypothalamic-pituitary-adrenal axis (HPA) and behavior [19]. Characterization of the bidirectional interactions between the CNS, the enteric nervous system (ENS ), and the GI tract has brought about results which convincingly suggest the role of the gut microbiota in these gut-brain interactions. This type of bidirectional communication which mediates the symbiotic and pathogenic relationships between the bacteria and the mammalian host is called “microbial endocrinology” [20] or “interkingdom signaling” [21]. The gut microbiota appears to influence the development of emotional behavior, stress and pain modulation systems, and brain neurotransmitter systems. Some of the recent researches explain the multiple mechanisms involved. Endocrine and neurocrine pathways facilitate gut microbiota-to-brain signaling, and the brain, in turn, alters microbial composition and behavior through the autonomic nervous system with the active involvement of immune and humoral system [22].
The enteric microbiota does assert a vital impact on “gut-brain axis” (GBA ), interacting locally with intestinal cells and the ENS and directly with the CNS through neuroendocrine and metabolic pathways (Fig. 13.2). To enumerate the main assistances rendered by bacterial colonization in terms of their absence or presence would include development and maturation of both the ENS and CNS [23], altered expression and turnover of neurotransmitters in both nervous systems [24], alterations of gut sensory-motor functions leading to delayed gastric emptying and intestinal transit [25], and enlarged cecal size [26]. The neuromuscular abnormalities caused via the intervention of microbiota results in downregulation of gene expression of enzymes related to the synthesis and transport of neurotransmitters and muscular contractile proteins [27]. These aberrations have been seen to be normalized once the experimental animals are colonized with specific bacterial species.
It has been observed that germ-free (GF) mice generally show decreased anxiety and an increased stress response. The organism with enhanced levels of ACTH and cortisol demonstrates that microbiota influences stress reactivity and anxiety-like behavior. The microbiota also regulates the set point for HPA activity and modulates the serotoninergic system. GF animals are also known to suffer from memory dysfunction with an altered expression of brain-derived neurotrophic factor (BDNF ) [28].
The main principal mechanisms by which the bidirectional brain-gut-microbiota axis works have been deduced. The gut microbiota asserts its role over the brain through its influence on production, expression, and turnover of neurotransmitters like serotonin and gamma-aminobutyric acid (GABA ) and neurotrophic factor (BDNF ), protection of the intestinal barrier and tight junction integrity, modulation of enteric sensory afferents, production of various bacterial metabolites, and mucosal immune regulation, while the brain claims its influence over microbiota through their capacity to bring about alterations in mucous and biofilm production, alteration in motility, alteration of intestinal permeability, and alteration in immune function [28]. The decisive role of the gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice is established. Catecholamines are crucial in regulation of various types of body functions such as cognitive abilities, mood, and gut motility. The principal catecholamines like norepinephrine (NE) and dopamine (DA) are utilized in the CNS and peripheral nervous systems [29]. A comparative study between specific pathogen-free mice (SPF-M ) and germ-free mice (GF-M) showed that substantial levels of free dopamine and norepinephrine were available in the gut lumen of SPF-M. Also the available catecholamine levels were found to be in a biologically inactive, conjugative form in GF-M. When GF-M was introduced with Clostridium species or SPF fecal flora, which are known to have abundant β-glucuronidase activity, drastic elevation of free catecholamines was visible indicating the role of gut microbiota and in particular the need of β-glucuronidase activity in these organisms [30]. The role of gut microbiota in regulation of sympathetic nervous system (SNS ), a component in bidirectional signaling, has also been reported to take place with the help of short-chain fatty acids and ketones produced by microbiota and their promotion of sympathetic outflow via G protein-coupled receptor 41 (GPR41 ) [31]. The effect of short-chain fatty acids on enterochromaffin cells promotes colonic serotonin production [32] and influences memory and learning process [33]. Another plausible mechanism of microbiota-GBA interaction could be through the release of biologically active peptides from enteroendocrine cells which in turn is regulated by the nutrient availability of the microbiota [34].
