Abstract
Feather waste is a promising protein biomass available as by-product from poultry processing was found to be rich in peptides, amino acids, and minerals like nitrogen, phosphorus, potassium, calcium, magnesium, iron, manganese, zinc, and copper. Soil and foliar application of these products, besides representing a sustainable solution to the problem of feather disposal, may also represent an effective strategy to tackle the environmental effluence. As a consequence, they were also found to be very attractive in elevating the protein, amino acids, reducing sugar, total chlorophyll, and proline content of plants. On the other side, fertilizing effect enhanced the antioxidant potential of banana fruit which was assessed using 2, 2-diphenyl-1-picrylhydrazyl, ferric reducing/antioxidant power, and N, N-dimethyl-p-phenylendiamine. This was associated with considerably higher antioxidant contents like total phenolics and flavonoids. Therefore, the application of this organic amendment could promote and improve the agro-ecosystem, human health; soil biological activities, and at the same time enhance the production of plant or products rich in bioactive substances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The feathers are the complex structural protein rich in disulphide bonds, hydrogen bonding, and hydrophobic interaction making it resistant to degradation by most plant, animal, and microbes (Coward-Kelly et al. 2006). Poultry feather contributes about 10 % to the broiler’s live body weight, while dried feather contains 85–99 % proteins (Papadopoulos 1985). Agrahari and Wadhwa (2010) estimated that about 24 billion chickens are killed annually worldwide through which 8.5 billion tons of poultry feather are produced, among which India alone contributes about 350 million tons annually. Currently, the feather waste is treated by using chemical (acid and alkali) and physical (cooking or burning) treatments which are expensive, hazardous, and also leads to the contamination of air, water, and soil (Gupta and Ramnani 2006). The microbial bioremediation offers great advantages over conventional methods. Much of the previous work has been focused only on degradation, enzyme purification, and analysis of the degradation products, but little is known about its effects and further applications. As feathers are rich source of organic nitrogen, they suggest an opportunity for the development of new nitrogen-rich organic amendments that will serve the dual purpose of improving plant growth and stimulating microbial activity in the soil, beneficial to human health and environment.
The feather degrading bacterium Chryseobacterium sp. RBT used in the present study was capable to hydrolyze the native chicken feathers within 30 h. According to our previous observation feather hydrolysate was rich source of amino acids like leucine, valine, methionine, glycine, cysteine, serine, phenylalanine, tryptophan, lysine, and tyrosine (Gurav and Jadhav 2012). Amino acids are well known as biostimulants as it promotes the vegetative growth, nutrient uptake in plants, abiotic and biotic stresses tolerance resulting in increased crop yield (Vernieri et al. 2006).
Material and methods
Chemicals and poultry feathers
The solvents, chemical, and reagents used in this study were of analytical grade obtained from local suppliers. Chicken feathers were collected as a waste by-product generated through poultry processing unit in Kolhapur, India. Raw feathers were washed under tap water, sun dried, and stored.
Biodegradation of native chicken feathers using Chryseobacterium sp. RBT
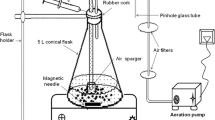
The feather-degrading bacterium previously isolated and identified as Chryseobacterium sp. RBT (accession number GU481093) was used in this study. The feather degradation was carried out in flask containing feather and basal salt medium (in grams per liter) KH2PO4 (4), Na2HPO4 (6), NaCl (5), MgSO4 (0.1), and native chicken feathers (10). The 2-ml seed culture of Chryseobacterium sp. RBT (1.12 O.D. at 660 nm) was transferred to feather basal salt medium and agitated on rotary shaker (140 rpm) at 37 °C for 30 h (Gurav and Jadhav 2012). The degraded broth was pasteurized and used for further studies.
Analysis of the feather degradation end products
The amount of total soluble protein was determined by Lowry method (Lowry et al. 1951) and amino acids using ninhydrin (Moore and Stein 1957). The total nitrogen (N) (Kjeldahl method), phosphorus (P), and potassium (K) (flame photometry) where determined. Also, the presence of metal-like copper (Cu), iron (Fe), calcium (Ca), manganese (Mn), magnesium (Mg), and zinc (Zn) were examined by means of atomic absorption spectroscopy (Perkin-Elmer analyst, USA), (APHA 1998).
