Abstract
This work presents the design of a bioprocess as an integral solution for adding value to whey by converting it into high value-added products for environmental/agronomical purposes as biostimulants for both soils and plants . The core of the bioprocess is a fermentation by Lactobacillus rhamnosus, a bacterial species within the group of plant growth promoting bacteria (PGPB), followed by a physicochemical separation of the valuable products obtained. The soil biostimulant products obtained are lactic acid, peptides and free amino acids and the biomass of Lactobacillus rhamnosus. All of these products were purified and the residual fraction, mainly comprising inorganic elements with high sodium content, was removed in order to avoid soil fertility problems. These products were evaluated on their soil biostimulant and biocontrol capacity, thus protein hydrolysates and lactic acid induced microbial activity, lactic acid also showed an effect modifying microbial biodiversity, favouring bacterial genera recognized as growth plants promoter, and L. rhamonsus presented biocontrol activity against some phytopathogenic microorganisms. These results give rise to the formulation of products for environmental/agronomic application.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The design of a new process for the valorization of organic by-products, specifically whey, leading to its total conversion into agricultural/environmental biostimulants is the main reason why this research was undertaken. Whey is produced in large volumes and presents problems of environmental management. This new process allows its valorization through its conversion into new products with high added value in the market of organic agriculture. This process can be the base of new industrial lines of production of biostimulants that would meet the growing agricultural demand sustainably, optimizing investments, and improving the crop yield and quality, with the increased benefit of reducing environmental impacts of inorganic fertilization.
Introduction
Whey is the watery part of milk (85–95% of the total volume) that results from separating curd when proteins coagulate during cheese production. It mainly consists of water, lactose (4–5%), soluble proteins (1%), and mineral salts (0.25%).
Annual whey production stands at approximately 180 million tons worldwide [1]. Due to its strong organic and saline content [2, 3] disposing of it as waste poses a great environmental problem and its correct treatment before discharging it into the receiving waters [4, 5] is a legal requirement.
An agronomic biostimulant is any substance or microorganism that is able to enhance plant nutrition efficiency, abiotic stress tolerance and/or crop quality traits [6].
Due to its potential as a fertiliser, applying whey to the land has long been an agronomical practice [7]. Whey contains beneficial nutrients for crops such as nitrogen, phosphorous, potassium, calcium, magnesium and sulphur. The quantities and proportions of these nutrients in whey make it a suitable substitute for, or supplement to, inorganic fertiliser [8]. Applying whey to soil has been reported to be an effective method of increasing organic matter, beneficial nutrients concentration and soil water holding capacity [9].
Phytochemical studies of whey-fertilised plants have proven its capacity to stimulate biosynthesis induction and plant active principles accumulation [10].
Applying whey to land does, however, have several drawbacks. Soil physical and chemical structure, and hence crop yield, may be affected—mainly by its suspended solids and high salinity content [11]. Suspended solids can obstruct soil pores, promoting soil fouling and negatively influencing infiltration rate [8]. Furthermore, whey’s salinity content decreases the plants’ ability to uptake water, thus affecting growth, fruit production, while it can also inhibit seed germination [7].
Moreover, it has been reported that continuously applying high rates of whey decreases crop yield, negatively affects soil microorganisms and even leads to more severe issues such as groundwater pollution [12].
Apart from its interest as fertiliser, it shows biocontrol activity against phytopathogenic fungi and bacteria [13]; it has also been used effectively against fungal infections [14] and for controlling powder mildew [15]. The main factors in this biocontrol activity are bioactive compounds, lactic acid and lactic acid bacteria (LAB) are [16,17,18]. The result of lactose fermentation, lactic acid is present in small quantities in whey. It is an organic acid produced naturally by roots under conditions of hypoxia and is exuded to prevent phytotoxicity problems in plant tissues [19]. It has been proven to be a soil prebiotic, showing a biostimulant effect, modulating the soil microbial community and enhancing the bioavailability of phosphorus in soil [20, 21]. LAB are also considered as PGPB since they protect plants from diseases and abiotic stresses. Their main trait as plant growth promoters is their biocontrol activity against phytopathogenic bacteria and fungi [18].
This work aims to enhance the production of these low-proportion compounds of interest, such as lactic acid and a specific LAB, Lactobacillus rhamnosus, through a whey fermentation process. It also aims simultaneously to transform other low-bioavailable compounds, such as proteins, into other high value-added agricultural products, such as protein hydrolysates. These three fractions with biostimulant properties were separated and assessed based on their biostimulant potential.
Materials and Methods
Chemicals and Microorganisms
Post-cheese production whey was obtained from Berrocales Trujillanos SL, Spain.
The enzyme bioprotease L-450 from Bacillus licheniformis obtained from Biocom (Spain).
MRS broth was prepared according to de Man, Rogosa and Sharpe’s indications [22].
Other chemicals and reagents employed in the study were of an analytical grade and used with no further purification.