When microbiota influences the brain in ways mentioned above, the GBA being a bidirectional signaling pathway, it should be an unavoidable occurrence that the brain dictates or influences microbiota composition and function through various other mechanisms. One major mechanism by which the brains assert its impact on the microbial population is exposure to stressors. A pyrosequencing study carried out to assess the effects of a single 2-hour exposure to a social stressor made a substantial alteration on colonic mucosa-associated microbial profiles of C57BL/6 mice, significantly reducing the relative proportions of the two genera and one family of highly abundant intestinal bacteria, including the genus Lactobacillus [35]. This can happen either directly via host-enteric microbiota signaling or indirectly via changes in the intestinal milieu. A more direct and evident influence on microbiota is facilitated by secretion of signaling molecules by neurons, immune cells, and enterochromaffin cells, under the regulation of the brain. As there are neurotransmitter receptors on bacteria, binding of neurotransmitters is capable of influencing the function of components of the microbiota that have influence on inflammatory and infection stimuli [21]. An example in this regard can be found in Pseudomonas fluorescens which is able to produce GABA and express GABA-binding proteins, and they also increase their necrotic-like activity on eukaryotic glial cells. This particular study also proved that GABA can regulate the lipopolysaccharide (LPS ) structure and cytotoxicity in specific strains of P. fluorescens [36]. Another interesting dimension is the microbial biofilms in which individual groups of bacteria are found to occupy different microhabitats and metabolic niches in the human gastrointestinal tract. The prominent role of the brain in modulation of gut functions, such as motility; secretion of acids, bicarbonates, and mucus; intestinal fluid handling; and mucosal response, is important for the maintenance of the mucus layer. The individual groups of bacteria exist in different microhabitats, and metabolic niches as biofilms in these areas are also modulated by this brain function [37]. The brain affects the microbiota through various stresses induced mainly by bringing about variation in size and quality of mucus secretion [38], by delaying the recovery of the migrating motor complex pattern and inducing a transient slowing of gastric emptying [39], by increasing the frequency of cecocolonic spike-burst activity through the central release of corticotrophin-releasing factor [40]. This causes regional and global changes in gastrointestinal transit which has a profound effect on the way nutrients are delivered to the enteric microbiota. The composition and biomass of the enteric microbiota get modulated by different types of psychological stressors both in adult [41] and newborn animals [42]. When postnatal stress was induced in animal models by separating the mothers, reduction in lactobacilli was observed, disrupting the integrity of the intestinal microbiota with the appearance of stress-indicative behaviors [42]. The animals in study were also more susceptible to opportunistic infection compared to unstressed control animals.
A microbial connection has been linked to anxiety and stress, the common forms of mood disorders. Germ-free mice exhibit increased motor activity and reduced anxiety, suggesting that gut-associated pathogens can exacerbate anxiety. Decreased neurotransmitter receptors and increased tryptophan metabolism observed in the GF condition tend to suggest that the gut microbiome regulates the set point for the HPA axis [24]. When rodents were infected with the food-borne pathogen Campylobacter jejuni , rodents showed perceptible anxiety-like behavior. Increased c-Fos expression as a result of infection with Campylobacter jejuni underpins the notion that gut-associated pathogens can intensify anxiety [43]. While Citrobacter rodentium followed a mechanism similar to Campylobacter jejuni [44], Trichuris muris elevated anxiety via immunological and metabolic mechanisms [45]. The anxiolytic properties exhibited by the specific species of the Lactobacillus and Bifidobacterium genera are almost in contrast to the pathogenic bacteria [46].
