Abstract
Activated carbon refers to a wide range of carbonised materials of high degree of porosity and high surface area. Activated carbon has many applications in the environment and industry for the removal, retrieval, separation and modification of various compounds in liquid and gas phases. Selection of the chemical activator agent is a major step controlling the performance and applicability of activated carbon. Here, we review chemical activators used to produce activated carbon. We compare the impregnation method with the physical mixing method used in activating with alkali hydroxides. We selected 81 articles from Google Scholar, PubMed, Scopus, Science Direct, Embase and Medlin databases. Eighteen articles report the activation with potassium hydroxide, 17 with phosphoric acid, 15 with zinc chloride, 11 with potassium carbonate, nine with sodium hydroxide, and 11 with new activating agents. Activation with phosphoric acid is commonly used for lignocellulosic material and at lower temperatures. Zinc chloride generates more surface area than phosphoric acid but is used less due to environmental concerns. Potassium carbonate, in comparison with potassium hydroxide, produces higher yields and a higher surface area for the adsorption of large pollutant molecules such as dyes. Activating with potassium hydroxide in terms of surface area and efficiency shows better results than sodium hydroxide for various applications. Also, the comparison of the physical mixing method and the impregnation method in activation with alkali metals indicates that the activated carbon obtained through physical mixing had a higher porosity than the activated carbon produced by the impregnation method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Activated carbon is a porous carbonaceous material with continually expanding applications in water treatment and desalination, wastewater treatment and air purification due to its unique characteristics (Fig. 1) (Kosheleva et al. 2019; Samsuri et al. 2014; Yousefi et al. 2019). Activated carbon is a very diverse adsorbent material including a high degree of porosity and high surface area, while up to 90% of it may be constituted from carbon (Gopinath and Kadirvelu 2018; Morin-Crini et al. 2019; Samsuri et al. 2014). Also, carbon structures contain the main functional groups such as carboxyl, carbonyl, phenol, lactone and quinone that are responsible for adsorbing contaminants. Oxygen, hydrogen, sulphur and nitrogen are also present in the form of functional groups or chemical atoms in the activated carbon structure. The unique adsorption properties depend on the existing functional groups of activated carbon, which are derived mainly from activation processes, precursors and thermal purification (Bhatnagar et al. 2013; Yousefi et al. 2019).
The advantages of activated carbon for zeolites or polymer-based adsorbents are high quality in wastewater treatment, simple process design, easy exploitation of the process, resistance to corrosive (acid and alkali) and toxic environments, high adsorption potential in gas and liquid purification and their use as supportive catalysts (Belala et al. 2011; Bhatnagar et al. 2013; Rambabu et al. 2015). Theoretically, all carbon-rich organic materials are commonly known as carbonised materials, which can be used to produce activated carbon. Activated carbon may be produced from agricultural waste, livestock and industrial by-products (Huang and Zhao 2016; Khadhri et al. 2019; Njoku et al. 2014).
The production of activated carbon around the world is estimated to be around 100,000 tonnes annually (Samsuri et al. 2014). The most common sources of activated carbon on a commercial scale are wood, anthracite and bitumen charcoal, lignite, peat shells and coconut. Alternative sources such as olive and almond shells are also used. The carbon content of these materials ranges from 40 to 90% (wt.), with a density of 0.4–1.45 g/m3 (Cui et al. 2011; Huang and Zhao 2016; Khadhri et al. 2019; Mishra et al.2010). Today, much effort has been devoted to exploiting waste as raw materials in activated carbon production (Crini et al. 2019). Activated carbon can also be produced from agricultural residues such as olive corn, biomass, rice rolls, corn stalks, bagasse, fruit stones (cherry and apricot stones, grape seeds), hard shells (pistachio, almond and pecan shell), fruit pulp, bones and coffee beans (Klasson et al. 2013). The raw material used for the preparation of activated carbon should be abundant, cheap and safe (Jolly et al. 2006). The mineral content of this material and its biodegradability during initial storage should be minimal (Prauchner et al. 2016; Samsuri et al. 2014; Zhu et al. 2010).
In addition, for the production of activated carbon, the presence of carbon materials, transportation of raw materials and the availability and seasonal changes in relation to the quality and availability of raw materials should be considered (Samsuri et al. 2014; Thitame and Shukla 2016). Also, to obtain good results, high adsorption of carbon and oxygen in adsorbents is very necessary. Other features include high abrasion resistance, high thermal strength and small pore diameters, which result in an increased exposure surface and thus an increase in adsorption capacity (Cui et al. 2011; Prauchner et al. 2016; Soleimani and Kaghazchi 2008). Also, the properties of the prepared activated carbon mainly depend on the type of activation agent (Sawant et al. 2017; Uysal et al. 2014).
Considering that the use of different activator substances culminates in producing different properties in activated carbon, and in many studies the selection of the best activated carbon is a priority for many researchers, a systematic study seems necessary to reach an overall conclusion. The purpose of this study was therefore to compare the properties of activated carbon, which were produced by various chemical activators and in two precursor/activator mixing modes—impregnation and physical mixing, through a systematic review of studies. This study will also mention the advantages and disadvantages of activating agents in selecting the best activated agent according to the potential of the activated carbon produced to adsorb various adsorbates from aqueous solutions.
Methodology
In order to conduct this study, international databases including Google Scholar, PubMed, Scopus, Science Direct, Embase and Medline were searched using the following Medical Subject Headings (MESH) keywords: activated carbon, chemical activation, impregnation, physical mixing, new activating agents, phosphoric acid, zinc chloride, potassium carbonate, potassium hydroxide, sodium hydroxide.
In this study, research articles published during 2000–2019 in valid journals in the field of activated carbon production from various agricultural bio-wastes through different activation procedures and used for the adsorption of various pollutants from aqueous solutions were included. On the other hand, other literatures including books, lectures, letter to editors, review articles and research articles published before 2000 were excluded. The articles with the following contents were excluded: i) activated carbon amended with nanoparticles, organic matters and bases/acids, ii) activated carbon used for application other than water and wastewater treatment and iii) accelerated adsorption onto activated carbon by ultrasound.
Study of article quality
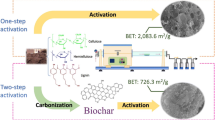
The resources utilised by the researchers were studied. At first, the title, abstract and method of work were examined if needed. Also, for further review of the full text of the related articles, the final articles were selected and studied. Finally, all the articles were studied by an expert group in the field of the adsorption of various pollutants with activated carbon produced by various activation agents. As shown in Fig. 2, a total of 201 articles were retrieved in the primary search, from which 156 articles were left after removing repetitive and completely unrelated articles. The focus of research was restricted to studies on the preparation of activated carbon with chemical activators and using the obtained adsorbents for the removal of various pollutants from aqueous solutions. After this filtering process, 76 articles were left for further investigation. The initial list of the articles was also manually reviewed, and five additional articles were selected. Finally, 81 articles were approved for full text review. Among the selected articles, the number of articles based on the chemical activator used for the preparation of activated carbon from agricultural residues is presented in Fig. 3.
Extracting the data
A checklist of the required information includes the name of the adsorbent, activation type, activation time, activation temperature (T), impregnation rate (IR), Brunauer, Emmett and Teller surface area (SBET), total pore volume (Vt), adsorption rate and maximum adsorption capacity (Qmax). The name of the adsorbent and the used precursor was prepared and completed. Comparison of the performance and properties of activated carbon with different chemicals showed that among different activation factors used in the process of the removal of large molecules such as dye, metal hydroxides produced activated carbon with a higher surface area than other activating agents, so the capacity adsorption of the adsorbent is higher with metal hydroxides (Ahmed and Theydan 2014; Njoku et al. 2013; Tongpoothorn et al. 2011).
In chemical activation, phosphoric acid and zinc chloride are used to activate lignocellulosic materials that have not previously been carbonised, while metal compounds such as potassium hydroxide are used to activate the precursors of charcoal and char. Phosphoric acid, in comparison with zinc chloride, has fewer restrictions in environmental and toxicological contamination and requires a lower activation temperature than potassium hydroxide (Al Bahri et al. 2012; de Yuso et al. 2014; Demiral and Şamdan 2016; Nowicki et al. 2013). The common ranges of activation temperatures in the process of producing activated carbon with phosphoric acid, zinc chloride, potassium carbonate, sodium hydroxide and potassium hydroxide were 450–600 °C, 400–900 °C, 700–1000 °C, 550–850 °C and 450–850 °C, respectively (Angın et al. 2013; Basta et al. 2009; Ibrahim et al. 2014; Liu et al. 2010a, 2012; Youssef et al. 2012). In chemical activation with alkali, in general activation with potassium hydroxide shows better results than sodium hydroxide in terms of surface area and performance in different applications. However, sodium hydroxide is cheaper and more environmentally friendly and does less harm than potassium hydroxide, and it is clear that it has more industrial applications because of its superiority to potassium hydroxide (Byamba-Ochir et al. 2016; Huang et al. 2014; Yahya et al. 2015). Also, the results of the comparison of the physical mixing method and the impregnation method in activating with metal hydroxides in different studies indicated that the activated carbon obtained through physical mixing had a higher porosity than the activated carbon produced by the impregnation method (Alabadi et al. 2015; Byamba-Ochir et al. 2016; Lee et al. 2015; Nowicki et al. 2013).
Activation of activated carbon methods
Activated carbon could be prepared through the direct activation of dry raw precursor or through a two-stage process including initial carbonisation and then activation. In the two-stage process, the dried raw organic materials such as walnut hulls, wood, bone and coal should be initially carbonised at high temperatures. In the carbonisation process, the material should be exposed to a red spot (less than 700 °C) temperature in the distillation apparatus in order to evaporate and remove the hydrocarbons from it in the absence of oxygen. Overall, the process of carbonisation is thus a pyrolytic process, and its product is known as carbonised material, char or biochar (Byamba-Ochir et al. 2016; Huang et al. 2014; Yahya et al. 2015). After activating the activated carbon, various activation methods are used to further develop porosity and create structures that lead to the formation of fine solid cavities in activated carbon (Yahya et al. 2015). The pores created on the surface of activated carbon could be categorised as macropores > 25 nm, 1 nm < mesopores < 25 nm, micropores < 1 nm (Huang et al. 2014).
In view of the nature of the activation process, activated carbon could be prepared in two ways: physical and chemical.
Activated carbon production through physical activation
Physical activation used commercially is a two-step process that involves the process of carbonisation (pyrolysis) in a neutral atmosphere and then activation in atmospheric oxidising gases such as steam, carbon dioxide, carbon dioxide and nitrogen or air mixtures with increasing temperature in the range of 800–1100 °C (Bouchelta et al. 2008). This method has the ability to produce activated carbon of porous structure and good physical power, which is an inexpensive method for activated carbon preparation and is considered a green approach because it is chemical-free (Byamba-Ochir et al. 2016; Pallarés et al. 2018; Yahya et al. 2015). However, in the process of the physical activation of activated carbon, the long activation time and low adsorption capacity of prepared activated carbon and its high energy consumption are the main disadvantages (Yahya et al. 2015).
