Abstract
Catalytic pyrolysis is an emerging process that can help eliminate the harmful effects of plastic wastes by turning them into liquid fuels. This chapter presents an overview of low-cost catalytic processing of plastic wastes with a focus on biomass-derived activated carbons (BACs) as low-cost catalysts. BACs are cost-effective, environmentally friendly and exhibit high porosity, flexibility of surface modification, and heteroatom surface functional groups, making them versatile as catalysts. Types of biomass, chemical reagents used for activation, reagent to biomass ratio and activation temperature influence the catalytic properties of BACs. Excessive reagent to biomass ratio leads to a high number of acid sites on the BAC that enhance cracking reactions and decrease liquid yield. Extreme activation temperature promotes degradation and volatilization of acid functional groups and thus, reduces catalytic activity. Overall, enhanced aromatization, hydrogen transfer and cracking reactions have been observed over biomass-derived BACs that exhibit strong acidity, large surface area, and large total pore volume. Coprocessing of plastic wastes with lignocellulosic biomass is a good option for reducing the activation energy of plastic waste decomposition and improving the composition of liquid fuels.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
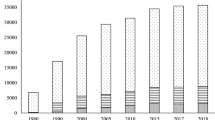
According to the World Bank, 2.01 billion tons of solid wastes including 242 million tons of plastic wastes were generated in 2016, of which more 33% were not properly managed, are responsible for 1.6 billion tons of CO2-equivalent greenhouse gas emissions , which is about 5 percent of global emissions [1]. Table 3.1 presents statistics about wastes generated around the world. It can be seen that large amounts of wastes are released in the world, and significant growth in generation is expected given the increasing population and industrial development. Statistics of each type of waste vary according to region due to different kinds of activities and regulation policies. In all regions presented, the wastes are disposed of mainly by open dumping and landfill , which could explain the higher greenhouse gas emissions associated with these wastes. In most regions, plastic wastes account for about 12% of all solid wastes-nevertheless, plastics contributing enormously to our daily activities. Durability, flexibility, strength, lightness, ability to be molded into different shapes, and endurance (thermal, electrical and chemical) are some physicochemical characteristics that make plastics attractive [2]. In 2015, polyolefins accounted for about 55% of global plastics materials demand, namely, 23% for Polypropylene (PP), 15% for high-density polyethylene (HDPE ) and 17% for low-density polyethylene (LDPE) and linear low-density polyethylene (LLDPE), followed by 16% for polyvinyl chloride (PVC ), 6% for polyurethane (PUR), 7% for polyethylene terephthalate (PET ) and 7% for polystyrene (PS) and expanded polystyrene (EPS ) [3]. The share of a total loss to the environment per year for PE , PP , PVC, PET, PS , PUR are estimated to be 20%, 14%, 3%, 6%, 4%, and 1%, respectively [4], which shows that the most abundant plastic materials in demand are among the most abundant fractions of plastic wastes ending up in the environment . In India, plastic wastes (daily generation of approximately 26,000 tons) accounts for 8% of the total solid waste annually generated, with more than 50% of these plastic wastes not recycled, and thus escaping into the environment [5]. In 2017, more than 70 million tons of plastic wastes were reported for China [6]. Hence, these plastic wastes required proper management.
Synthetic organic polymers in most plastic wastes are generally non-biodegradable. The large proportion of monomers used to produce plastics, such as ethylene and propylene, are made from fossil hydrocarbons. Thus, disposal of plastic wastes in landfills not only creates significant environmental issues, such as soil leaching and contamination of groundwater but also constitutes a major waste of fossil fuel resources. Although incineration can help to reduce the amount of plastic waste, costly treatment of large amounts of flue gases is required [7]. Although direct recycling processes are being developed, they are limited, in contrast to single component plastic waste. Real-world plastic waste is a mixture of many components, including PVC, PE T, PE , PP , PS , and other types of waste that can be difficult to separate. Most of these components are not compatible with each other for processing together during direct recycling. They vary in polymer type, intermolecular bonding, and added inorganic fillers, stabilizers, and pigments that affect their mechanical properties [8]. Another reason is that they are made of different resin compounds and have different degrees of transparency and colors [9]. Subsequently, a significant fraction of plastic wastes collected for recycling cannot be processed, which is estimated to be 40% for post-consumer plastic wastes collected in the European Union in 2012 [10]. Given these limitations, new technologies that can turn plastic wastes into valuables resources in an optimized way are needed. These technologies would eliminate not only the harmful effects of plastic wastes but also create an opportunity to recover resources such as fuels and chemicals. Catalytic pyrolysis is one of these emerging technologies that can turn plastic wastes into high-quality liquid fuels in an environmentally friendly way, and that can help to alleviate energy shortages that the world is facing. However, widely used catalysts are expensive, and their deactivation readily occurs with waste plastics. Therefore, recent works are exploring new ways of low-cost catalysts design, including biomass-derived activated carbon (BAC).
This chapter aims to highlight the positive impact that can provide low-cost catalysts such as BAC and some industrial byproducts in the pyrolysis processing of plastic wastes for liquid fuels production and to provide areas of future research for further development. The advantages of the co-pyrolysis of plastic wastes with lignocellulosic biomass and sewage sludge are briefly introduced to illustrate challenges in the pyrolysis of real-world plastic wastes.
2 Pyrolysis Processing of Plastic Wastes: Why and How?
2.1 Motivation Behind Pyrolysis Processing of Waste Plastics
Results of elemental and proximate analysis and higher heating value (HHV) of different types of plastic wastes are presented in Table 3.2. In fact, plastic wastes contain high-value chemicals and high energy density [11], with an HHV of (15–49) MJ/kg (Table 3.2). Ash is composed of inorganic matter that comes from materials employed for plastics manufacturing. Halogens in plastics include Cl, Br, and F, while the most important metals include Pb, Al, Sb, Ti, Sn, Zn, Fe, Ni, Cu, Cd, Cr, and Co. Sulfur and alkaline earth metal compounds such as Ca, Ba, Mg, are also present. Typical concentration ranges of these elements in each type of plastic are summarized in Ref. [12]. PE , PP , and PS have the highest volatile matter content and HHV, with almost no ash and fixed carbon, while PET has high carbon content but low hydrogen content [13]. Given the properties of plastic wastes and that large proportions of monomers for plastics manufacturing are made from fossil hydrocarbons, pyrolysis is a suitable method to recover fossil hydrocarbons from plastic wastes. A comparison of typical recycling and pyrolysis of plastic wastes is presented in Fig. 3.1. Pyrolysis processes can significantly increase the recycling rate as it can use a wider range of plastic wastes than traditional recycling [14]. Although pyrolysis is a mature technology for char generation from solid material [15, 16], it has been recently used extensively to produce liquid fuels (Fig. 3.2). As depicted in Fig. 3.2, the liquid oil obtained can be used as transportation fuels, burned to generate heat/electricity or used to synthesize value-added chemicals that can be used as fertilizers, resins, and light aromatics such as benzene, xylene, toluene and dl-limonene. These chemicals can also be obtained directly from the pyrolysis process, depending on chosen conditions, the catalyst employed, and feedstock type. By-products, including gaseous and solid char products, can be used in many fields, as showed in Fig. 3.2. During pyrolysis, the ash is melted, and inorganic compounds such as glass and heavy metals are mainly fixed in the solid product. They can be recovered as carbon black and reused for several applications such as additives or fillers for other plastics.