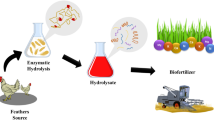
Degraded feather waste as biofertilizer
Plantation and dose application
The tissue cultured banana plantlets (Musa sp. known as Grand niene or G9) were generous gift from Callus Biotech Pvt. Ltd, Kolhapur, India. Plantlets obtained were of uniform height and age. The 30 × 30 m land was selected near Akluj, India, which was divided into three sets each having 10 × 10 m area. Plantation was done on 5 September 2011 in the selected plot with spacing of 2 × 2 m between two plants and rows consisting 36 plantlets for each treatment. Each set was amended with FDP as 20 % root dose (RD) and 5 % shoot dose (SD) through fertigation and foliar spray at the interval of 15 days. The control plants without treatment were maintained. All the plants were irrigated regularly through drip irrigation and the experiment was carried out in triplicate. Regular practices required for the cultivation like weeding and earthing up were done routinely.
Chemical analysis of banana plant and fruit
Chlorophyll estimation
The fresh leaves of treated and control plants were collected randomly after 4 months of plantation; washed thoroughly and blotted. Then, 1 g of leaf tissue was crushed in acetone (80 % v/v) and the final volume was adjusted to 20 ml and stored overnight under refrigeration. This extract was then filtered through glass wool followed by centrifugation. The absorbance of the supernatant was recorded at 663 and 645 nm using spectrophotometer (Shimadzu UV-1800, Japan) and the total chlorophyll content was determined as the mean of all readings (Arnon 1949).
Quantification of free protein, amino acids and reducing sugars
The fresh fruit pulp/vegetative tissue (1 g) was crushed in 100 mM phosphate buffer (pH 7; for protein) and in 80 % ethanol (for amino acids); this extracts were centrifuged and the aliquots were pooled for the estimation of free protein (Lowry et al. 1951) and amino acids (Moore and Stein 1957). The fruit pulp (1 g) was homogenized in 90 % ethanol and then centrifuged at 10,000 rpm. The residue was again extracted and subjected for evaporation and dissolved in distilled water and reducing sugar was measured by glucose standard curve (Miller 1959).
Estimation of free proline
The assessment of the free proline content in vegetative tissue was determined using method of Bates et al. (1973). Briefly, fresh tissue (1 g) was homogenized in 3 % (w/v) aqueous 5-sulfosalicylic acid, centrifuged, and the supernatant was reacted with equal amount of acid ninhydrin and glacial acetic acid. After 1 h of incubation, the reaction mixture was extracted with toluene. The chromophore containing toluene was aspirated and optical density was measured at 520 nm (UV-1800, Shimadzu, Japan) using toluene as a blank. The amount of proline was determined using standard l-proline and expressed as milligram of proline per gram fresh weight (FW).
Preparation of extract
The peeled pulp/vegetative tissue were crushed in food processor to produce uniform slurries. In extraction process, about 2 g of slurry was homogenized in different solvents (methanol, ethanol, and water) in the ratio 1:10. Extraction was carried out on orbital shaker for 24 h followed by centrifugation at 15,000 rpm and the supernatant was used for the analysis of antioxidants, phenolics, and flavonoids.
Analysis of total phenolics
The total phenolics of treated and control plants were determined using Folin–Ciocalteu’s reagent (Singleton and Rossi 1965). An aliquot (500 μl) of methanolic extract (ME), ethanolic extract (EE), and aqueous extract (AE) of vegetative tissue/fruit was added to 500 μl of distilled water and 1,800 μl of Folin–Ciocalteu reagent, followed by the addition of 1200 μl 15 % (w/v) sodium carbonate. After 90 min incubation in the dark, the absorbance was recorded at 765 nm against blank. Total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per gram of fresh weight using calibration curve.
Determination of total flavonoids
A total flavonoid content of ME, EE, and AE of the fruit and vegetative tissue was measured using colorimetric method (Chang et al. 2002). In brief, 500 μl of plant extract was mixed with distilled water (2,000 μl) followed by 10 % (w/v) aluminum chloride (100 μl), 1 M potassium acetate (100 μl), and distilled water (2,800 μl). After 30 min incubation, the absorbance was recorded at 415 nm (Shimadzu UV-1800, Japan). The total flavonoids content was quantified according to the standard curve and was reported as milligrams of rutin equivalents per gram fresh weight.
