Abstract
Sustainable management of organic solid wastes (OSW) within environmental, economic, and social standards is becoming an increasingly important and hot topic. This chapter gives a brief introduction to the types, sources and properties of OSW and then outlines technologies for sustainable recycle or conversion of OSW into biofuels and chemicals. In this chapter, features of biological, chemical, thermochemical, and photo-chemical technologies are described. An overview of databases used in life cycle assessment (LCA) of OSW and related topics are given. Advantages and scope of each technology are given for converting OSW into valuable products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the industrial revolution , world population and economic development have entered a period of rapid development. By 2030, the world’s population will increase to 8.6 × 109 people [1] and with the bloom of modern science and technology, large percentages of society will have migrated from rural areas to urban areas, which now account for more than 55% of the population (>4 × 109 persons) in 2017 [2]. The GDP of the world economy has been increasing at an annual rate of about 2% [3] such that world health and prosperity depend on large amounts of basic materials needed for survival, such as food, clean water, air, shelter, clothing, clean cooking facilities and energy [4]. The World Bank (2020) reported that an average individual generates nearly 0.74 kg of solid waste per day and that generation of solid waste in 2025 can be expected to increase to 2.2 × 109 tons per year [5]. Hence, appropriate methods for processing solid waste with the aim of reducing and conserving resources are requirements for achieving sustainable society.
A considerable part of solid waste contains carbon compounds and is referred to as organic solid waste (OSW) in this book. According to the source and characteristics of OSW , it can be divided into municipal solid waste (MSW), organic sludge, polymer solid waste and agricultural waste [6, 7]. OSW has a complex composition and creates environmental pollution due to its wide variety, changeable shape, and properties. However, OSW still has some common characteristics, such as being mainly composed of elements, C, H, and O and containing highly volatile compounds and having high calorific value [8, 9]. Therefore, OSW cannot simply be regarded as another type of polluting waste, but rather as an under-utilized resource. In other words, OSW should be regarded as a new type of renewable resource that contains large amounts of carbon and hydrogen. OSW has the potential to be used as a renewable carbon source for producing energy and chemicals provided that efficient and sustainable methods can be developed for its conversion. The concept of OSW as a renewable resource for sustainable development should become an essential element necessary for achieving ecological harmony.
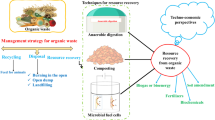
Waste-to-energy and chemicals (WTEC) strategy is an economically viable and environmentally sustainable proposal for recovering carbon from waste resources through production of fuel , chemical, heat, and electricity [10, 11]. WTEC strategy has a vital role for sustainable waste management and mitigation of environmental issues and can also address global warming and climate change [12]. To date, many techniques have been proposed and applied for the recovery of OSW . According to the strategy, processing conditions, technology maturity, product , recycle methods can be divided into conventional treatment and advanced treatment categories. Conventional technologies are incineration, gasification, pyrolysis, hydrothermal treatment [13,14,15,16] and bio-chemical methods (e.g., anaerobic fermentation, aerobic fermentation, and enzymatic hydrolysis ) [7, 17, 18]. Advanced technologies include chemolysis, mechanochemical degradation, photodegradation and microbial fuel cells [19,20,21,22].
This chapter provides a brief introduction to multiple solid wastes (type, sources, and properties) and introduces conventional and advanced strategies for sustainable recycle of OSW into biofuels and chemicals.
2 Classification, Properties of Organic Solid Waste
The origin and characteristics of OSW can be divided into four categories: (i) MSW , (ii) organic sludge, (iii) polymer solid waste and (iv) agricultural wastes . OSW has complex composition, wide variability and consists of an aggregate of many kinds of materials that can have very different physical properties which means that much more attention needs to be given to its source for effective reuse and recycle. Properties and characteristics of four typical OSW along with their variations according to source will be introduced in the following sections.