2.2 Overview of Pyrolysis Processing of Plastic Wastes
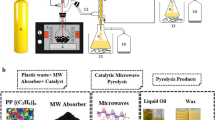
The pyrolysis process refers to organic matter decomposition in an oxygen-free environment . The main factors impacting the distribution and properties of the resulting liquid product include reactor type, process temperature, residence time, heating rate , pressure, turbulence, and feedstock characteristics. Ranges of values and combined factors that are commonly reported are presented in Table 3.3. Although many works have reported on the influence of these factors on the products, less information is available on the effect of turbulent flow conditions. Lower temperatures (T < 700 °C) yield solids and waxes/oil, while higher temperatures favor light molecular weight hydrocarbons and non-condensable gases . The effect of pressure is dominant at lower temperatures and reduces with increasing temperature. Char formation is predominant at a lower heating rate, while a higher heating rate enhances the bond cleavage. Computational models reveal that high turbulence promotes heat and mass transfer in the core flow, leading to a uniform distribution reaction [22]. Therefore, medium turbulence would promote liquid oil yield , while higher turbulence would result in extensive destruction of the tar [23]. Further investigations are needed to confirm reported observations. The low thermal conductivity and melting temperatures of plastics make the design of reactors very important, mainly from the viewpoint of heat and mass transfer [24]. TGA , TG-MS, TG-FTIR, and Py-GCMS studies can help to confirm the degradation ranges of plastics and the product trends and further set the pyrolytic conditions for macro-scale operations [25, 26]. Although batch reactors can provide information in terms of yields and quality on a lab scale, they are limited on an industrial scale given the relatively high operation cost associated with feedstock loading, product discharge and heat loss between different batches [24]. Reactors that can be adapted to continuous operation mode are more promising than batch mode reactors. Reactors commonly reported for plastics pyrolysis include bubbling fluidized bed, conical spouted bed, vacuum, stirred tank and screw/rotary kiln reactors [24, 27,28,29]. These reactors can provide high heat and mass transfer and sufficient solid mixing regimes. Although microwave pyrolysis is an attractive way for providing efficient heat transfer via core volumetric internal heating, this method has some disadvantages such as poor mixing, uncertain scale-up factors and requirements for mixing plastics with heat adsorbent materials such as graphitic carbon or inorganic oxides [25, 29]. However, a continuous microwave-assisted pyrolysis system has been proposed that combines microwave heating with a mixed SiC ball-bed as a promising system for energy recovery from plastic waste pyrolysis on an industrial scale [24]. For PVC, cascade reactors with two steps (a low-temperature step for dechlorination and a higher temperature step for degradation) are convenient [27].
Besides the above studies, many research works focus on the use of catalysts that can improve the composition and yield of the liquid oil. Over a high acidic catalyst and well-selected reaction conditions, long-chain polymers of plastic wastes can be easily degraded into smaller chain molecules via random chain scission, thereby improving oil quality. The mixing of plastic waste with other feedstocks is also another option that is being examined to promote liquid oil yield and to control the oil liquid composition .
3 Progress in Catalytic Pyrolysis of Plastic Wastes
3.1 Catalytic Pyrolysis Mechanism
Most plastic waste pyrolysis plants use high temperatures (700 °C) to moderate temperatures (500 °C) in the presence of a catalyst [2]. The use of catalysts can alter reaction kinetics during pyrolysis, helping to improve the properties of the pyrolysis oil. Many types of catalysts have been examined for plastic wastes pyrolysis. Metals impregnated fluid-cracking catalysts and acid silica-alumina and zeolites catalysts exhibit enhanced selectivity for aromatics and alkenes, while others such as MCM-41 and Al-MCM-41 promote yield and content of aliphatic compounds in the oil [34,35,36,37]. Further improved performance has been achieved in staged catalysis combining MCM-41 and ZSM-5 [34]. These catalysts promote carbocationic cracking of volatiles, and subsequent isomerization, cyclisation, oligomerization, aromatization and hydrogen transfer reactions [34, 35] to help achieve high quality and high yield oil at relatively low temperatures compared with non-catalytic pyrolysis. Acid strength, high specific surface area, and porosity of a catalyst are decisive factors for achieving high catalytic performance [35]. The high acidity and microporous structure of zeolites catalysts are responsible for forming lighter hydrocarbons [17, 38]. In fact , the microporous structure limits the range of higher molecular weight hydrocarbons that can enter the pores of the catalyst for the reaction [34]. Some recent papers have extensively discussed these catalysts [39, 40].
3.2 In-Situ and Ex-Situ Catalytic Pyrolysis
As shown in Fig. 3.3, catalytic pyrolysis of plastic wastes can be implemented as an in-situ or ex-situ process. For the in-situ process, the catalyst is mixed into the feedstock, and the mixture is pyrolyzed. The ex-situ process includes two main steps. In the first step, the plastic waste is separately pyrolyzed, which causes thermal degradation to produce relatively short chains radicals that undergo H shift and rearrangement reactions during cooling down to room temperature to form stable short polymers [35]. In the second step, products from thermal degradation are conveyed to a catalytic bed for upgrading into fuel compounds. Although the in-situ process is simple and does not require mechanical modification to existing reactors as well as offering low degradation temperatures, it is difficult to recover the catalyst from its mixture with the biochar . Also, high ash or metal elements contents in the plastic waste feedstock can promote catalyst deactivation [41]. The ex-situ catalytic pyrolysis requires an external catalytic bed and moderately high temperatures. However, ex-situ catalytic pyrolysis is a good option when processing high ash content plastic wastes as it favors the cracking reactions of pyrolytic volatiles and facilitates subsequent separation between catalyst and pyrolysis solid residues [35].