Evaluation of antioxidant activity
DPPH radical scavenging activity
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging capacity was determined according to Thaipong et al. (2006). In its radical form, DPPH absorbs at 517 nm, but upon reduction with an antioxidant, its absorption decreases due to the formation of its nonradical form, DPPH-H. The ME, EE, and AE (200 μl) of the treated and control fruits was reacted with DPPH solution (2.8 ml) in the final reaction volume of 3 ml. The decrease in absorbance of resulting solution was then measured at 517 nm using spectrophotometer (UV 1800, Shimadzu, Japan) against methanol. The results were expressed as percent radical scavenging activity (%RSA) and were calculated using the following equation.
Where A = absorbance at 517 nm.
FRAP assay
The ferric reducing/antioxidant power (FRAP) assay was performed as described by Benzie and Strain (1996). Briefly, the working FRAP reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2, 4, 6-tripyridyl-s-triazine in 40 mM HCl and 20 mM FeCl3 · 6H2O in 10:1:1 ratio prior to use. The ME, EE, and AE (200 μl) of fruit were react with 3 ml of the FRAP reagent. The final reaction volume mixture was made up to 4 ml. After incubation (30 min), the absorbance was measured at 593 nm using (UV 1800, Shimadzu, Japan). The results were recorded as absorbance where higher absorbance indicates higher reducing power.
DMPD scavenging capacity
The N, N-dimethyl-p-phenylendiamine (DMPD) assay was performed according to the method described by Fogliano et al. (1999) with some modifications. Briefly, 100 mM DMPD solution was prepared by dissolving 209 mg DMPD in 10 ml distilled water. Of this solution, 1 ml was transferred to 100 ml 0.1 M acetate buffer (pH 5.2) followed by addition of 0.2 ml of a 0.05 M ferric chloride solution which resulted in the colored radical cation DMPD. The 200 μl of ME, EE, and AE were added to 2 ml of DMPD·+ solution and the total volume was adjusted to 3 ml. After 10 min, the absorbance was recorded at 505 nm by UV-visible spectrophotometer using buffer as blank. The DMPD·+ scavenging capacity was calculated by following equation (Jagtap et al. 2011).
Where, A 0 is the absorbance of the initial concentration of DMPD and A 1 is the absorbance of the remaining concentration of DMPD.
Soil and plant analysis
The total N (Kjeldahl method), P and K (flame photometry) content of the soil was determined. Similarly, soil and plant samples were subjected to wet digested in nitric acid/perchloric acid (3:1, v/v). After dilution, samples were filtered and analyzed for the presence of Cu, Fe, Ca, Mn, Mg, and Zn using atomic absorption spectrophotometer (Perkin-Elmer analyst, USA). All the analytical techniques were performed according to APHA (1998).
Results and discussion
Flowering, harvesting, and yield
The flowering in the RD-treated plant was observed after 249.6 days, SD of 256.2 days, and control of 274.3 days of plantation. The harvesting of bunch of plants treated as RD and SD was done on 27 August 2012. Whereas, the untreated plants harvesting was delayed by 28.3 days. The data regarding hands per bunch, fingers per bunch, and bunch weight of the treated and control plants has shown in Table 1.
Evaluation of the banana plant and fruit
Photosynthetic pigment
The chlorophylls are the most essential pigments of photosynthesis. The banana plants treated with the FDP as RD showed higher total chlorophyll content (1.435 ± 0.08 mg g−1 FW) followed by SD (1.213 ± 0.01 mg g−1 FW). The control plants showed less total chlorophyll content (0.896 ± 0.05 mg g−1 FW). The data is shown in Table 1.
According to Neals (1956), formation of chlorophyll molecule is dependent on nutrients like N, Mg, S, Ca, Fe, Mn, as well as Zn available to the plants. These metals were present in good amount in feather hydrolysate were applied to the plants as RD and SD may have enhanced the chlorophyll content. Previously, it is well stated that higher chlorophyll content is the indication of physiologically active healthy plants and those which are deprived in their nutritional condition showed poor growth and chlorosis (Zarco-Tejada et al. 2004).