The Lactobacillus rhamnosus used to carry out whey fermentations was identified by gene sequencing the 16S rDNA after being isolated from the whey microbial consortium and stored at − 80 °C.
Phytopathogenic strains Botryotinia fuckeliana, Fusarium oxysporum and Pseudomona syringae for biocontrol essays were obtained from the Spanish Collection of Type Cultures, Valencia, Spain (CECT).
Analytical Techniques
Lactose and Lactic Acid Determination
Lactose and lactic acid concentration in whey were determined using their respective enzymatic test kits according to the manufacture’s recommendations (Lactose Assay kit and d-/l-Lactic Acid (d-/l-Lactate) (Rapid) Assay Kit, both from Megazyme Int. Wicklow, Ireland).
Protein Analysis
HPLC Molecular Size Exclusion Chromatography
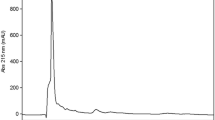
Molecular-mass distribution of protein and peptides were determined by HPLC size exclusion chromatography using a JASCO LC-4000 system, with a Superdex Peptide™ 10/300 GL column (optimum separation range 0.1–7 kDa) [23]. Proteins/peptides were detected at 280 and 215 nm with a JASCO UV-4075 UV/Vis detector module coupled to the column.
SDS-PAGE
Protein and peptide profiles were analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% polyacrylamide gels in Tris-Glycine SDS running buffer (25 mM Tris-HCl pH 8.4, 190 mM Glycine, 0.1% SDS).
Fermentation of Whey
Selection of the Microbiological Tool from the Whey Microbial Consortium
Microbiological Characterisation: Isolation and Identification
A microbial characterisation of the main cultivable microbiological strains was performed. Serial dilutions of whey were sown in MRS agar broth in order to find morphological differences between the colonies of the microbial species present in the original consortium of whey. Those colonies that showed significant differences were then isolated and identified by gene sequencing [24]. To identify yeast, samples were amplified with ITS5 and LR6 primers and sequenced with 4 primers: ITS1, NL1, NL4 y ITS4. To identify bacterial species, samples were amplified with 27F and 1492R primers and sequenced with 4 primers: 27F, 518F, 800R and 1492R.
PCR products were amplified, purified and sequenced by STAB VIDA (Oeiras, Portugal). PCR products were purified with magnetic beads; the purified PCR products were then sequenced with a Bigdye Terminator V3.1 and run on the 3730XL DNA analyser.
Screening of the Major Lactic-Acid-Producing Strain
The capacity of the different microorganisms isolated to produce lactic acid has been tested. Each microorganism was grown in 100 mL of MRS medium in a 250-mL Erlenmeyer flask. The medium was previously sterilised by being autoclaved at 121 °C for 20 min, and then inoculated with a loopful of cells from a single colony grown on an agar plate, and incubated with shaking at 150 rpm, at 37 °C for 72 h. Lactose and lactic acid isomers content were measured at the end of the fermentation time.
In order to obtain the starter culture for the following fermentations, the best lactic acid-producing strain already known was grown in a previously-sterilised flask with 100 mL of MRS medium.
Whey Fermentations
Whey fermentations were performed in a 2-L Biobundle System (Applikon Inc., Foster City, Calif.) under previously-optimised, controlled conditions of pH (pH 5.5, using calcium hydroxide as alkaline base), temperature (37 °C) and agitation (300 rpm).
Whey was previously pasteurised and inoculated with a 2% v/v culture of L. rhamnosus.
In order to increase whey protein bioavailability, 0.1% of protease (Bioproteasa LA-450 from Biocon Española, S.A.) was added to fermentations as an inductor.
Separation of Biostimulants
Different fractions of fermented whey were separated by molecular weight using an MMS triple system membrane device. A 0.2-µm PVDF membrane was used to separate L. rhamnosus biomass and a 200-Da MW cut-off TFM membrane was used to separate the protein hydrolysate.
Lactic acid was purified from the permeate using the esterification-distillation method described by Kwak et al. [25].
Testing the Biostimulant Capacity of Products Obtained from Fermented Whey
Lactobacillus rhamnosus Biocontrol Activity Test
Lactobacillus rhamnosus biocontrol activity was tested against different phytopathogenic species. Plate confrontation method in PDA plates was used for fungi assays. Fungal cakes were placed in the centre of a PDA plate, and pure bacterial colonies were cultured at an equal distance (3 cm) from pathogenic cakes (72 h, 28 °C) and inhibition diameter were calculated. Negative control plates had no bacteria [26].
The agar spot method in MRS plates was used to test L. rhamnosus antagonist activity against Pseudomona syringae. In this case, 50 µL of a culture of P. syringae were sown by the spread-plate method and a small strip of an 18-h culture of L. rhamnosus was sown in the centre of the plate using a sterile stick [27].
Soil Biostimulant Capacity of Lactic Acid and Protein Hydrolysate
Both whey-hydrolysed protein and lactic acid were tested in separate trials based on its edaphological stimulation capacity. In both cases, a soil-stimulation test was carried out, and the effect on soil microbiota was based on the dehydrogenase activity, measured by a reduction of 2-p-iodo-3-nitrophenyl 5-phenyl tetrazolium chloride to iodonitrophenyl formazan [20, 28].
