Abstract
For the actual climate crisis, resilient agriculture is required to guarantee access to enough food, in quality and quantity, for a growing population. To face these challenges, innovative agricultural practices under organic or biological concepts for a sustainable crop production are required. To achieve the goal of sustainable agriculture, it is necessary to incorporate new models, agricultural supplies, and biotechnologies to enhance crop productivity. Plant biostimulants emerge as an innovative option to conventional chemical plant nutrition schemes. Active molecules in these compounds trigger complex physiological and metabolic responses in plants, enhancing plant performance and stress adaptation traits that ultimately result in an increased yield. Biostimulants based on protein hydrolysates (PH) are particularly relevant in the concept of plant stimulation. PH-based biostimulants are produced from different protein by-products and wastes by enzymatic processing, and the mixture of oligopeptides released during these proteolytic events is the main active compound associated with the stimulatory effects observed in different crops. This chapter describes the fundamentals in the technologies used in PH-based biostimulant productions, enzymatic processing, and recent advances in biostimulant research and development, as well as the incorporation of new phenomics and transcriptomic technologies to elucidate the mode of action of these biostimulants in a concept of rational design.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Since the past decade, serious questions are being raised about the overuse of agrochemicals in agricultural systems and their adverse effects on the environment and soil biochemistry. An increase in soil salinization, toxicity, and loss of soil microbial diversity are the most common problems around this production concept that ultimately result in poor crop productivity (Ganguly et al. 2021). This concern has led to an increasing interest in new agroecological alternatives for crop nutrition and management, especially under a climate change scenario. As a result of different research efforts, plant biostimulants emerge as an innovative option to chemicals in agriculture; additionally, it has been proven that the use of these compounds also promotes interesting changes in crop physiology, enhancing environmental resilience, yield, and quality (du Jardin 2015; Colla et al. 2017). Biostimulants differ in their chemical nature, stimulatory mechanism, and efficiency; particularly protein hydrolysates (PH) are considered one of the most complex in composition, as well as in the mode of action, triggering intricate plant responses at the cellular level. Conceptually, PH-based biostimulants must be produced from any protein source (food, waste, or by-product); however, some technical issues arise during its manufacturing, characterization, and testing (Moreno-Hernández et al. 2020). Given the increasing use of PH-based biostimulants in food production, regulation of the market is necessary to provide accurate pieces of evidence of experimental biostimulants and their primary function (Ricci et al. 2019; EBIC 2021). Recently, numerous PHs are recognized as plant biostimulants by improving specific traits in crops, and others are under continuous evaluation by phenomics and omic-based approaches. This chapter highlights some fundamentals involved in the science of PH-based biostimulants, focusing on the technology for its production and evaluation for agricultural purposes.
6.2 Proteins from By-Products for Hydrolysates Production
By-products represent an excellent source of valuable compounds for organic agriculture. Some of these resources are especially rich in protein content that might be recovered, isolated, and bioconverted into add-value products. Annually, large volumes of waste effluents and solid by-products are produced worldwide, only 54 billion pounds correspond animal-derived, and similar volume to cereal-processing waste, under-utilized foods, and unoptimal edible horticultural fruits (one-third of food for human consumption), unfortunately only a small part of these resources are properly exploited, and transformed into commodities (Martínez-Alvarez et al. 2015; FAO 2021). Table 6.1 indicates the most representative by-products produced by agriculture, livestock, and seafood industries as well as its protein component and summarizes protein recovery principles applied for waste processing. Solid or effluent by-products from vegetable or animal sources are complex matrices with a differentiated composition, not only in protein content but also other macromolecules, including other aggregated proteins, fatty acids, starch, fibers, gums, and polyphenols, rendering difficult its extraction and utilization for PH production. Protein solubility is a key feature for successful protein recovery in general; highly soluble proteins are extracted-solubilized easily by washing process or maceration with low ionic strength solutions (<0.05 M NaCl, pH 4.5–5.5) that include sarcoplasmic fraction in minced meal and green leaves proteins (chloroplastic, cytosolic protein) accounting high recovery yields (70–80%) by combining centrifugation process (Kim et al. 2005; Tamayo Tenorio et al. 2016). Similar approaches have been used in aqueous two-phase partitioning (ATP) for a proper resolution of whole blood proteins (hemoglobin, plasmin, albumin) employing polyethylene glycol/sal combination, recovering around 85% of albumin from blood suspension in the aqueous phase (Rito-Palomares et al. 1998). In contrast, extraction of stromal, myofibrillar, and structural proteins requires the combination of several extractions and fractionation principles. In meat wastes, both terrestrial and aquatic organisms, myofibrillar contractile proteins (myosin-actin complex) represent over 70% (w/w) of total protein content, its separation requires consecutive washing protocols with concentrated chaotropic salt solutions (0.3–0.6 M NaCl or KCl) improving protein extractability in different meat systems (Dara et al. 2021).
Particularly, fish processing waste represents an important source of proteins with biotechnological potential, typically 60–70% of the fish weight is discarded in the form of frames, heads, tails, and guts, representing a suitable resource for myofibrillar, collagen, and elastin protein isolated manufacturing. The pH-shifts process improves the separation of myofibrillar proteins from collagen-enriched structures, by employing isoelectric solubilization/precipitation (ISP). ISP-disruption induces selective solubilization in conditions away from isoelectric point (pI) of proteins, and precipitation near to protein pI (pH 5.5 for myofibrillar proteins) increasing over 90% the concentration of crude hydrolyzable protein (Matak et al. 2015). After separation of collagen-containing tissues (bones, skin, cartilage) from other proteins, insoluble collagen might be extracted by a combination of acid-saline-enzymatic conditions to increase collagen solubilization, the amount of protein recovered vary according to cross-linking grade in collagen molecule, source, and method for processing. Generally, higher collagen yields (80-84%) are obtained by pepsin-solubilized methods in comparison with acid-assisted extraction (Ahmed et al. 2020). Solubility problems are observed in keratin, which is the main component in chicken feathers and horn wastes. Sinkiewicz et al. (2017) describe a method for the preparation of soluble feather keratin, coupling ether pre-treatment (defatting), and chemical alkaline-hydrolysis reaction in presence of reducing agents (2-mercaptoethanol, sodium m-bisulfite, sodium bisulfite, or dithiothreitol) obtaining over 80% of keratin yield. Microbial fermentation, microwave irradiation, and superheat processing have been also discussed in detail for keratin extraction from different natural resources (Chilakamarry et al. 2021).
Agrofood wastes offer extraordinary potential as sources of protein substrates in PH manufacturing. Waste from wet-milling, soy paste, and legumes are excellent sources of albumins, globulins, glutenins, and prolamin proteins, the last one represents around 80% of crude protein content. Prolamins extraction protocols include alcoholic solubilization of cereal meal in 70% ethanol aqueous solution in continuous stirring system, after centrifugation the supernatant containing prolamins is recovered and concentrated by lyophilization. This approach is employed practically for different cereal meals, including barley, sorghum, wheat, and corn, with minor modifications to obtain between 60 and 80% of prolamin protein (Tapia-Hernández et al. 2019). Recently, ultrasonic and hydrothermal processing have been proposed to improve protein/peptide extraction from soy, legume, algal material as an alternative to chemical-based processes (Rahman et al. 2020; Rico et al. 2020).
6.3 Fundamentals in Protein Hydrolysates Production
Protein hydrolysates (PH) are considered as mixtures of polypeptides, oligopeptides, and amino acids released by partial hydrolysis of proteins (Schaafsma 2009). Due to the importance of peptides and amino acids as basic building blocks of proteins and their multiple physiological functions in the plant, the selection of protein source is a key factor to obtain PH-based biostimulant with attractive functions (Popko et al. 2018; Moreno-Hernández et al. 2020). In addition, since amino acid synthesis is a highly energy-consuming process, its presence in PH allows plants to save energy and increase the metabolic rate or de-novo reconstruction (Popko et al. 2014).