Estimation of free proline
The results revealed highest proline accumulation in banana plants supplemented as SD 0.123 ± 0.002 mg g−1 FW, whereas the RD showed 0.111 ± 0.003 mg g−1 FW and control 0.078 ± 0.002 mg g−1 FW proline content. The results are represented in Table 1.
Previously, it has been reported that under stress conditions, many plant species accumulate proline as an adaptive response to the adverse conditions (Verbruggen and Hermans 2008). However, there are also some circumstantial evidences suggesting that proline accumulation may occur in physiological nonstressed conditions for the development purposes. The proline accumulation may play an important role in flowering and developmental both as a metabolite and as a signal molecule or in the increase demand of protein synthesis (Mattioli et al. 2009).
Evaluation of the reducing sugar
The reducing sugar contents of the treated and control fruit was evaluated. The substantial increase in reducing sugar content of ripe banana fruits was higher in RD-treated plants (6.752 ± 0.12 mg g−1 FW), followed by SD (6.261 ± 0.09 mg g−1 FW) and control (5.812 ± 0.11 mg g−1 FW). All the results are quoted in Table 1.
Banana fruit is a cost-effective energy source and used by endurance athletes because of the perception that they are a good source of energy. The elevated sugar content may be due to higher concentrations of the photosynthetic pigments which favored the synthesis of carbohydrates on application with FDP. During the ripening process, this starch were converted into sugars through enzymatic breakdown process which showed accumulation of reducing sugars as reported by Beaudry et al. (1989).
Peptides and amino acid as bioenhancer
The feathers are rich source of protein (90 %) which was solublized into peptides and amino acids using microbial keratinase. Banana plants, supplemented with this mixture showed growth promoting activity by increasing protein and amino acid content of the plant. It was found that RD was most effective for elevating the protein content of ripe fruit (16.13 ± 0.63 mg g−1 FW) and vegetative part (6.48 ± 0.19 mg g−1 FW). Furthermore, RD was also effective in enhancing the level of amino acids in fruit (2.96 ± 0.11 mg g−1 FW) and vegetative tissue (1.31 ± 0.02 mg g−1 FW). Similarly, the SD was also effective for enhancing the protein and amino acid content compared to the control plants. The result of the protein and amino acid contents are quoted in Table 2.
Currently, most of the hydrolyzed protein fertilizers available in the market are obtained by strong chemical hydrolytic process resulting in high contents of unwanted d-amino acids (Cavani et al. 2003). The biodegraded keratin waste contained amino acids which may have chelating action on micronutrients (Veselá and Friedrich 2009). According to Cao et al. (2012), peptide and amino acid mixture is the ideal fertilizer for growth and development of plants. Similarly, Endres and Mercier (2003) have also stated that plants can absorb and assimilate amino acids not only from the soil but also directly through leaves so we have also tried FDP as a foliar spray which showed interesting results. The synthetic growth regulators used in agriculture system have detrimental effects on living beings and environment (Abou Dahab and Abd El-Aziz 2006). Thus, it was clear that good characterisation of such organic residues is obliging to enhance the growth, particularly in terms of yield and nutritional quality of fruits is important in being able to understand and predict the value of FDP as biofertilizer.
Total phenolics and flavonoids content
Polyphenolics are broadly distributed in the plant kingdom and considered as the most potent natural antioxidants (Karaman et al. 2010). The banana plants supplemented with FDP showed increase in their content of total phenolics and flavonoids. Root dose was most effective in enhancing the phenolic contents in ME of fruit (1.072 ± 0.009 mg GAE g−1 FW) and vegetative part (2.343 ± 0.050 mg GAE g−1 FW). Similar trend was observed in total flavonoids for root dose; the ME of treated plants exhibited maximum readings, i.e., (0.612 ± 0.005 mg RE g−1 FW) and (1.910 ± 0.020 mg RE g−1 FW) in fruit and vegetative extract, respectively. The data regarding total phenolic and flavonoid contents of ME, EE, and AE of the treated and control plants has been noted in Table 2.