3.3 Limits of Widely Used Catalysts and New Concepts About Catalyst Design for Waste Plastics Pyrolysis
Despite the appreciable results with catalysts shown in Sect. 3.3.1, most of those approaches are expensive. Quick deactivation induced by coke formation and limited adaptability to feedstocks severely retard performance of many catalysts [36, 38]. Therefore, it still a challenge to develop cost-efficient catalysts. In recent years, activated carbons have received increased interest as catalysts [36, 42], due to their relatively low production cost, high porosity structure , and surface modification flexibility [36]. Enhanced aromatization, hydrogen transfer and cracking reactions can be observed over an activated carbon that exhibits at the same time strong acidity , large Brunauer-Emmett-Teller (BET ) surface area, large total pore volume, and low percentage of micropores [35].
3.4 Biomass-Derived activated Carbons as Catalysts for Plastic Waste Pyrolysis
3.4.1 Preparation of Biomass-Derived Activated Carbons
As a material with a well-developed amorphous and porous texture, activated carbon is commonly used as an adsorbent in industries [43, 44]. These porous carbon materials constitute a large part of the support materials that are used to prepare heterogeneous catalysts [45]. Their inert nature, especially under strongly acidic and basic conditions, is an advantage as they do not decompose or only decompose very slowly, and given that the interaction between carrier and active phase such as noble metals is small and that their pore size distribution and the chemical properties of their surface can be adjusted (polarity and hydrophobicity) according to the intended application, the possibility to recover the metal particles by simply burning the carbon support, are some of the motivations behind their application as catalyst supports. However, commercial activated carbons, having a small surface area and poor adsorption properties, cannot provide good performance in the aforementioned applications [46]. Thus, authors have explored many kinds of biomass feedstocks, including coal , wood, and agriculture wastes, to produce BACs for catalyst or catalyst support applications [45]. Besides being cost-effective and environmentally friendly, BACs are characterized by high porosity and heteroatom surface functional groups, making them suitable as catalysts or as catalyst supports [47].
As depicted in Fig. 3.4, activation of biomass for BAC preparation is commonly performed by physical or chemical activation or a combination of the two methods [48]. In physical activation, biomass is subjected to carbonization, and the obtained char is activated at high temperatures in the second stage with steam, air or CO2. In chemical activation, carbonization and activation processes occur in a single step and at relatively lower temperatures making the process faster [48, 49]. In the third method that can be used for activation, the biomass feedstock is subjected to carbonization, and the resulting biochar is impregnated with a chemical reagent for activation. This can be qualified as a mixture of physical and chemical activation processes and is usually applied to biochar obtained from thermochemical treatment of biomass where other products are expected besides the biochar .
The BAC produced via chemical activation usually has a high specific surface area, good pore development, and the method gives a high carbon yield compared with that of physical activation [48, 49]. In fact, in chemical activation methods, suitable modifications are made by the addition of chemical dehydrating reagents to the biomass and the entire feedstock is then decomposed in an inert atmosphere [49, 50]. To date , the chemical method is widely employed for BAC preparation for plastic waste pyrolysis. To achieve good quality BAC (textural and surface properties), careful attention is required to a selection of the type of biomass and to the activation conditions.
Biomass used for BAC should contain high carbon and low inorganic (ash) content [43, 50,51,52], and it should be cheap, abundant, and able to be easily activated. A wide variety of BAC with different characteristics can be obtained from low-cost biomass, including cherry stones [51], nutshells and fruit stones [50], bamboo, wheat straw, corn cobs, almond shells, sewage sludge, sugar cane bagasse, grape processing industry waste, date stones, and coconut shells [49]. Differences in cellulose, lignin and holocellulose content in the biomass impact the pore structure and pore size distribution of the resulting BAC [43]. Biomass with a low-density and a high volatile matter content can promote pore volume but decrease bulk density [50]. Biomass with high bulk density , such as coconut shells or fruit stones, provides a non-graphitizable activated carbon in granular form with a large pore volume and can be used in many applications such as catalysts or catalyst support or materials for supercapacitor electrodes. As shown in Table 3.4, at appropriate activation conditions, corn cob, chestnut shell and wood chips provide BACs that exhibit good catalytic properties on liquid oil obtained from pyrolysis of plastic wastes.
Activation with H3PO4 is commonly used for lignocellulosic material and at lower temperatures [55]. Other reagents, such as zinc chloride (ZnCl2), potassium hydroxide (KOH) and iron chloride (FeCl3) have been examined with promising results [44]. For each type of biomass, there is an optimal value of activation agent (H3PO4) to biomass ratio to keep for activation. At a very low ratio , biomass is hardly destroyed, and pores are barely formed. Increasing the H3PO4 to biomass ratio below the optimal value enhances BET surface area, micropore volume and acidity of the resulting BAC. In contrast, at a ratio higher than the optimal value, micropores react with excessive H3PO4 to convert into mesopores and macropores [56], or some compounds such as phosphorus pentoxide are formed and block the pore structure , decreasing the area and volume of micropores [54]. Especially for liquid oil production, a very high number of acid sites on the BAC promotes C-C random scission and cracking and rearrangement reactions, which would decrease the liquid yield .
Activation temperature is responsible for the carbonization of the biomass and is an important factor in the production of BAC. Carbonization leads to a decrease in volatile matter content of the biomass and increases elemental carbon content and the formation of pores [49]. Compared with conventional heating, microwave heating offers advantages such as uniform heating [46], rapid heating, negligible energy loss and an easily controllable heating process [52]. Increasing temperature enhances the devolatilization process, destroying BAC’s surface to a different extent with more pores that can enlarge BET surface area resulting in more active sites. Above the optimal temperature (e.g., 850 °C for chestnut shell [54]; 500 °C for corncob [56]), increasing temperature enhances the reaction rate of C-H3PO4 and breaks up some acid functional groups that are volatilized.