13.3 Autism and Gut Microbiome
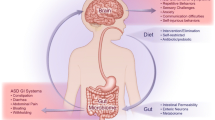
Autism is a non-immune-mediated CNS disorder in which the role of gut microbiome has been implicated in its exacerbation. Among many non-immune-mediated CNS disorders, autism stands out due to its prevalence and bewildering increase in epidemic levels throughout the world. As per Centers for Disease Control and Prevention data, 1 in 500 to 1 in 166 children has an autism spectrum disorder. Various physiological parameters observed in autism include autoimmune reactions, food reactions, diagnostic connection of upper GI disease, abnormal stools, autistic enterocolitis, leaky gut syndrome, excessive inflammation, aberrant glutathione levels, irregular metal, mineral levels, etc. [47], all supporting the importance of the gut microbiome. The aforementioned parameters are either influenced by microbiota or vice versa, revealing the tantalizing link between autism and gut microbiome.
Seemingly pervasive, autism spectrum disorders (ASD) are characterized by impaired communication, difficulty in social interactions, and stereotyped behavioral patterns [48]. People with autism most frequently tend to display unusual ways of learning, paying attention, and reacting to different sensations. Their imaginative skills are also known to suffer [49]. Coupling genetic predisposition with environmental factors seems to raise the risk involved in autistic children.
Considered as a psychiatric disorder, many physical symptoms common in patients with autism have largely gone unnoticed or even ignored by the medical establishment, and coinciding GI symptoms are a major example. Anecdotal reports as well as various studies indicate that gastrointestinal problems are frequently associated with autism [50] and presumed to have a correlation with autism severity [48]. Various independent studies have verified that the GI dysfunction in these children includes diarrhea, unformed stools, constipation, bloating, flatulence, etc. [51, 52]. It was Wakefield and colleagues at the Royal Free Hospital in London who described a new variant of inflammatory bowel disease, named as autistic enterocolitis which is characterized by chronic patchy inflammation and lymphonodular hyperplasia in the terminal ileum or in the colon [53]. Intestinal permeability is another grave issue faced by children with autism, suggesting a leaky gut which can lead to neurological disability as these children are forced to absorb neurotoxic molecules across a gut membrane damaged by inflammation [54]. An abnormal level of intestinal permeability has also been documented in independently carried out studies [55, 56]. A leaky gut allows molecules to enter the bloodstream, otherwise kept at bay. Immune activation, tissue damage, and effects on the brain, including damage to brain tissue, are a few corollary problems that could emerge over time. The opioid peptides that are produced from certain diets can disrupt the normal neurotransmitter function in the brain, causing certain typical behavioral patterns observed in children with autism such as decreased socialization, decreased response to pain, abnormal language, and self-abusive or repetitive behaviors. Direct effects on the neuronal structure of the brain tissue and on the immune system by these opiate-like molecules cannot be ruled out. We need to investigate if neurotoxic and cytotoxic molecules produced by microorganisms can contribute to intensify the abovementioned situations in autistic enterocolitis and leaky gut and their possibility to assert greater damage by working independently or in tandem with each other.
Studies undertaken at different research labs indicate a link between the gut microbiome and ASD, although a direct causality or indirect consequence of atypical patterns of feeding and nutrition has yet to be proved. Children with ASD tend to show food selectivity with strong preferences for starches, snack, and processed food, while most tend to reject fruits, vegetables, and/or proteins [57, 58]. A chronic imbalance of gut microbiota known as intestinal dysbiosis is suspected in children with autism, and many investigators have found evidence of this imbalance in autistic patients [12]. However, most of the gut microorganisms are beneficial to host, and dozens of species of pathogenic organisms, if allowed to thrive due to the compromised immune system, can cause disease. Apart from causing local effects on gut tissue, abnormal bacteria can have an effect on the brain. The toxins that are produced by the harmful bacteria are not properly metabolized. They can build up in the brain by way of the bloodstream, resulting in confusion, delirium, and even coma. The GI inflammation and abnormal immune functions observed in children with autism may increase the abnormal levels of harmful bowel organisms, and metabolites produced by the harmful bacteria can create havoc intensifying GI inflammation, gut permeability, and abnormal immune functions. Yeasts also seem to play a negative role in vulnerable children, as yeasts are known producers of chemicals that have neurological effects and children with autism indicate elevated levels of chemicals that are found in yeasts [59].