The soil used in this work is a Plagic Antrosol soil [29]. Assays were carried out in triplicate, kept in semi-closed microcosms of 200 g of dried and sieved (< 2 mm) soil at 25 ± 2 °C for 28 days. Soil samples were mixed with 0.5% and 1% v/w protein hydrolysate or 0.5% v/w lactic acid, and 60% of water-holder capacity was maintained during the time of the experiment by adding distilled water. No product was added to the control pots.
DNA Metabarcoding Analysis
Total genomic DNA was extracted from soil samples using the DNeasy Power-Soil DNA isolation kit (Qiagen) according to manufacturer’s instructions.
The V3–V4 hypervariable regions of the bacterial 16S rRNA were amplied by PCR using the primers Bakt_341F and Bakt_805R [30] to prepare libraries. Libraries were purified, pooled and sequenced in a fraction of a MiSeq PE300 run (Illumina).
Sequencing data were performed using the bioinformatic tool Qiime 1.9.0 [31]. 16S reads were clustered into OTUs using the de novo approach, and each OTU was assigned to amicrobial taxon using the RDP classifier [32] with a confidence threshold of 97%.
Results and Discussion
Whey Chemical Composition
The whey used as raw material in this work presents the typical chemical composition (Table 1), mainly comprising carbohydrates—mostly lactose—and proteins.
While lactose is the major component in whey (50 ± 3.9 g/L, Table 1), soluble protein is the second main component (10 ± 2 g/L, Table 1). Protein composition has been analysed, showing the typical protein profile of whey obtained from raw milk [33], mainly composed of high molecular weight proteins such as β-lactoglobulin, α-lactalbumin, and minor amounts of casein. Whey also contains minor quantities of lactic acid (5.2 ± 0.6 g/L, Table 1), a product of the spontaneous microbial fermentation of lactose during storage [34].
The inorganic composition of the cheese whey used in this work consists of mineral salts (0.24 ± 0.2 g/L, Table 1), mainly comprising K (1.28 g/L), Ca (0.30 g/L), Na (0.28 g/L) and P (0.34 g/L), primarily in the form of phosphatic salts; similar results have been reported previously [35, 36].
In order to assimilate lactose and proteins, soil microorganisms need hydrolytic enzymes [3, 37]. Our work aims to skip this hydrolytic stage in soil by applying more bioavailable products obtained from the previous compounds, such as lactic acid and protein hydrolysates, as soil prebiotics.
Whey Fermentation and Separation of Biostimulants
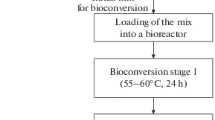
A bioprocess for the production of biostimulants has been designed (Fig. 1). The core of this bioprocess consists of a fermentation with L. rhamnosus that is LAB previously isolated from the whey microbial consortium. Operational fermentation parameters were optimised and established in order to achieve the greatest production of lactic acid and of L. rhamnosus biomass, as well as the total conversion of proteins into a protein hydrolysate. These products are also separated and purified through physicochemical process.
Fermentation Process
The biological phase is based on a microbial fermentation process coupled with a process of enzymatic protein hydrolysis. The optimisation of the process is described below. It comprises selecting the microbiological tool and optimising the fermentation operational parameters.
Microbiological Tool Selection
Six major cultivable species were reported from a microbial isolation carried out within the consortium present in whey. Once they were identified, screening was undertaken in order to search for the maximum lactic acid-producing strain (results shown in Table 2). Presenting the highest production yield of lactic acid, Lactobacillus rhamnosus was chosen as biological the tool to perform the fermentations (Table 2). Selecting a locally-adapted strain would be more beneficial compared to a bacterial strain obtained from a culture collection.
Operational Fermentation Parameters
Operational fermentation parameters were optimised based on maximising the production of lactic acid and L. rhamnosus biomass: pH 5.5, temperature 40 °C, and an aeration rate of 0.1 vvm were chosen.
Nitrogen availability is a limiting factor in whey fermentation. The poor proteolytic system of LAB [38] and the fact that whey’s nitrogenous fraction is mainly composed of hardly-available high-molecular-weight globular proteins make nutrient supplementation (yeast extract, protein hydrolysates, etc.) necessary in order to obtain good bacterial growth and high lactic acid productivity [39,40,41]. The use of protease as a proteolytic agent to enhance nitrogen bioavailability could solve the nutritional deficiency of whey [38]. Subtilisin was therefore used in order to carry out a simultaneous fermentation and protein hydrolysis and achieve an optimal lactic acid production yield, avoiding the need for nutritional inputs, which in turn reduces fermentation time. The HPLC chromatographic analysis of the molecular exclusion of both whey and fermented whey (Table 3) reveals that protein hydrolysis leads to an inversion of the protein profile in favour of low molecular weight peptides. The majority of the peptides obtained have a molecular weight of between 1000 and 300 Da, which corresponds to peptides of between 8 and 10 amino acids that efficiently support LAB growth [38]. Confirming the above, the results of the electrophoretic analysis show that soluble proteins remain unchanged after the enzyme-free fermentation process compared with unfermented whey (Fig. 2, lane b). Subtilisin produces a drastic change in protein size, making the bands corresponding to the typical whey proteins, β-lactoglobulin and α-lactalbumin (Fig. 2, lane c) disappear.