3.4.2 Pyrolysis of Plastic Wastes Over Biomass-Derived Activated Carbon Catalysts
The use of BACs as catalysts is attracting attention in the processing of plastic wastes. Good results have been pointed out by authors, making this way of catalyst design a promising way for enhanced recycling of plastics wastes. The significant catalytic effect of these alternatives low-cost catalysts is mainly attributed to the conditions of activation and carbonization. From Table 3.4, one can see that at some given conditions, BAC exhibits good quality and can promote yield and improved properties of the liquid oil. Wan et al. [53] examined LDPE pyrolysis in a fixed bed reactor at 500 °C over corncob BACs (activation with H3PO4). Without a catalyst , the liquid produced by pyrolysis of LDPE (86.7% yield ) turned into solid white wax at room temperature. BACs prepared through different temperatures (400 °C to 700 °C) and H3PO4 to corncob ratios (0.2–1.6) had different pore size distributions and acidities, corresponding to changes in their product distributions. At a catalyst to LDPE ratio of 1, the BAC prepared at 500 °C and H3PO4 to corncob ratio of 0.8 provided the highest liquid yield with 93.13 area % of jet fuel-range hydrocarbons. In comparison , BACs obtained at 400 °C and 600 °C with H3PO4 to corncob ratio of 0.8 produced a liquid yield of 60% (highest alkane content, 48.2 area %) and 49% (highest aromatic content, 59.2 area %), respectively. Overall, BACs prepared at 500 °C and ratio of 0.8 were found suitable, which can be attributed to the relative stronger acidity (0.4422 mmol/g) of the material . By varying the catalyst to LDPE ratio from 0.3 to 1.5, liquid yield gradually decreased from 82% to 42%. The content of C8-C23 alkanes in the liquid also decreased (C17-C23 completely absent at a ratio of 1.5). In contrast, the aromatic content gradually increased to reach a maximum of 72.8 area % at a ratio of 1.5. These results imply that a higher catalyst to LDPE ratio increased the number of acid sites, which enhances aromatic cyclization, and scission reactions converting long-chain hydrocarbons into lighter ones. An excessive amount of acid sites decreases liquid yield at the expense of increasing the amount of gaseous product . Duan et al. [54] pyrolyzed LDPE at 550 °C over chestnut shell BAC (H3PO4/ chestnut shell ratio of 0.8) at a catalyst to LPDE ratio of 1. The authors observed that when using BACs activated at 550 °C and 650 °C, the obtained liquid oils remained in the solid phase at room temperature. For BACs at high activation temperatures (750 °C to 950 °C), the BET surface area and total volume of the BAC were enhanced, promoting the catalytic reaction of volatiles and liquid yields decreased from 55.5 (thermal process) to 38%, while quality was improved. The best results were found for BACs activated at 850 °C, which led to 44% of the liquid with 63.5 and 32.5 area % of mono-aromatics and polyaromatics, respectively. It was also found that the liquid yield decreased for BACs obtained with increasing H3PO4/ chestnut shell ratio from 0.4 to 1 (catalytic pyrolysis at 550 °C and catalyst to LPDE ratio of 1). The mono-aromatics and polyaromatics content in the liquid increased gradually for ratios from 0.4 to 0.8 and decreased thereafter. Zhang et al. [35] investigated the catalytic effect of corn stover BAC (H3PO4 to corn stover ratio of 0.85) for pyrolysis of LDPE in a fixed bed at 500 °C. At a catalyst to LDPE ratio of 2.5, the prepared BACs produced more content of aromatic hydrocarbons (<C16) and C17-C23 alkanes, and lower contents of C8-C16 alkanes, in comparison to five commercial activated carbons, except one that was also activated with H3PO4 and produced high aromatic hydrocarbon content (36.4 area %). The selectivity of alkanes and aromatic hydrocarbons in the liquid obtained when the prepared BACs were used accounted for 48.0 and 28.7 area %, respectively.
These results show that biomass-derived AC rich in P-containing functional groups are favorable for aromatization reactions, and moreover, P-containing functional groups can also provide effective catalytic acid sites (such as -C=O and -PO) that could promote the C-C and C-H bond cracking of LDPE , resulting in the production of relatively light alkanes. For each type of AC, there is an optimal catalyst to plastic wastes ratio . Ratios lower than this optimal value promote catalytic activity that favors liquid product quality, while at ratios higher than this optimal value, a very high number of acid sites are offered, which accelerates C-C random scission and cracking and rearrangement reactions [54], which increases the content of aliphatics while decreasing aromatics.
The desire to achieve a more eco-efficient process has lead researchers to investigate the mixing of alternative BAC catalysts with other low-cost catalysts. In this regard, Huo et al. [42] explored ex-situ catalytic pyrolysis of LDPE over a mixture of corncob BAC (activation with H3PO4 at 600 °C) and MgO. The placement of the catalysts and catalyst to LDPE ratio (0.1, 1, 2, and 3) were examined at 500 °C. Although homogeneous mixing of these catalysts provided a high liquid yield (81%), the liquid was, unfortunately, mainly composed of waxes. The base catalyst MgO blocks the pore structure of the acidic BAC, inhibiting its catalytic effect for the pyrolysis process. When pyrolytic volatiles first flowed through MgO and then BAC, the liquid yield was 72%, and almost 100% of the liquid belonged to fuel hydrocarbons with the selectivity of aromatic hydrocarbons (<C16), and C8-C16 and C17–C23 alkanes accounting for (33.4, 65.3, and 1.3) area %, respectively. The large average pore size of MgO favors long-chain hydrocarbons, which further undergoes cracking and aromatization over-acidic sites of BAC to produce C8-C16 alkanes along with aromatic hydrocarbons(<C16). In contrast, when pyrolytic volatiles first flowed through AC and then MgO, the selectivity of aromatic hydrocarbons (<C16), and C8-C16 and C17–C23 alkanes were (18.1, 58.9 and 20.8) area %, respectively, which suggests that the remaining long-chain hydrocarbons from the reaction over AC flowed through the large pore of MgO to form diesel range alkanes. The authors also noticed that at low catalyst to LDPE ratios (0, 0.5, and 1) for AC to MgO ratio =1, waxes were the main product . With increasing catalyst to LDPE ratio from 2 to 3, no waxes were formed, but the liquid yields decreased. The content of alkanes decreased from 66.6 to 61.2 area %, while the content of aromatic hydrocarbons (<C16) increased from 33.4 to 38.8 area %. The selectivity of mono-ring aromatic hydrocarbons increased to reach a maximum of 29.3 area % at a ratio of 3, which can be attributed to an increase in acidic sites according to the amount of catalyst that promotes cleavage of C-C bonds.
BAC activated with ZnCl2 has also been tested for pyrolysis of plastic wastes. Sun et al. [36] conducted catalytic pyrolysis of mixed plastic wastes over wood chip BAC (activated with ZnCl2 at 600 °C, ZnCl2/ wood chip ratio of 1). The non-catalytic process yielded 61.6% of the liquid with the high alkenes (40.9 area %), while alkanes and aromatics accounted for 23.4 and 35.1 area %, respectively. The use of BAC reduced the liquid yield to 51.8% and its alkenes, while the selectivity of alkanes and aromatics were improved to 27 and 47.5 area %, respectively. The selectivity of two-ring aromatics was enhanced to 90.7 area % of aromatics, with 1,3-diphenylpropane occupying the highest area (40.9%). Zn species introduced on the BAC during activation promotes the formation of Lewis acid sites , which enhances the transformation of alkenes into aromatic and alkanes through dehydrogenation, hydrogen transfer, alkylation and Diels–Alder reactions.