6.3.1 Amino Acid Content and Profile Analysis
Amino acids are one of the main bioactive ingredients in PHs applied as biostimulants, the accurate determination of its content is important. Generally, protein substrates are hydrolyzed at acidic conditions to their constituent amino acids and consequently are separated by chromatographic techniques, mainly Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC). Once the amino acids are separated, its detection and quantification involve the reaction of the amine portion with a derivatizing reagent (Klampfl 2005). The selection of hydrolysis conditions of samples and the kind of derivatization (pre- or post-column) is an important aspect to be considered to obtain reliable results of amino acid composition. For instance, the most common method for protein hydrolysates involves the usage of strong monoprotic acid at high concentration (HCl 6 M) in combination with high temperature (110 °C) under vacuum for 20–24 h; however, this method might lead to loss of Ser, Thr, and Tyr (Jajić et al. 2013). Regarding derivatization reagents, the most common is o-phthaldialdehyde (OPA), which reacts with primary amines producing a fluorophore that is able to be excited at 302–395 nm and detected at 420–650 nm (Roth and Hampaǐ 1973). However, the OPA reagent is only suitable to detect primary amines, therefore, other reagents such as 9-Fluorenylmethyl chloroformate (FMOC) is utilized for the derivatization of secondary amino acids, including Hyp and Pro (Turnell and Cooper 1982). The main issue of derivatives reagents like OPA is their limited stability and so must be prepared routinely before each run to avoid interferences (Halket et al. 2005). More recently, the application of high-performance liquid chromatography (HPLC), coupled with tandem mass spectrometry (LC–MS/MS) using hydrophilic columns, offers a suitable method that doesn’t need the usage of unstable derivatives reagents and has been used for successful resolution of amino acids profile for animal and plant protein matrices (Kambhampati et al. 2019).
Amino acids are crucial for plant biochemistry, participating as anti-stress (Hyp, Pro) and chelating agents (Cys, Glu, Gly, His, Lys), as well as stimulating chlorophyll synthesis (Ala, Lys, Ser), seed germination (Asp, Glu, Lys, Met, Phe, Thr), and signaling process in hormone metabolism. (Ala, Pro) (Paleckiene et al. 2007; Popko et al. 2018); however, some consideration must be taken into account during protein source processing in order to guarantee amino acid integrity and functionality. In practice, chemical (strong acids or alkalis) and enzymatic methods are used for hydrolysate production, and both strongly affect the amino acidic composition of the final product (Colla et al. 2015). For instance, acid hydrolysis is a low-cost process, but causes the destruction of Trp and a partial loss of Met. Alkaline hydrolysis (using NaOH or KOH) has the advantage of low cost and full recovery of Trp, but can generate the loss of most amino acids (Hou et al. 2017). Also, chemical hydrolysis combining high temperatures (121–137 °C) and acid or alkaline treatment, is considered a drastic process that results in the conversion from L-forms to D-form of amino acids, and the hydrolyzed product is composed by free amino acids and to a lesser extent by soluble peptides, limiting their metabolism and causing other toxic effects in plants (Cerdán et al. 2008). On the other hand, enzymatic hydrolysis can be performed under mild conditions with precise control of the degree of hydrolysis, minimizing side reactions and the presence of toxic chemicals in the products; thus, the final PH contains higher peptides: free amino acids ratio, and proportion of L-amino acids (proteinogenics) in comparison with those obtained by chemical hydrolysis (Colla et al. 2017; Álvarez-Viñas et al. 2020).
Related to the protein source (animal or vegetal) and amino acid composition of PH-based biostimulants, it has been observed that animal protein hydrolysates from collagen possess elevated concentrations of Gly and Pro, whereas in legume derived PH, Asp and Glu acids are predominant (Ertani et al. 2014; Colla et al. 2015, 2017). In this sense, comparing several protein sources applied as plant biostimulants, it has been observed that Gly concentration of fish and chicken hydrolysates per 100 g of protein is higher than that of alfalfa but less than that of animal gelatin, although this parameter must be strongly influenced by the source (Table 6.2). Legume-derived PH biostimulant Trainer® (Italpollina S.P.A., Italy) comprises mainly amino acids and soluble peptides (75% of free amino acids and peptides), 22% of carbohydrates, and 3% of mineral nutrients (Di Mola et al. 2020; Lucini et al. 2020). The foliar application of Trainer® in spinach and lamb’s lettuce (sprayed four times at 21, 27, 33, and 39 days after sowing, at a concentration of 4 mL/L), induced a major improved N uptake/use efficiency compared to untreated plants (Di Mola et al. 2020). Feather keratins have low bio-availability, and it is deficient in amino acids His, Lys, Met, and Trp (Callegaro et al. 2019), while collagen has high assimilation by its significant amounts of Gly, Pro, and Lys (Colla et al. 2015) with distinctive assimilation/functions in plant metabolism and development. The deficiency of some amino acids could limit the biostimulant activity for some PH.
6.3.2 Hydrolysis Degree
Protein hydrolysates are composed of a complex mixture of free amino acids and peptides of different chain lengths. Generally, it is assumed that the degree which indicates that a protein substrate has been hydrolyzed is proportional to the number of peptide bonds broken and, consequently, to the average size and molecular mass of the peptides present. In this regard, the number of peptide bonds broken as a proportion of the total number of peptide bonds present is defined as the percentage degree of hydrolysis (DH) (Rutherfurd 2010).
Protein enzymatic hydrolysis is frequently preferred over chemical hydrolysis for several reasons. For instance, it preserves the nutritional quality of the amino acids better than chemical hydrolysis. In addition, the choice of the enzyme allows the control of protein breakdown at desired DH and drives the hydrolysis toward the desired hydrolysis products (Friedman 1996; Wouters et al. 2016). Although, a high percentage of PHs used as biostimulants are produced by chemical hydrolysis of proteins from animal origin and their production processes are considered harmful to the environment. In this sense, due to enzymatic hydrolysis being ecologically safe, this kind of PH is gaining acceptance for organic agriculture, being the preferred option for farmers and informed consumers (Bradshaw et al. 2012; Colla et al. 2015; Caruso et al. 2019; Madende and Hayes 2020).
Several methods exist to determine DH during protein hydrolysis, but there is no standard technique to accomplish reliable results for samples that have been produced by chemical or enzymatic hydrolysis (Spellman et al. 2003). For instance, DH can be measured by determining the amount of nitrogen released during hydrolysis, which becomes soluble in the presence of a precipitating agent such as trichloroacetic acid (Hung et al. 1984). Another approach to determine DH is by quantification of the free amino groups released during hydrolysis using compounds that react specifically with amino groups such as trinitrobenzene-sulphonic acid (TNBS) and o-phthaldialdehyde (OPA); several modifications of these techniques exist (Polychroniadou 1988; Caer and Colas 1993; Nielsen et al. 2001). Another technique used to measure DH is the pH-stat method, having the advantage of monitoring the hydrolytic process in real-time, taking advantage of the dissociation of protons from the free amino groups that occurs when hydrolysis is carried out at neutral or alkaline conditions (Adler-Nissen 1986).
Since hydrolysis on protein structure causes a decrease of molecular weight (MW) and also increases the number of ionizable groups and the accessibility of hydrophobic regions in the protein structure (Panyam and Kilara 1996) the biostimulant effect of PH can be affected. For example, Lucini et al. (2020) analyzed the effect of peptide fractions on the performance of a legume-derived PH biostimulant in tomato; interestingly, the smallest (MW <1 kDa) peptides showed the most active stimulatory activity.
6.4 Protein Hydrolysate Production
The production of protein hydrolysates has been increased in the last three decades (CAGR of 6.5% and a market size value of $844.2 m by 2019), and are specially used as additives in food products and feed for animals. This process converts raw agricultural materials or pure proteins into value-added products for use in several agro-industries However, recently, its uses as plant biostimulants (PB) in agricultural practices has gained relevance for improving nutrition, quality, yield, and abiotic tolerance in different crops (Colla et al. 2015).
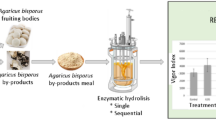
PH-based biostimulants can be manufactured from agro-industrial by-products or by using pure proteins. The use of isolated proteins results in better quality products; however, it increases the costs of production. Therefore, the use of protein-rich by-products is more attractive. Protein sources are pre-treated by either heating them with acid, fermented with specific microorganisms, applying separation procedures (e.g., pressing, defatting, sedimentation, centrifugation, filtration, etc.), or adding enzymes to remove undesirable material, as was discussed in previous sections. PH is a complex mixture of polypeptides of different sizes and free amino acids, and their composition and properties are highly variable depending on the protein source, type of hydrolytic method, degree of hydrolysis, fractionation, etc. (Moreno-Hernández et al. 2020). Protein hydrolysate production by enzymatic methods has been the preferred process (around 70% of the PH is produced by this procedure) due to its higher efficiency than acid and alkaline treatments since these last can destroy essential amino acids such as lysine, serine, arginine, and threonine (Fiormarket 2020). On the contrary, enzymatic methods are eco-friendly and hydrolytic-conditions controllable, which yields products with characteristics and quality reproducibles.