Activated carbon production through chemical activation
Chemical activation, known as wet oxidation, is usually used for raw materials containing cellulose, such as wood, sawdust or fruit pits. These materials are also called biomass resources. In chemical activation for the preparation of activated carbon, organic precursors are activated in the presence of chemicals at high temperatures (Njoku et al. 2014; Samsuri et al. 2014; Yahya et al. 2015). For chemical activation, the raw material, in the first stage, is saturated with oxidising and highly dehydrated chemicals. After impregnation, the suspension is dried and the remaining mixture is heated for a given time. Depending on the activating material and the properties of the final product, activation can take place at temperatures ranging from 400 to 900 °C, at which cellulose is degraded. Eventually, activated carbon is obtained from the repeated washing of the resulting mixture. Another purpose of the final rinse is the recovery of active substances (Samsuri et al. 2014). Chemical activation agents are dehydrating agents that influence pyrolytic decomposition and, by inhibiting the formation of bitumen, increase the activated carbon content and, with subsequent changes in the thermal degradation of precursors, result in the development of the porous structure of carbon materials (Gratuito et al. 2008; Molina-Sabio and Rodrıguez-Reinoso 2004). These activating agents with deep penetration into the carbon structure lead to the development of small pores in the activated carbon, thereby increasing its surface area (Gratuito et al. 2008). Unlike thermal physical activation, the carbonisation and activation phenomena occur simultaneously in the chemical activation so, in contrast to physical activation where carbonisation and activation processes are typically performed in two different furnaces, chemical activation can be performed in a single furnace (Samsuri et al. 2014).
In the process of chemical activation, the variables that affect the characteristics of the final activated carbon are the amount of impregnation and the weight ratio of chemical agents to dry precursor (Gratuito et al. 2008). Compared to physical activation, this type of activation is more economical because it requires a lower activation temperature, shorter processing time and higher carbon efficiency (Rambabu et al. 2015). Also, the activated carbon prepared through chemical activation has a more porous structure than that of physical activation (Cui et al. 2011). Activated chemicals react with carbon matrices and liberate gas products to form a porous structure (Molina-Sabio and Rodrıguez-Reinoso 2004). However, the need for a repeated and long washing step to eliminate the spent activator agent from the final mixture at the end of activation process is one of the disadvantages of this method. In addition, toxic wastewater is produced at the washing step, which causes water pollution and therefore requires secondary treatment (Wang et al. 2016; Yorgun et al. 2016).
The precise selection of the parameters of the chemical activation process is important to the quality of activated carbon production. In addition, in the production of activated carbon, the efficiency of the process is also considered an important factor (Molina-Sabio and Rodrıguez-Reinoso 2004; Wang et al. 2016; Yorgun et al. 2016). In the chemical activation method, the parameters of the chemical agent effect, impregnation ratio and method, temperature, final temperature of carbonisation, carbonisation time, activation space (under atmospheric conditions) and activation method have been investigated (Cui et al. 2011; Wang et al. 2016; Yorgun et al. 2016). Different types of chemicals have different reactions with precursors and thus affect the adsorption behaviour. The main chemicals which have been used as potential activators are alkaline groups such as potassium hydroxide (KOH), sodium hydroxide (NaOH), calcium chloride (CaCl2) and potassium carbonate (K2CO3), acidic groups such as phosphoric acid (H3PO4) and sulphuric acid (H2SO4), intermediate metal salts such as ZnCl2 and other activating agents (Balajii and Niju 2019; Yahya et al. 2015). Based on the physical nature of the activating agent, the activator and precursor could be mixed through two approaches: the physical mixing of the activator and precursor in dry conditions and impregnation (Nowicki et al. 2013; Yorgun et al. 2016).
Activated carbon activators
Activated carbon preparation through activation with phosphoric acid
Among the activating agents, phosphoric acid with the chemical formula H3PO4 is widely used in the activation of various lignocellulosic materials (Yahya et al. 2015; Yorgun et al. 2016). In the reaction of phosphoric acid with lignocellulose since cellulose is resistant to hydrolysis of acid, at the beginning of the mixing of the compounds the acid first reacts with the cellulose and lignin. Activation with phosphoric acid is used in the preparation of activated carbon from various forms of biomass (Jadhav and Mohanraj 2016; Nowicki et al. 2013). Some studies on activation with phosphoric acid and related details about activated carbon prepared with this acid are listed in Table 1.
During the impregnation stage and due to the high polarity of phosphoric acid, controlling the physical and chemical interactions occurring in the bulk of the solution and with the substratum is essential. In this regard, the solution concentration is a primary factor of the activation process with this acid agent (Yahya et al. 2015; Yakout and El-Deen 2016; Yorgun et al. 2016). In a study by Demiral and Samdan (2016) on the chemical activation of pumpkin skin by phosphoric acid, it was found that the pores and cavities formed on the active surface of activated carbon are created due to the evaporation of phosphoric acid during the carbonisation process (Demiral and Şamdan 2016; Yakout and El-Deen 2016). The main mechanisms of activation with phosphoric acid are the depolymerisation, dehydration and redistribution of biopolymers in lignocellulosic materials (Abdelnaeim et al. 2016; de Yuso et al. 2014). Also, during the activation process, the reaction of phosphoric acid with the active carbon-based precursor leads to the formation of products in the form of particles or volatile substances, which as a result create pores or increase the number of pores in the sites previously occupied by this material (de Yuso et al. 2014; Liu et al. 2010b). In addition, phosphoric acid leads to the expansion of microporous and mesoporous pores in activated carbon (Demiral and Şamdan 2016), so the activated carbon produced from lignocellulose wastes through activation with phosphoric acid is very porous (de Yuso et al. 2014; Demiral and Şamdan 2016).
Generally, acid refinement leads to an increase in acid groups, eliminates mineral elements and improves the hydrophilic nature of the surface, so the carbon surface will have more access to the aqueous phase (Abdelnaeim et al. 2016; de Yuso et al. 2014). Phosphoric acid has two important functions: It improves the pyrolytic decomposition of the starting material and the formation of a grid structure (net or lattice) (de Yuso et al. 2014; Liu et al. 2010b). However, the excessive amount of phosphoric acid due to the formation of an insulating layer on the activated carbon does not result in the enhancement of porosity on the activated carbon surface (Zhong et al. 2012). According to a study conducted by Liu et al. (2010b), at higher phosphoric acid doses, more potential sites could be created and occupied by the activating agent, which are beneficial to the subsequent pore-opening and -widening processes (Liu et al. 2010b). However, an excessive increase in phosphoric acid leads to the formation of an insulating layer on the activated carbon (Liu et al. 2010b; Zhong et al. 2012).
Vicinisvarri et al. (2014) investigated the effects of phosphoric acid concentration on the morphology of the activated carbon derived from the core and shells of nuts. The results showed that by increasing phosphoric acid (80% by weight), the highest porosity surface in activated carbon was observed due to the increase in the velocity of the formation of cavities. The outer surface of activated carbon has different vents, while the pore size depends on the amount of carbonisation and impregnation. In this case, the whole surface of activated carbon is full of holes and irregular shapes (Vicinisvarri et al. 2014; Yakout and El-Deen 2016). In a study of activated carbon production from olive stone through activation by 60, 70 and 80% w/w of phosphoric acid, Yakout and El-Deen (2016) reported that the surface area of the activated carbon increased by increasing the concentration of the acid. It was found that the activated carbon prepared with 80% phosphoric acid had the highest surface area (1218 m2/g) and pore volume (0.63 cm3/g) (Yakout and El-Deen 2016).
Shamsuddin et al. 2016 carried out a study of the synthesis and characterisation of H3PO4-activated carbon prepared from hemp fibre. The results indicated that the increases in the surface area and porosity of activated carbon fibre with acid activation were higher than crude activated carbon, and Fourier-transform infrared spectroscopy (FTIR) showed the significant presence of peaks from different frequencies before and after activation. The results of BET analysis indicated an increase in the surface area and porosity of activated carbon after acid activation (Shamsuddin et al. 2016). It has been shown that the activated carbon prepared with phosphoric acid has less C = O groups than the raw materials. The reduction of carbonyl groups may be due to the effect of H3PO4 hydrolysis, which decomposes these groups and other products such as volatile substances (Baccar et al. 2009). Phosphorus groups are among the most important substances for the adsorption of heavy metals from acidic solutions. H3PO4-activated carbon may therefore be considered a cation exchanger for the removal of heavy metal cations from aqueous solutions in the future (Xu et al. 2014).
Acidic groups are the most derivative of the reaction between phosphoric acid and activated carbon precursor (Liu et al. 2010b). Activation with phosphoric acid leads to the composition of the phosphorus element in the carbon structure (Baccar et al. 2009). Phosphoric acid is the most commonly used chemical activator, can produce high-porous activated carbon from raw materials and has fewer environmental and toxicological contaminants than potassium hydroxide and zinc chloride. Moreover, phosphoric acid requires a lower activation temperature (Al Bahri et al. 2012; de Yuso et al. 2014), is not volatile and can form a large number of alkaline or acid-soluble phosphates with elements such as iron, nickel and boron and others that can be incorporated into carbon precursors (Zou et al. 2016).
Yorgun et al. (2016) and Yorgun and Yildiz (2015) confirmed that H3PO4 was an effective activator agent, and they observed via carbon electron micrograph scanning electron microscope images that the pores created on the surface of activated carbon are tunnel-shaped and generally have a honeycomb structure. The honeycomb holes of the activated carbon have been fully developed as the corners of the cavities were clearly visible (Yorgun and Yildiz 2015). Acidic purification of activated carbon leads to an increase in the adsorption of various pollutants on the surface due to changes in the chemical surfaces of activated carbon as observed in various articles (de Yuso et al. 2014; Shamsuddin et al. 2016; Zhong et al. 2012), so purification can lead to the removal of hydroxides and the creation of reactive oxygen species groups on activated carbon. Also, the number of acidic functional groups is strongly associated with activated carbon capacity to absorb metal compounds (Bhatnagar et al. 2013; Shamsuddin et al. 2016; Yorgun and Yıldız 2015).
Activated carbon preparation through activation with zinc chloride
Among the activating reagents, zinc chloride is widely used to produce activated carbon, especially lignocellulosic and cellulosic precursors (Arami-Niya et al. 2010). Table 2 illustrates the detailed properties of activated carbon prepared through activation with zinc chloride (Zou et al. 2016).
Zinc chloride acts as a dampening agent for samples impregnated with this material during activation. Movement of volatile substances through zinc chloride-saturated pores is not disrupted, and after that, during the activation process, volatile substances are released from the surface of activated carbon. Increasing the mass ratio of zinc chloride causes easier release of volatile substances, so the absorption of nitrogen increases on the activated carbon (Arami-Niya et al. 2010). Zinc chloride activation induces an electrolytic action called swelling in the molecular structure of cellulose. Inflation causes a breaking down of the cellulose molecules and leads to an increase in different intra- and inter-coated cavities, which causes a higher surface area in the activated carbon (Saka 2012). During the activation process, lignocellulosic materials are converted into carbon, and hydrogen atoms, oxygen, carbon monoxide, carbon dioxide, methane and aldehydes are liberated, and diatomaceous distillates are produced (Anisuzzaman et al. 2016). Zinc chloride prevents the formation of bitumen and other fluids that block the surface of carbon monoxide and prevent the movement of volatile substances, and volatile substances are subsequently released from the surface of activated carbon (Deng et al. 2009).