3.5 Pyrolysis of Waste Plastics with Other Low-Cost Material Catalysts
Another means to increase the efficiency and the rate of waste recycling is to make all kinds of waste profitable. In this regard, some industrial and municipal wastes are being employed as catalyst or co-feedstock in the pyrolysis processing of waste plastics. López et al. [57] used red mud, which is an inexpensive by-product of the alumina industry, as a catalyst to pyrolyze a mixture of plastic wastes with mass fractions of 40% PE , 35% PP , 18% PS , 4% PET and 3% PET. The red mud was mostly composed of metal oxides (Fe2O3 (36.5%), Al2O3 (23.8%), TiO2 (13.5%), SiO2 (8.5%), CaO (5.3%), Na2O (1.8%)). The catalytic effect of red mud was significant at 500 °C, while no positive contribution was observed at 440 °C. At 500 °C, red mud catalyst provided more liquid yield (57%) than ZSM-5 (39.8 wt.%), while the non-catalytic process provided 65.2%. The selectivity of aromatic compounds in the liquid obtained at 500 °C with red mud was 89.6 area %, higher than that obtained from the non-catalytic run (73.9 area %) and lower than that from ZSM-5 (98.4 area %). The range of the liquid carbons from the red mud catalytic run was the same as the zeolite (C7–C16), while the non-catalytic run liquid reached C19 compounds. The Fe2O3 and TiO2 present in red mud promoted hydrogenation of styrene to produce ethyl-benzene, while the acid nature of Al2O3 and SiO2 promoted cracking reactions of styrene to form toluene. Na2O may hinder the catalytic activity of red mud. Overall, the cracking ability of red mud is lower than that of the zeolite . Fekhar et al. [27] also mentioned a significant reduction of the concentration of chlorinated compounds and the acid number at higher ratios of red mud:Ca(OH)2:Ni/ZSM-5 (0.5:0.25:0.25 and 0.25: 05:0.25) mixed catalyst , after having pyrolyzed a mixture of HDPE , LDPE , and PVC at 550 °C. These ratios promoted light oil and HHV while decreasing heavy oil content. A slight improvement in the liquid oil properties was reported by Luo et al. [17], who pyrolyzed a reworked PP at 600 °C over HCl-modified low-cost kaolin as a catalyst . Compared to the non-catalytic process and the use of non-modified kaolin, the HCl-modified kaolin improved the cracking of heavy components into diesel range components (alkanes and alkenes). HCl modification enhances the catalytic effect via the increase of the number of Al-O and Si-O bonds on the kaolin, which not only promotes the decomposition of PP through b-scission reaction and carbonium ion mechanisms. But also enhances secondary cracking of diesel components and aromatization and Diels-Alder reaction of alkanes and alkenes. The liquid oil yields for this case decreased, while aromaticity increased with high naphthalene content. A significant increase was observed for a fraction of C6-C11 compounds, with reduced content of straight alkenes and cycloparaffins being obtained.
4 Co-pyrolysis Processing of Plastic Waste with Lignocellulosic Biomass and Sewage Sludge
Many works report on the co-pyrolysis of plastic wastes and biomass as a promising way to improve the properties of the oil from biomass pyrolysis, with synergetic effects being discussed in several reviews [26, 39, 40, 58]. In fact, hydrocarbons from the hydrogen-rich plastics provide hydrogen for biomass-derived oxygenates, which may decrease coke formation from dehydration and other deoxygenation reactions of hydrogen deficient oxygenates [59]. Notable findings have been reported for improving plastic wastes conversion . Through thermogravimetric analysis (TGA), Salvilla et al. [60] observed a significantly reduced activation energy of decomposition of waste PP , LDPE and HDPE when pyrolyzed with wood and corn stover in the temperature range of (30 to 850) °C. Similarly, after examination of the co-pyrolysis of bamboo sawdust (BSD) and LLDPE using TGA at (30 to 900) °C, Alam et al. [61] reported average apparent activation energies (isoconversional method) of pure BSD, LLDPE and their mixtures at LLDPE :BSD ratios of 3 to be 294 kJ/mol, 204 kJ/mol, and 188 kJ/mol, respectively. It is believed that hydroxyl groups resulting from the degradation of cellulose from lignocellulosic biomass react with vinyl groups from polyolefin bond cleavage to produce alcohols, while furan and its derivatives combine with unsaturated hydrocarbons to produce aromatic hydrocarbons through dehydration and Diels-Alder reactions [58]. During co-pyrolysis of PVC with biomass, the degradation of PVC will generate HCl as an intermediate that can act as an acid catalyst to accelerate cleavage of intra-ring in glycosidic units promoting dehydration, remove -COOH from carbohydrates, promote depolymerization, and thereby providing high liquid yields with reduced oxygenated compounds [62]. The presence of solid-solid and solid-gas interactions affects synergism, as the biochar formed during the co-pyrolysis process is able to act as a catalyst [63].
The co-pyrolysis of sewage sludge and waste LDPE through TGA was investigated [63], where the authors observed significant synergetic effects for co-pyrolysis and found mixture ratios of 1:1 to be optimal, as observed by lower activation energy and lower char formation. The activation energies for sewage sludge, LDPE and mixture , were 30.01 kJ/mol, 187.40 kJ/mol and 37.2 kJ/mol, respectively. In this work, the active pyrolysis zone of sewage sludge and LDPE were in the same temperature range of (200 to 600) °C, while in the case of lignocellulosic biomass in the work of Salvilla et al. [60], biomass degradation occurred at (200 to 400) °C and plastic degradation at (400 to 500) °C.
Although the above-mentioned works present co-pyrolysis as a possible alternative for enhancing the overall efficiency and economic feasibility of plastic wastes and biomass pyrolysis, several issues have yet to be addressed. The chlorine content in waste plastics, the increased viscosity of the produced oil can be listed among the obstacles that need to be overcome [64]. Higher concentrations of intermediate HCl can decrease aromatic yields at the expense of gaseous products, causing poor oil quality , and can also promote the production of highly toxic chemicals such as dioxins and furans [62]. Therefore, it is necessary to select an effective catalyst and convenient reaction conditions that can address these limitations. As eco-effectiveness of the co-pyrolysis process is expected for mixed wastes that already contain both plastics and biomass or other wastes that are difficult to be separated [64], such as real-world plastic wastes, further research is needed on the processing of plastic wastes using inexpensive BAC catalysts.