6.4.1 Protein Substrates Treatment
Most commercial protein hydrolysate-based biostimulants are plant protein-derived (P-PH); however, animal protein-derived (A-PH) have also gained acceptance due to their satisfactory results and their lower cost (Lucini et al. 2020). Several plants (e.g., legume seeds, alfalfa hay, corn wet-milling, and vegetable by-products) and animal sources (e.g., leather by-products, collagen, blood meal, fish by-products, chicken feathers, and milk proteins), have been used for this purpose. Protein-rich plant material for PH production can be used either minimal processed (raw) or pre-treated to concentrate the protein before its proteolytic enzymatic processing. For example, sunflower defatted seed meal (SDSM) (a by-product from oil production), is concentrated by a sedimentation/flotation fractionation procedure. If required, a further alkaline extraction followed by precipitation at the isoelectric point (pH 4.3) is used to obtain a protein isolate (PI) (Ugolini et al. 2015).
A legume-derived PH-PB, known as Trainer®, is a commercial product manufactured by Italpollina (Rivoli Veronese, Italy) and has become the focus of several studies due to its high activity as a plant biostimulant. It contains 27–31% of amino acids and soluble peptides and has been obtained through a process of enzymatic hydrolysis of proteins derived from legume seed flour, followed by separation of insoluble residual compounds by centrifugation and concentration to obtain a product with a final acid pH (Colla et al. 2015; Lucini et al. 2020).
Proteins from animal sources (e.g., leather by-products, collagen, blood meal, fish by-products, chicken feathers, and milk proteins) have been also used for PH production. Collagen, elastin, and keratins are the prevalent fibrous proteins found in animal by-products generated from meat production. It is estimated that approx. five million tons chicken feather are generated worldwide by the poultry industry, representing an attractive source of protein (approx. 90% of keratin) to convert into PH-PB. However, due to the insolubility and hydrolysis resistance efficient hydrothermal, chemical, biological, or enzymatic processes are required (Callegaro et al. 2019). The use of microorganisms with high keratinolytic activity has been one of the preferred processes for keratin feather hydrolysis. Bacillus licheniformis, B. subtilis, B. pumilus, and B. cereus are among the most effective feather-degrading microorganisms. However, other bacteria genera such as Chryseobacterium, Serratia, and Stenotrophomonas, and the fungi Chrysosporium spp. and Aspergillus spp. have been also considered (Callegaro et al. 2019; Gurav et al. 2020).
Feather microbial fermentation (whole or milled) is usually produced through submerged cultivations with mesophilic (5–20 g feathers/L, 30–40 °C, 24–96 h) or thermophilic bacteria (30–50 g milled feathers /L, 45–50 °C, pH 10.0, 48 h). The hydrothermal and enzymatic process, alone or combined, has been also used for this purpose (Callegaro et al. 2019).
Bryndina et al. (2019) describe a procedure to hydrolyze non-ground pen keratin by applying a pre-treatment with sodium sulfide, urea, sodium thioglycolate, or sodium tetraborate (0.3% by weight) using a ratio of 1:20 (solid: liquid) and pressure of 0.15 MPa for 2 h. Then a protease preparation from Str. chromogenes s.g. 0832 at a concentration of 3 U/g of protein was used and the enzymatic hydrolysis was carried out for 6 h with continuous stirring, at 40 °C, pH 8. The degree of hydrolysis (DH) after enzymatic treatment was higher in pen keratin pre-treated with sodium tetraborate (DH 80%), followed by sodium thioglycolate (DH 50%) (Bryndina et al. 2019).
A recent strategy for whey valorization has been the production of PH-based biostimulant. A fermentation process using Lactobacillus ramnosus (considered as a plant growth-promoting bacterium, PGPB) under controlled conditions (pH 5.5, 37 °C and agitation at 300 rpm with 0.1% of protease added as inductor), was developed. Lactic acid, peptides, and free amino acids and the biomass of Lactobacillus rhamnosus were fractionated with a triple system membrane device (MMS AG membrane System) using a 0.2-μm PVDF membrane to separate L. rhamnosus biomass (microfiltration) and a 200-Da MW cut-off TFM membrane to separate the protein hydrolysate (nanofiltration), and the lactic acid recovered by distillation. L. rhamonsus presented biocontrol activity against some phytopathogenic microorganisms and the PH and the lactic acid were used as soil biostimulant which induced microbial activity and had a modifying effect on microbial biodiversity, favoring the growth of plant growth-promoting bacterial (Caballero et al. 2020).
6.4.2 Enzymatic Proteolysis Performance
To maximize bioactive peptide/oligopeptide proportion and yield, proteolytic enzymes (proteases) must be added at specific ratios and under controlled conditions. Enzymatic hydrolysates have been developed by utilization of pure proteases, enzymatic mixtures, and raw aqueous preparations extracted from food or by-products themselves (Salazar-Leyva et al. 2017). However, proteolytic enzymes such as Alcalase, Flavourzye, Neutrase, Pepsin, and Protamex are used frequently in both commercial and experimental PH manufacture (Mazorra-Manzano et al. 2018). The selection of proteolytic enzyme is based on operative conditions and specificity. Generally, fermentation-produced enzymes by specific microbial strains (i.e., Bacillus subtilis, Bacillus lichenifromis, Aspergillus flavus Asperguillus niger) show thermostability in comparison with animal proteases (pepsin, chymotrypsin, trypsin) improving hydrolysis rates (dos Santos Aguilar and Sato 2018). The chemical nature of active sites in protease structure provides remarkable insights into its proteolysis mechanism (enzyme-substrate interaction), pH conditions, and specificity. Specificity parameter determines the positions of clave sites in which the enzyme catalyzes peptide bond break, as well as reveals the nature of amino and carboxylic terminal groups of the released peptides. For instance, flavourzyme ad alcalase preferentially hydrolyses peptide bonds between aromatic residues (Phe, Trp, and Tyr), while papain preferentially cleavages peptide bonds containing large hydrophobic side chains (Tavano 2013). It has been observed that acceptable DH (20–50%) percentage is obtained by using 1–5% w/v of E/S ratio respect protein basis. Higher doses of proteases, especially in their purified forms, induce excessive proteolysis, increasing the content of free amino acids, and might reduce bioactivity by overhydrolysis of oligopeptides. Actually, there are a lot of proteases from animal, microbial, and plant sources employed in the production of a protein hydrolysate, and extensive literature have been generated around protein hydrolysates manufacture and characterizations, as well as a hydrolysis optimization parameter, protease selection, enzyme/substrate ratio, pH, temperature, DH, additives, etc. (dos Santos Aguilar and Sato 2018; Mazorra-Manzano et al. 2018; Etemadian et al. 2021). Most of these enzymes are actually explored in the production of PH-based biostimulant at experimental and commercial levels (Moreno-Hernández et al. 2020). The releases of peptides and amino acids will depend on the extent of hydrolysis and appropriated protease selection, since the specificity of the protease, protein substrate digestibility, DH, among other factors, determining the final characteristics of PH.
6.5 Effects of PH-Based Biostimulant on Crops’ Traits
Agro-industry and farmers search for products that promote plant growth, productivity, and quality crop. Biostimulant are other than fertilizer, induce growth and resistance of biotic and abiotic stress; many biostimulants are made from diverse agro and seafood sources, these have macromolecules or chemicals substances, that can modify physiological processes of plants that enhance nutrient uptake, resistance to biotic or abiotic stresses, and remarkable improvement on crop yield and quality (Xu and Geelen 2018; Ricci et al. 2019; Shukla et al. 2019). Additionally, the fertilizers supplemented with biostimulants (protein hydrolysate, chitosan, algal extract, humic acid, or microorganism) might reduce the drawbacks of agrochemicals fertilizers or pesticides (Drobek et al. 2019; Aktsoglou et al. 2021).
6.5.1 Foliar and Radicular Administration of Hydrolysates
Crops exhibit different physiological responses to the application of PH, which seems to be affected by the protein source and amino acid composition of hydrolysates, application mode, and dosage used (Aktsoglou et al. 2021). PH as liquid, soluble powder, and granular forms promotes macro and micronutrient assimilation when these compounds are applied in a foliar spray and radicular manner (Fig. 6.1), stimulating plant metabolism with a potential effect on the quality and yield, in many crops (Calvo et al. 2014; Nardi et al. 2016; Colla et al. 2017). Amino acid composition is a key feature in the stability of PH employed in crop nutritional programs. It has been proposed that PH incorporated into fertigation solutions requires a high balance of hydrophilic/hydrophobic oligopeptides (with high solubility) to prevent insoluble aggregates or undesirable interactions with other nutrients, especially mineral, due to chelating properties of some amino acids (Schiavon et al. 2008; Ertani et al. 2013b). Moreover, radicular plant systems, root secretions (hydrolytic enzymes), soil biochemistry, and root absorption coefficient are some critical aspects for optimal utilization of peptides through the root system (Moreno-Hernández et al. 2020). While leaves’ porosity (stomata activity) is critical for proper peptide or amino acid acquisitions during the foliar application, other factors involved are peptide size/sequence, relative humidity, temperature, evaporation parameters, and leaf area to improve diffusion (Koukounararas et al. 2013). For instance, Sestili et al. (2018) showed that the application of PH is more effective in improving plant growth and total N uptake than foliar sprays. This is because free amino acids in PHs have been reported to activate nitrate transporters.