In activation with zinc chloride, the yield of activated carbon increases due to polymerisation by zinc chloride and the creation of a few large-ring aromatic compounds (Anisuzzaman et al. 2016). Since zinc chloride does not react with carbon, the obtained activated carbon has a higher yield than activated carbon produced with potassium hydroxide. Using of zinc chloride leads to the removal of the hydrogen and oxygen atoms from the activated carbon structure (Gundogdu et al. 2013). The effect of temperature and amount of zinc chloride on various atoms is that the content of hydrogen and oxygen decreases while nitrogen increases (Alothman et al. 2011). Zinc chloride acts as a Lewis acid and enhances the condensed aromatic reactions by facilitating molecular hydrogen deformation from the hydro-aromatic structure of precursors so, through the exclusion of some of the active sites from the adjacent molecules, polymerisation reactions occur and are affected (Alothman et al. 2011; Gundogdu et al. 2013).
By increasing the amount of zinc chloride, more cracks may occur in the structure of activated carbon, so the productivity may decline, resulting in an increase in the mesoporosity of the activated carbon structure. It can be said that by increasing the weight ratio of zinc chloride, the structure breaks down and the micropores deform and become mesopores (Donald et al. 2011; Gundogdu et al. 2013). Increasing the amount of zinc chloride leads to the removal of volatile compounds from the activated carbon structure, so the number of acidic groups is reduced. It has also been reported that by increasing zinc chloride, the phenolic and carboxylic groups are also affected, while the lactone groups are not. Then, during activation with zinc chloride, phenolic and carboxylic groups are reduced, while lactone groups are increased (Angin 2014; Gundogdu et al. 2013). By increasing the amount of zinc chloride activating agent, the percentage of carbon and mesopores in the structure of the prepared activated carbon is increased (Gundogdu et al. 2013). Cavities on the surface of the activated carbon result from the evaporation of spaces occupied by zinc chloride during the carbonisation process, so chloride is an active agent in producing activated carbon with a high surface area and provides higher adsorption capacity (Angin 2014; Gundogdu et al. 2013).
In a study conducted by Arami-Niya et al. (2010), it was observed that the BET surface area and micropore and mesopore volumes of activated carbon prepared increased by increasing the amount of zinc chloride in the initial activator/precursor mixture. Moreover, by increasing the amount of zinc chloride, the removal of tar from the activated carbon structure was increased, as was the release of volatiles (Arami-Niya et al. 2010). Although zinc chloride is an excellent activating agent in activated carbon preparation, it is seldom used in the food and pharmaceutical industries due to its health-related problems (Anisuzzaman et al. 2016; Saka 2012).
Activated carbon preparation through activation with potassium carbonate
Potassium carbonate with the chemical formula K2CO3 is a well-known activating agent in the production of activated carbon (Abbas and Ahmed 2016; Budinova et al. 2008). Table 3 shows the performance and properties of activated carbon produced from various agricultural materials and activated with K2CO3. Potassium and sodium hydroxide have adverse effects, but potassium carbonate is not harmful if used for food supplements (often used as supplementary food supplements) (Budinova et al. 2008). Potassium carbonate is known to be a better activating agent than potassium hydroxide due to the production of activated carbon with higher yield, higher surface and pore volume, and higher capacity for adsorbing large molecules like methylene blue from aqueous solutions (Abbas and Ahmed 2016).
A study by Tay et al. (2009) showed that the activated carbon produced by potassium carbonate had higher yields than the activated carbon produced by potassium hydroxide. Also, under the same conditions, the specific surface area of the activated carbon produced from potassium carbonate is more than the carbon produced from potassium hydroxide. In addition, the activated carbon produced from potassium carbonate has lower ash and sulphur content than the activated carbon produced from potassium hydroxide (Tay et al. 2009). Foo and Hameed (2012c) reported that by increasing the ratio of potassium carbonate to char, the adsorptive capability of prepared orange peel-based activated carbon was enhanced (Foo and Hameed 2012c). Moreover, the presence of potassium carbonate prevents the formation of tar and other liquids such as acetic acid and methanol during the process (Adinata et al. 2007). The main reactions that may occur between the activating agent of potassium carbonate and activated carbon under gasification conditions (during the activation process) are presented in Eqs. (1)–(3) as follows (Adinata et al. 2007; Foo and Hameed 2012c; Horikawa et al. 2010):
According to these equations, the development of porosity during activation with K2CO3 is attributed to its reduction under inert condition to form K, K2O, CO2 and CO. The potassium compound formed during the activation stage penetrates the internal structure of char matrix, expands the existing pores and creates new pores (Adinata et al. 2007; Foo and Hameed 2012c; Horikawa et al. 2010). Also, due to the evaporation of potassium carbonate, the cavities on the activated carbon surface could be produced from the occupied spaces by the activating agent. These cavities create channels that provide the adsorbent molecules with access to the micro- and mesopores of the activated carbon (Abbas and Ahmed 2016). In K2CO3-activated carbons, phenolic groups in the surface area are more specific than the other groups and, with the increase in the activation temperature, the number of functional groups decreases (Horikawa et al. 2010). Also, by increasing the concentration of potassium carbonate, the dehydration effect decreases and leads to the degradation of mesopores, which reduces the adsorption efficiency. By increasing the carbonisation temperatures from 600 to 800 °C, the microporous pores increase on the surface of the activated carbon (Tay et al. 2009).
Activated carbon preparation through activation with sodium hydroxide
Studies show that chemical activation using alkaline materials such as sodium hydroxide and potassium hydroxide produces large amounts of microspores on the activated carbon surface (Martins et al. 2015). Sodium hydroxide is widely known as an effective activating agent for the production of activated carbon (Table 4) (Pezoti et al. 2016). The proposed reactions during activation by NaOH are presented in Eqs. (4)–(6) as follows (Martins et al. 2015; Pezoti et al. 2016):
The possible reactions between active substances and the surface of the organic precursor result in the creation of micropores on the activated carbon surface due to: i) the release of CO, CO2, H2 gases [Eqs. (4)–(6)], which are produced by Na2CO3 decomposition at high temperature and hydroxyl reduction, respectively, and ii) alkali metal intercalation into the carbon structure (Martins et al. 2015; Pezoti et al. 2016). As a result, the evaporation of sodium hydroxide and other compounds derived from activated carbon gives rise to a rugged surface with different pore sizes, indicating that the porous structure is well developed and that these canals are suitable channels for adsorbent materials to penetrate the surface of the activated carbon (Liou et al. 2016; Martins et al. 2015; Pezoti et al. 2016).
Through the oxidation reduction process, sodium hydroxide causes the separation and destruction of the graphite layers and thereby makes the pores expand (Liou et al. 2016; Pezoti et al. 2016). The production of carbonates and alkali metals in the carbon matrix leads to the sustainability and expansion of the spaces between the carbon atomic layers so, by increasing the sodium hydroxide-to-char ratio in the activation process, it plays a key role in the development of pores and increasing the surface area and pore volume (Foo and Hameed 2012a). However, according to Eq. (7), excess sodium hydroxide promotes a vigorous gasification reaction, which destroys the walls between the pores, leading to a dramatic decrease in accessible area. Moreover, excess sodium hydroxide molecules deposited in the carbon pore wall might cause catalytic oxidation and decomposition, lowering the adsorption capacity and carbon yield (Foo and Hameed 2012a):
In chemical activation with alkali, activation with potassium hydroxide generally shows better results than sodium hydroxide in terms of surface area and performance in different applications. However, sodium hydroxide is cheaper, more environmentally friendly and less harmful than potassium hydroxide, and it is clear that sodium hydroxide has industrial applications due to its superiority to potassium hydroxide (Gratuito et al. 2008). At high temperatures, the reaction between carbon and sodium hydroxide leads to the reduction of sodium cation to a sodium metal, the formation of sodium carbonate and the reduction of hydroxyl anion to hydrogen gas (Mestre et al. 2007). The results of the Byamba-Ochir et al. (2016) study showed that the activation of carbon by sodium hydroxide through physical mixing of precarbonised precursor and solid sodium hydroxide resulted in more porous activated carbon than that prepared using the impregnation procedure (Byamba-Ochir et al. 2016). Also, Foo and Hameed (2012b) studied the application of different activating agents in the preparation of activated carbon from langsat (Lansium domesticum) empty fruit bunch waste. In that study, sodium hydroxide was selected as the best activating agent due to its higher adsorption capacity, lower cost, lower environmental pollution during its life cycle and lower level of corrosiveness (Foo and Hameed 2012b). According to a review of various articles, activation with metal hydroxides such as sodium hydroxide and potassium can be used for the preparation of activated carbon with a high surface area in the range of 3500–2300 m2/g (Tongpoothorn et al. 2011).
Activated carbon preparation through activation with potassium hydroxide
In recent years, potassium salts such as KOH and K2CO3 have been widely used in the production of low-cost activated carbon (Hui and Zaini 2015). Among the various activators, potassium hydroxide has been extensively used, due to its ability to produce activated carbon with a high surface area, its distribution of fine pore size under the same conditions, low environmental pollution, less corrosiveness and lower cost (Chayid and Ahmed 2015; Zuo et al. 2016). The chemical activation of phosphoric acid and zinc chloride is used to activate lignocellulosic materials that have not previously been carbonised, while metal compounds such as potassium hydroxide are used to activate the precursors of coal (Yakout and El-Deen 2016). Several reports have been presented on the effectiveness of KOH-activated carbon in the adsorption of various organic chemicals such as phenol, dyes, heavy metals and pesticides (Table 5).
Tounsadi et al. (2016) reported that activated carbon with potassium hydroxide has the highest efficiency in the adsorption of heavy metals compared to other activators (Tounsadi et al. 2016). Cavities formed in activated carbon are the result of the evaporation of potassium hydroxide from places previously occupied by this activator (Njoku et al. 2013). KOH activator is an activating agent rapidly saturated with precursors and does not evaporate completely, so its activation temperature is generally lower than the boiling point of KOH (1327 °C) (Hui and Zaini 2015). KOH-activated carbon has a higher surface area and pore volume, but typically has lower yield (10–40%) compared to other activators such as ZnCl2 and H3PO4 (Ahmed and Theydan 2014). During activated carbon activation with alkali substances, alkali metals and carbonates are created which, in the carbon matrix, are responsible for the stability and expansion of the spaces between the carbon-atom layers and, as a result, increase the efficiency and adsorption capacity of activated carbon (Ahmed and Theydan 2014; Njoku et al. 2013). Activated carbon produced from potassium hydroxide has a higher microporous structure than activated carbon produced from sodium hydroxide (Ahmed and Theydan 2014; Wu et al. 2010). By increasing the dosage of potassium hydroxide, microporous pores develop on the surface of the activated carbon, while the mesoporous pores decrease due to the characteristics of the potassium hydroxide activators (Wu et al. 2010).