A succession of microbial consortia studies in infants has proven that the microbiome gets enriched as associated with life events and as per the diet being introduced. A healthy microbiome is observed to be capable of assisting in the breakdown of complex plant polysaccharides, promoting digestion and overall host health [60]. Therefore, it could be predicted that deviations in the establishment and maintenance of the gut microbiome could lead to pain and discomfort. This hypothesis has been corroborated by William et al. whose study revealed that children with ASD tend to have less of Bacteroidetes which play a prominent role in the digestion of polysaccharides. This study could also underpin the suggestion that children with autism have anomalous carbohydrate digestion capacity and mucosal dysbiosis in the intestines. Metagenomic analysis revealed that they have a decrease in Bacteroidetes, an increase in the ratio of Firmicutes to Bacteroidetes, and an increase in Betaproteobacteria [49]. A pyrosequencing study of fecal microflora of children with autism showed significant differences in the Actinobacteria and Proteobacterium phyla in comparison with healthy controls. The same study also showed higher prevalence of Desulfovibrio species and Bacteroides vulgatus in stools of severely autistic children [12]. Basic anaerobic culturing techniques to count and isolate microorganisms, followed by polymerase chain reaction (PCR) targeting the 16S rDNA in the isolates cultivated in children with late-onset autism against neurotypical children, showed that the number and type of Clostridium and Ruminococcus species significantly differed from normal children [12]. A follow-up study by Song et al. using quantitative real-time PCR found that Clostridium cluster groups I and XI and Clostridium bolteae were significantly higher in children with autism [61]. Another culture-independent study by Parracho et al. using fluorescence in situ hybridization (FISH ) reported elevated levels of Clostridium hystolyticum in the ASD children compared to typical children [49]. Disruption of gut microbiota might contribute for the over-colonization of neurotoxin-producing bacteria, exacerbating autistic symptoms. Clostridium tetani is looked upon as an organism that could induce autism [62]. Clostridia are known propionate producers [63], and the property of propionate to bring upon neurological effects in rats [64] has led to the hypothesis that incidence of autism is related to extensive exposure to Clostridium spores. A study into investigation of prevalence of four types of beneficial bacteria including Bifidobacteria, Lactobacillus spp., E.coli, and Enterococcus revealed that the children with autism had much lower levels of Bifidobacterium, slightly lower levels of Enterococcus, and much higher levels of Lactobacillus [65]. Given that all Lactobacillus strains are beneficial, their higher levels seem to be paradoxical and need to be understood. With regard to commensal bacteria which are neither beneficial nor harmful, the same study found that the autism group was more likely to have Bacillus spp. and less likely to have Klebsiella oxytoca. However, a significant piece of information procured by the same study was the finding of significantly lower levels of short-chain fatty acids observed in autistic children. This was thought to occur due to lower saccharolytic fermentation by beneficial bacteria further substantiating the suspected link between autism and gut microbiome [50].
The finding of distinctive gut microbes associated with ASD was brought about in a small pilot study using a high-throughput sequencing of the 16S rDNA gene by Kang et al. An overall less diverse gut microbiome with a lower abundance of the bacterial genera Prevotella, Coprococcus, and unclassified Veillonellaceae was reported in this study. The said organisms are versatile carbohydrate-degrading and/or fermenting bacteria, and the changes in the spectrum of metabolites produced from a given diet could be greatly influenced by them [66]. The possibility cannot be ruled out that the differences in microbiota composition in ASD may have negatively influenced the microbial interactions so as to result in a decreased overall diversity and reduced function. Another intriguing observation is that Prevotella is a genus highly enriched in populations of agrarian societies, and its depletion in ASD shall be probed if certain environmental factors like industrialization contribute to the prevalence of autism. Individuals with untreated HIV infection are reported to have uniformly high Prevotella [67], and a reverse pattern is observed in ASD. The rationale behind this observation can be explained as HIV patients with a suppressed adaptive immune system tend to have a higher number of Prevotella, whereas those with ASD have a hyperactive adaptive immune system with less Prevotella occurrence.