Schematic view of the effect of foliar and root application of protein hydrolysate effects on tomato plants. (Created with BioRender.com)
Amino acids in PH represent an important source of nitrogen that could be equally effective such as inorganic nitrogen fertilizers when they are used as a nutrient hydroponic solution (Aktsoglou et al. 2021). The hydroponic cultivation of peppermint and spearmint has not affected plant growth either positively or adversely by the addition of PH Amino16® (Evyp LLP, Greece) in the nutrient solution and was attributed to either the increased root growth on or to the low rate of PHs applied (lower than 0.5%) (Aktsoglou et al. 2021). Pieces of evidence suggest that amino acids and small peptides derivated from PH, are uptake and translocated by amino acid transport proteins involved in phloem loading and unloading, xylem-phloem transfer, import into seed, and intracellular transport in plants from leaves or root tissues (Yang et al. 2020). Foliar application of PH can increase amino acid and peptide availability for plant uptake by reducing the competition with a microorganism (Colla et al. 2015). Glu is rapidly absorbed by creeping bentgrass foliage and directly utilized as a precursor to synthesize gamma-aminobutyric acid and proline, two important metabolites with well-known roles in plant stress adaptation (Rouphael and Colla 2020). Tryptophan is considered a fundamental amino acid for the synthesis of indoleacetic acid (IAA), a hormone with important functions on plant growth. However, its activity can be affected when it is applied separately. In a study, L-methionine stimulated lettuce growth parameters; however, distinct effects have been observed when L-Gly and L-Trp were applied radicular on butterhead lettuce hydroponically grown (Rouphael and Colla 2020). Paul et al. (2019a) suggest that foliar application of PH reach mesophyll cells by absorption trough cuticle, epidermal cells, and stomata, while in drench or hydroponically application, the absorption occurs through root epidermal cells via ABC membrane transport and gets redistributed through the xylem. Most PH-based biostimulant induces positive physiological effects as growth and development; moreover, it enhances uptake nutrient from soil or microorganism of the rhizosphere; however, to perform the desired effect, PH must be able to penetrate the plant tissue at low dosages, depending species, cultivars, and vegetative stage, but also depend on environmental condition, stomata, and cuticle that act as a barrier (Pecha et al. 2012).
6.5.2 Effects in Crop Growth and Quality
Biostimulants have significant effects on many crop traits related to productivity and quality, including root architecture, change in endogen phytohormone levels, photosynthetic rate, increased pigment content, protein, phenolic contents, stimulate the growth, antioxidant activity, and enhance macro and microelements in vegetal tissues (Yakhin et al. 2017; Drobek et al. 2019; Ertani et al. 2019; Ambrosini et al. 2021). Table 6.3 includes a description of the most applied PH-based biostimulants, their intended use, and primary functions.
PH-based biostimulants probably contain molecules that display phytohormone-like activities as has been proposed in a recent revision (Moreno-Hernández et al. 2020). PH-containing peptides might act as auxin-like and gibberellin-like elicitors, triggering signaling as naturally occurring peptides in plants and promoting vegetative plant growth, and early maturation of fruits (Drobek et al. 2019), effects triggered by some endogenous regulatory signaling peptides and protein-like hormones (e.g., phytosulfokine) influencing productive traits such as fruit maturation, root length, and thickness of stem, or inducing primary and secondary metabolism biosynthesis through the activation of multiple signaling pathways that involve second messengers that stimulate enzymes of the nitrate assimilation pathway, like nitrate reductase and glutamine synthetase which catalyze a rate-limiting step in nitrogen assimilation (Ertani et al. 2013a). In this context, Ertani et al. (2019) reported indole-3-acetic acid (IAA)-like and gibberellin (GA)-like activities of PH, obtained from Cicer arietinum L. and Spirulina platensis, and that they induced plant growth and accumulation of N-compounds (proteins, chlorophylls, and phenols) on hydroponically Zea mays L. culture; furthermore, PH from C. arietinum and S. platensis increased the activity of two enzymes (peroxidase and esterase) related with plant growth and differentiation of organogenesis; in the same way, Casadesús et al. 2020 reported hormonal signaling for improving root growth in tomato (Solanum lycopersicum, var. Ailsa Craig) plants, mediated by chorismate-derived hormones, in particular by salicylic acid.
Recently, Ceccarelli et al. (2021) reported foliar application of vegetal-derived PH on tomato cutting-promoted rooting and biomass density, length, and the number of lateral root branching, with promoting plant growth and development owing to stimulation of auxins (particularly precursors as 4-(indol-3-yl) butanoate and tryptamine), cytokinin, and gibberellin biosynthesis or IAA precursors. The foliar application (spraying) of animal-derived PH (from fish by-products) on lettuce, showed significant effects on the total number and area of leaves, carbohydrate, proline, shoot-fresh weight of plants, dry matter, total soluble solids, and total yield (Al-Malieky and Jerry 2019). In celery, Plant-PH Tyson® obtained from soy protein extract, Trainer® (legume-derived PH), and animal-PH Aswell® (bovine epithelium hydrolysate) improved plant growth and nutritional balance in both foliar and radicular applications (Consentino et al. 2020). Recently, the biostimulant activity of five plant-derived PH on tomatoes was evaluated for their ability to promote rooting in tomato cuttings following quick dipping. All PHs increased root length (45–93%) and root number (37–56%) (Ceccarelli et al. 2021).
Since, in addition to its biostimulant activity, PH also mediates plant adaptation to several stress conditions, including mineral depletions, cold, thermal, drought, and saline. StresSal® and Trainer® have been employed as osmoregulator in both fruit trees (persimmon) and horticultural crops (lettuce), to avoid the negative impact of saline stress (Visconti et al. 2015; Luziatelli et al. 2019). Experimental blood-derived PH and commercial Amino 16®, displayed protective effect in lettuce cultivated under extreme climate conditions, hydrolysates showed thermo-protective functionality toward warm and chilling temperatures, respectively (Polo et al. 2006; Tsouvaltzis et al. 2014). Some studies suggest that the accumulation of glycine betaine and proline is associated with increased stress tolerance, and the exogenous application of protein-based compounds in maize, barley, soybean, alfalfa, and rice has been highly correlated (Ahmad et al. 2013). Ambrosini et al. (2021) evaluated on hydroponic culture the capacity of a commercial PH derived from bovine collagen to mitigate Fe deficiency stress in roots of Zea mays, and observed that PH exhibited an increased growth and absorption by chelation of Fe area of the roots compared with control treatment; these studies show how PH have a positive effect by foliar or hydroponic culture; in another way, PH drench application on Solanum lycopersicum L. plants enhance transpiration rate and transpiration use efficiency with a positive impact on the biomass and metabolic profile (Paul et al. 2019a). Protective effects in some PH-based biostimulants have been attributed to proline or proline-precursors compounds (glutamate an/or ornithine) in hydrolysates, due their osmolyte and chemical chaperone roles under various stressful sceneries during plant development.
A clear mechanism for peptides found in PH-PB remains uncertain, but most pieces of evidence suggest that PH does not only provide nutrients directly to plants but these compounds also stimulate plant nutrient acquisition processes and is an alternative to diminishing chemical fertilizers. Protein hydrolysates induce a positive effect on plant crops, containing signaling peptides and free amino acid that enhance germination, seedling growth, fruits, and vegetable quality as well as crop productivity (Rouphael and Colla 2020). Table 6.4 shows some examples of PH whose potential as plant biostimulants has been tested for many plant traits such as morphophysiological parameters (stem, leaves number, foliar area), flowering time, fruit set-filling, crop productivity, and nutrient use efficiency. Until now, clear identification and characterization of the peptides (or amino acids) in PH related to the PB activity and associated mechanism of action has not been determined. Therefore, an important characteristic to be included in PH-PB characterization could be the peptidic size fraction, in addition to the common parameter considered such as DH, chemical composition, and amino acids content (Moreno-Hernández et al. 2020).
6.6 Approaches to Elucidate PH Mode of Action
High crop productivity is the ultimate goal of agricultural systems that employ PH-based biostimulants in their production practices. Farmer decision about the application of a particular biostimulant must be supported by accurate information about biostimulant quality and safety, effectivity, and also by the mode of action of the active ingredients or any other parameter about primary-secondary function (EBIC 2021). A collection of evidence about PH function as plant biostimulant has been recovered experimentally under different production systems and are based on the agronomic parameter; however, the trend around these biostimulants is turned into a more multidisciplinary approach, to validate a new generation of PH-based biostimulants employing precision testing technologies and genomic-based tools.