2.1 Multiple Solid Wastes (MSW )
MSW refers to the materials that are discarded as a result of urban daily life or activities, mainly coming from urban households, urban commerce, catering industry, hotel industry, tourism, service industry, municipal sanitation, transportation, industrial enterprises, water supply and drainage treatment sludge activities [23,24,25]. MSW has the characteristics of being huge in quantity, wide in variety, and complex in composition. Annual MSW generation can be expected to continue to increase with changing lifestyles and increasing population [5]. As shown in Fig. 1.1, 2.01 × 109 tons of MSW were generated in 2016, and 2.59 × 109 tons of global MSW are expected to be generated annually by 2030. Moreover, MSW generation across the world is expected to reach 3.40 × 109 tons in 2050.
Forecast of global MSW generation based on historical trends [5]
Typical MSW includes organic wastes (kitchen discards, yard, or garden related, paper, plastic ) and inorganic wastes (glass, metal, electronics, construction) [26, 27]. The composition of MSW not only depends on its region of generation, but also on socioeconomic status and stage of human development (infancy, adolescence, adulthood). Table 1.1 illustrates the composition of MSW collected from different regions according to income level. Global food loss and green waste (weeds, leaves, and grass cuttings) account for a large proportion of wastes for middle- and low-income level households with the percentage of food and green waste being over 50% [5]. The percentage of OSW in MSW decreases as income levels increase. Compared with low-income countries, consumption waste, such as paper and plastic , has higher percentage in MSW collected from high-income level households. Moreover, the granularity of waste composition, such as detailed rubber and wood waste, increases as household income levels increase. The percentage of “Other” in MSW from low-income level households is up to 27%, which is much higher than high-income level households, and implies that MSW from low-income level households is less-defined than MSW from higher-income level households. Nevertheless, MSW has value as a resource and it could be used to produce useful chemical products within a certain scope, time, and conditions, rather than being treated as something to be discarded.
2.2 Organic Sludge
Organic sludge is a solid residue generated from wastewater treatment operations. Sources of organic sludge can be divided into three major categories: (i) sewage, (ii) paper, and (iii) dye [28]. Sewage sludge, which is the largest source of solids, is generated in quantities of more than 1.50 × 108 tons each year [29]. The main component of sewage sludge is functional microbes and secreted extracellular polymeric substances (EPS) that are suspended in wastewater [30]. Paper sludge is derived from wastewater treatment operations in the pulp and paper industry for which approximately (40–50) kg of paper sludge is generated per 1000 kg paper produced in typical mills [31]. Paper sludge is rich in cellulose with low lignin content, which makes it a useful raw material for renewable production of hydrogen, bio-fuels and chemicals. Dye sludge is a waste stream generated by wastewater treatment plants of the textile industry [32]. Currently, more than 2.1 × 107 tons of dye sludge are generated each year in China, and its quantity continues to increase annually [33]. In addition, many toxic components (dyes, pathogens, additives, and heavy metals) are contained in dye sludge , which makes it a high priority to properly dispose and treat the waste to prevent serious hazards to the natural environment and public health [34]. The main characteristics of dye sludge are high water content, high organic content, and low calorific value. Dye sludge is rich in nitrogen, phosphorus, and other nutrients, but it can contain heavy metals ions. Table 1.2 shows the proximate analysis of organic sludge derived from three major sources. Considerable opportunities exist for developing suitable recycle and reuse technologies for organic sludge.
2.3 Polymer Solid Waste
Polymer solid waste is generated from human daily life and industrial production for which the source materials are mainly derived from petroleum [35, 36]. Polymer solid waste can be divided into two categories, (i) plastics and (ii) rubber [37] and they are mainly composed of carbon, hydrogen, oxygen (C, H and O) and some metals. Polymer solid waste is difficult to degrade by nature according to its design for durability in the environment, thus it causes environmental pollution, including ecosystem disturbance and toxic substance release, with common examples being microplastics and heavy metals [38, 39].