5 Conclusions and Perspectives
Low cost and easily manufacturable BACs with high porosity and heteroatoms surface functional groups are gaining more attention in pyrolysis processing of plastic waste for liquid fuels production. However, catalytic properties of a BAC depend on type of biomass, type of reagent, reagent to biomass ratio and activation temperature, all of which can impact the BET surface area, total pore volume, and acidity of the resulting BAC. Biomass having high carbon content and low ash content is preferable among the many forms of biomass. H3PO4 is a common reagent that can be used advantageously for activation of lignocellulosic biomass . When activating with H3PO4, increasing carbonization temperature and H3PO4 to biomass ratio promote the catalytic activity of the BAC by enhancing its BET surface area, volume of micropores and acidity . However, excessive values of each of these parameters can be counterproductive because of enhanced devolatilization of acid functional groups and reduction of micropores. When an excessive H3PO4 to biomass ratio is used, a high number of acid sites on the BAC enhances scission, cracking, and rearrangement reactions, thus decreasing liquid oil yield . Overall, BAC rich in P-containing functional groups is favorable for aromatization and C-C and C-H bond cracking reactions, resulting in the production of aromatics and light alkanes. Red mud as a low-cost additive , has the ability to improve hydrogenation and cracking reactions, and dechlorination of the liquid oil. During co-pyrolysis of plastic waste and lignocellulosic biomass, Diels-Alder reactions are promoted between furan or its derivatives from cellulose and unsaturated hydrocarbons from plastic waste, promoting aromatic hydrocarbons in the liquid oil.
The reported works that use BACs and the above mentioned low-cost wastes as additives for catalytic pyrolysis of plastic wastes mainly examine single plastic wastes or simulated mixtures of a given number of plastic wastes. More works are expected to examine the co-pyrolysis process over BACs, as the real-world plastic wastes contain both plastics and other types of waste that are difficult to separate. New studies in this area would enhance the effectiveness of technology for plastic wastes valorization. BACs prepared from high-carbon biomass activated with different reagents, including KOH, ZnCl2 and FeCl3, should also be examined for catalytic pyrolysis of plastic wastes. BACs with high selectivity of high value-added compounds such as naphtha can be expected one of the focal points in future research.
References
Kaza S, Yao L, Bhada-Tata P, Woerden FV (2018) What a Waste 2.0: A global snapshot of solid waste management to 2050. In: Urban Development series. Washington, DC: World Bank. https://openknowledge.worldbank.org/handle/10986/30317
Das P, Tiwari P. The effect of slow pyrolysis on the conversion of packaging waste plastics (PE and PP) into fuel. Waste Manag. 2018;79:615–24. https://doi.org/10.1016/j.wasman.2018.08.021.
PlasticsEurope World plastics materials demand 2015 by types. https://committee.iso.org/files/live/sites/tc61/files/The%20Plastic%20Industry%20Berlin%20Aug%202016%20-%20Copy.pdf
Ryberg MW, Laurent A, Hauschild M. Mapping of global plastics value chain and plastics losses to the environment (with a particular focus on marine environment). Nairobi, Kenya: United Nations Environment Programme; 2018. https://sites.miis.edu/bluepioneers/files/2019/06/Mapping-of-global-plastics-value-chain-and-hotspots.pdf
DCC- Zero waste recycler. Plastic Waste Management. https://www.zerowasterecycler.com/plastic-waste-management/ (accessed on May 28th, 2021).
Cai N, Yang H, Zhang X, Xia S, Yao D, Bartocci P, Fantozzi F, Chen Y, Chen H, Williams PT. Bimetallic carbon nanotube encapsulated Fe-Ni catalysts from fast pyrolysis of waste plastics and their oxygen reduction properties. Waste Manag. 2020;109:119–26. https://doi.org/10.1016/j.wasman.2020.05.003.
Parku GK, Collard FX, Görgens JF. Pyrolysis of waste polypropylene plastics for energy recovery: Influence of heating rate and vacuum conditions on composition of fuel product. Fuel Process Technol. 2020;209:36–8. https://doi.org/10.1016/j.fuproc.2020.106522.
Götze R (2016) Composition of waste materials and recyclables. Ph.D. thesis, department of environmental engineering, technical university of Denmark. https://orbit.dtu.dk/en/publications/composition-of-waste-materials-and-recyclables
Anuar Sharuddin SD, Abnisa F, Wan Daud WMA, Aroua MK. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers Manag. 2017;148:925–34. https://doi.org/10.1016/j.enconman.2017.06.046.
Shen L, Worrell E. Plastic recycling. In: Worrell E, Reuter MA, editors. Handbook of recycling: state-of-the-art for practitioners, analysts, and scientists. Elsevier Inc.; 2014. p. 179–90. https://doi.org/10.1016/B978-0-12-396459-5.00013-1.
Uzoejinwa BB, He X, Wang S, El-Fatah Abomohra A, Hu Y, Wang Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers Manag. 2018;163:468–92. https://doi.org/10.1016/j.enconman.2018.02.004.
Ranta-Korpi M, Vainikka P, Konttinen J, Saarimaa A, Rodriguez M. Ash forming elements in plastics and rubbers. In: VTT Technology series; 2014. http://www.vtt.fi/inf/pdf/technology/2014/T186.pdf
Zhou H, Meng A, Long Y, Li Q, Zhang Y. Classification and comparison of municipal solid waste based on thermochemical characteristics. J Air Waste Manag Assoc. 2014;64:597–616. https://doi.org/10.1080/10962247.2013.873094.
Qureshi MS, Oasmaa A, Pihkola H, Deviatkin I, Tenhunen A, Mannila J, Minkkinen H, Pohjakallio M, Laine-ylijoki J. Pyrolysis of plastic waste : Opportunities and challenges. J Anal Appl Pyrolysis. 2020;152:104804. https://doi.org/10.1016/j.jaap.2020.104804.
Abdel-Shafy HI, Mansour MSM. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt J Pet. 2018;27:1275–90. https://doi.org/10.1016/j.ejpe.2018.07.003.
Chanashetty VB, Patil BM. Fuel from plastic wastes. Int J Emerg Technol. 2015;6(2):121–8. https://www.researchtrend.net/ijet/ijet61/24%20NCRIET.pdf
Luo W, Hu Q, Fan Z, Wan J, He Q, Huang S, Zhou N, Song M, Zhang J, Zhou Z. The effect of different particle sizes and HCl-modified kaolin on catalytic pyrolysis characteristics of reworked polypropylene plastics. Energy. 2020;213:119080. https://doi.org/10.1016/j.energy.2020.119080.
Yao D, Yang H, Chen H, Williams PT. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl Catal B Environ. 2018;239:565–77. https://doi.org/10.1016/j.apcatb.2018.07.075.