6.6.1 High-Throughput Phenotyping Characterization
Biostimulants induce significant changes in crop development at different physiological levels. Most results on biostimulant efficiency have been based on agronomical traits (germinations, adaptation, flowering time, fruit set, and yield), monitoring phenotypical changes to identify biostimulant candidates, and also provide clues about its modes of action. However, conventional phenotyping protocols for reporting these traits might prove time-consuming, laborious, with low reproducibility, and strong subjectivity. Additionally, some of these methods are destructive and unsuitable for large-scale probes. This has driven the development of new tools for the automatic management of crops, and the continuous monitoring of plants treated with biostimulants. The concept of high-throughput phenotyping (HTP) in agriculture emerged with the necessity of high-precision systems for data recovery in agronomy; these powerful robotic tools can analyze broad scenarios and their influences on plant traits, also known as plant phenomics. In the field of biostimulants, HTP has been used to evaluate the influence of active components in biostimulants on physiological plant traits quantitatively and enables to compare dynamic plant-environment responses in a real-time manner (Dalal et al. 2019).
During the development of new biostimulants, HTP platforms have provided an accurate assessment of different active products contained in protein hydrolysates. An interesting feature of this platform is that a set of biostimulants can be assayed in a wide range of conditions, including water stress, nutrient deficiency, temperature stress conditions (heat/cold), and light intensity, in a continuous lab-field-lab cycle (Rouphael et al. 2018). In a drought model, this technology was used to analyze the morphophysiological traits of Trainer®-treated (spray or substrate drench) tomato plants, under semi-controlled greenhouse conditions. By employing imaging sensors, visible red, green, blue images for digital biomass increase, and fluorometers to report photosynthetic performance, HTP identified the best-performing plants as an effect of biostimulant applications. Moreover, analysis of spectral data revealed the most active photosynthetic tissues and their correlation with biomass accumulation, and the metabolomics profiling of stimulated plants, annotated over 1900 compounds associated with ROS signaling, sterols, carotenoids, membrane lipids, phytohormones, polyamines, and chlorophyll-related molecules (Paul et al. 2019a, b). Similar approaches have been applied to understand the role of biostimulants on peppers’ productivity and survival under drought conditions (Dalal et al. 2019). Recent advances in imaging acquisition technology as spectrograph (hyperspectral analysis) offers new opportunities in the field of biostimulant research by analysis of whole plants; till date, around 16 crops, including barley, maize, potato, grapevines, wheat, oak, and peppers have been analyzed with this technology (Mishra et al. 2020). Although these studies do not contemplate the use of biostimulants, the tests evidence the efficacy of these systems for the high-throughput phenotyping in a variety of conditions.
6.6.2 Metabolomic Analysis
Plant metabolite profiling is an emerging field to describe cellular plant response to a wide range of biotic or abiotic conditions. The metabolomic approach seeks to establish a relationship between cellular metabolites and a specific variation factor, as well as its influence on particular traits shown by plants (Schauer and Fernie 2006). In the field of plant sciences, metabolomics is a powerful tool to integrate new metabolic pathways to omic-data, for high-throughput analysis, combining analytical techniques like Liquid/Gas Chromatography-MS/MS systems to explore primary/secondary plant metabolites in many economically important crops like maize, rice, tomato (Sharma et al. 2021). Metabolomics analyses have been applied to profile the metabolite change of plant to HP-based biostimulants.
Plant crops treated with protein hydrolysates derivatives from animal, plant, or algae sources induce growth and development of fruits, leaf, roots, and phytochemicals metabolites—in tomato, the application of PH-enhanced root and quality of fruits with increased diameter, weight, and volume—these effects were reported in diverse crops such as, kiwifruit, papaya, banana, passionfruit, and vegetables such as lettuce and pepper (Rodrigues et al. 2020; Ceccarelli et al. 2021). Furthermore, several studies show that application of PHs stimulate secondary metabolites with antioxidant activity such as carotenoids, polyphenols, and flavonoids, as well as defense metabolites like alkaloids, salicylic acid, jasmonates, and ethylene, as well as phytoalexins as indole-3-carboxyl and psoralen (Casadesús et al. 2020; Lucini et al. 2020; Ambrosini et al. 2021), thus promoting crop productivity. The benefit of PH is not only over crops but also has beneficial effects on the microbiome of the rhizosphere, improving physiological and development processes in plants, favoring greater nutrient and water uptake as well as enhanced resistance against biotic and abiotic stress. PH also promotes nitrogen assimilation via coordinate regulation of carbon and nitrogen metabolism, by inducing activity enzymes as nitrate reductase, nitrite reductase, glutamine synthase, glutamate synthase, and aspartate aminotransferase (Ertani et al. 2009, 2019; Sestili et al. 2018; Paul et al. 2019a), and carbon metabolism as malate dehydrogenase, isocitrate dehydrogenase, and citrate synthase (Ertani et al. 2013a), or esterase and peroxidase enzymes that have a role in meristematic growth that induces vegetative development (Ertani et al. 2019), as well HP drench, foliar, or hydroponic application induce high-affinity nitrate transporters belonging to NRT2 family: NRT2.1 and NRT2.3, that play a key role in the coordination of root development, acting on lateral root initiation and nitrate uptake and long-distance transport system from root to shoot (Sestili et al. 2018; Paul et al. 2019a), as well as iron transporters (ZmTOM1 and ZmIRT1) involved in phytosiderophores and FeII assimilation (Ambrosini et al. 2021), the PH application favoring minerals absorption and transport, known as nutrient acquisition response (Rouphael et al. 2021).
Metabolomic data of four plant-derived PH indicate a reprogramming of phytohormones profile by modulating gibberellin and cytokinin biosynthesis with a lower effect on auxins and brassinosteroids biosynthesis. The hierarchical cluster analysis (HCA) revealed that metabolomic profiles in roots were significantly influenced by PHs foliar application, in a PH-dependent specific manner, providing evidence that such hormone-like activity of PHs depends on the protein source (Ceccarelli et al. 2021).
6.6.3 Differential Gene Expression Analysis
In the last decade, improvements in Next-Generation Sequencing (NGS) technology have led to a substantial increase of data about plant genetics, providing relevant knowledge about genome functionality. This information is fundamental in the construction of transcriptomes, employing RNA-seq analysis. Due to the transcription process is the very first genome response to biotic and abiotic stimulus; this provides a complete scenario about changes in expression patterns, genomic information flux, and gene network enrichment in plant cells for a particular time-lapse. Fundamentally, RNA-seq reflects the total RNA identities produced by a single cell or tissue that has been successfully sequenced and mapped to an annotated genome or curated transcriptome dataset. Coupled with bioinformatics exploration, RNA-seq tells us about a group of genes and gene networks significantly upregulated or downregulated as a response to certain factors (Differential Gene Expression Analysis; DGEA) like variations in nutritional status, phenological stage, light/dark cycle, environmental conditions, and also biostimulant application.
Advances in NGS have reduced the cost of sequencing services and allowed access to this technology by biostimulant developers. This approach has gained relevance in the field of biostimulant research, providing not only accurate evidence about the effects of these compounds in crop development but also recovering robust data to elucidate the mode of action of biostimulant at the molecular level. Although NGS is relatively time-consuming and costly in comparison with non-omic approaches (limiting its application to some horticultural crops), the number of available genomes in databases is growing faster than ever, and this is impacting the number of scientific reports documenting biostimulant uses. Transcriptomic-wide identification of DEG has been used to explore the mechanistic process of plant growth-promoting bacteria (González-Morales et al. 2021), humic substances (Galambos et al. 2020), and PH-based biostimulants (Trevisan et al. 2017). For instance, DGEA has been used to understand the influence of PH-based biostimulants at the transcriptional level in crops like cucumber, maize, soybean, and tomato (Table 6.5). Wilson et al. (2015) evidenced the complexity of transcriptional regulation on two-week-old cucumber seedling by hydrolyzed collagen (gelatin) capsules, employing the RNA-seq data to coexpress gene network construction. In this spot trial controlled conditions, gelatin biostimulant induced 620 differentially expressed genes (DEGs), grouped in five modules by Weighted Gene Coexpression Network Analysis (WGCNA) with interconnected hub networks. An upregulation of phloem amino acid, N-transporter genes (amino acid transporter, amino acid permease, ammonium transporter), and nitrogen metabolism, as well as detoxifying mechanism (Glutathione S-transferase), was evidenced, indicating its relevance at first-level of plant response during the early stage of biostimulation process (Wilson et al. 2015). Other reports have evidenced new target molecules in tomato seedlings exposed to alfalfa hydrolysates, inducing overexpression of stress-related genes as phytohormone modulation, antioxidant-related genes, phenylpropanoid pathways, detoxification process, and MAPK signaling pathway in crosstalk between biotic and abiotic stress responses (Ertani et al. 2017). A recent study reveals insights on the mechanistic action of PH-stimulant by combining transcriptomic and quantitative proteomics approaches. By examining maize seedlings exposed to commercial PH-stimulant under chamber controlled conditions, Ebinezer et al. (2020) identified 608 DEGs and 242 differentially abundant proteins (DAP) at a high dosage of biostimulant. The bioinformatics to construct Gene Ontology Enrichment (GO terms), clustered DEG and DAP into 20 categories associated with stimulus responses, including osmotic, salt, hormone regulation, brassinosteroid metabolic, and biosynthesis, phenylpropanoid, lignin, siderophore, and homeostasis maintenance. Pathway Enrichment Analysis discriminates significant metabolic pathways impacted by PH, mainly metabolic pathways, biosynthesis of secondary metabolites, glutathione metabolism, and amino acid metabolism, all with up/down-regulated genes under essay conditions (Ebinezer et al. 2020).