Waste plastic is a general term for plastics that have been used and eventually eliminated or replaced in civil, industrial, and other applications. Due to their favorable properties (e.g., lightweight, good processing characteristics, easy application , chemical stability), plastics are widely used in consumer products [40,41,42]. Plastic products are an indispensable part of daily life and are widely applied in construction, healthcare, electronic components, agriculture, automotive, and packaging industries. From 1950 to 2015, it is estimated that over 9 × 109 tons of virgin plastic have been produced all over the world [43]. In 2018, approximately 3.60 × 108 tons of plastics were produced globally with increasing trends so that production can be expected to increase to 5 × 108 tons in 2025 at an annual global production growth rate of 8.4% [44]. Demand for plastics is mainly for polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC ), polyurethane (PUR), polyethylene terephthalate (PET ), and polystyrene (PS) [43]. Correspondingly, generated plastic waste is proportional to the above major plastic products. Waste plastics that do not easily degrade in nature can persist in the environment for decades or hundreds of years and can cause problems that affect the ecosystem and living creatures [45,46,47].
Rubber is widely used as a raw material for producing many flexible polymeric products that are widely used in transportation, industrial, agricultural, medical treatment, and construction [48,49,50]. Rubber has become the second most used polymer after plastics. In 2018, global rubber consumption reached 2.94 × 107 tons and has an annual growth rate of 3.4% which means that much waste rubber will continue to be generated in the future [51]. Rubber differs from polymers in that a vulcanization step or curing step is needed to make the product useful. Over 60% of rubber is used as a raw material for producing tires, with more than 2 × 107 tons of waste tires being generated in 2020, such that rubber has an annual growth rate of 8% [52, 53]. Waste tires are challenging to recycle because of their complex ingredients and additives that make them non-biodegradable by design, and furthermore, they have lead to the phrase, “black pollution” that has become a global scale environmental, public health and safety issue [54]. Waste rubber has high volatile content, low ash content, high calorific value with a higher heating value (HHV) of (26–36) MJ/kg that is similar to high rank coals, so that waste rubber can be considered as a rich source of fuel and chemicals through technologies such as pyrolysis and combustion [48].
2.4 Agricultural and Forestry Solid Waste
Agricultural and forestry solid waste refers to unwanted materials generated in farming, forest management and animal husbandry that are required to maintain the life of about 7 × 109 people all over the world [55,56,57]. Agricultural and forestry solid waste can be divided into two categories: (i) lignocellulosic waste , such as straw, bagasse, discarded branches and palm kernel shells, and (ii) animal manure waste , such as that generated from the production of poultry, dairy, or pig farming. The properties and characteristics of lignocellulosic waste and animal manure waste will be introduced next.
Lignocellulosic waste refers to biomass-like solid wastes such as rice straw , wheat straw, bagasse, or wood sawdust some of which are also generated in agricultural and forestry production processes [58]. Lignocellulosic waste is the most abundant form of OSW and is composed of carbon, hydrogen, and oxygen, but it can also include inorganics such as silica, potassium, calcium, sodium, magnesium, and aluminum oxides [59]. Every year, more than 2 × 109 tons of lignocellulose solid waste are generated worldwide [60]. Lignocellulosic waste is composed of cellulose, hemicellulose, and lignin [61]. Cellulose is composed of D-glucose monomers through the linear polymerization of β(1˗4) glycoside bonds and makes up about 40–80% of the content of lignocellulosic waste [62]. Hemicellulose is composed of sugar monomers (C5 and C6) including glucose, galactose, mannose, xylose, arabinose, with xylose (C5) monomers or mannose (C5) monomers being in larger proportions than C6 monomeric units with the content of hemicellulose being about 25–35% in lignocellulosic biomass [63]. Lignin is a complex three-dimensional polymer of propyl-phenol groups bound together by C-O (β-O-4, α-O-4, 4-O-5 linkage) and C-C (β-5, 5–5, β-1, β–β linkage) bonds with its content in lignocellulosic biomass being about 10–36% [64]. More than 5 × 107 tons of industrial lignin are generated annually in the paper and pulp industry [65]. Lignocellulosic waste has the characteristics of being renewable, having low pollution potential, being widely distributed and being available in large amounts as a carbon neutral resource . Valorization of lignocellulosic waste into high value-added, eco-friendly, eco-efficient, and recyclable products (e.g., biofuels, biochemical , materials) with sustainable methods is an important goal for realizing renewable carbon circulation for a zero-waste society [66,67,68].