The metabolites produced by various microorganisms could be performing the same or different functions affecting the system in manifold ways, depending upon their differential abundance. It has been proven that differential abundance of bacteria-produced metabolites has the potential to directly affect neural processes. Increased urinary excretion of an abnormal phenylalanine metabolite of Clostridia species, namely, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA ), has been confirmed in urine samples from patients with autism. This tyrosine analogue is thought to be responsible for depletion of catecholamines, and, thus, it is believed to be a chief contributor in the exacerbation of typical autistic symptoms like stereotypical behavior, hyperactivity, and hyper-reactivity in experimental animals [59]. P-Cresol is another microbial metabolite which can initiate damage in cases of autism as they compete with neurotransmitters for enzymes and cofactors needed for sulfonation reactions in the liver [68]. A recent study using a maternal immune activation (MIA) model of ASD in mice showed a significant increase in 4-ethylphenylsulfate (4EPS ), a metabolite produced by gut bacteria [69]. Bacterial tag-encoded FLX-titanium amplicon pyrosequencing carried out by De Angelis et al. showed that the highest microbial diversity presented with autism. The same study also identified higher abundance of Caloramator, Sarcina and Clostridium genera in autistic children. Alistipes and Akkermansia species were higher in autism along with almost all of the identified Sutterellaceae and Enterobacteriaceae . Concomitantly, the levels of free amino acids and volatile organic compounds of fecal samples were markedly different in autism [70]. Notably, lower levels of Prevotella, Coprococcus, and unclassified Veillonellaceae were observed by Kang et al. in autistic children in a study carried out using 16 s rRNA gene pyrosequencing analysis from fecal DNA samples [66].
13.3.1 Mineral Elements and Gut Microbiome
Higher average levels of several toxic metals are evident in autism severity. Lead, mercury (Hg), arsenic (As) [71], thallium, tin, and tungsten [72] are among a few of the metals which correlate with autism severity. The role of environmental pollutants such as these heavy metals in the alteration of physiological functions causing detrimental effects on health has been established. Recognized as neurotoxicants with known effects on neurodevelopment, their role in microbial dysbiosis is inferred adding to the exacerbation of autism symptoms. Although, the exact mechanism has not been deduced, the essential role of the intestinal microbiome in limiting the heavy metal body burden has been established using GF mouse studies [73]. An interesting conclusion of this particular study was that genes relevant for divalent metal transporters and oxidative pathways were expressed with significant differences depending on the microbial status of the animal along with the dose and type of metals present, suggesting the complex host-microbe interplay induced by the environmental pollutants inside the gut. The ability of metals such as Hg and As to exert toxic effects on human health has been well characterized. The volatile derivatives of these metals interact directly with host cells causing irreversible damage and aggravate the diseased state by disturbing the physiological microflora [74]. The critical role of gut microbiota in intestinal homeostasis is characterized by the fact that different types of dysbiosis cause diseases outside and inside the intestine. A study by Breton et al. proved that oral exposure to heavy metals does lead to specific changes in bacterial commensal communities. Their study showed that, relative to the control animals, test animals had notably lower numbers of Lachnospiraceae and higher numbers of Lactobacillaceae and Erysipelotrichaceae [73].