The results of a study by Jin et al. (2014) showed that chemical activation for the preparation of activated carbon from municipal waste with potassium hydroxide (2 M) significantly (Jin et al. 2014):
1. Increased the surface area and the total pore volume.
2. Modified the number of functional groups on the surface.
3. Increased the removal of arsenic in a shorter time.
4. Improved the arsenic adsorption capacity.
The possible reactions that may occur during the activation process with potassium hydroxide are presented in Eqs. (8)–(11) as follows (Meng and Park 2010; Jin et al. 2014):
Activated carbon with potassium hydroxide is oxidised in alkaline medium with high oxygen content (Huang and Zhao 2016). In strong activation with a high amount of potassium hydroxide, carbon atoms are eliminated from the internal structure of carbon, and the BET surface area increases with the formation of a porous structure (Vukčević et al. 2015). Oxygenated functional groups as active sites are able to interact with other molecules in adsorption applications (Bedin et al. 2016). By increasing the activation temperature of potassium hydroxide, the surface area of activated carbon and the number of oxygen groups of activated carbon increase (Vukčević et al. 2015). Generally, by increasing the impregnation ratio of potassium hydroxide to char, the surface area of the activated carbon increases, but if the amount of potassium hydroxide is about eight times greater, the walls between pores formed on activated carbon are further degraded so the surface area is reduced (Huang et al. 2015). Increasing the concentration of potassium hydroxide activator, the dehydration and degradation of the mesopores and their conversion to larger pores probably lead to a decrease in the adsorption capacity of activated carbon (Ahmed and Theydan 2014). The reactions of carbon with alkali metal activators are presented in Eqs. (12) and (13) as follows (Cha et al. 2016):
Correspondingly, K2CO3, along with carbon, is reduced to K, K2O, CO and CO2 in accordance with the following Eqs. (1)–(3).
Potassium metal is thought to be introduced into the internal structure of the carbon matrix during the gasification process, leading to the expansion of existing pores and the creation of new ones (Ahmed and Theydan 2014). Therefore, increasing the amount of potassium hydroxide plays a key role in porosity modelling. Porosity expands successfully, and micro- and mesopores are formed in the off-centre walls of the pores, which increases the Brunauer, Emmett and Teller surface area and pore volume (Chayid and Ahmed 2015).
Activation with potassium hydroxide can also be accomplished through direct chemical activation (physical mixing) or impregnation with activated chemicals. In direct chemical activation, in the first stage activated carbon precursors get saturated, moisture is removed and activation occurs at the desired ratio (KOH weight is greater than precursor weight). The impregnation is solid and then placed in the furnace at a specified temperature and time to heat it. Precursor carbonisation is often eliminated when the solid impregnation method is considered (Chayid and Ahmed 2015; Hui and Zaini 2015). By increasing activation, a large amount of potassium hydroxide is typically used, and the weight ratio of KOH to carbon is in the range of 3–7 in most cases. This not only increases the cost of preparation of materials but also increases the potential for environmental hazards caused by potassium hydroxide, the corrosiveness of the process of washing with acid solutions, which results in using other appropriate methods (such as potassium induction as an activation agent by ion exchange) for activation with potassium hydroxide (Ahmed and Theydan 2014; Chayid and Ahmed 2015; Hui and Zaini 2015; Wang et al. 2016).
Also, in the char impregnation method with KOH, potassium hydroxide molecules are readily in contact with the surface of the char, thus leading to a higher degree of micro- and mesoporosity. At the stage of washing the activated carbon, a significant amount of potassium hydroxide is introduced into the aquatic media before use. Some studies have focused on the significant concerns regarding the release of spent potassium hydroxide residues during activation, in terms of either environmental risks or their recovery potential after activation (Chayid and Ahmed 2015; Hui and Zaini 2015; Wang et al. 2016).
The mechanism of the potassium hydroxide reaction is described by Radovic and Rodriguez-Reinoso. In this mechanism (Fig. 4), potassium hydroxide is converted to K2O at the beginning of the dehydration process (step 1), and then K2O is converted to metallic potassium (step 2) (Tounsadi et al. 2016). The free potassium then penetrates the graphene layers and causes the structural expansion of the graphene layers. Moreover, after a series of reactions during activation with potassium hydroxide, oxidation (step 3) and hydration (step 4), various potassium compounds form. The carbon produced with chloride acid 0.1 N and water is then washed to remove K, K2O, K2CO3 and KOH residues from the graphene layers. However, potassium carbonate will decompose during the activation process and CO2 will be emitted. The reaction between the activating agent and the precursor of carbon materials results in the decomposition of volatile organic compounds, thus creating a porous surface on the surface of the activated carbon samples (Nur 2015; Tounsadi et al. 2016).

Adapted by kind permission of Noureddine Barka (Tounsadi et al. 2016)
Mechanisms of KOH activation.
Among the alkali metals, potassium hydroxide and sodium hydroxide are effective activators in producing activated carbon. Among the alkaline metals, KOH is the most effective factor for producing activated carbon. Researchers have been able to provide convincing descriptions of the activated carbon activation process with KOH (Rambabu et al. 2015). The results showed that sodium hydroxide has a lower efficiency than potassium hydroxide in the chemical activation of activated carbon, which is due to the different performances of metal hydroxides in the activation of activated carbon (Rambabu et al. 2015; Tay et al. 2009; Tounsadi et al. 2016).
Although potassium hydroxide increases the pore surface, potassium hydroxide-saturated activated carbon is less efficient than activated carbon saturated with zinc chloride or phosphoric acid, so activating with potassium hydroxide requires a high temperature (greater than 650 °C) and carbon content is less than constant carbon in the precursor. In this condition, the potassium metal is placed in the carbon matrix, with activated carbon efficiency lower than the carbon content of the raw material (Prauchner et al. 2016; Rambabu et al. 2015; Thitame and Shukla 2016; Yakout and El-Deen 2016). The use of KOH as an active agent is to produce activated carbon with a narrow pore size distribution and the development of effective porosity. It is believed that the activation mechanism with alkali metals such as KOH relies on the fact that alkali metals act as an input catalyst in the carbon network, an electron donor, during the reaction to gas (gasification) (Rambabu et al. 2015; Yahya et al. 2015).
Also, using KOH as an activator has been proposed for environmental compatibility with ZnCl2 (Yahya et al. 2015; Yakout and El-Deen 2016). Generally, chemical activation with alkaline groups leads to an increase in the positive charge on activated carbon, which is desirable to absorb contaminants with a negative charge (Bhatnagar et al. 2013; Thitame and Shukla 2016).
Activated carbon preparation through activation with other activating agents
Table 6 shows the performance and properties of prepared activated carbon from various agricultural materials and activated with various uncommon activating materials.
Phytic acid with the chemical formula C6H18O24P6 (Fig. 5) is one of the new activator materials. Phytic acid is a strong type of acid with the ability to chemically react with proteins in the form of a partial depolymerisation. In addition, phytic acid can easily enter into the spaces of raw material and cause dehydration on hemicelluloses and cellulose (Cheng et al. 2016).
Phytic acid decomposition can simultaneously lead to the release of some of the radicals that can accelerate oxidative decomposition and the process of carbonisation at lower temperatures. As a result, some cracks and pores are generated on the activated carbon surface after activation. By increasing the amount of impregnation, the pores on the activated carbon surface are reduced because they are further degraded by increasing the amount of impregnation, and more organic matter is produced, so it is deposited on the activated carbon surface and the pores are blocked (Al-Qodah and Shawabkah 2009; Cheng et al. 2016). In other studies, it was found that, by increasing the impregnation of activated carbon created with phytic acid, the acid decomposes into di-ester or monoester and eventually turns into phosphorus oxoacids, which can create organic materials with high amounts of free radicals, as well as increasing the efficiency. Sulphuric acid is a chemical activating agent that is able to dissolve many minerals and impurities from activated carbon precursor (Al-Qodah and Shawabkah 2009; Cheng et al. 2016; Olivares-Marín et al. 2012). An alternative method is chemical purification of dry carbonisation with concentrated sulphuric acid. Sulphuric acid is a highly reactive material that can be combined with organic compounds (such as carbohydrates and other organic materials) to remove water and break down organic precursors into carbon elements according to Eq. (14) (Olivares-Marín et al. 2012):
In addition, sulphuric acid reacts with the mineral compounds of lignocellulosic material. In fact, sulphuric acid is used as a cleaning and de-ashing agent from activated carbon precursors, so using H2SO4 for carbonisation has some advantages in addition to low cost (Olivares-Marín et al. 2012). Activation with sulphuric acid has been used for various porous structures, in which the sulphuric acid activation process enters into the material and leads to large and medium porosity on the surface of the activated carbon (Al-Qodah and Shawabkah 2009; Cheng et al. 2016; Olivares-Marín et al. 2012).
Di-ammonium hydrogen with a molecular formula of (NH4)2HPO4 obtained from ammonium phosphate ((NH4)3PO4) decomposition at temperatures above 155 °C is one of the activating agents for the production of activated carbons with various pore distribution and surface area (Benaddi et al. 2000). In a study by Li et al. (2016a) conducted on activated carbon preparation with different ammonium phosphate groups, it was found that the activated carbon prepared with (NH4)3PO4 (activated carbon –(NH4)3PO4) had much higher surface area and pore volume than those prepared with (NH4)2HPO4 (activated carbon –(NH4)2HPO4) and NH4H2PO4 (activated carbon –NH4H2PO4), while activated carbon –(NH4)2HPO4 had the largest micropore volume ratio. Activated carbon –NH4H2PO4 had a lower specific surface area. The different micropore sizes distribution in the carbons was attributed to the different molecular sizes of these (NH4)x HyPO4. In this regard, activated carbon –(NH4)3PO4 had the highest pore volume because (NH4)3PO4 can be converted to lower-sized NH4H2PO4. Overall, activated carbon –(NH4)2HPO4 had higher nickel adsorption capacity than other adsorbents (Li et al. 2016a). The results of the study obtained by Benaddi et al. (2000) showed that the maximum obtained surface area in activated carbon prepared by phosphoric acid was 1800 m2/g (Benaddi et al. 2000), while in the results of the study Liodakis et al. (2009), the highest surface area obtained in activated carbon prepared by (NH4)2HPO4 was about 1350 m2/g, but the pore structure of (NH4)2HPO4-activated carbon was mainly composed of micropores (Liodakis et al. 2009).
Ferric chloride is an activating agent that has rarely been used as such. The small size of iron cations enables ferric chloride to produce activated carbon of small pore size. Various reports indicate that impregnation with ferric chloride has a significant effect on increasing the specific surface area and development of micropores (Theydan and Ahmed 2012).
During the activation process of potassium hydroxide with activated carbon, potassium acetate (C2H3O2K) is produced, which is known as a good activator (Auta and Hameed 2011a). Potassium acetate is converted to potassium and acetate ions when dissolved in deionised water. Under optimal conditions, the adsorption of opposite charges in the solution of both potassium acetate and water leads to the formation of new compounds such as KOH from the hydrolysis of metal ions (Auta and Hameed 2011b).
The effect of physical mixing and impregnation methods on chemical activation
In the chemical activation process, the initial mixing of dry precursor and alkali hydroxides (such as sodium hydroxide and potassium hydroxide) could be performed in two ways (Lillo-Ródenas et al. 2007):
- 1.
Impregnation In this method, precursors are mixed with suitable volumes of hydroxide solutions and then the samples are filtered and dried at 110 °C.
- 2.
Physical mixing In this method, the precursors are mixed directly with solid hydroxide lentils at room temperature and other activation steps are performed (it should be emphasised that this process is done in the absence of water).