In plants, polyphenols are generally involved in defense mechanism against biotic and abiotic stress as well as contributing to plant colors. They are ubiquitous in all plant organs and therefore a vital part of the human diet including fruits which are considered as a supplementary dietary source of various antioxidant phytochemicals. The antioxidant activities of polyphenols may be related to their redox properties, which allow them to act as a reducing agents or hydrogen/electron donors, scavenge free radicals, and terminate radical chain reactions (Le et al. 2007). Antioxidants promotes possible beneficial roles in human health, such as reducing the risk of cancer, cardiovascular disease, may prevent or repair cell damage caused by reactive oxygen species (Tepe et al. 2006). Currently, the natural polyphenol antioxidant from plant sources has gained enormous attention as their protective effects against different degenerative diseases and also due to toxicity and carcinogenicity of the synthetic antioxidants. Previously it has been also reported that increase in the polyphenols in different crops amended with organic wastes, suggested that organic waste may cause change that favors the accumulation of antioxidants (McGrath et al. 1994). Likewise, the plants treated with the FDP enhanced the contents of the polyphenolic antioxidants. Ren et al. (2001) has also demonstrated that organically grown spinach contained 120 % higher antioxidant activity and Chinese cabbage showed about 20–50 % higher antioxidant activity compared to their conventionally grown plants.
Antioxidant potential of banana fruits
Antioxidants are defined as compounds that can delay, inhibit, or prevent the oxidation of enzymes, proteins, DNA, and lipids by scavenging free radicals and diminishing oxidative stress caused due to reactive oxygen and/or nitrogen species, superoxide anion, hydrogen peroxide, hydroxyl radical, and peroxynitrite (Ames et al. 1993). DPPH is a free radical assay often used to measure the radical scavenging activity of natural compounds. The DPPH radical scavenging activity (in percent) of different fruit extracts (ME, EE, and AE) of treated and control plants were evaluated (Fig. 1). The RD was most effective which showed higher %RSA in ME of fruit (26.47 %). On the other hand plants supplemented with SD also demonstrated %RSA in ME (22.85 %). The control plants showed less %RSA of ME (16.45 %).
FRAP assay is widely used to determine the efficiency of antioxidant compounds to compete with the FRAP reagent and reduce the ferric to ferrous. This assay is one of the most simple, rapid, inexpensive direct tests of total antioxidant power of a sample. Antioxidant compounds that are able to function in this approach are categorized as secondary antioxidants where they suppress the radical formation and prevent oxidative damage. The antioxidant capacity of ripe banana fruit using FRAP assay is shown in Fig. 2. The reducing power in ME of fruit was found to be higher in plants treated as RD (0.424 ± 0.001) followed by SD (0.386 ± 0.002). Control plants did not showed any significant results (ME is 0.334 ± 0.005).
The DMPD assay has some advantages like high stability end point, quick reaction time, cost effectiveness, and is less cumbersome. At acidic pH in presence of a suitable oxidant solution (FeCl3), DMPD can form a stable red radical cation (DMPD·+). Antioxidant compounds, which transfer a hydrogen atom to DMPD·+, quench the red color, and produce a bleaching of the solution proportional to their amounts (Fogliano et al. 1999). As shown in Fig. 3, it was demonstrated that DMPD radical scavenging activity (in percent) of the plant extract treated as RD showed higher level of scavenged DMPD·+ radicals in ME (68.96 %). Whereas, SD showed 63.40 % RSA in the methanolic extract. The control plants showed less RSA, i.e., 54.25 % in ME.
The highest antioxidant activity was observed in methanol revealed that methanol soluble factor was most likely responsible for reducing potential of the fruit extract. The difference in management of soil fertility affects the soil dynamics and plant metabolism, which may result in differences in plant composition (Martins et al. 2005). In fact, several authors reported a similar increase in the antioxidant potential of different crops amended with organic wastes, suggesting that organic matter causes changes that favor the accumulation of antioxidants (Martins et al. 2005). So it is well stated here that the FDP application was helpful for the enhancement of antioxidant potential of the fruit.
Macronutrients and micronutrients of soil and plants
Organic wastes are utilized in agriculture mainly for improving the soil physical and chemical properties and as well as nutrient source for growing crops. The potential of degraded chicken feather products as renewable source of fertilizer was investigated. It was found that feather hydrolysate showed presence of the macro (N, P, K, Ca, and Mg) and micro (Fe, Mn, Zn, and Cu) nutrients as reported in Table 3. The fertilizing with FDP in the form of RD may have increased both soil and plant mineral contents. The SD and control plants showed less metals accumulation in vegetative part and as well as in the soil.