These studies evidence all possible target molecules in broad metabolic pathways for biostimulant design; however, this scenario evokes a major challenge in the development of active molecules as plant biostimulants. Information about the size, sequences, and functionality of peptides are characteristics that need to be included in PH characterization to associate their putative role as plant biostimulants (Lucini et al. 2020; Moreno-Hernández et al. 2020). Until now, a clear identification of peptides found in PH (with diverse composition, characteristics, and properties) responsible for the plant biostimulation activity has not been completed and established. Figure 6.2 proposes an interdisciplinary approach to biostimulant rational design to provide accurately integrated evidence on the effectivity of plant stimulation phenom. To accelerate the re-investigation on the first and new generation of plant biostimulant based on protein hydrolysates.
Rational design for PH-based biostimulant production. Schematic representation of multidisciplinary approach for “ad-hoc” design bioactive peptide for plants. DGEA Differential Gene Expression Analysis, DH% hydrolysis degree, E/S enzyme-substrate ratio, GSEA Gene Set Enrichment Analysis, HPLC-MS/MS High-Performance Liquid Chromatography-Mass Spectrometry, HTP High-Throughput Phenotyping, PPI Protein-Protein Interaction, RNAseq RNA-sequencing, (RNAseq)
6.7 Conclusions
Emerging agriculture is necessary to meet the millennium goal of food security. Biostimulants are a strategic issue to break with limitations of traditional crop production practices and overcome the challenges of the climate crisis. PH-based biostimulants offer broad opportunities for the development of efficient and eco-friendly systems for food production. These compounds can be produced from a variety of protein substrates from food wastes or by-products discarded by industries, promoting revalorization of these residues and mitigating their environmental impacts. The collective efforts on biostimulant development and research have to lead to the identification of functional PH, experimentally and commercially probed, with agricultural applications. Many of these bioactive PH with attractive improvements on crop vegetative growth, plant nutrition, stress adaptation, yield, and quality.
Advances in high-throughput phenotyping and genomics have increased our understanding of the mode of action of some PH-based biostimulants at the physiological and molecular levels. Nonetheless, only a few studies report the evaluation of biostimulants under these approaches, partially due to the availability of reference genomes and transcriptomes, as well as the inherent limitations on the technology transferences process. To face the challenges of PH-based biostimulant production, the structure-function relationship of peptides released during the hydrolytic process must be conducted, to link specific peptide sequences to particular bioactivity detected in plants. Coupled to phenomic-metabolomic-transcriptomic studies, structural data will lead us to a rational design for “ad-hock” production of biostimulants based on proteins, by developing specific peptide mixtures, biologically or synthetically produced, to improve target traits for a particular crop, and finally migrate toward more sustainable agriculture.
References
Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science Publishers, New York, pp 110–169
Ahmad R, Lim CJ, Kwon S-Y (2013) Glycine betaine: a versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol Rep 7:49–57
Ahmed M, Verma AK, Patel R (2020) Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: a review. Sustain Chem Pharm 18:1–12
Aktsoglou D-C, Kasampalis DS, Sarrou E et al (2021) Protein hydrolysates supplement in the nutrient solution of soilless grown fresh peppermint and spearmint as a tool for improving product quality. Agronomy 11:1–13
Al-Malieky HM, Jerry AN (2019) Preparation protein hydrolysates from fish by-product and study effected on lettuce (Lactuca sativa L.) growth, yield, quality and enhanced salt tolerance. Basrah J Agric Sci 32:246–255
Álvarez-Viñas M, Rodríguez-Seoane P, Flórez-Fernández N et al (2020) Subcritical water for the extraction and hydrolysis of protein and other fractions in biorefineries from agro-food wastes and algae: a review. Food Bioprocess Technol 14:373–387
Ambrosini S, Sega D, Santi C et al (2021) Evaluation of the potential use of a collagen-based protein hydrolysate as a plant multi-stress protectant. Front Plant Sci 12:1–14
Bioiberica. Data sheet-StresSal. https://www.bioiberica.com/en/products/plant-health/biostimulants/stressal. Accessed 13 May 2021a
Bioiberica. Data sheet-Terra-Sorb. https://www.bioiberica.com/en/products/plant-health/biostimulants/terra-sorb-radicular. Accessed 13 May 2021b
Bradshaw T, Berkett L, Griffith M et al (2012) Assessment of kelp extract biostimulants on tree growth, yield, and fruit quality in a certified organic apple orchard. Proceedings of the II International Organic Fruit Symposium 1001
BrownsFish Genesis Brown’s fish hydrolysate. https://brownsfishfertilizer.com/products-page/. Accessed 12 May 2021
Bryndina L, Ilyina N, Baklanova O, Moiseyeva E (2019) Comparative evaluation of biostimulator efficiency on corn seeds germination: keratin protein and preparation Ribav extra. IOP Conf Ser Earth Environ Sci 392:012068
Caballero P, Rodríguez-Morgado B, Macías S et al (2020) Obtaining plant and soil biostimulants by waste whey fermentation. Waste Biomass Valor 11:3281–3292
Caer D, Colas B (1993) Protease susceptibility and amino group accessibility to trinitrobenzenesulfonic acid of legumin during its glycosylation. J Agric Food Chem 41:544–546
Callegaro K, Brandelli A, Daroit DJ (2019) Beyond plucking: feathers bioprocessing into valuable protein hydrolysates. Waste Manag 95:399–415
Calvo P, Nelson L, Kloepper J (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Campobenedetto C, Mannino G, Agliassa C et al (2020) Transcriptome analyses and antioxidant activity profiling reveal the role of a lignin-derived biostimulant seed treatment in enhancing heat stress tolerance in soybean. Plants 9(10):1308
Caruso G, De Pascale S, Cozzolino E et al (2019) Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 8:208
Casadesús A, Pérez-Llorca M, Munné-Bosch S, Polo J (2020) An enzymatically hydrolyzed animal protein-based biostimulant (Pepton) increases salicylic acid and promotes growth of tomato roots under temperature and nutrient stress. Front Plant Sci 11:1–12
Ceccarelli AV, Miras-Moreno B, Buffagni V et al (2021) Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 10:326
Cerdán M, Sánchez-Sánchez A, Oliver M et al (2008) Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Proceedings of the IV Balkan symposium on vegetables and potatoes 830
Chilakamarry CR, Mahmood S, Saffe SNBM et al (2021) Extraction and application of keratin from natural resources: a review. 3 Biotech 11:220
Cifo Srl Data sheet-SINERGON 300. https://www.cifo.it/en/product/professional-agriculture/pa-products/specialties/sinergon-3000/. Accessed 13 May 2021
Colla G, Nardi S, Cardarelli M et al (2015) Protein hydrolysates as biostimulants in horticulture. Sci Hortic 196:28–38
Colla G, Hoagland L, Ruzzi M et al (2017) Biostimulant action of protein hydrolysates: unraveling their effects on plant physiology and microbiome. Front Plant Sci 8:1–14
Consentino BB, Virga G, La Placa GG et al (2020) Celery (Apium graveolens L.) performances as subjected to different sources of protein hydrolysates. Plants 9:1633
Cristiano G, Pallozzi E, Conversa G et al (2018) Effects of an animal-derived biostimulant on the growth and physiological parameters of potted snapdragon (Antirrhinum majus L.). Front Plant Sci 9:1–12
Dalal A, Bourstein R, Haish N et al (2019) Dynamic physiological phenotyping of drought-stressed pepper plants treated with “productivity-enhancing” and “survivability-enhancing” biostimulants. Front Plant Sci 10:1–14
Dara PK, Geetha A, Mohanty U et al (2021) Extraction and characterization of myofibrillar proteins from different meat sources: a comparative study. J Bioresour Bioprod 6(4):367–378. https://doi.org/10.1016/j.jobab.2021.04.004
Di Mola I, Cozzolino E, Ottaiano L et al (2020) Nitrogen use and uptake efficiency and crop performance of baby spinach (Spinacia oleracea L.) and lamb’s lettuce (Valerianella locusta L.) grown under variable sub-optimal n regimes combined with plant-based biostimulant application. Agronomy 10:1–15