These two simple processes are useful for activating with sodium hydroxide and potassium hydroxide. In addition, it has been proven that the method of physical mixing is much more efficient in the case of sodium hydroxide (Lillo-Ródenas et al. 2007). A study by Ros et al. (2006) on the preparation of activated carbon from sewage sludge showed that activation with sodium hydroxide through physical mixing, compared to impregnation, led to a higher Brunauer, Emmett and Teller surface area in the final activated carbon. This finding was attributed to better physical contact between the precursor and the sodium hydroxide powder in the physical mixing method (Ros et al. 2006). Overall, the physical mixing method is good for the development of porosity in activated carbon (Lillo-Ródenas et al. 2007; Ros et al. 2006). The behaviour of NaOH and KOH is very similar, although activation with KOH produces a slightly higher porosity (Lillo-Ródenas et al. 2007; Ros et al. 2006). The general reaction of metal hydroxides during the activation stage of activated carbon production is according to Eq. (15) (Lillo-Ródenas et al. 2007; Ros et al. 2006):
Activation after physical mixing is rarely studied (Lillo-Ródenas et al. 2001). In a study by Lillo-Ródenas et al. (2001) to compare the impregnation and physical mixing methods of the sodium hydroxide activator, the results indicated that the activated carbon produced by the physical mixing had a higher porosity than the activated carbon produced by impregnation (Lillo-Ródenas et al. 2001). Also, the activated carbon obtained from physical mixing has a higher surface area and greater pore volume than the activated carbon obtained by impregnation. Moreover, since the physical mixing method requires less time and work than the impregnation method, physical mixing is appropriate to produce activated carbon on an industrial scale (Alabadi et al. 2015; Lillo-Ródenas et al. 2001).
In a study by Byamba-Ochir et al. (2016) on the activation of activated carbon induced by anthracite with NaOH, both activation methods (physical mixing and impregnation) were used. The results indicated that the activation process under the same conditions by physical mixing resulted in higher Brunauer, Emmett and Teller surface area and pore volume than by impregnation, so the surface areas of the activated carbon obtained by physical mixing and impregnation were reported to be 2063–1357 and 1763–816 m2/g, respectively. In this regard, the adsorption capacity of activated carbon prepared through physical mixing was higher than that prepared through impregnation (Byamba-Ochir et al. 2016). Studies showed that the activation process by the impregnation method creates both micro- and mesopores, while in the physical mixing method, mesopores were formed on activated carbon. Activated carbon derived from impregnation resulted in more oxygenated functional groups and less carbon bonds than that produced by physical mixing (Alabadi et al. 2015; Byamba-Ochir et al. 2016; Lillo-Ródenas et al. 2001).
Other studies also reported that, through impregnation, more micropores are created on the activated carbon surface and a small fraction of the pores are mesopores. It seems that, in the impregnation method, small micropores are developed during the activation process, because the chemical activating agent is better distributed into the pores of raw material than by the physical mixing method (Byamba-Ochir et al. 2016; Lee et al. 2015; Lillo-Ródenas et al. 2001).
Basically, the two approaches mentioned in the process of activation of carbon precursors using alkali metal hydroxides depend on the physical state of the activating agent (Alabadi et al. 2015). In the mechanism of pore formation by physical mixing, a solid activating agent such as KOH pellet is mixed with activated carbon precursor and it seems that, during the carbon oxidation process, KOH is converted into potassium metal and carbonate. In the impregnation method, where the activating agent is the liquid phase and precursor in solid form such as KOH in the first step, KOH is broken down as Eq. (16) (Alabadi et al. 2015; Nur 2015):
The alkali metal ions have the power to bind to different materials. In aqueous solutions, potassium ions are randomly distributed and free to move. During activation of these ions, this leads to the formation of pores in the structure of the activated carbon. Then, in the washing stage, the potassium ions are removed from the activated carbon (Nur 2015; Tounsadi et al. 2016). The pore formation processes at lower and higher KOH concentrations are presented in Figs. 6 and 7.
Pore formation processes at lower KOH concentrations (< 2.0M): carbon sphere (CS) surrounded by free K+ in aqueous solution (a), penetration of energised potassium ion, *K + into the carbon sphere (b), removal of the ions to produce porous carbon sphere (c).

Adapted by kind permission of Sanagi (Nur 2015)
Pore formation and structural collapse of carbon spheres (CS) at higher KOH concentrations (above 2.5 M): penetration/intercalation of *K + into the carbon sphere (a); formation of through and blind pores (b); distortion of the sphere structure by the interconnecting through pores (c).
In the study of Nowicki et al. (2013), comparing the activating methods of activated carbon with sodium hydroxide by physical mixing and impregnation shows that the activated carbon produced by physical mixing is of more porosity and larger pore volume than that of activated carbon produced by impregnation. Also, oxygenated functional groups on activated carbon produced by physical mixing are greater in number and have higher adsorption capacity (Nowicki et al. 2013).
Conclusion
Activated carbon contains a wide range of carbonised materials that have a high degree of porosity and surface area. Due to its unique characteristics, it has various applications in water purification, domestic and industrial wastewater treatment, desalination, refining and separating gases, odour and pollutant removal and medical applications in many parts of the world. Today, various industrial wastes are used to produce activated carbon. The activation of activated carbon is done both physically and chemically. Compared with physical activation, chemical activation is more economical because it requires lower activation temperature, shorter processing time and higher carbon efficiency. Also, in the chemical activation method, the development of porous structures in activated carbon is greater.
Various chemicals used as potential activators include alkaline groups such as potassium hydroxide (KOH), sodium hydroxide (NaOH), calcium chloride (CaCl2), potassium carbonate (K2CO3), acidic groups such as phosphoric acid (H3PO4) and sulphuric acid (H2SO4), intermediate metal salts such as ZnCl2 and other activating agents. In this study, the role of different activation factors was studied in the performance and efficiency of activated carbon produced from various precursors. Utilising activated carbon in developed countries is very important due to the production cost and adsorption capacity of various pollutants, so the selection of the best activating agents in activated carbon production is of particular importance. The discussion of the present study showed that some activating chemicals create more adsorption capacity in the activated carbon.
Activation with phosphoric acid has less environmental and toxicological contamination than with zinc chloride and also requires a lower activation temperature than potassium hydroxide. Generally, in chemical activation with alkali materials, activation with potassium hydroxide gives a better result than sodium hydroxide in terms of surface area and performance in different applications. However, sodium hydroxide is cheaper, more environmentally friendly and is less harmful than potassium hydroxide, and it is clear that sodium hydroxide has industrial applications due to its superiority to potassium hydroxide. Also, activated carbon with potassium hydroxide is more efficient in adsorption than other activators.
On the other hand, the salts of alkali metals are used on the basis of the two methods of physical mixing and impregnation method for the activation of activated carbon. Comparison of these two approaches has shown that activated carbon produced by physical mixing is of a more porous structure and larger pore volume than activated carbon produced through impregnation. Despite the fact that a large number of articles have been written on the removal of various pollutants using activated carbon, few have compared the performance of different activation factors in the production of activated carbon using various primary precursors. As noted, several factors affect the activation of activated carbon, so lots of studies are necessary to better understand the adsorption mechanism and improve the adsorption of pollutants using activated carbon produced on various scales worldwide.
Abbreviations
- IR:
-
Impregnation rate
- MESH:
-
Medical subject headings
- q max :
-
Maximum adsorption capacity
- S BET :
-
Brunauer, Emmett and Teller surface area
- t :
-
Adsorption time
- T :
-
Activation temperature
- V t :
-
Total pore volume
- FTIR:
-
Fourier-transform infrared spectroscopy
References
Abbas AF, Ahmed MJ (2016) Mesoporous activated carbon from date stones (Phoenix dactylifera L.) by one-step microwave assisted K2CO3 pyrolysis. J Water Process Eng 9:201–207. https://doi.org/10.1016/j.jwpe.2016.01.004
Abdelnaeim MY, El Sherif IY, Attia AA et al (2016) Impact of chemical activation on the adsorption performance of common reed towards Cu (II) and Cd (II). Int J Miner Process 157:80–88. https://doi.org/10.1016/j.minpro.2016.09.013
Abdulkarim M, Al-Rub FA (2004) Adsorption of lead ions from aqueous solution onto activated carbon and chemically-modified activated carbon prepared from date pits. Adsorp Sci Technol 22:119–134. https://doi.org/10.1260/026361704323150908
Acosta R, Fierro V, De Yuso AM et al (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 149:168–176. https://doi.org/10.1016/j.chemosphere.2016.01.093
Adinata D, Daud WMAW, Aroua MK (2007) Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour Technol 98:145–149. https://doi.org/10.1016/j.biortech.2005.11.006
Ahmad MA, Puad NAA, Bello OS (2014) Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour Ind 6:18–35. https://doi.org/10.1016/j.wri.2014.06.002
Ahmed MJ, Theydan SK (2012) Physical and chemical characteristics of activated carbon prepared by pyrolysis of chemically treated date stones and its ability to adsorb organics. Powder Technol 229:237–245. https://doi.org/10.1016/j.powtec.2012.06.043
Ahmed MJ, Theydan SK (2014) Optimization of microwave preparation conditions for activated carbon from Albizia lebbeck seed pods for methylene blue dye adsorption. J Anal Appl Pyrolysis 105:199–208. https://doi.org/10.1016/j.jaap.2013.11.005