Chronic dietary depletion of elements like iron (Fe) [75] and zinc (Zn) [76] is found to induce significant taxonomic alterations in the gut microbial profile. Understanding the effects of Zn deficiency on the host may help to elucidate the influence of gastrointestinal microbiota on physiology from a novel perspective. The need of Zn, almost in double the amount, in conventionally raised mice against their germ-free counterparts as indicated in studies points out the role of the host microbiota in Zn homeostasis [77]. It has also been seen that optimal levels of Zn administration on various animal models had the benefits of increased concentration of short-chain fatty acids (SCFAs ) [78], increased overall bacterial species richness and diversity [79], and favorable change in metabolic activity [80]. Studies have also revealed that gut microbial diversity of Zn-deficient organisms bear a resemblance to that of GI microbiota in pathological states. The mentioned study also suggested that chronic Zn deficiency may reshape the gut microbiome. The metagenome predictive analysis showed that cecal microbiome metabolism was perturbed in Zn-deficient organisms since aberrant pathways involving lipid metabolism, carbohydrate digestion, and mineral absorption were prominent [76].
With practical concern for human health, Fe is also an important trace element to study. Low Fe conditions are seen to cause a decrease in Roseburia spp. and an increase in Lactobacillus spp., along with a parallel decrease in butyrate and propionate [75]. Thus low Fe conditions may contribute to weaken the barrier effect of microbiota by strong dysbiosis and decreased production of beneficial metabolites.
13.3.2 Glutathione Metabolism and Gut Microbiota
One of the most important molecules, glutathione, is popularly called the mother of all antioxidants. This prototype antioxidant is capable of protecting cells from the harmful effects of oxidative stress and can act as a defensive agent against toxic xenobiotics [81]. Although the role of gut microbiota and dysbiosis is inferred as a cause for many pathological situations, the mechanistic insight into how the specific microbial populations lead to the progression of such disorders has not been studied extensively. It has been assumed that microbiota in the small intestine consumes glycine as well as other amino acids to support its growth and survival, curtailing the availability to the host and thus causing decreased levels of the amino acids and glutathione metabolism [82]. This could underlie the mechanism of how the gut microbiome plays a pivotal role in the exacerbation of certain metabolism-related disorders.
13.4 Conclusions
The realization is that the microbes that live inside and on us outnumber our somatic and germ cells by an estimated tenfold. This has given them the status of a supraorganism with their capacity of providing traits in human beings and has significantly increased the importance of gut microbiome in health and disease. This concept is expected to lead to a paradigm shift in the strategies involved in diagnosis, prognosis, and treatment of a few disorders involving the gut-brain axis in which autism claims a position. Without a cure so far and with limited knowledge on the etiology of the disorder, autism is a topic that needs immediate attention from the researchers. As some of the studies indicate that the gut microbiome modulates the glutathione and certain minerals like zinc, copper, and iron in the experimental organism, studies on the gut microbiome in autism can give some serendipitous insights into the etiology, diagnosis, and treatment of this condition.
References
Lederberg J, McCray AT (2001) Ome SweetOmics--A Genealogical Treasury of Words. The Scientist 15(7):8–8
Turnbaugh PJ, Gordon JI (2009) The core gut microbiome, energy balance and obesity. J Physiol 587(17):4153–4158
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65
Glendinning L, Free A (2013) Supra-organismal interactions in the human intestine. Front Cell Infect Microbiol 4:47–47
Walker AW, Lawley TD (2013) Therapeutic modulation of intestinal dysbiosis. Pharmacol Res 9(1):75–86
Sommer F, Bäckhed F (2013) The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11(4):227–238
Wang Y, Kasper LH (2014) The role of microbiome in central nervous system disorders. Brain Behav Immun 38:1–12
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immun 9(5):313–323
Collins SM, Bercik P (2009) The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136(6):2003–2014
Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J et al (2012) Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol 8(7):e1002606. https://doi.org/10.1371/journal.pcbi.1002606
MacDonald TT, Monteleone G (2005) Immunity inflammation and allergy in the gut. Science 307(5717):1920–1925
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E et al (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35(Supplement 1):S6–S16