dos Santos Aguilar JG, Sato HH (2018) Microbial proteases: production and application in obtaining protein hydrolysates. Food Res Int 103:253–262
Drobek M, Frąc M, Cybulska J (2019) Plant biostimulants: importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—a review. Agronomy 9:3351–3318
du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
EBIC Function defines biostimulant products: [European Biostimulants Industry Council]. https://biostimulants.eu/issue/function-defines-biostimulant-products/. Accessed 18 May 2021
Ebinezer LB, Franchin C, Trentin AR et al (2020) Quantitative proteomics of maize roots treated with a protein hydrolysate: a comparative study with transcriptomics highlights the molecular mechanisms responsive to biostimulants. J Agric Food Chem 68:7541–7553
Ebru G, Atici Ö (2019) Chicken feather protein hydrolysate as a biostimulant improves the growth of wheat seedlings by affecting biochemical and physiological parameters. Turk J Bot 43:67–79
Ertani A, Cavani L, Pizzeghello D et al (2009) Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J Plant Nutr Soil Sci 172:237–244
Ertani A, Pizzeghello D, Altissimo A, Nardi S (2013a) Use of meat hydrolyzate derived from tanning residues as plant biostimulant for hydroponically grown maize. J Plant Nutr Soil Sci 176:287–295
Ertani A, Schiavon M, Muscolo A, Nardi S (2013b) Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 364:145–158
Ertani A, Pizzeghello D, Francioso O et al. (2014) Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: chemical and metabolomic approaches. Front Plant Sci 5:1–12
Ertani A, Schiavon M, Nardi S (2017) Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front Plant Sci 8:1–19
Ertani A, Nardi S, Francioso O et al (2019) Effects of two protein hydrolysates obtained from chickpea (Cicer arietinum L.) and Spirulina platensis on Zea mays (L.) plants. Front Plant Sci 10:1–13
Etemadian Y, Ghaemi V, Shaviklo AR et al (2021) Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: state of the art. J Clean Prod 278:123219
FAO Food loss and food waste: Food and Agriculture Organization of the United Nations. http://www.fao.org/food-loss-and-food-waste/flw-data. Accessed 21 May 2021
FEMSSA Pepton 85/16 biological biostimulant with high nutritional value. http://www.femssa.com.mx/pepton/pepton8516-femssa.php. Accessed 13 May 2021
Fiormarket Protein hydrolysate market by product type (plant protein, animal protein and milk protein), form (liquid and powder), process, application, region, global industry analysis, market size, share, growth, trends, and forecast 2021 to 2028. https://www.fiormarkets.com/report/protein-hydrolysate-market-by-product-type-plant-protein-419219.html. Accessed 20Apr 2020
Friedman M (1996) Nutritional value of proteins from different food sources. A review. J Agric Food Chem 44:6–29
Galambos N, Compant S, Moretto M et al (2020) Humic acid enhances the growth of tomato promoted by endophytic bacterial strains through the activation of hormone-, growth-, and transcription-related processes. Front Plant Sci 11:1–18
Ganguly RK, Mukherjee A, Chakraborty SK, Verma JP (2021) Chapter 2—impact of agrochemical application in sustainable agriculture. In: New and future developments in microbial biotechnology and bioengineering. Elsevier, p 15–24
González-Morales S, Solís-Gaona S, Valdés-Caballero MV et al (2021) Transcriptomics of biostimulation of plants under abiotic stress. Front Genet 12:1–24
Gurav R, Jadhav J (2013) A novel source of biofertilizer from feather biomass for banana cultivation. Environ Sci Pollut Res 20:4532–4539
Gurav R, Nalavade V, Aware C et al (2020) Microbial degradation of poultry feather biomass in a constructed bioreactor and application of hydrolysate as bioenhancer to vegetable crops. Environ Sci Pollut Res Int 27:2027–2035
Halket JM, Waterman D, Przyborowska AM et al (2005) Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J Exp Bot 56:219–243
Horii A, McCue P, Shetty K (2007) Seed vigour studies in corn, soybean and tomato in response to fish protein hydrolysates and consequences on phenolic-linked responses. Bioresour Technol 98:2170–2177
Hou Y, Wu Z, Dai Z et al (2017) Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol 8:1–13
Hung N, Vas M, Cseke E, Szabolcsi G (1984) Relative tryptic digestion rates of food proteins. J Food Sci 49:1535–1542
Hydro Fert Catalog of products: hydrostim. https://www.hydrofert.it/Catalogo%20Digital%202019_IT-ES_HYDROFERT_001.pdf. Accessed 12 May 2021
Ilsagroup Products: ilsadrim forte. https://www.ilsagroup.com/en/prodotti/prodotto/44/ilsadrip-forte.htm. Accessed 12 May 2021
INTERMAG Crop-farming-product: AMINOPRIM. https://intermag.eu/crop-farming/product/aminoprim. Accessed 12 May 2021
Isagro Data sheet-Siapton. https://www.isagro.com/en/products/biostimulants.html. Accessed 13 May 2021
ITALPOLLINA Data sheet-Trainer SP. https://www.hello-nature.com/us/product/trainer-sp/. Accessed 13 May 2021
Jajić I, Krstović S, Glamočić D et al (2013) Validation of an HPLC method for the determination of amino acids in feed. J Serb Chem Soc 78:839–850
Kambhampati S, Li J, Evans BS, Allen DK (2019) Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods 15:461–412
Kim YS, Yongsawatdigul J, Park JW, Thawornchinsombut S (2005) Characteristics of sarcoplasmic proteins and their interaction with myofibrillar proteins. J Food Biochem 29:517–532
Klampfl CW (2005) Determination of underivatized amino acids by capillary electrophoresis and capillary electrochromatography. J Chromatography Library 70:269–296
Kocira S (2019) Effect of amino acid biostimulant on the yield and nutraceutical potential of soybean. Chil J Agr Res 79:17–25
Koukounararas A, Tsouvaltzis P, Siomos AS (2013) Effect of root and foliar application of amino acids on the growth and yield of greenhouse tomato in different fertilization levels. J Food Agric Environ 11:644–648
Liu H, Zhang H, Liu Q et al (2020) Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrasonics Sonochem 67:105160
Lucini L, Rouphael Y, Cardarelli M et al (2015) The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci Hortic 182:124–133
Lucini L, Miras-Moreno B, Rouphael Y et al (2020) Combining molecular weight fractionation and metabolomics to elucidate the bioactivity of vegetal protein hydrolysates in tomato plants. Front Plant Sci 11:976
Luziatelli F, Ficca AG, Colla G et al (2019) Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Front Plant Sci 10:601–616
Madende M, Hayes M (2020) Fish by-product use as biostimulants: an overview of the current state of the art, including relevant legislation and regulations within the EU and USA. Molecules 25:1–20
Marfa O, Cáceres R, Polo J, Ródenas J (2008) Animal protein hydrolysate as a biostimulant for transplanted strawberry plants subjected to cold stress. VI international strawberry symposium 842
Martínez-Alvarez O, Chamorro S, Brenes A (2015) Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: a review. Food Res Int 73:204–212
Martinez-Esteso MJ, Vilella-Anton MT, Selles-Marchart S et al (2016) A DIGE proteomic analysis of wheat flag leaf treated with TERRA-SORB® foliar, a free amino acid high content biostimulant. J Integr OMICS 6:9–17
Matak KE, Tahergorabi R, Jaczynski J (2015) A review: protein isolates recovered by isoelectric solubilization/precipitation processing from muscle food by-products as a component of nutraceutical foods. Food Res Int 77:697–703
Mazorra-Manzano MA, Ramírez-Suarez JC, Yada RY (2018) Plant proteases for bioactive peptides release: a review. Cirt Rev Food Sci Nutr 58:2147–2163
Mishra P, Lohumi S, Ahmad Khan H, Nordon A (2020) Close-range hyperspectral imaging of whole plants for digital phenotyping: recent applications and illumination correction approaches. Comput Electron Agric 178:105780
Moreno-Hernández JM, Benítez-García I, Mazorra-Manzano MA et al (2020) Strategies for production, characterization and application of protein-based biostimulants in agriculture: a review. Chil J Agric Res 80:274–289
Nardi S, Pizzeghello D, Schiavon M, Ertani A (2016) Plant biostimulants: physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci Agric 73:18–23
Nielsen P, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66:642–646