Al Bahri M, Calvo L, Gilarranz MA, Rodríguez JJ (2012) Activated carbon from grape seeds upon chemical activation with phosphoric acid: application to the adsorption of diuron from water. Chem Eng J 203:348–356. https://doi.org/10.1016/j.cej.2012.07.053
Alabadi A, Razzaque S, Yang Y et al (2015) Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem Eng J 281:606–612. https://doi.org/10.1016/j.cej.2015.06.032
Alothman Z, Habila M, Ali R (2011) Preparation of activated carbon using the copyrolysis of agricultural and municipal solid wastes at a low carbonization temperature. Carbon 24:67–72
Al-Qodah Z, Shawabkah R (2009) Production and characterization of granular activated carbon from activated sludge. Braz J Chem Eng 26:127–136. https://doi.org/10.1590/S0104-66322009000100012
Altenor S, Carene B, Emmanuel E et al (2009) Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J Hazard Mater 165:1029–1039. https://doi.org/10.1016/j.jhazmat.2008.10.133
Angin D (2014) Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 115:804–811. https://doi.org/10.1016/j.fuel.2013.04.060
Angın D, Altintig E, Köse TE (2013) Influence of process parameters on the surface and chemical properties of activated carbon obtained from biochar by chemical activation. Bioresour Technol 148:542–549. https://doi.org/10.1016/j.biortech.2013.08.164
Anisuzzaman S, Joseph CG, Krishnaiah D et al (2016) Removal of chlorinated phenol from aqueous media by guava seed (Psidium guajava) tailored activated carbon. Water Resour Ind 16:29–36. https://doi.org/10.1016/j.wri.2016.10.001
Arami-Niya A, Daud WMAW, Mjalli FS (2010) Using granular activated carbon prepared from oil palm shell by ZnCl2 and physical activation for methane adsorption. J Anal Appl Pyrolysis 89:197–203. https://doi.org/10.1016/j.jaap.2010.08.006
Attia AA, Girgis BS, Fathy NA (2008) Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: batch and column studies. Dyes Pigm 76:282–289. https://doi.org/10.1016/j.dyepig.2006.08.039
Auta M, Hameed B (2011a) Optimized waste tea activated carbon for adsorption of Methylene Blue and Acid Blue 29 dyes using response surface methodology. Chem Eng J 175:233–243. https://doi.org/10.1016/j.cej.2011.09.100
Auta M, Hameed B (2011b) Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem Eng J 171:502–509. https://doi.org/10.1016/j.cej.2011.04.017
Baccar R, Bouzid J, Feki M, Montiel A (2009) Preparation of activated carbon from Tunisian olive-waste cakes and its application for adsorption of heavy metal ions. J Hazard Mater 162:1522–1529. https://doi.org/10.1016/j.jhazmat.2008.06.041
Balajii M, Niju S (2019) Biochar-derived heterogeneous catalysts for biodiesel production. Environ Chem Lett. https://doi.org/10.1007/s10311-019-00885-x
Başar CA (2006) Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J Hazard Mater 135:232–241. https://doi.org/10.1016/j.jhazmat.2005.11.055
Basta A, Fierro V, El-Saied H, Celzard A (2009) 2-Steps KOH activation of rice straw: an efficient method for preparing high-performance activated carbons. Bioresour Technol 100:3941–3947. https://doi.org/10.1016/j.biortech.2009.02.028
Bedin KC, Martins AC, Cazetta AL et al (2016) KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem Eng J 286:476–484. https://doi.org/10.1016/j.cej.2015.10.099
Belala Z, Jeguirim M, Belhachemi M et al (2011) Biosorption of copper from aqueous solutions by date stones and palm-trees waste. Environ Chem Lett 9:65–69. https://doi.org/10.1007/s10311-009-0247-5
Benaddi H, Bandosz T, Jagiello J et al (2000) Surface functionality and porosity of activated carbons obtained from chemical activation of wood. Carbon 38:669–674. https://doi.org/10.1016/S0008-6223(99)00134-7
Bhatnagar A, Hogland W, Marques M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511. https://doi.org/10.1016/j.cej.2012.12.038
Bouchelta C, Medjram MS, Bertrand O, Bellat J-P (2008) Preparation and characterization of activated carbon from date stones by physical activation with steam. J Anal Appl Pyrolysis 82:70–77. https://doi.org/10.1016/j.jaap.2007.12.009
Boudrahem F, Soualah A, Aissani-Benissad F (2011) Pb (II) and Cd (II) removal from aqueous solutions using activated carbon developed from coffee residue activated with phosphoric acid and zinc chloride. J Chem Eng Data 56:1946–1955. https://doi.org/10.1021/je1009569
Brudey T, Largitte L, Jean-Marius C et al (2016) Adsorption of lead by chemically activated carbons from three lignocellulosic precursors. J Anal Appl Pyrolysis 120:450–463. https://doi.org/10.1016/j.jaap.2016.06.018
Budinova T, Petrov N, Parra J, Baloutzov V (2008) Use of an activated carbon from antibiotic waste for the removal of Hg(II) from aqueous solution. J Environ Manage 8:165–172. https://doi.org/10.1016/j.jenvman.2007.02.005
Byamba-Ochir N, Shim WG, Balathanigaimani M, Moon H (2016) Highly porous activated carbons prepared from carbon rich Mongolian anthracite by direct NaOH activation. Appl Surf Sci 379:331–337. https://doi.org/10.1016/j.apsusc.2016.04.082
Cabal B, Budinova T, Ania CO et al (2009) Adsorption of naphthalene from aqueous solution on activated carbons obtained from bean pods. J Hazard Mater 161:1150–1156. https://doi.org/10.1016/j.jhazmat.2008.04.108
Cabrita I, Ruiz B, Mestre AS et al (2010) Removal of an analgesic using activated carbons prepared from urban and industrial residues. Chem Eng J 163:249–255. https://doi.org/10.1016/j.cej.2010.07.058
Cazetta AL, Vargas AM, Nogami EM et al (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174:117–125. https://doi.org/10.1016/j.cej.2011.08.058
Cha JS, Park SH, Jung S-C et al (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15. https://doi.org/10.1016/j.jiec.2016.06.002
Chayid MA, Ahmed MJ (2015) Amoxicillin adsorption on microwave prepared activated carbon from Arundo donax Linn: isotherms, kinetics, and thermodynamics studies. J Environ Chem Eng 3:1592–1601. https://doi.org/10.1016/j.jece.2015.05.021
Chen Y, Huang B, Huang M, Cai B (2011) On the preparation and characterization of activated carbon from mangosteen shell. J Taiwan Inst Chem Eng 42:837–842. https://doi.org/10.1016/j.jtice.2011.01.007
Chen Y-D, Chen W-Q, Huang B, Huang M-J (2013) Process optimization of K2C2O4-activated carbon from kenaf core using Box–Behnken design. Chem Eng Res Des 91:1783–1789. https://doi.org/10.1016/j.cherd.2013.02.024
Cheng C, Liu H, Dai P et al (2016) Microwave-assisted preparation and characterization of mesoporous activated carbon from mushroom roots by phytic acid (C6H18O24P6) activation. J Taiwan Inst Chem Eng 67:532–537. https://doi.org/10.1016/j.jtice.2016.08.032
Crini G, Lichtfouse E, Wilson LD, Morin-Crini N (2019) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17:195–213. https://doi.org/10.1007/s10311-018-0786-8
Cui X, Jia F, Chen Y, Gan J (2011) Influence of single-walled carbon nanotubes on microbial availability of phenanthrene in sediment. Ecotoxicology 20:1277–1285. https://doi.org/10.1007/s10646-011-0684-3
de Yuso AM, Rubio B, Izquierdo MT (2014) Influence of activation atmosphere used in the chemical activation of almond shell on the characteristics and adsorption performance of activated carbons. Fuel Process Technol 119:74–80. https://doi.org/10.1016/j.fuproc.2013.10.024
Demiral H, Gündüzoğlu G (2010) Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour Technol 101:1675–1680. https://doi.org/10.1016/j.biortech.2009.09.087
Demiral İ, Şamdan CA (2016) Preparation and characterisation of activated carbon from pumpkin seed shell using H3PO4. Anadolu Univ J Sci Technol A Appl Sci Eng 17:125–138. https://doi.org/10.18038/btda.64281
Deng H, Yang L, Tao G, Dai J (2009) Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—application in methylene blue adsorption from aqueous solution. J Hazard Mater 166:1514–1521. https://doi.org/10.1016/j.jhazmat.2008.12.080
Din ATM, Hameed B, Ahmad AL (2009) Batch adsorption of phenol onto physiochemical-activated coconut shell. J Hazard Mater 161:1522–1529. https://doi.org/10.1016/j.jhazmat.2008.05.009
Donald J, Ohtsuka Y, Xu CC (2011) Effects of activation agents and intrinsic minerals on pore development in activated carbons derived from a Canadian peat. Mater Lett 65:744–747. https://doi.org/10.1016/j.matlet.2010.11.049
Dural MU, Cavas L, Papageorgiou SK, Katsaros FK (2011) Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: kinetics and equilibrium studies. Chem Eng J 168:77–85. https://doi.org/10.1016/j.cej.2010.12.038
El-Hendawy A-NA (2009) An insight into the KOH activation mechanism through the production of microporous activated carbon for the removal of Pb2+ cations. Appl Surf Sci 255:3723–3730. https://doi.org/10.1016/j.apsusc.2008.10.034
Erdem M, Orhan R, Şahin M, Aydın E (2016) Preparation and characterization of a novel activated carbon from vine shoots by ZnCl2 activation and investigation of its rifampicine removal capability. Water Air Soil Pollut 227:226. https://doi.org/10.1007/s11270-016-2929-5
Erdoğan S, Önal Y, Akmil-Başar C et al (2005) Optimization of nickel adsorption from aqueous solution by using activated carbon prepared from waste apricot by chemical activation. Appl Surf Sci 252:1324–1331. https://doi.org/10.1016/j.apsusc.2005.02.089
Fierro V, Muñiz G, Basta A et al (2010) Rice straw as precursor of activated carbons: activation with ortho-phosphoric acid. J Hazard Mater 181:27–34. https://doi.org/10.1016/j.jhazmat.2010.04.062
Foo K, Hameed B (2012a) Potential of jackfruit peel as precursor for activated carbon prepared by microwave induced NaOH activation. Bioresour Technol 112:143–150. https://doi.org/10.1016/j.biortech.2012.01.178
Foo K, Hameed B (2012b) Preparation of activated carbon by microwave heating of langsat (Lansium domesticum) empty fruit bunch waste. Bioresour Technol 116:522–525. https://doi.org/10.1016/j.biortech.2012.03.123
Foo K, Hameed B (2012c) Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour Technol 104:679–686. https://doi.org/10.1016/j.biortech.2011.10.005
Galhetas M, Mestre AS, Pinto ML et al (2014a) Carbon-based materials prepared from pine gasification residues for acetaminophen adsorption. Chem Eng J 240:344–351. https://doi.org/10.1016/j.cej.2013.11.067
Galhetas M, Mestre AS, Pinto ML et al (2014b) Chars from gasification of coal and pine activated with K2CO3: acetaminophen and caffeine adsorption from aqueous solutions. J Colloid Interface Sci 433:94–103. https://doi.org/10.1016/j.jcis.2014.06.043
Gao P, Liu Z-h, Xue G et al (2011) Preparation and characterization of activated carbon produced from rice straw by (NH4) 2HPO4 activation. Bioresour Technol 102:3645–3648. https://doi.org/10.1016/j.biortech.2010.11.080
Gao J-j, Qin Y-b, Zhou T et al (2013a) Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: kinetics, equilibrium, and thermodynamics studies. J Zhejiang Univ Sci B 14:650–658. https://doi.org/10.1631/jzus.B12a0225