Hume G, Radford-Smith GL (2002) The pathogenesis of Crohn's disease in the 21st century. Pathology-Abingdon 34(6):561–567
McGarr SE, Ridlon JM, Hylemon PB (2005) Diet anaerobic bacterial metabolism and colon cancer: a review of the literature. J Clin Gastroenterol 39(2):98–109
Hartstra AV, Bouter KE, Bäckhed F, Nieuwdorp M (2015) Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 38(1):159–165
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W et al (2012) Host-gut microbiota metabolic interactions. Science 336(6086):1262–1267
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen L (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26. https://doi.org/10.3402/mehd.v26.26191
Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6(5):306–314
Sudo N (2014) Microbiome HPA axis and production of endocrine hormones in the gut. Adv Exp Med Biol 817:177–194
Lyte M (2004) Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol 12(1):14–20
Hughes DT, Sperandio V (2008) Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6(2):111–120
Mayer EA, Tillisch K, Gupta A (2015) Gut/brain axis and the microbiota. J Clin Invest 125(3):926–938
Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R et al (2005) Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol 100(11):2560–2568
Clarke G, Grenham S, Scully P, Fitzgeral P, Moloney RD, Shanahan F et al (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18(6):666–673
Abrams GD, Bishop JE (1967) Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med 126(1):301–304
Iwai H, Ishihara Y, Yamanaka J, Ito T (1973) Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn J Exp Med 43(4):297–305
Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291(5505):881–884
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56(3):331–349
Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K (2012) Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol-Gastrointest Liver Physiol 303(11):G1288–G1295
Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S et al (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 108(19):8030–8035
Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL et al (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29(4):1395–1403
Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA (2009) Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A 106(23):9447–9452
Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E (1994) Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 107(5):1259–1269
Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS et al (2014) Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 14(1):189. https://doi.org/10.1186/1471-2180-14-189
Guthrie GD, Nicholson-Guthrie CS (1989) gamma-Aminobutyric acid uptake by a bacterial system with neurotransmitter binding characteristics. Proc Natl Acad Sci U S A 86(19):7378–7381
Macfarlane S, Dillon JF (2007) Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol 102(5):1187–1196
Rubio CA, Huang CB (1991) Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo 6(1):81–84
Gue M, Peeters T, Depoortere I, Vantrappen G, Bueno L (1989) Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology 97(5):1101–1107
Gue M, Junien JL, Bueno L (1991) Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology 100(4):964–970
Schaedler RW, Dubos RJ (1962) The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med 115(6):1149–1160
Bailey MT, Coe CL (1999) Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 35(2):146–155
Gaykema RP, Goehler LE, Lyte M (2004) Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun 18(3):238–245
Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE (2006) Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 89(3):350–357
Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X et al (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139(6):2102–2112
Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E et al (2013) Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology 38(9):1738–1747
Jepson B, Johnson J (2007) Changing the course of autism: A scientific approach for parents and physicians. 1st edn, Sentient Publications Boulder. ISBN-10: 1591810612
Williams BL, Hornig M, Parekh T, Lipkin WI (2012) Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio 3(1). https://doi.org/10.1128/mBio.00261-11
Parracho HM, Bingham MO, Gibson GR, McCartney AL (2005) Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54(10):987–991
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA (2011) Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11(1):22. https://doi.org/10.1186/1471-230X-11-22
Ming X, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC (2008) Autism spectrum disorders: concurrent clinical disorders. J Child Neurol 23(1):6–13
Valicenti-McDermott M, McVicar K, Rapin I, Wershil BK, Cohen H, Shinnar S (2006) Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J Dev Behav Pediatr 27(2):S128–S136
Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M et al (1998) Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 352(9123):234–235