Komilis D, Evangelou A, Giannakis G, Lymperis C. Revisiting the elemental composition and the calorific value of the organic fraction of municipal solid wastes. Waste Manag. 2012;32:372–81. https://doi.org/10.1016/j.wasman.2011.10.034.
Kumar S, Singh RK. Pyrolysis kinetics of waste high-density polyethylene using thermogravimetric analysis. Int J ChemTech Res. 2014;6:131–7. https://sphinxsai.com/2014/ChemTech/JM14CT1_50/CT=16(131-137)JM14.pdf
Adrados A, de Marco I, Caballero BM, López A, Laresgoiti MF, Torres A. Pyrolysis of plastic packaging waste: a comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012;32:826–32. https://doi.org/10.1016/j.wasman.2011.06.016.
Feng Y, Liu S, Qin J, Cao Y, Jiang Y, Zhang S. Numerical study on the influence of turbulence on the pyrolysis of hydrocarbon fuel in mini-channel. Int J Heat Mass Transf. 2018;119:768–76. https://doi.org/10.1016/j.ijheatmasstransfer.2017.12.002.
Mellin P, Yu X, Yang W, Blasiak W. Influence of reaction atmosphere (H2O, N2, H2, CO2, CO) on fluidized-bed fast pyrolysis of biomass using detailed tar vapor chemistry in computational fluid dynamics. Ind Eng Chem Res. 2015;54:8344–55. https://doi.org/10.1021/acs.iecr.5b02164.
Zhou N, Dai L, Lyu Y, Li H, Deng W, Guo F, Chen P, Lei H, Ruan R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem Eng J. 2021;418:129412. https://doi.org/10.1016/j.cej.2021.129412.
Das P, Gabriel JCP, Tay CY, Lee JM. Value-added products from thermochemical treatments of contaminated e-waste plastics. Chemosphere. 2021;269:129409. https://doi.org/10.1016/j.chemosphere.2020.129409.
Wang Z, Burra KG, Lei T, Gupta AK. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals: a review. Prog Energy Combust Sci. 2021;84:100899. https://doi.org/10.1016/j.pecs.2020.100899.
Fekhar B, Zsinka V, Miskolczi N. Value added hydrocarbons obtained by pyrolysis of contaminated waste plastics in horizontal tubular reactor: In situ upgrading of the products by chlorine capture. J Clean Prod. 2019;241:118166. https://doi.org/10.1016/j.jclepro.2019.118166.
Butler E, Devlin G, McDonnell K. Waste polyolefins to liquid fuels via pyrolysis: Review of commercial state-of-the-art and recent laboratory research. Waste Biomass Valorization. 2011;2:227–55. https://doi.org/10.1007/s12649-011-9067-5.
Qureshi MS, Oasmaa A, Lindfords C (2019) Thermolysis of plastic waste: reactor comparison. In: Pyroliq 2019: Pyrolysis and liquefaction of biomass and wastes, Engineering Conferences International Digital Archives. Cork, Ireland, https://dc.engconfintl.org/cgi/viewcontent.cgi?article=1015&context=pyroliq_2019
Djandja OS, Wang Z, Wang F, Xu Y-P, Duan P-G. Pyrolysis of municipal sewage sludge for biofuel production: a review. Ind Eng Chem Res. 2020;59:16939–56. https://doi.org/10.1021/acs.iecr.0c01546.
Li L, Rowbotham JS, Christopher Greenwell H, Dyer PW. An introduction to pyrolysis and catalytic pyrolysis: Versatile techniques for biomass conversion. In: Suib SL, editor. New and future developments in catalysis: catalytic biomass conversion. Elsevier B.V.; 2013. p. 173–208. https://doi.org/10.1016/B978-0-444-53878-9.00009-6.
Collard FX, Carrier M, Görgens JF. Fractionation of lignocellulosic material with pyrolysis processing. In: Mussatto SI, editor. Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier Inc.; 2016. p. 81–101. https://doi.org/10.1016/B978-0-12-802323-5.00004-9.
Arabiourrutia M, Lopez G, Artetxe M, Alvarez J, Bilbao J, Olazar M. Waste tyre valorization by catalytic pyrolysis – a review. Renew Sust Energ Rev. 2020;129:109932. https://doi.org/10.1016/j.rser.2020.109932.
Ratnasari DK, Nahil MA, Williams PT. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. J Anal Appl Pyrolysis. 2017;124:631–7. https://doi.org/10.1016/j.jaap.2016.12.027.
Zhang Y, Duan D, Lei H, Villota E, Ruan R. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl Energy. 2019;251:113337. https://doi.org/10.1016/j.apenergy.2019.113337.
Sun K, Huang Q, Chi Y, Yan J. Effect of ZnCl2-activated biochar on catalytic pyrolysis of mixed waste plastics for producing aromatic-enriched oil. Waste Manag. 2018;81:128–37. https://doi.org/10.1016/j.wasman.2018.09.054.
Li K, Lee S, Yuan G, Lei J, Lin S, Weerachanchai P, Yang Y, Wang J-Y. Investigation into the catalytic activity of microporous and mesoporous catalysts in the pyrolysis of waste polyethylene and polypropylene mixture. Energies. 2016;9:431. https://doi.org/10.3390/en9060431.
Khalil U, Vongsvivut J, Shahabuddin M, Samudrala SP, Srivatsa SC, Bhattacharya S. A study on the performance of coke resistive cerium modified zeolite Y catalyst for the pyrolysis of scrap tyres in a two-stage fixed bed reactor. Waste Manag. 2020;102:139–48. https://doi.org/10.1016/j.wasman.2019.10.029.
Ryu HW, Kim DH, Jae J, Lam SS, Park ED, Park YK. Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour Technol. 2020;310:123473. https://doi.org/10.1016/j.biortech.2020.123473.
Ahmed MHM, Batalha N, Mahmudul HMD, Perkins G, Konarova M. A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock: insights into synergistic effect, catalyst development and reaction mechanism. Bioresour Technol. 2020;310:123457. https://doi.org/10.1016/j.biortech.2020.123457.
Xue Y, Johnston P, Bai X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers Manag. 2017;142:441–51. https://doi.org/10.1016/j.enconman.2017.03.071.
Huo E, Lei H, Liu C, Zhang Y, Xin L, Zhao Y, Qian M, Zhang Q, Lin X, Wang C, Mateo W, Villota EM, Ruan R. Jet fuel and hydrogen produced from waste plastics catalytic pyrolysis with activated carbon and MgO. Sci Total Environ. 2020;727:138411. https://doi.org/10.1016/j.scitotenv.2020.138411.