Gao Y, Yue Q, Gao B et al (2013b) Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni (II) adsorption. Chem Eng J 217:345–353. https://doi.org/10.1016/j.cej.2012.09.038
Gopinath A, Kadirvelu K (2018) Strategies to design modified activated carbon fibers for the decontamination of water and air. Environ Chem Lett 16:1137–1168. https://doi.org/10.1007/s10311-018-0740-9
Gratuito MKB, Panyathanmaporn T, Chumnanklang R-A et al (2008) Production of activated carbon from coconut shell: optimization using response surface methodology. Bioresour Technol 99:4887–4895. https://doi.org/10.1016/j.biortech.2007.09.042
Gundogdu A, Duran C, Senturk HB et al (2013) Physicochemical characteristics of a novel activated carbon produced from tea industry waste. J Anal Appl Pyrolysis 104:249–259. https://doi.org/10.1016/j.jaap.2013.07.008
Hameed B, Din AM, Ahmad A (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825. https://doi.org/10.1016/j.jhazmat.2006.07.049
Hameed B, Tan I, Ahmad A (2009) Preparation of oil palm empty fruit bunch-based activated carbon for removal of 2, 4, 6-trichlorophenol: optimization using response surface methodology. J Hazard Mater 164:1316–1324. https://doi.org/10.1016/j.jhazmat.2008.09.042
Heidarinejad Z, Rahmanian O, Fazlzadeh M, Heidari M (2018) Enhancement of methylene blue adsorption onto activated carbon prepared from Date Press Cake by low frequency ultrasound. J Mol Liq 264:591–599. https://doi.org/10.1016/j.molliq.2018.05.100
Horikawa T, Kitakaze Y, Sekida T et al (2010) Characteristics and humidity control capacity of activated carbon from bamboo. Bioresour Technol 101:3964–3969. https://doi.org/10.1016/j.biortech.2010.01.032
Huang Y, Zhao G (2016) Preparation and characterization of activated carbon fibers from liquefied wood by KOH activation. Holzforschung 70:195–202. https://doi.org/10.1515/hf-2015-0051
Huang F-C, Lee C-K, Han Y-L et al (2014) Preparation of activated carbon using micro-nano carbon spheres through chemical activation. J Taiwan Inst Chem Eng 45:2805–2812. https://doi.org/10.1016/j.jtice.2014.08.004
Huang Y-P, Hou C-H, His H-C et al (2015) Optimization of highly microporous activated carbon preparation from Moso bamboo using central composite design approach. J Taiwan Inst Chem Eng 50:266–275. https://doi.org/10.1016/j.jtice.2014.12.019
Hui TS, Zaini MAA (2015) Potassium hydroxide activation of activated carbon: a commentary. Carbon Lett 16:275–280. https://doi.org/10.5714/CL.2015.16.4.275
Ibrahim T, Moctar BL, Tomkouani K et al (2014) Kinetics of the adsorption of anionic and cationic dyes in aqueous solution by low-cost activated carbons prepared from sea cake and cotton cake. Am Chem Sci J 4:38–57. https://doi.org/10.9734/ACSJ/2014/5403
Islam MA, Benhouria A, Asif M, Hameed B (2015) Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes. J Taiwan Inst Chem Eng 52:57–64. https://doi.org/10.1016/j.jtice.2015.02.010
Islam MA, Ahmed M, Khanday W et al (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285. https://doi.org/10.1016/j.ecoenv.2017.01.010
Jadhav A, Mohanraj G (2016) Synthesis of activated carbon from Cocos nucifera leaves agrowaste by chemical activation method. J Chem Eng 10:201–208
Jin H, Capareda S, Chang Z et al (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As (V) removal: adsorption property and its improvement with KOH activation. Bioresour Technol 169:622–629. https://doi.org/10.1016/j.biortech.2014.06.103
Jolly G, Dupont L, Aplincourt M, Lambert J (2006) Improved Cu and Zn sorption on oxidized wheat lignocellulose. Environ Chem Lett 4:219–223. https://doi.org/10.1007/s10311-006-0051-4
Karagöz S, Tay T, Ucar S, Erdem M (2008) Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour Technol 99:6214–6222. https://doi.org/10.1016/j.biortech.2007.12.019
Khadhri N, Saad MEK, ben Mosbah M, Moussaoui Y (2019) Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J Environ Chem Eng 7:102775. https://doi.org/10.1016/j.jece.2018.11.020
Khezami L, Capart R (2005) Removal of chromium (VI) from aqueous solution by activated carbons: kinetic and equilibrium studies. J Hazard Mater 123:223–231. https://doi.org/10.1016/j.jhazmat.2005.04.012
Klasson KT, Ledbetter CA, Uchimiya M, Lima IM (2013) Activated biochar removes 100% dibromochloropropane from field well water. Environ Chem Lett 11:271–275. https://doi.org/10.1007/s10311-012-0398-7
Kosheleva RI, Mitropoulos AC, Kyzas GZ (2019) Synthesis of activated carbon from food waste. Environ Chem Lett 17:429–438. https://doi.org/10.1007/s10311-018-0817-5
Kumar A, Jena HM (2016) Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J Clean Prod 137:1246–1259. https://doi.org/10.1016/j.jclepro.2016.07.177
Kyzas GZ, Deliyanni EA, Matis KA (2016) Activated carbons produced by pyrolysis of waste potato peels: cobalt ions removal by adsorption. Colloids Surf A Physicochem Eng Asp 490:74–83. https://doi.org/10.1016/j.colsurfa.2015.11.038
Lee H-C, Byamba-Ochir N, Shim W-G et al (2015) High-performance super capacitors based on activated anthracite with controlled porosity. J Power Sources 275:668–674. https://doi.org/10.1016/j.jpowsour.2014.11.072
Lemraski EG, Sharafinia S (2016) Kinetics, equilibrium and thermodynamics studies of Pb2+ adsorption onto new activated carbon prepared from Persian mesquite grain. J Mol Liq 219:482–492. https://doi.org/10.1016/j.molliq.2016.03.031
Li Y, Du Q, Wang X et al (2010) Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. J Hazard Mater 183:583–589. https://doi.org/10.1016/j.jhazmat.2010.07.063
Li J, Ng DH, Song P et al (2015) Preparation and characterization of high-surface-area activated carbon fibers from silkworm cocoon waste for Congo red adsorption. Biomass Bioenergy 75:189–200. https://doi.org/10.1016/j.biombioe.2015.02.002
Li G, Wang M, Huang J et al (2016a) Preparation of activated carbon from Iris tectorum with different ammonium phosphates activation and removal of nickel from aqueous solution. J Taiwan Inst Chem Eng 59:341–347. https://doi.org/10.1016/j.jtice.2015.08.013
Li H, Sun Z, Zhang L et al (2016b) A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloids Surf A Physicochem Eng Asp 489:191–199. https://doi.org/10.1016/j.colsurfa.2015.10.041
Lillo-Ródenas M, Lozano-Castelló D, Cazorla-Amorós D, Linares-Solano A (2001) Preparation of activated carbons from Spanish anthracite: II. Activation by NaOH. Carbon 39:751–759. https://doi.org/10.1016/S0008-6223(00)00186-X
Lillo-Ródenas M, Marco-Lozar J, Cazorla-Amorós D, Linares-Solano A (2007) Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J Anal Appl Pyrolysis 80:166–174. https://doi.org/10.1016/j.jaap.2007.01.014
Liodakis S, Fetsis I, Agiovlasitis I (2009) The fire-retarding effect of inorganic phosphorus compounds on the combustion of cellulosic materials. J Therm Anal Calorim 98:285–291. https://doi.org/10.1007/s10973-009-0307
Liou T-H, Wang PY, Liou YH (2016) An effective method to enhance adsorption capacity and mesoporosity of activated carbon by pre-pyrolysis and chemical activation procedures. BioResources 11:6110–6124. https://doi.org/10.15376/biores.11.3.6110-6124
Liu Q-S, Zheng T, Li N et al (2010a) Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl Surf Sci 256:3309–3315. https://doi.org/10.1016/j.apsusc.2009.12.025
Liu Q-S, Zheng T, Wang P, Guo L (2010b) Preparation and characterization of activated carbon from bamboo by microwave-induced phosphoric acid activation. Ind Crops Prod 31:233–238. https://doi.org/10.1016/j.indcrop.2009.10.011
Liu Y, Guo Y, Gao W et al (2012) Simultaneous preparation of silica and activated carbon from rice husk ash. J Clean Prod 32:204–209. https://doi.org/10.1016/j.jclepro.2012.03.021
Liu J, Li Y, Li K (2013) Optimization of preparation of microporous activated carbon with high surface area from Spartina alterniflora and its p-nitroaniline adsorption characteristics. J Environ Chem Eng 1:389–397. https://doi.org/10.1016/j.jece.2013.06.003
Liu B, Gu J, Zhou J (2016) High surface area rice husk-based activated carbon prepared by chemical activation with ZnCl2–CuCl2 composite activator. Environ Prog Sustain Energy 35:133–140. https://doi.org/10.1002/ep.12215
Martins AC, Pezoti O, Cazetta AL et al (2015) Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: kinetic and equilibrium studies. Chem Eng J 260:291–299. https://doi.org/10.1016/j.cej.2014.09.017
Meng L-Y, Park S-J (2010) Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J Colloid Interface Sci 325:498–503. https://doi.org/10.1016/j.jcis.2010.08.048
Mestre A, Pires J, Nogueira J, Carvalho A (2007) Activated carbons for the adsorption of ibuprofen. Carbon 45:1979–1988. https://doi.org/10.1016/j.carbon.2007.06.005
Mestre AS, Bexiga AS, Proença M et al (2011) Activated carbons from sisal waste by chemical activation with K2CO3: kinetics of paracetamol and ibuprofen removal from aqueous solution. Bioresour Technol 102:8253–8260. https://doi.org/10.1016/j.biortech.2011.06.024
Mishra SB, Mishra AK, Khan MA (2010) Decolourization of pulp and paper mill effluents using heat-treated coal: a comparison with activated charcoal. Environ Chem Lett 8:231–235. https://doi.org/10.1007/s10311-009-0211-4
Mohammadi SZ, Karimi MA, Afzali D, Mansouri F (2010) Removal of Pb(II) from aqueous solutions using activated carbon from Sea-buckthorn stones by chemical activation. Desalination 262:86–93. https://doi.org/10.1016/j.desal.2010.05.048
Mohammadi SZ, Karimi MA, Yazdy SN et al (2014) Removal of Pb(II) ions and malachite green dye from wastewater by activated carbon produced from lemon peel. Quim Nova 37:804–809. https://doi.org/10.5935/0100-4042.20140129
Molina-Sabio M, Rodrıguez-Reinoso F (2004) Role of chemical activation in the development of carbon porosity. Colloids Surf A Physicochem Eng Asp 241:15–25. https://doi.org/10.1016/j.colsurfa.2004.04.007
Momčilović M, Purenović M, Bojić A et al (2011) Removal of lead (II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 276:53–59. https://doi.org/10.1016/j.desal.2011.03.013
Moreno-Barbosa JJ, López-Velandia C, del Pilar Maldonado A et al (2013) Removal of lead (II) and zinc (II) ions from aqueous solutions by adsorption onto activated carbon synthesized from watermelon shell and walnut shell. Adsorption 19:675–685. https://doi.org/10.1007/s10450-013-9491-x
Morin-Crini N, Loiacono S, Placet V et al (2019) Hemp-based adsorbents for sequestration of metals: a review. Environ Chem Lett 17:393–408. https://doi.org/10.1007/s10311-018-0812-x
Mouni L, Merabet D, Bouzaza A, Belkhiri L (2011) Adsorption of Pb(II) from aqueous solutions using activated carbon developed from Apricot stone. Desalination 276:148–153. https://doi.org/10.1016/j.desal.2011.03.038
Njoku V, Foo K, Hameed B (2013) Microwave-assisted preparation of pumpkin seed hull activated carbon and its application for the adsorptive removal of 2, 4-dichlorophenoxyacetic acid. Chem Eng J 215:383–388. https://doi.org/10.1016/j.cej.2012.10.068