As shown in Fig. 3a and b, protease enables proteins to be converted into a protein hydrolysate. As a fermentation inductor, it also has an effect on fermentation performance, increasing the speed of the process, achieving a faster total lactose consumption (Fig. 3a) and consequently a faster and higher lactic acid production (Fig. 3b). The highest hydrolytic rate was reached with 0.5% v/v of protease. In comparison with the control where no protease was added, at 24 h of fermentation, the lactose depletion and lactic acid production were around 21% and 12% higher respectively.
At the end of this biological process, a fermented product is obtained which composition is shown in Table 1. It is mainly composed of lactic acid (42 ± 2.7 g/L), followed by protein hydrolysates (9.16 ± 1.8 g/L) whose molecular weight is mainly between 1000 and 300 Da (Table 3). Proteins are totally converted into peptides and free amino acids, thus increasing nitrogen bioavailability.
Finally, the insoluble fraction is composed of the of L. rhamnosus bacterial biomass, which has increased from 0.07 ± 0.01 to 3.2 ± 0.7 g/L of dry weight after fermentation (Table 1).
In brief, the biological process developed consisted of a whey fermentation using an L. rhamnosus strain as the inoculant. Under optimum fermentation parameters (40 °C, pH 5.5, aeration 0.1 vvm and protease 0.5% v/v) a fermented whey product is obtained, with 42 ± 2.7 g/L of lactic acid (Table 1).
Physico-chemical Stage: Separation of Biostimulant Products
A sequential separation process was designed in order to separate these new products from the fermented whey (Fig. 1). It starts with a microfiltration step (0.2 µm PVDF membrane) to separate the biomass fraction. The nitrogenous fraction, comprising of low molecular weight peptides and amino acids, was then separated using a 200-Da MW cut-off TFM membrane.
Due to the high lactic acid concentration and the absence of other organic molecules in the permeate, lactic acid could be separated from the remaining salt solution by a distillation process. However, due to the high boiling point of lactic acid, conventional distillation processes would not be effective. In order to decrease its boiling point, it was necessary to resort to a solvent-esterification process [25, 42, 43]. The lactic acid was, therefore, purified using the esterification-distillation method described by Kwak et al. [25].
Due to its high content in K, Ca and P, one of the potential uses of this inorganic fraction would be for mineral plant nutrition. However, its high saline (NaCl) content might affect physically and chemically the structure of soil when it is applied [11, 44], decreasing in turn the availability of water for plants. One approach to solving this problem, however, would be to dilute this fraction to adequate salinity levels [8]. In this work the saline fraction was discarded, due to it not being considered as a biostimulant.
The mass balance of the products obtained from the process is shown in Table 1. Briefly, the products (Fig. 1) show the following representation with respect to the initial dry matter content of the whey: (A) probiotics (4.73%), composed only by L. rhamnosus at a concentration of 1011 CFU/g, (B) protein hydrolysates (13.54%), (C) lactic acid (62.07%), (D) minerals (3.55%), and 16.11% of the whey organic matter was metabolically consumed.
Testing of Biostimulant Products
Once every valuable product had been purified from the fermented whey, their biostimulant capacities were evaluated (see scheme of the process, Fig. 1).
Biocontrol Activity of Lactobacillus rhamnosus
The bacterial insoluble fraction is composed of L. rhamnosus. Among the LAB, which are collected within the group of PGPB for influencing positively plant growth and development [45], L. rhamnosus has been widely described for its biological properties such as bacteriocins production [46, 47] and biocontrol activity [48].
The plate tests carried out to assess the biocontrol activity of this fraction showed the antagonistic activity of L. rhamnosus against Phytophthora cactorum, Phytophthora cinamomi and Pseudomonas syringae. It did not, however, show any antagonistic activity against Botryotinia fuckeliana, Fusarium oxysporum nor Verticillium dahlia. The average radius size of the inhibition zones is shown in the Table 4.
Although more detailed in-vivo studies are needed, these results confirm the biocontrol activity of this product against several phytopathogenic species.
Soil Biostimulant Capacity of Protein Hydrolysate
The protein hydrolysate is mainly composed of peptides and free amino acids. Given its positive effects on crop performances, it is considered an important plant biostimulant [49]. There are studies that report an improvement in growth, yield and fruit quality when applying protein hydrolysates to crops [50]. They have been described as increasing nutrient uptake by specific enzymatic activity stimulation [51], acting as chelating agents [52], or by improving the tolerance to salinity [53].