Paleckiene R, Sviklas A, Šlinkšiene R (2007) Physicochemical properties of a microelement fertilizer with amino acids. Russ J Appl Chem 80:352–357
Panyam D, Kilara A (1996) Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Tech 7:120–125
Parrado J, Escudero-Gilete ML, Friaza V et al (2007) Enzymatic vegetable extract with bio- active components: influence of fertiliser on the colour and anthocyanins of red grapes. J Sci Food Agric 87:2310–2318
Paul T, Halder SK, Das A et al (2013) Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal Agric Biotechnol 2:50–57
Paul K, Sorrentino M, Lucini L et al (2019a) A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front Plant Sci 10:1–18
Paul K, Sorrentino M, Lucini L et al (2019b) Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: a case study on tomato. Front Plant Sci 10:1–17
Pecha J, Fürst T, Kolomazník K et al (2012) Protein biostimulant foliar uptake modeling: the impact of climatic conditions. AICHE J 58:2010–2019
Polo J, Mata P (2018) Evaluation of a biostimulant (Pepton) based in enzymatic hydrolyzed animal protein in comparison to seaweed extracts on root development, vegetative growth, flowering, and yield of gold cherry tomatoes grown under low stress ambient field conditions. Front Plant Sci 8:2261–2268
Polo J, Barroso R, Ródenas J et al (2006) Porcine hemoglobin hydrolysate as a biostimulant for lettuce plants subjected to conditions of thermal stress. Horttechnology 16:483–487
Polychroniadou A (1988) A simple procedure using trinitrobenzenesulphonic acid for monitoring proteolysis in cheese. J Dairy Res 55:585–596
Popko M, Wilk R, Gorecki H (2014) New amino acid biostimulators based on protein hydrolysate of keratin. Przem Chem 93:1012–1015
Popko M, Michalak I, Wilk R et al (2018) Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 23:4701–4713
Rahman MM, Byanju B, Grewell D, Lamsal BP (2020) High-power sonication of soy proteins: hydroxyl radicals and their effects on protein structure. Ultrasonics Sonochem 64:1050191–1050110
Ricci M, Tilbury L, Daridon B, Sukalac K (2019) General principles to justify plant biostimulant claims. Front Plant Sci 10:1–8
Rico X, Gullón B, Yáñez R (2020) Environmentally friendly hydrothermal processing of melon by-products for the recovery of bioactive pectic-oligosaccharides. Foods 9:1–21
Rito-Palomares M, Dale C, Lyddiatt A (1998) Aqueous two-phase fractionation of biological suspensions for protein recovery from bovine blood. Biotechnol Tech 12:711–714
Rodrigues M, Baptistella JLC, Horz DC et al (2020) Organic plant biostimulants and fruit quality—a review. Agronomy 10:1–16
Roth M, Hampaǐ A (1973) Column chromatography of amino acids with fluorescence detection. J Chromatogr A 83:353–356
Rouphael Y, Colla G (2020) Toward a sustainable agriculture through plant biostimulants: from experimental data to practical applications. Agronomy 10:1461
Rouphael Y, Spíchal L, Panzarová K et al (2018) High-throughput plant phenotyping for developing novel biostimulants: from lab to field or from field to lab? Front Plant Sci 9:1–6
Rouphael Y, Carillo P, Cristofano F et al (2021) Effects of vegetal-versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci Hortic 284:110123
Rutherfurd SM (2010) Methodology for determining degree of hydrolysis of proteins in hydrolysates: a review. J AOAC Int 93:1515–1522
Salazar-Leyva JA, Lizardi-Mendoza J, Ramirez-Suarez JC et al (2017) Catalytic and operational stability of acidic proteases from Monterey sardine (Sardinops sagax caerulea) immobilized on a partially deacetylated chitin support. J Food Biochem 41:e12287
Santi C, Zamboni A, Varanini Z, Pandolfini T (2017) Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci 8:433
Schaafsma G (2009) Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur J Clin Nutr 63:1161–1168
Schauer N, Fernie AR (2006) Plant metabolomics: towards biological function and mechanism. Trends Plant Sci 11:508–516
Schiavon M, Ertani A, Nardi S (2008) Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J Agric Food Chem 56:11800–11808
Schmidt CS, Mrnka L, Frantík T et al (2020) Impact of protein hydrolysate biostimulants on growth of barley and wheat and their interaction with symbionts and pathogens. Agric Food Sci 29:222–238
Sestili F, Rouphael Y, Cardarelli M et al (2018) Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front Plant Sci 9:1–11
Sharma V, Gupta P, Priscilla K et al (2021) Metabolomics intervention towards better understanding of plant traits. Cell 10:3461–3432
Shukla PS, Mantin EG, Adil M et al (2019) Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci 10:1–22
SICIT Data sheet-PROTIFERT LMW. http://ferbainternacional.com/fichas/FICHA%20TECNICA%20DE%20PROTIFERT%20LMW.pdf. Accessed 13 May 2021
Sinkiewicz I, Śliwińska A, Staroszczyk H, Kołodziejska I (2017) Alternative methods of preparation of soluble keratin from chicken feathers. Waste Biomass Valor 8:1043–1048
Soppelsa S, Kelderer M, Casera C et al (2018) Use of biostimulants for organic apple production: effects on tree growth, yield, and fruit quality at harvest and during storage. Front Plant Sci 9:1342
Spellman D, McEvoy E, O’cuinn G, FitzGerald R (2003) Proteinase and exopeptidase hydrolysis of whey protein: comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int Dairy J 13:447–453
Tamayo Tenorio A, Gieteling J, de Jong GAH et al (2016) Recovery of protein from green leaves: overview of crucial steps for utilisation. Food Chem 203:402–408
Tapia-Hernández JA, Del-Toro-Sánchez CL, Cinco-Moroyoqui FJ et al (2019) Prolamins from cereal by-products: classification, extraction, characterization and its applications in micro- and nanofabrication. Trends Food Sci Technol 90:111–132
Tavano OL (2013) Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym 90:1–11
Tejada M, Rodríguez-Morgado B, Paneque P, Parrado J (2018) Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur J Agron 96:54–59
Trevisan S, Manoli A, Ravazzolo L et al (2017) mRNA-sequencing analysis reveals transcriptional changes in root of maize seedlings treated with two increasing concentrations of a new biostimulant. J Agric Food Chem 65:9956–9969
Tsouvaltzis P, Koukounaras A, Siomos AS (2014) Application of amino acids improves lettuce crop uniformity and inhibits nitrate accumulation induced by the supplemental inorganic nitrogen fertilization. Int J Agric Biol 16:951–955
Turnell D, Cooper J (1982) Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin Chem 28:527–531
Ugolini L, Cinti S, Righetti L et al (2015) Production of an enzymatic protein hydrolyzate from defatted sunflower seed meal for potential application as a plant biostimulant. Ind Crop Prod 75:15–23
USAGRO Biostimulants-FERTITECH AMINO HORT. https://www.usagro.com.tr/en/fertitech-amino-hort. Accessed 12 May 2021
Venugopal V, Sasidharan A (2021) Seafood industry effluents: environmental hazards, treatment and resource recovery. J Environ Chem Eng 9:104758
Visconti F, de Paz JM, Bonet L et al (2015) Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. “Rojo Brillante” grafted on Diospyros lotus L. Sci Hortic 185:129–138
Wilson HT, Xu K, Taylor AG (2015) Transcriptome analysis of gelatin seed treatment as a biostimulant of cucumber plant growth. Sci World J 2015:1–14
Wilson HT, Amirkhani M, Taylor AG (2018) Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front Plant Sci 9:1006
Wouters AG, Rombouts I, Fierens E et al (2016) Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compr Rev Food Sci Food Saf 15:786–800
Xu L, Geelen D (2018) Developing biostimulants from agro-food and industrial by-products. Front Plant Sci 9:1–13
Xu C, Mou B (2017) Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Horttechnology 27:539–543
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:1–32
Yang G, Wei Q, Huang H, Xia J (2020) Amino acid transporters in plant cells: a brief review. Plants 9:967
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Martín, MH.J., Ángel, MM.M., Aarón, SL.J., Israel, BG. (2022). Protein Hydrolysates as Biostimulants of Plant Growth and Development. In: Ramawat, N., Bhardwaj, V. (eds) Biostimulants: Exploring Sources and Applications. Plant Life and Environment Dynamics. Springer, Singapore. https://doi.org/10.1007/978-981-16-7080-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-7080-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7079-4
Online ISBN: 978-981-16-7080-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)