Njoku V, Foo K, Asif M, Hameed B (2014) Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem Eng J 250:198–204. https://doi.org/10.1016/j.cej.2014.03.115
Norouzi S, Heidari M, Alipour V et al (2018) Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour Technol 258:48–56. https://doi.org/10.1016/j.biortech.2018.02.106
Nowicki P, Kuszyńska I, Przepiórski J, Pietrzak R (2013) The effect of chemical activation method on properties of activated carbons obtained from pine cones. Open Chem 11:78–85. https://doi.org/10.2478/s11532-012-0140-0
Nunthaprechachan T, Pengpanich S, Hunsom M (2013) Adsorptive desulfurization of dibenzothiophene by sewage sludge-derived activated carbon. Chem Eng J 228:263–271. https://doi.org/10.1016/j.cej.2013.04.067
Nur H (2015) Understanding pore formation and structural deformation in carbon spheres during KOH activation.pdf. Sains Malays 44(4):613–618
Olivares-Marín M, Fernández-González C, Macías-García A, Gómez-Serrano V (2012) Preparation of activated carbon from cherry stones by physical activation in air. Influence of the chemical carbonisation with H2SO4. J Anal Appl Pyrolysis 94:131–137. https://doi.org/10.1016/j.jaap.2011.11.019
Oliveira LC, Pereira E, Guimaraes IR et al (2009) Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J Hazard Mater 165:87–94. https://doi.org/10.1016/j.jhazmat.2008.09.064
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 115:64–73. https://doi.org/10.1016/j.biombioe.2018.04.015
Patnukao P, Pavasant P (2008) Activated carbon from Eucalyptus camaldulensis Dehn bark using phosphoric acid activation. Bioresour Technol 99:8540–8543. https://doi.org/10.1016/j.biortech.2006.10.049
Pezoti O, Cazetta AL, Bedin KC et al (2016) NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies. Chem Eng J 288:778–788. https://doi.org/10.1016/j.cej.2015.12.042
Prauchner MJ, Sapag K, Rodríguez-Reinoso F (2016) Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO2. Carbon 110:138–147. https://doi.org/10.1016/j.carbon.2016.08.092
Rambabu N, Rao B, Surisetty V et al (2015) Production, characterization, and evaluation of activated carbons from de-oiled canola meal for environmental applications. Ind Crops Prod 65:572–581. https://doi.org/10.1016/j.indcrop.2014.09.046
Reddy KSK, al Shoaibi A, Srinivasakannan C (2012) A comparison of microstructure and adsorption characteristics of activated carbons by CO2 and H3PO4 activation from date palm pits. New Carbon Mater 27:344–351. https://doi.org/10.1016/S1872-5805(12)60020-1
Reffas A, Bernardet V, David B et al (2010) Carbons prepared from coffee grounds by H3PO4 activation: characterization and adsorption of methylene blue and Nylosan Red N-2RBL. J Hazard Mater 175:779–788. https://doi.org/10.1016/j.jhazmat.2009.10.076
Ros A, Lillo-Ródenas M, Fuente E et al (2006) High surface area materials prepared from sewage sludge-based precursors. Chemosphere 65:132–140. https://doi.org/10.1016/j.chemosphere.2006.02.017
Saka C (2012) BET, TG–DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. J Anal Appl Pyrolysis 95:21–24. https://doi.org/10.1016/j.jaap.2011.12.020
Samsuri A, Sadegh-Zadeh F, Seh-Bardan B (2014) Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals. Int J Environ Sci Technol 11:967–976. https://doi.org/10.1007/s13762-013-0291-3
Sawant SY, Munusamy K, Somani RS et al (2017) Precursor suitability and pilot scale production of super activated carbon for greenhouse gas adsorption and fuel gas storage. Chem Eng J 315:415–425. https://doi.org/10.1016/j.cej.2017.01.037
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004. https://doi.org/10.1016/j.jclepro.2015.12.055
Shamsuddin M, Yusoff N, Sulaiman M (2016) Synthesis and characterization of activated carbon produced from kenaf core fiber using H3PO4 activation. Procedia Chem 19:558–565. https://doi.org/10.1016/j.proche.2016.03.053
Soleimani M, Kaghazchi T (2008) Adsorption of gold ions from industrial wastewater using activated carbon derived from hard shell of apricot stones—an agricultural waste. Bioresour Technol 99:5374–5383. https://doi.org/10.1016/j.biortech.2007.11.021
Spagnoli AA, Giannakoudakis DA, Bashkova S (2017) Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: the role of surface and structural parameters. J Mol Liq 229:465–471. https://doi.org/10.1016/j.molliq.2016.12.106
Sun Y, Yue Q, Gao B et al (2012) Preparation of activated carbon derived from cotton linter fibers by fused NaOH activation and its application for oxytetracycline (OTC) adsorption. J Colloid Interface Sci 368:521–527. https://doi.org/10.1016/j.jcis.2011.10.067
Sun Y, Li H, Li G et al (2016) Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour Technol 217:239–244. https://doi.org/10.1016/j.biortech.2016.03.047
Tang Y-b, Liu Q, Chen F-y (2012) Preparation and characterization of activated carbon from waste ramulus mori. Chem Eng J 203:19–24. https://doi.org/10.1016/j.cej.2012.07.007
Tay T, Ucar S, Karagöz S (2009) Preparation and characterization of activated carbon from waste biomass. J Hazard Mater 165:481–485. https://doi.org/10.1016/j.jhazmat.2008.10.011
Theydan SK, Ahmed MJ (2012) Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: equilibrium, kinetics, and thermodynamic studies. J Anal Appl Pyrolysis 97:116–122. https://doi.org/10.1016/j.jaap.2012.05.008
Thitame P, Shukla S (2016) Adsorptive removal of reactive dyes from aqueous solution using activated carbon synthesized from waste biomass materials. Int J Environ Sci Technol 13:561–570. https://doi.org/10.1007/s13762-015-0901-3
Tongpoothorn W, Sriuttha M, Homchan P et al (2011) Preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation and characterization of their physico-chemical properties. Chem Eng Res Des 89:335–340. https://doi.org/10.1016/j.cherd.2010.06.012
Tounsadi H, Khalidi A, Farnane M et al (2016) Experimental design for the optimization of preparation conditions of highly efficient activated carbon from Glebionis coronaria L. and heavy metals removal ability. Process Saf Environ Prot 102:710–723. https://doi.org/10.1016/j.psep.2016.05.017
Tseng R-L (2007) Physical and chemical properties and adsorption type of activated carbon prepared from plum kernels by NaOH activation. J Hazard Mater 147:1020–1027. https://doi.org/10.1016/j.jhazmat.2007.01.140
Tseng R-L, Tseng S-K (2006) Characterization and use of high surface area activated carbons prepared from cane pith for liquid-phase adsorption. J Hazard Mater 136:671–680. https://doi.org/10.1016/j.jhazmat.2005.12.048
Uysal T, Duman G, Onal Y et al (2014) Production of activated carbon and fungicidal oil from peach stone by two-stage process. J Anal Appl Pyrolysis 108:47–55. https://doi.org/10.1016/j.jaap.2014.05.017
Vicinisvarri I, Kumar S, Aimi N et al (2014) Preparation and characterization of phosphoric acid activated carbon from Canarium Odontophyllum (Dabai) nutshell for methylene blue adsorption. Res J Chem Environ 18:57–62
Vukčević MM, Kalijadis AM, Vasiljević TM et al (2015) Production of activated carbon derived from waste hemp (Cannabis sativa) fibers and its performance in pesticide adsorption. Microporous Mesoporous Mater 214:156–165. https://doi.org/10.1016/j.micromeso.2015.05.012
Wang Y, Ngo H, Guo W (2015) Preparation of a specific bamboo based activated carbon and its application for ciprofloxacin removal. Sci Total Environ 533:32–39. https://doi.org/10.1016/j.scitotenv.2015.06.087
Wang B, Zhu C, Zhang Z et al (2016) Facile, low-cost, and sustainable preparation of hierarchical porous carbons from ion exchange resin: an improved potassium activation strategy. Fuel 179:274–280. https://doi.org/10.1016/j.fuel.2016.03.088
Wu F-C, Tseng R-L, Juang R-S (2005) Preparation of highly microporous carbons from fir wood by KOH activation for adsorption of dyes and phenols from water. Sep Purif Technol 47:10–19. https://doi.org/10.1016/j.seppur.2005.03.013
Wu F-C, Wu P-H, Tseng R-L, Juang R-S (2010) Preparation of activated carbons from unburnt coal in bottom ash with KOH activation for liquid-phase adsorption. J Environ Manage 91:1097–1102. https://doi.org/10.1016/j.jenvman.2009.12.011
Xu J, Chen L, Qu H et al (2014) Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl Surf Sci 320:674–680. https://doi.org/10.1016/j.apsusc.2014.08.178
Yahya MA, Al-Qodah Z, Ngah CZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Yakout S, El-Deen GS (2016) Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab J Chem 9:S1155–S1162. https://doi.org/10.1016/j.arabjc.2011.12.002
Yorgun S, Yıldız D (2015) Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J Taiwan Inst Chem Eng 53:122–131. https://doi.org/10.1016/j.jtice.2015.02.032
Yorgun S, Yıldız D, Şimşek YE (2016) Activated carbon from paulownia wood: yields of chemical activation stages. Energy Sources Part A 38:2035–2042. https://doi.org/10.1080/15567036.2015.1030477
Yousefi M, Arami SM, Takallo H et al (2019) Modification of pumice with HCl and NaOH enhancing its fluoride adsorption capacity: kinetic and isotherm studies. Hum Ecol Risk Assess 25:1508–1520. https://doi.org/10.1080/10807039.2018.1469968
Youssef A, Ahmed A, El-Bana U (2012) Adsorption of cationic dye (MB) and anionic dye (AG 25) by physically and chemically activated carbons developed from rice husk. Carbon Lett 13:61–72. https://doi.org/10.5714/CL.2012.13.2.061
Zhang D, Yin J, Zhao J et al (2015a) Adsorption and removal of tetracycline from water by petroleum coke-derived highly porous activated carbon. J Environ Chem Eng 3:1504–1512. https://doi.org/10.1016/j.jece.2015.05.014
Zhang Z, Luo X, Liu Y et al (2015b) A low cost and highly efficient adsorbent (activated carbon) prepared from waste potato residue. J Taiwan Inst Chem Eng 49:206–211. https://doi.org/10.1016/j.jtice.2014.11.024
Zhong Z-Y, Yang Q, Li X-M et al (2012) Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Ind Crops Prod 37:178–185. https://doi.org/10.1016/j.indcrop.2011.12.015
Zhu J, Yang J, Deng B (2010) Ethylenediamine-modified activated carbon for aqueous lead adsorption. Environ Chem Lett 8:277–282. https://doi.org/10.1007/s10311-009-0217-y
Zou Z, Zhang Y, Zhang H, Jiang C (2016) A combined H3PO4 activation and boron templating process for easy synthesis of highly porous, spherical activated carbons as a superior adsorbent for rhodamine B. RSC Adv 6:15226–15233
Zuo L, Ai J, Fu H et al (2016) Enhanced removal of sulfonamide antibiotics by KOH-activated anthracite coal: batch and fixed-bed studies. Environ Pollut 211:425–434. https://doi.org/10.1016/j.envpol.2015.12.064
Acknowledgements
This research has been supported by the Hormozgan University of Medical Sciences (Code # 980071) and Tehran University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heidarinejad, Z., Dehghani, M.H., Heidari, M. et al. Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18, 393–415 (2020). https://doi.org/10.1007/s10311-019-00955-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-019-00955-0