In order to assess the biostimulant capacity of the protein hydrolysate, a biostimulant soil essay was performed. In the essay, this product was evaluated at different concentrations: The one obtained in the purification process, and a dilution of the product to half its concentration, both compared with the control, where the protein hydrolysate was replaced by water. Dehydrogenase activity, which reflects the total range of oxidative activity of soil microorganisms, and which can be considered a good indicator of oxidative metabolism in soil, was measured as an indicator of microbiological activity [54]. The results revealed strong microbial stimulation by both concentrations of the protein hydrolysate (0.5 and 1% v/w). Both showed the same pattern, mainly enhanced on day 5, followed by a gradual decrease of the dehydrogenase activity until the end of the essay (Fig. 4). However, the control did not show any stimulation of the dehydrogenase activity.
Lactic Acid Biostimulant Activity
The biostimulant effect of the lactic acid obtained by a whey fermentation on soil microbiota has previously been tested. Results are shown in a work recently published by the authors of this paper [20] which shows the effect of lactic acid on stimulating soil enzymatic activities such as dehydrogenase and acid phosphomonosesterase activities, inducing in turn the release of soluble phosphate and shifting the composition of soil bacterial communities towards an enrichment of PGPBs such as the genera Pseudomonas, Bacillus, Azotobacter and Rhizobium.
In order to confirm the results reported by Rodriguez Morgado et al. [20] we repeated the same experiments analysing them using DNA metabarcoding. The biostimulation profile of lactic acid, which is reflected in the soil dehydrogenase activity, was similar to the one found by them (Fig. 4), and the DNA metabarcoding analysis revealed a similar modification of the soil taxonomic composition, which resulted in a decrease of bacterial biodiversity due to the favouring of some specific taxa. Specifically, as shown in Fig. 5, 7 days after lactic acid application, the relative abundance of the family Bacillaceae, which corresponds entirely to the relative abundance of genus Bacillus, and the family Veillonellaceae, which corresponds entirely to the relative abundance of the genus Pelosinus, were 13.8% and 15.2% respectively, not being present in the control samples. This can be explained by the fact that microorganisms belonging to this genera are able of proliferating in acidic environments [55] and using lactate as a source of C [56, 57]. At this time of the essay, the abundance of the family Micrococcaceae increased 22.4% compared to the control, reaching 28.9% of the total relative abundance. Also noteworthy is the enrichment of the families Pseudomonadacea (4.6%), Rizhobiaceae (2.8%), which corresponds entirely to the relative abundance of the genus Sinorhizobium, and Microbacteriaceae (2.6%), which corresponds entirely to the relative abundance of genus Agromyces, in lactic acid samples, none of them having any presence in the relative abundance of control samples on day 7.
Abundance of the 15 most abundant bacterial families. Those sequences that are not classified into any known family group were designated as “NA”. C-T7 control soil sample at day 7, C-T28 control soil sample at day 28, L-T7 lactic acid treated soil sample at day 7, L-T28 lactic acid treated soil sample at day 28
On day 28th of the essay, when lactic acid had disappeared from the soil due to its consumption by soil microorganisms [20], taxonomic changes were maintained for the families Microbacteriaceae, Rizhobiaceae, Micrococcaceae and Pesudomonas, being their relative abundances 4.3%, 6.9%, 14.2%, and 13.3% respectively. However, the families Bacilliaceae and Veillonellaceae seem to be more dependent on lactic acid since they were not present once it was consumed. Finally, it is interesting to note that the family Xanthomonadaceae, which 100% corresponds to the relative abundance of the genus Lysobacer, was favoured when the lactic acid disappeared from the soil, assuming a relative abundance 7.5% higher in lactic acid samples at day 28 than in control samples at day 28 and 6.4% higher than lactic acid samples at day 7.
Conclusions
This paper provides the design of a process for whey valorisation in order to obtain products whose biostimulant capacity has been tested. The products are probiotics (the biomass of L. rhamnosus), and prebiotics such as protein hydrolysates and lactic acid. The biomass of L. rhamnosus has shown biocontrol activity against several phytopathogenic species such as Phytophthora cactorum, Phytophthora cinamomi and Pseudomona syringae. Moreover, both the protein hydrolysate and the lactic acid showed a stimulatory effect on soil microorganisms. This effect was reflected in the stimulation of soil enzymes. Lactic acid in particular has also shown to have an effect on shaping the composition of bacterial communities in soil, leading to an enrichment of PGPBs.