Animal manure is a solid waste obtained from livestock farming that has a wide range of applications [69]. In 2014, the total mass of animal manure waste was 3.9 × 109 tons, and this amount will continue to grow at a rate of about 2% per year [70]. By 2030, generation of animal manure is expected to reach at least 4.6 × 109 tons. Unlike lignocellulosic wastes, animal manure waste has high nitrogen content and intrinsic metal content, which makes it highly recyclable as a source of organic fertilizer or nutrients or as a source of energy and metals [71]. The carbon/nitrogen (C/N) ratio, which is a key organic fertilizer quality parameter, is inversely proportional to nitrogen immobilization in soil and favorable for animal manure waste. The overall C/N ratio in the composting process is in the range of 10–30, but the appropriate C/N ratio for different substrates is different and requires further research [72]. Apart from its use as an organic fertilizer , animal manure can be used as an energy resource in the production of biogas. However, there are risks in animal manure waste processing due to its potential as a biohazard, so that management technologies are needed to realize its effective use [73].
3 Thermochemical Recycling Technology
Technologies for recovery of OSW are highly dependent on factors related to technological, social, environmental, and economic impact. Moreover, properties of OSW , such as type, composition, volume, and energy content, also depend on region, population, and economy [5]. Thermochemical recycle technology, which includes incineration, gasification, pyrolysis, and liquefaction, is widely applicable for converting OSW into valuable products (e.g., biofuels, chemicals, biochar, and heat) under appropriate conditions [74,75,76]. Figure 1.2 summarizes selected thermochemical conversion technologies for recycling OSW along with their corresponding products that will be discussed in detail below.
3.1 Incineration/Combustion
Incineration or combustion is a treatment technology that can convert OSW into heat and electricity through oxidation in air at short reaction times [77]. Due to the many advantages of the technology, such as large processing capacity, small space requirements, low labor requirements, low time consumption and low investment and operating costs, incineration or combustion is the most common recycling method for converting waste into energy and preferred in many cases over landfilling wastes [78, 79]. During incineration, OSW is completely converted into flue gas , bottom ash, fly ash, and slag that are accompanied with the release of a large amount of heat at high temperatures that can be further used for generating electrical power. Two types of incineration systems in general use are: (i) mass incineration and (ii) modular incineration and they depend on process scale [26]. Mass incineration systems are the most widely applied thermal treatment, in which unprocessed or unsorted MSW is burned in large furnaces in the presence of excess air, which is coupled to a boiler and a generator for producing electricity [80]. Modular incineration systems are mainly composed of a rotary kiln and fluidized bed, which are an important supplement to mass incineration [81]. The compact nature of modular systems makes it easier to transport and act as a mobile solid waste treatment device than mass incineration systems.
Compared with landfilling, incineration has several important merits: (i) direct treatment of virtually any waste without pretreatment, (ii) weight reduction by 80–85%, and (iii) volume reduction by 95–96%, thereby realizing low amounts and volumes of solid waste while generating energy [82, 83]. Moreover, incineration reduces maintenance and eliminates biohazards due to rodents, pests, flies, and foul odors from microbial decomposition, and thereby increasing safety and health for much of the surroundings. However, incineration also generates fly ash (solid residue ) or potentially releases dioxins (extremely harmful exhaust gases) depending on the constituents of the substrates which can cause serious health problems to humans and living creatures [84]. Therefore, the tail gas and tailings generated by incineration must be treated with effective techniques to remove solid and gaseous pollutants. Incineration process generates large amounts of CO2 [85], so that carbon capture and storage (CCS) or carbon capture and utilization (CCU) strategies must be implemented.
3.2 Pyrolysis
Pyrolysis is a thermochemical transformation technology that depolymerizes OSW into solid, liquid and gas products in an inert atmosphere [86]. Pyrolysis is one of the main thermochemical technologies for converting OSW into energy-dense bio-oils [87]. Transformation of OSW to bio-oils under pyrolysis conditions occurs via free radical mechanisms, concerted mechanisms, and ionic mechanisms [88]. During the pyrolysis process, many chemical reactions occur simultaneously, such as decomposition, dehydration, cracking, isomerization, and hydrothermal reforming [89]. Although pyrolysis is a complex thermodynamic degradation process and the detailed pyrolysis product distributions are also complicated, much progress has been made in elucidating its reaction schemes [90].