Previous literature reported that feather waste by-product of poultry slaughter house can be directly used as slow-release nitrogen fertilizer for organic farming (Hadas and Kautsky 1994). Nevertheless, the disadvantage of this process was very low bioavailability of protein nitrogen to plants, because the feathers are not degraded by most common proteolytic enzymes. So we have exploited the potentiality of Chryseobacterium sp. RBT that can hydrolyse the chicken feathers into peptide and amino acids which played remarkable role as ideal crop bioenhancer. Beside the potent application as root dose, the plants also responded well for foliar fertilization which involves the application of a high nitrogen-content material to leaves at the time of active growing season, thereby inducing rapid vegetative growth and crop yield.
The growing poultry industries are producing about millions of tons of noxious organic solid by-products like feathers. Although chicken feather contains high nitrogen content, they cannot be used directly as fertilizer due to the non-available form of nitrogen (Górecki et al. 2006). Therefore, recycling of this burdensome feather wastes into renewable source of nutrients is the subject of interest among animal breeders (Bertsch and Coello 2005). So there was a need of appropriate treatment to make them bioavailable to crops as a source of the organic nutrients. The biodegradation of chicken feather was performed by Chryseobacterium sp. RBT within 30 h, and the degraded product contains soluble proteins (3.28 mg ml−1) and amino acids (4.83 mg ml−1).
Banana is the most important commercial fruit crop grown all over the tropical regions of world, hence was selected as model crop for dose application. This crop requires a substantial amount of fertilizers for commercial cultivation, which is costly and may be hazardous when used excessively. Thus, there was a need of substitute like feather hydrolysate rich in active biochemicals, organic nitrogen, as well as other important macro- and microelements as source of organic nutrients. Previously, Cao et al. (2012) has reported the foliar application of the feather degradation products to Chinese cabbage and observed only the morphological changes in plants. In the present study we have amended these degraded products in horticulture practices for fertigation and foliar spray and assessment of plant biochemicals related to the growth, nutritional quality of fruit and soil metals was performed.
To the best of our knowledge and literature survey, this is the first report highlighting field studies and nutritional quality enhancement using low-cost feather-based medium using biological method as a first step on the way to the industrial scaling strategy.
Conclusions
The present study highlighted a successful process for the application of feather degradation products as a source of organic nutrient to banana plants. These feather degradation products constituted a promising approach in sustaining soil properties and promoted the production of plant sources rich in health beneficial compounds like antioxidants. The results indicated that this method will play the dual role as a bioenhancer to reduce use of chemical addition to agriculture and to eliminate the poultry waste treatments or disposal problem. Such cost effective practices could reduce resistant waste and obtain a valuable product that can open several avenues for further research and large-scale commercial production.
References
Abou Dahab TAM, Abd El-Aziz NG (2006) Physiological effect of diphenylamin and tryptophan on the growth and chemical constituents of Philodendron erubescens plants. World J Agric Sci 75:75–81
Agrahari S, Wadhwa N (2010) Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at Ghazipur poultry processing plant. Int J Poult Sci 9:482–489
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90:7915–7922
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. Am Public Health Association, Washington, DC
Arnon D (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Beaudry RM, Ray FS, Clanton CB, Stanley JK (1989) Banana ripening: implications of changes in glycolytic intermediate concentration, glycolytic and gluconeogenic carbon flux and fructose 2,6-bisphosphate concentration. Plant Physiol 91:1436–1444
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Bertsch A, Coello N (2005) A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour Technol 96:1703–1708
Cao Z, Lu D, Luo L, Deng Y, Bian Y, Zhang X, Zhou M (2012) Composition analysis and application of degradation products of whole feathers through a large scale of fermentation. Environ Sci Pollut Res. doi:10.1007/s11356-012-0763-x