References
Ghasemi, M., Ahmad, A., Jafary, T., Azad, A.K., Kakooei, S., Daud, W., Sedighi, W.R.: M.: Assessment of immobilized cell reactor and microbial fuel cell for simultaneous cheese whey treatment and lactic acid/electricity production. Int. J. Hydrogen Energy. 42, 9107–9115 (2017). https://doi.org/10.1016/j.ijhydene.2016.04.136
Marwaha, S.S., Kennedy, J.F.: Whey—pollution problem and potential utilization. Int. J. Food Sci. Technol. 23, 323–336 (1988). https://doi.org/10.1111/j.1365-2621.1988.tb00586.x
Siso, M.I.G.: The biotechnological utilization of cheese whey: a review. Bioresour. Technol. 57, 1–11 (1996). https://doi.org/10.1016/0960-8524(96)00036-3
Kalyuzhnyi, S.V., Martinez, E.P., Martinez, J.R.: Anaerobic treatment of high-strength cheese-whey wastewaters in laboratory and pilot UASB-reactors. Bioresour. Technol. 60, 59–65 (1997). https://doi.org/10.1016/S0960-8524(96)00176-9
Mawson, A.J.: Bioconversions for whey utilization and waste abatement. Bioresour. Technol. 47, 195–203 (1994). https://doi.org/10.1016/0960-8524(94)90180-5
du Jardin, P.: Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. (Amsterdam). 196, 3–14 (2015). https://doi.org/10.1016/J.SCIENTA.2015.09.021
Prazeres, A.R., Carvalho, F., Rivas, J.: Cheese whey management: a review. J. Environ. Manage. 110, 48–68 (2012). https://doi.org/10.1016/j.jenvman.2012.05.018
Robbins, C.W., Lehrsch, G.A.: Cheese whey as a soil conditioner. Handb. Soil Cond. Subst. Enhanc. Phys. Prop. Soil. 1, 167–185 (1998)
Aboukila, E., Abdelraouf, E., Gomma, I.: Effects of cheese whey on some chemical and physical properties of calcareous and clay soils. Int. J. Plant Soil Sci. 21, 1–12 (2018). https://doi.org/10.9734/IJPSS/2018/39082
Grosu, L., Fernandez, B., Grigoras, C.G., Patriciu, O.I., Grig-Alexa, I.-C., Nicuta, D., Ciobanu, D., Gavrila, L., Finaru, A.L.: Valorization of whey from dairy industry for agricultural use as fertiliser: effects on plant germination and growth. Environ. Eng. Manag. J. 11, 2203–2210 (2012). https://doi.org/10.1016/j.ancene.2014.05.002
Dragone, G., Mussatto, S.I., Oliveira, J.M., Teixeira, J.A.: Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 112, 929–935 (2009). https://doi.org/10.1016/J.FOODCHEM.2008.07.005
Peterson, A.E., Walker, W.G., Watson, K.S.: Effect of whey applications on chemical properties of soils and crops. J. Agric. Food Chem. 27, 654–658 (1979). https://doi.org/10.1021/jf60224a064
Clément, M., Tremblay, J., Lange, M., Thibodeau, J., Belhumeur, P.: Purification and identification of bovine cheese whey fatty acids exhibiting in vitro antifungal activity. J. Dairy Sci. 91, 2535–2544 (2008). https://doi.org/10.3168/jds.2007-0806
Pane, C., Celano, G., Villecco, D., Zaccardelli, M.: Control of Botrytis cinerea, Alternaria alternata and Pyrenochaeta lycopersici on tomato with whey compost-tea applications. Crop Prot. 38, 80–86 (2012). https://doi.org/10.1016/J.CROPRO.2012.03.012
Bettiol, W., Silva, H.S.A., Reis, R.C.: Effectiveness of whey against zucchini squash and cucumber powdery mildew. Sci. Hortic. (Amsterdam). 117, 82–84 (2008). https://doi.org/10.1016/j.scienta.2008.03.010
Caplice, E., Fitzgerald, G.F.: Food fermentations: Role of microorganisms in food production and preservation. (1999). http://www.ncbi.nlm.nih.gov/pubmed/10488849,
Yadav, J.S.S., Yan, S., Pilli, S., Kumar, L., Tyagi, R.D., Surampalli, R.Y.: Cheese whey: a potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 33, 756–774 (2015). https://doi.org/10.1016/J.BIOTECHADV.2015.07.002
Shrestha, A., Kim, B.S., Park, D.H.: Biological control of bacterial spot disease and plant growth-promoting effects of lactic acid bacteria on pepper. Biocontrol Sci. Technol. 24, 763–779 (2014). https://doi.org/10.1080/09583157.2014.894495
Jones, D.L.: Organic acids in the rhizosphere—a critical review. Plant Soil. 205, 25–44 (1998). https://doi.org/10.1023/A:1004356007312
Rodríguez-Morgado, B., Jiménez, P.C., Moral, M.T., Rubio, J.P.: Effect of l-lactic acid from whey wastes on enzyme activities and bacterial diversity of soil. Biol. Fertil. Soils. 53, 389–396 (2017). https://doi.org/10.1007/s00374-017-1187-z
Bolan, N.S., Naidu, R., Mahimairaja, S., Baskaran, S.: Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol. Fertil. Soils. 18, 311–319 (1994). https://doi.org/10.1007/BF00570634