Wakefield AJ, Puleston JM, Montgomery SM, Anthony A, O'leary JJ, Murch SH (2002) The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Aliment Pharmacol Ther 16(4):663–674
d'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M et al (1996) Abnormal intestinal permeability in children with autism. Acta Paediatr 85(9):1076–1079
Horvath K, Perman JA (2002) Autistic disorder and gastrointestinal disease. Cur Opin Pediatr 14(5):583–587
Sharp WG, Jaquess DL, Lukens CT (2013) Multi-method assessment of feeding problems among children with autism spectrum disorders. Autism Spectr Disord 7(1):56–65
Field D, Garland M, Williams K (2003) Correlates of specific childhood feeding problems. J Paediatr Child Health 39(4):299–304
Shaw W (2010) Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 13(3):135–143
Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaug J, Knight R et al (2011) Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108(Supplement 1):4578–4585
Song Y, Liu C, Finegold SM (2004) Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 70(11):6459–6465
Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML et al (2000) Short-term benefit from oral vancomycin treatment of regressive-onset autism. JChild Neurol 15(7):429–435
Elsden SR, Hilton MG (1978) Volatile acid production from threonine, valine, leucine and isoleucine by clostridia. Arch Microbiol 117(2):165–172
Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R et al (2008) Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology 54(6):901–911
Pulikkan J, Maji A, Dhakan DB, Saxena R, Mohan B, Anto MM et al (2018) Gut microbial dysbiosis in Indian children with autism spectrum disorders. Microb Ecol 76(4):1102–1114
Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB et al (2013) Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8(7):e68322. https://doi.org/10.1371/journal.pone.0068322
Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK et al (2014) An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 7(4):983–994
Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK (2009) Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci 106(34):14728–14733
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Patterson PH (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463
De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI et al (2013) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8(10):e76993. https://doi.org/10.1371/journal.pone.0076993
Dickerson AS, Rahbar MH, Bakian AV, Bilder DA, Harrington RA, Pettygrove S et al (2016) Autism spectrum disorder prevalence and associations with air concentrations of lead, mercury, and arsenic. Environ Monit Assess 188(7):407. https://doi.org/10.1007/s10661-016-5405-1
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E et al (2013) Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol Trace Elem Res 151(2):171–180
Breton J, Daniel C, Dewulf J, Pothion S, Froux N, Sauty M et al (2013) Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol Lett 222(2):132–138
Meyer J, Michalke K, Kouril T, Hensel R (2008) Volatilisation of metals and metalloids: an inherent feature of methanoarchaea? Syst Appl Microbiol 31(2):81–87
Dostal A, Fehlbaum S, Chassard C, Zimmermann MB, Lacroix C (2013) Low iron availability in continuous in vitro colonic fermentations induces strong dysbiosis of the child gut microbial consortium and a decrease in main metabolites. FEMS Microbiol Ecol 83(1):161–175
Reed S, Neuman H, Moscovich S, Glahn RP, Koren O, Tako E (2015) Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 7(12):9768–9784
Smith JC, McDaniel EG, McBean LD, Doft FS, Halsted JA (1972) Effect of microorganisms upon zinc metabolism using germfree and conventional rats. J Nutr 102(6):711–719
Pieper R, Vahjen W, Neumann K, Van Kessel AG, Zentek J (2012) Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. J Anim Physiol Anim Nutr 96(5):825–833
Vahjen W, Pieper R, Zentek J (2010) Bar-coded pyrosequencing of 16S rRNA gene amplicons reveals changes in ileal porcine bacterial communities due to high dietary zinc intake. Appl Environ Microbiol 76(19):6689–6691
Højberg O, Canibe N, Poulsen HD, Hedemann MS, Jensen B (2005) Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol 71(5):2267–2277
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66(8):1499–1503
Mardinogl A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E et al (2015) The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 11(10):834. https://doi.org/10.15252/msb.20156487
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pulikkan, J., Mazumder, A., Grace, T. (2019). Role of the Gut Microbiome in Autism Spectrum Disorders. In: Guest, P. (eds) Reviews on Biomarker Studies in Psychiatric and Neurodegenerative Disorders. Advances in Experimental Medicine and Biology(), vol 1118. Springer, Cham. https://doi.org/10.1007/978-3-030-05542-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-05542-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05541-7
Online ISBN: 978-3-030-05542-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)