The main products produced via pyrolysis processes are bio-oil (liquid product ), bio-char (solid product ), and gases [88]. The product distribution is greatly influenced by catalyst , temperature, heating rate , vapor residence time, inert gas flow rate, reactor geometry, feedstock type and properties such as moisture, particle size , and elemental composition [91]. According to the heating rate and vapor residence time, pyrolysis can be divided into several main types: (i) slow pyrolysis, (i) fast pyrolysis, (iii) intermediate pyrolysis and (iv) flash pyrolysis [92]. Slow pyrolysis is distinguished by its moderate temperature, low heating rate and longer residence time compared with other types of pyrolysis, thus increasing the yield of bio-char. Fast and flash pyrolysis processes are characterized by high temperatures, high heating rates, and short residence times, which promote higher yields of bio-oil than the other pyrolysis types. Pyrolysis can also be applied as a catalytic or non-catalytic process [93]. Catalysts used in the pyrolysis process can improve bio-oil quality and reduce costs [94]. Moreover, product distribution of bio-oil can also be simplified with catalytic pyrolysis processes. Catalytic pyrolysis can be applied as an in-situ or ex-situ process [95]. When the catalyst is mixed directly with the feedstock in the pyrolysis reactor , the process is referred to as in-situ catalytic fast pyrolysis (in-situ CFP ), whereas when the catalysts are only contacted with pyrolysis gases, the process is referred to as ex-situ catalytic fast pyrolysis (ex-situ CFP). Many reactor types are used in pyrolysis processes, including fixed-bed, rotary kiln, fluidized-bed, and tubular, which depend on the properties of the raw materials and target products [96].
Pyrolysis has several advantages and disadvantages in its application [97, 98]. Pyrolysis processes are applicable to a variety of OSW , such as waste biomass, waste plastics, waste rubber, or MSW in which different targeted products (e.g., bio-char, bio-oil and biogas) can be selectively produced by optimizing pyrolysis conditions. Pyrolysis processes have low operating cost and high flexibility making them simple and reliable for large-scale commercial applications. On the other hand, pyrolysis processes tend to have complicated reactions, especially for the case of non-catalytic pyrolysis, which results in a complex distribution of liquid products along with a large amount of coke being formed, such that if catalysts are used, they are prone to deactivation. Since the price of catalysts used in pyrolysis processes tend to be relatively expensive, developing stable and inexpensive catalysts are necessary for future development. Furthermore, the obtained bio-char and pyrolysis gas can contain toxic components, which require further treatment before use.
3.3 Gasification
Gasification technology is a thermal conversion process that can break OSW into gaseous products such as syngas [99, 100]. Compared with pyrolysis processes, the products of gasification process are much simpler with the main components being CO, H2, CH4 and CO2 [101]. Syngas products can be further processed to produce green diesel, higher alcohols, long-chain hydrocarbons, and gasoline-like products via Fischer-Tropsch reaction [102].
According to the operating conditions, gasification processes can be divided into two main types: (i) high temperature thermo-gasification and (ii) hydrothermal gasification [26]. High temperature thermo-gasification involves partial or incomplete oxidation in presence of controlled amounts of oxidants (air, oxygen, steam) at very high temperatures (550 °C–1200 °C) [103]. Hydrothermal gasification involves cracking reactions under subcritical or supercritical water conditions [104]. For hydrothermal gasification, water not only serves as reaction medium but also as reactant. In hydrothermal gasification, water can be in a supercritical thermodynamic state or a subcritical thermodynamic state [105, 106]. Water in a thermodynamic state that is higher than its critical temperature (Tc ≥ 374.1 °C) and critical pressure (Pc ≥ 22.1 MPa) is referred to as supercritical water; water in a thermodynamic liquid state below its critical temperature (Tc < 374.1 °C) and critical pressure (Pc < 22.1 MPa) is referred to as subcritical water . Gasification processes can be catalytic or non-catalytic depending on the product requirements. Catalysts used in gasification processes are generally nickel-based, because conditions can be mild and reaction pathways can be regulated to form more H2 and CO than other products [107]. Gasification processes can be implemented as downdraft or updraft fixed bed, fluidized bed, entrained flow, or twin bed reactors depending on the product requirements [108]. Among the above gasification processes, fluidized bed reactors and twin bed reactors have great potential for application to form syngas of high-quality in scale-up.