De man, rogosa and sharpe (MRS) agar. Prog. Ind. Microbiol. 34, 362–363 (1995). https://doi.org/10.1016/S0079-6352(05)80056-6
Parrado, J., Rodriguez-Morgado, B., Tejada, M., Hernandez, T., Garcia, C.: Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzyme Microb. Technol. 57, 1–7 (2014). https://doi.org/10.1016/j.enzmictec.2014.01.001
Tavares, N., Penedo, P.: Molecular identification of Monascus purpureus NART001 isolated from commercially available Chinese red fermented rice. Biomed. Biopharm. Res. 14, 88–94 (2017). https://doi.org/10.19277/bbr.14.1.152
Kwak, H., Hwang, D.W., Hwang, Y.K., Chang, J.S.: Recovery of alkyl lactate from ammonium lactate by an advanced precipitation process. Sep. Purif. Technol. 93, 25–32 (2012). https://doi.org/10.1016/j.seppur.2012.03.025
Shan, H., Zhao, M., Chen, D., Cheng, J., Li, J., Feng, Z., Ma, Z., An, D.: Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Prot. 44, 29–37 (2013). https://doi.org/10.1016/J.CROPRO.2012.10.012
Spelhaug, S.U.E.R., Harlander, S.K.: Inhibition of foodborne bacterial pathogens by bacteriocins from Lactococcus lactis and Pediococcus pentosaceous 1. J. Food Prot. 52, 856–862 (1989). https://doi.org/10.4315/0362-028X-52.12.856
von Mersi, W., Schinner, F.: An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils. 11, 216–220 (1991). https://doi.org/10.1007/BF00335770
FAO: World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. FAO. (2015)
Herlemann, D.P., Labrenz, M., Jürgens, K., Bertilsson, S., Waniek, J.J., Andersson, A.F.: Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5, 1571–1579 (2011). https://doi.org/10.1038/ismej.2011.41
Caporaso, J.G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F.D., Costello, E.K., Fierer, N., Peña, A.G., Goodrich, J.K., Gordon, J.I., Huttley, G.A., Kelley, S.T., Knights, D., Koenig, J.E., Ley, R.E., Lozupone, C.A., McDonald, D., Muegge, B.D., Pirrung, M., Reeder, J., Sevinsky, J.R., Turnbaugh, P.J., Walters, W.A., Widmann, J., Yatsunenko, T., Zaneveld, J., Knight, R.: QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7, 335–336 (2010). https://doi.org/10.1038/nmeth.f.303
Wang, Q., Garrity, G.M., Tiedje, J.M., Cole, J.R.: Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007). https://doi.org/10.1128/AEM.00062-07
Chen, W.L., Hwang, M.T., Liau, C.Y., Ho, J.C., Hong, K.C., Mao, S.J.T.: Beta-lactoglobulin is a thermal marker in processed milk as studied by electrophoresis and circular dichroic spectra. J. Dairy Sci. 88, 1618–1630 (2005). https://doi.org/10.3168/jds.S0022-0302(05)72833-2
Panesar, P., Kennedy, J., Gandhy, D., Bunko, K.: Bioutilisation of whey for lactic acid production. Food Chem. 105, 1–14 (2007). https://doi.org/10.1016/j.foodchem.2007.03.035
Dangin, M., Guillet, C., Garcia-Rodenas, C., Gachon, P., Bouteloup-Demange, C., Reiffers-Magnani, K., Fauquant, J., Ballèvre, O., Beaufrère, B.: The rate of protein digestion affects protein gain differently during aging in humans. J. Physiol. 549, 635–644 (2003). https://doi.org/10.1016/j.parco.2007.12.005
Venetsaneas, N., Antonopoulou, G., Stamatelatou, K., Kornaros, M., Lyberatos, G.: Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour. Technol. 100, 3713–3717 (2009). https://doi.org/10.1016/j.biortech.2009.01.025
Ladd, J.N., Paul, E.A.: Changes in enzymic activity and distribution of acid-soluble, amino acid-nitrogen in soil during nitrogen immobilization and mineralization. Soil Biol. Biochem. 5, 825–840 (1973). https://doi.org/10.1016/0038-0717(73)90028-X
Vasala, A., Panula, J., Neubauer, P.: Efficient lactic acid production from high salt containing dairy by-products by Lactobacillus salivarius ssp. salicinius with pre-treatment by proteolytic microorganisms. J. Biotechnol. 117, 421–431 (2005). https://doi.org/10.1016/j.jbiotec.2005.02.010
Fitzpatrick, J.J., O’Keeffe, U.: Influence of whey protein hydrolysate addition to whey permeate batch fermentations for producing lactic acid. Process Biochem. 37, 183–186 (2001). https://doi.org/10.1016/S0032-9592(01)00203-5
Kadam, S.R., Patil, S.S., Bastawde, K.B., Khire, J.M., Gokhale, D.V.: Strain improvement of Lactobacillus delbrueckii NCIM 2365 for lactic acid production. Process Biochem. 41, 120–126 (2006). https://doi.org/10.1016/J.PROCBIO.2005.06.007