Arami-Niya A, Daud WMAW, Mjalli FS. Using granular activated carbon prepared from oil palm shell by ZnCl2 and physical activation for methane adsorption. J Anal Appl Pyrolysis. 2010;89:197–203. https://doi.org/10.1016/j.jaap.2010.08.006.
Bedia J, Peñas-Garzón M, Gómez-Avilés A, Rodriguez JJ, Belver C. Review on activated carbons by chemical activation with FeCl3. C - J Carbon Res. 2020;6:21. https://doi.org/10.3390/c6020021.
Iwanow M, Gärtner T, Sieber V, König B. Activated carbon as catalyst support: precursors, preparation, modification and characterization. Beilstein J Org Chem. 2020;16:1188–202. https://doi.org/10.3762/bjoc.16.104.
Ji Y, Li T, Zhu L, Wang X, Lin Q. Preparation of activated carbons by microwave heating KOH activation. Appl Surf Sci. 2007;254:506–12. https://doi.org/10.1016/j.apsusc.2007.06.034.
Veerakumar P, Panneer Muthuselvam I, Hung C Te, Lin KC, Chou FC, Liu S Bin (2016) Biomass-derived activated carbon supported Fe3O4 nanoparticles as recyclable catalysts for reduction of nitroarenes. ACS Sustain Chem Eng 4:6772–6782. doi: https://doi.org/10.1021/acssuschemeng.6b01727
Hui TS, Zaini MAA. Potassium hydroxide activation of activated carbon: a commentary. Carbon Lett. 2015;16:275–80. https://doi.org/10.5714/CL.2015.16.4.275.
Ateş F, Özcan Ö. Preparation and characterization of activated carbon from poplar sawdust by chemical activation: comparison of different activating agents and carbonization temperature. Eur J Eng Res Sci. 2018;3:6–11. https://doi.org/10.24018/ejers.2018.3.11.939.
Rodríguez-Reinoso F, Sepúlveda-Escribano A. Porous carbons in adsorption and catalysis. In: Nalwa HS, editor. Handbook of surfaces and interfaces of materials, vol. 5. Elsevier Inc.; 2001. p. 309–55. https://doi.org/10.1016/b978-012513910-6/50066-9.
Olivares-Marín M, Fernández-González C, Macías-García A, Gómez-Serrano V. Preparation of activated carbon from cherry stones by chemical activation with ZnCl2. Appl Surf Sci. 2006;252:5967–71. https://doi.org/10.1016/j.apsusc.2005.11.008.
Gerçel Ö, Gerçel HF. Preparation and characterization of activated carbon from vegetable waste by microwave-assisted and conventional heating methods. Arab J Sci Eng. 2016;41:2385–92. https://doi.org/10.1007/s13369-015-1859-7.
Wan K, Chen H, Zheng F, Pan Y, Zhang Y, Long D. Tunable production of jet-fuel range alkanes and aromatics by catalytic pyrolysis of LDPE over biomass-derived activated carbons. Ind Eng Chem Res. 2020;59:17451–61. https://doi.org/10.1021/acs.iecr.0c02482.
Duan D, Feng Z, Dong X, Chen X, Zhang Y, Wan K, Wang Y, Wang Q, Xiao G, Liu H, Ruan R. Improving bio-oil quality from low-density polyethylene pyrolysis: effects of varying activation and pyrolysis parameters. Energy. 2021;232:121090. https://doi.org/10.1016/j.energy.2021.121090.
Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpää M. Methods for preparation and activation of activated carbon: a review. Environ Chem Lett. 2020;18:393–415. https://doi.org/10.1007/s10311-019-00955-0.
Idumah CI, Nwuzor IC. Novel trends in plastic waste management. SN Appl Sci. 2019;1:1402. https://doi.org/10.1007/s42452-019-1468-2.
López A, de Marco I, Caballero BM, Laresgoiti MF, Adrados A, Aranzabal A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl Catal B Environ. 2011;104:211–9. https://doi.org/10.1016/j.apcatb.2011.03.030.
Gin AW, Hassan H, Ahmad MA, Hameed BH, Mohd Din AT. Recent progress on catalytic co-pyrolysis of plastic waste and lignocellulosic biomass to liquid fuel: the influence of technical and reaction kinetic parameters. Arab J Chem. 2021;14:103035. https://doi.org/10.1016/j.arabjc.2021.103035.
Li X, Zhang H, Li J, Su L, Zuo J, Komarneni S, Wang Y. Improving the aromatic production in catalytic fast pyrolysis of cellulose by co-feeding low-density polyethylene. Appl Catal A Gen. 2013;455:114–21. https://doi.org/10.1016/j.apcata.2013.01.038.
Salvilla JNV, Ofrasio BIG, Rollon AP, Manegdeg FG, Abarca RRM, de Luna MDG. Synergistic co-pyrolysıs of polyolefin plastics with wood and agricultural wastes for biofuel production. Appl Energy. 2020;279:115668. https://doi.org/10.1016/j.apenergy.2020.115668.
Alam M, Bhavanam A, Jana A, Viroja S, Rao N. Co-pyrolysis of bamboo sawdust and plastic: aynergistic effects and kinetics. Renew Energy. 2020;149:1133–45. https://doi.org/10.1016/j.renene.2019.10.103.
Lee DJ, Lu JS, Chang JS. Pyrolysis synergy of municipal solid waste (MSW): a review. Bioresour Technol. 2020;318:123912. https://doi.org/10.1016/j.biortech.2020.123912.
Zaker A, Chen Z, Zaheeruddin M, Guo J. Co-pyrolysis of sewage sludge and low-density polyethylene – a thermogravimetric study of thermo-kinetics and thermodynamic parameters. J Environ Chem Eng. 2020;9:104554. https://doi.org/10.1016/j.jece.2020.104554.
Johansson AC, Sandström L, Öhrman OGW, Jilvero H. Co-pyrolysis of woody biomass and plastic waste in both analytical and pilot scale. J Anal Appl Pyrolysis. 2018;134:102–13. https://doi.org/10.1016/j.jaap.2018.05.015.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Djandja, O.S., Chen, D., Yin, LX., Wang, ZC., Duan, PG. (2022). Roadmap to Low-Cost Catalytic Pyrolysis of Plastic Wastes for Production of Liquid Fuels. In: Fang, Z., Smith Jr., R.L., Xu, L. (eds) Production of Biofuels and Chemicals from Sustainable Recycling of Organic Solid Waste. Biofuels and Biorefineries, vol 11. Springer, Singapore. https://doi.org/10.1007/978-981-16-6162-4_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-6162-4_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6161-7
Online ISBN: 978-981-16-6162-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)