Gasification processes are applicable to the same types of OSW as those used in incineration and pyrolysis processes [13, 99, 100, 108], but they are much cleaner than incineration and moreover, purification steps used for syngas products can be designed to eliminate emissions of poisonous gaseous impurities such as dioxins, allowing gasification processes to be much simpler than those of pyrolysis. Furthermore, in gasification of OSW , there is less liquid and coke produced than either pyrolysis or incineration, which make the technology easy to scale-up and to operate on a continuous basis. On the other hand, operating conditions for gasification processes are more severe regarding high temperatures for thermo-gasification and high pressures for hydrothermal gasification and some preprocessing steps (shredding, torrefaction, pelletization) are necessary to ensure uniform heat transfer and product quality. Catalysts, purification steps instruments, and high standard gasification reactors make the costs of gasification processes much higher than the other methods, such that further technology development is still needed.
3.4 Liquefaction
Liquefaction is a thermochemical technology for producing high energy-density liquid bio-oil from OSW by liquid phase decomposition of organic compounds under high-temperatures and high-pressures [109]. In addition to bio-oil, adhesives or epoxy resins may also be produced in the liquefaction process. Operating conditions for liquefaction are usually in the range of (200–370) °C and (4–20) MPa, which are milder than hydrothermal gasification processes [26]. Typical liquid phase mediums used in liquefaction are water or organic solvents , such as methanol, ether, butanol, or octanol [110]. Processes that use water as medium are referred to as hydrothermal liquefaction, while those that use organic solvents as medium are referred to as solvothermal liquefaction. Decomposition mechanisms in liquefaction are complicated and involve a series of chemical reactions that may include degradation, hydrolysis, cracking, steam reforming and isomerization [111]. Liquefaction processes can be operated with or without catalyst depending on the target products. Introducing catalysts into the liquefaction process can lower reaction temperature, increase product selectivity , enhance reaction kinetics , reduce reaction time, and improve bio-oil yields.
Liquefaction processes are suitable to treat high-moisture substrates, such as algae, sewage sludge, kitchen waste, animal manure , and aquatic biomass when water medium is used [110, 111]. When using water medium, raw materials do not need to be dried, which lowers operating cost and saves much energy. Bio-oil yields obtained from liquefaction processes are higher than those obtained from pyrolysis processes, and OSW can be transformed into energy at nearly complete conversion. On the other hand, liquefaction processes require somewhat harsh operating conditions, which means that high standards for liquefaction reactors must be used resulting in high equipment costs. The harsh conditions of high-pressure liquefaction make scale-up less certain than other methods and for continuous production, solid formation may limit OSW throughput.
4 Biochemical Recycling Technology
Biochemical recycling technology is a process that can convert OSW into chemical products through the presence of microorganisms or active enzymes, including anaerobic fermentation, aerobic fermentation, and enzymatic hydrolysis [6, 9, 10, 12]. Figure 1.3 summarizes OSW biochemical recycle technologies and their corresponding products. The processes shown in Fig. 1.3 will be discussed in detail in the following sections.
4.1 Anaerobic Digestion (Biomethanation )
Anaerobic digestion, which is also referred to as biomethanation, is one of the most important methods for converting OSW into energy [16]. In anaerobic digestion, microorganisms degrade OSW into dissolved organic matter and produce gaseous products such as CH4, H2 and CO2 in the absence of free oxygen [17, 26]. MSW , animal manure , sewage sludge, food processing waste, fats, grease, agricultural crop residues are among the wide ranges of biodegradable substrates that can be treated with anaerobic digestion.