Cavani L, Ciavatta C, Gessa C (2003) Determination of free l and d-alanine in hydrolysed protein fertilizers by capillary electrophoresis. J Chromatogr A 985:463–469
Chang C, Yang M, Wen H, Chen J (2002) Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Coward-Kelly G, Chang VS, Agbogbo FK, Holtzapple MT (2006) Lime treatment of keratinous materials for the generation of highly digestible animal feed: 1 Chicken feathers. Bioresour Technol 97:1337–1343
Endres L, Mercier H (2003) Amino acid uptake and profile in bromeliads with different habits cultivated in vitro. Plant Physiol Biochem 41:181–187
Fogliano V, Verde V, Randazzo G, Rittieni A (1999) Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem 47:1035–1040
Górecki H, Górecka H, Chojnacka K (2006) The technological applicability of renewable phosphorus sources. Przem Chem 85:814–818
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70:21–33
Gurav RG, Jadhav JP (2012) Biodegradation of keratinous waste by Chryseobacterium sp. RBT isolated from soil contaminated with poultry waste. J Basic Microbiol 52:1–8
Hadas A, Kautsky L (1994) Feather meal, a semi-slow-release nitrogen fertilizer for organic farming. Nutr Cycl Agroecosys 38:165–170
Jagtap UB, Waghmare SR, Lokhande VH, Suprasanna P, Bapat VA (2011) Preparation and evaluation of antioxidant capacity of Jackfruit (Artocarpus heterophyllus Lam.) wine and its protective role against radiation induced DNA damage. Ind Crop Prod 34:1595–1601
Karaman S, Tutem E, Baskan KS, Apak R (2010) Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chem 120:1201–1209
Le K, Chiu F, Ng K (2007) Identification and quantification of antioxidants in Fructus lycii. Food Chem 105:353–363
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Martins M, Ordway D, Kristiansen M, Viveiros M, Leandro C, Molnar J, Amaral L (2005) Inhibition of the Carpobrotus edulis methanol extract on the growth of phagocytosed multidrug-resistant Mycobacterium tuberculosis and methicillin resistant Staphylococcus aureus. Fitoterapia 76:96–99
Mattioli R, Costantino P, Trovato M (2009) Proline accumulation in plants: not only stress. Plant Signal Behav 11:1016–1018
McGrath SP, Chang AC, Page AL, Witter E (1994) Land application of sewage sludge: scientific perspectives of heavy metal loading limits in Europe and the United States. Environ Rev 2:108–118
Miller GL (1959) Dinitrosalicyclic acid reagent for determination of reducing sugar. J Anal Chem 3:426–428
Moore S, Stein W (1957) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913
Neals TF (1956) Components of total magnesium content within the leaves of white clover and perennial rye grass. Nature 177:388–389
Papadopoulos MC (1985) Processed chicken feathers as feedstuff for poultry and swine. A review. Agric Wastes 14:275–290
Ren H, Bao H, Endo H, Hayashi T (2001) Antioxidative and antimicrobial activities and flavonoid contents of organically cultivated vegetables. Nippon Shokuhin Kagaku Kogaku Kaishi 48:246–252
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Tepe B, Sokmen M, Akpulat H, Sokmen A (2006) Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem 95:200–204
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH (2006) Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal 19:669–675
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Vernieri P, Borghesi E, Tognoni F, Serra G, Ferrante A, Piagessi A (2006) Use of biostimulants for reducing nutrient solution concentration in floating system. Acta Hort 718:477–484
Veselá M, Friedrich J (2009) Amino acid and soluble protein cocktail from waste keratin hydrolysed by a fungal keratinase of Paecilomyces marquandii. Biotechnol Bioproc Eng 14:84–90
Zarco-Tejada PJ, Miller JR, Morales A, Berjón A, Agüera J (2004) Hyperspectral indices and model simulation for chlorophyll estimation in open canopy tree crops. Remote Sens Environ 9:463–476
Acknowledgments
Mr. R.G. Gurav wishes to express gratitude to the Council of Scientific and Industrial Research (CSIR), New Delhi, India for Senior Research Fellowship (CSIR-SRF) and Dr. (Mrs) J.P. Jadhav is grateful to the Department of Biotechnology (DBT), New Delhi, India for Interdisciplinary Programme on Life Science for Advanced Research and Education (IPLS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Gurav, R.G., Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ Sci Pollut Res 20, 4532–4539 (2013). https://doi.org/10.1007/s11356-012-1405-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1405-z