Schepers, A.W., Thibault, J., Lacroix, C.: Continuous lactic acid production in whey permeate/yeast extract medium with immobilized Lactobacillus helveticus in a two-stage process: model and experiments. Enzyme Microb. Technol. 38, 324–337 (2006). https://doi.org/10.1016/j.enzmictec.2004.07.028
Sun, X., Wang, Q., Zhao, W., Ma, H., Sakata, K.: Extraction and purification of lactic acid from fermentation broth by esterification and hydrolysis method. Sep. Purif. Technol. 49, 43–48 (2006). https://doi.org/10.1016/j.seppur.2005.08.005
Khunnonkwao, P., Boontawan, P., Haltrich, D., Maischberger, T., Boontawan, A.: Purification of l-(+)-lactic acid from pre-treated fermentation broth using vapor permeation-assisted esterification. Process Biochem. 47, 1948–1956 (2012). https://doi.org/10.1016/j.procbio.2012.07.011
Saddoud, A., Hassaïri, I., Sayadi, S.: Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Bioresour. Technol. 98, 2102–2108 (2007). https://doi.org/10.1016/j.biortech.2006.08.013
de Souza, R., Ambrosini, A., Passaglia, L.M.P.: Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38, 401–419 (2015). https://doi.org/10.1590/S1415-475738420150053
Todorov, S.D., Dicks, L.M.T.: Screening for bacteriocin-producing lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria: comparison of the bacteriocins. Process Biochem. 41, 11–19 (2006). https://doi.org/10.1016/j.procbio.2005.01.026
Dimitrijević, R., Stojanović, M., Živković, I., Petersen, A., Jankov, R.M., Dimitrijević, L., Gavrović-Jankulović, M.: The identification of a low molecular mass bacteriocin, rhamnosin A, produced by Lactobacillus rhamnosus strain 68. J. Appl. Microbiol. 107, 2108–2115 (2009)
Bueno, D.J., Silva, J.O., Oliver, G., GonzáLez, S.N.: Lactobacillus casei CRL 431 and Lactobacillus rhamnosus CRL 1224 as biological controls for Aspergillus flavus strains. J. Food Prot. 69, 2544–2566 (2006)
Colla, G., Nardi, S., Cardarelli, M., Ertani, A., Lucini, L., Canaguier, R., Rouphael, Y.: Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. (Amsterdam). 196, 28–38 (2015). https://doi.org/10.1016/j.scienta.2015.08.037
Parrado, J., Bautista, J., Romero, E.J., García-Martínez, A.M., Friaza, V., Tejada, M.: Production of a carob enzymatic extract: potential use as a biofertilizer. Bioresour. Technol. 99, 2312–2318 (2008). https://doi.org/10.1016/j.biortech.2007.05.029
Cerdán, M., Sánchez-Sánchez, A., Oliver, M., Juárez, M., Sánchez-Andreu, J.J.: Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Acta Hortic. 830, 481–488 (2009). https://doi.org/10.17660/ActaHortic.2009.830.68
Ashmead, H.D., Ashmead, H.H., Miller, G.W., Hsu, H.H.: Foliar feeding of plants with amino acid chelates. (1986). http://agris.fao.org/agris-search/search.do?recordID=XF2015033080
Ertani, A., Schiavon, M., Muscolo, A., Nardi, S.: Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil. 364, 145–158 (2013). https://doi.org/10.1007/s11104-012-1335-z
Nannipieri, P., Grego, S., Ceccanti, B.: Ecological significance of the biological activity in soil. Soil Biochem. 6, 293–355
Hansel, C.M., Fendorf, S., Jardine, P.M., Francis, C.A.: Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol. 74, 1620–1633 (2008). https://doi.org/10.1128/AEM.01787-07
Beller, H.R., Han, R., Karaoz, U., Lim, H., Brodie, E.L.: Genomic and physiological characterization of the chromate-reducing, aquifer-derived firmicute Pelosinus sp. strain HCF1. Appl. Environ. Microbiol. 79, 63–73 (2013). https://doi.org/10.1128/AEM.02496-12
Mosher, J.J., Phelps, T.J., Podar, M., Hurt, R.A., Campbell, J.H., Drake, M.M., Moberly, J.G., Schadt, C.W., Brown, S.D., Hazen, T.C., Arkin, A.P., Palumbo, A.V., Faybishenko, B.A., Elias, D.A.: Microbial community succession during lactate amendment and electron acceptor limitation reveals a predominance of metal-reducing Pelosinus spp. Appl. Environ. Microbiol. 78, 2082–2091 (2012). https://doi.org/10.1128/AEM.07165-11
Acknowledgements
This work was supported by the Ministry of Science and Innovation (Spain), Plan Estatal 2013–2016 Retos—Proyectos I + D + i CTM2015-64354-C3-1-R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caballero, P., Rodríguez-Morgado, B., Macías, S. et al. Obtaining Plant and Soil Biostimulants by Waste Whey Fermentation. Waste Biomass Valor 11, 3281–3292 (2020). https://doi.org/10.1007/s12649-019-00660-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00660-7