Anaerobic digestion process is mainly composed of four stages: (i) hydrolysis, (ii) acidogenesis, (iii) acetogenesis, and (iv) methanogenesis [112]. In the hydrolysis stage, OSW is hydrolyzed to form simple soluble monomers or oligomers (e.g., glucose, amino acids , glycerol, fatty acids) via promotion by a diverse population of bacteria. In the acidogenesis stage, volatile fatty acids (e.g., acetic acid, propionic acid, butyric acid, and other minor products) are produced by fermentative bacteria that promote formation of small water-soluble molecules. In the acetogenesis stage, volatile fatty acids are transformed into acetate, CO2 and H2 by acetogenic bacteria. In the methanogenesis stage, acetic acid, H2 and CO2 are converted into CH4 and CO2 by strictly anaerobic methanogenic bacteria. Biogas yield is highly affected by factors, such as substrate composition, microbial loading, and operating parameters (temperature, pH, C/N ratio, water content, oxygen content, nutrition elements).
Anaerobic digestion has many advantages such as being able to: (i) treat many types of OSW , especially those with high-moisture or high nitrogen content, (ii) prevent manure and odor missions and (iii) improve air and water quality [113, 114]. Biogas from anaerobic digestion can be harvested to generate energy and thus reduce greenhouse-gas emissions, while solid residues can be used as fertilizer to improve soil nutrient content and to reduce the use of chemical fertilizers. Anaerobic digestion uses mild conditions and is a very simple process, which allows the technology to be readily implemented on a large scale with low treatment cost. However, anaerobic digestion has some weak points as a conversion technology [115]. In anaerobic digestion, the proliferation of anaerobic microorganisms is slow, making the entire treatment process require several weeks or even months. In the anaerobic digestion process, much wastewater is generated, which must be treated and so this increases the cost. Finally, the content of methane in the gas mixtures produced by anaerobic digestion is typically below 70%, thus making the methane gas purity very low. Moreover, because methane is an explosive gas, the reactor needs to be explosion-proof and to have subsequent gas purification steps [116].
4.2 Aerobic Composting
Aerobic composting uses the natural decomposition of organic matter in the presence of air to form a semi-solid product with the help of living microorganisms, worms and insects and their derived enzymes [42, 117]. Composting is a traditional, widely used, cheap and simple technology that can effectively treat and valorize OSW into useful products. Aerobic composting should be carried out in the presence of sufficient oxygen, and good ventilation should be maintained [118]. The semi-solid product formed is a type of organic fertilizer that typically has rich plant-available nutrients and is widely applicable to agriculture, horticulture, landscape, and other fields. In aerobic digestion processes, many types of substrates can be used such as MSW , animal manure , sewage sludge, food processing waste, fats, grease, and agricultural crop residues, showing that it has many similarities with anaerobic digestion [119].
Aerobic composting process is regarded as a biological and chemical coupling process that relies on aerobic bacteria to degrade organic matter [117, 120, 121]. In the process of aerobic composting, small water-soluble organic molecules penetrate microorganism cell walls and are absorbed, and then utilized by the microorganisms. Insoluble macromolecular organic substances become attached to the microorganism and are decomposed into small water-soluble molecules that further support microorganism bioactivity through the secretion of extracellular enzymes. Aerobic composting is affected by many parameters, including organisms, use of cultures, nutrients (C/N ratio), aeration, addition of sewage, characteristics of sewage sludge and operating parameters, such as temperature, pH, moisture, oxygen content, and compost (container or pile) flip frequency [122].
Aerobic composting is a mature technology that can reduce the amount of waste on a large scale, save much space, produce fertilizers, recycle humus and nutrients into the soil, protect and improve microbiological diversity and generally improve the quality of cultivated soils [26, 123]. Aerobic composting is an easily used technology that has minimal equipment requirements and low operating costs, however it carries the risk of secondary pollution, such as odor and potential contamination [117]. Moreover, greenhouse gas emissions (CH4, CO2) can be large for aerobic composting and if the OSW contains heavy metals or antibiotics, there is risk for soil and groundwater pollution along with the transfer of pathogens or chemicals into the life cycle [117]. Treatment of waste plastics and rubber is generally ineffective with aerobic composting.
4.3 Enzyme Hydrolysis
Enzymatic hydrolysis breaks down macromolecules into small molecules or monomeric compounds via biocatalysts (enzymes) that become active in water [124, 125]. In enzymatic hydrolysis, enzymes act on specific chemical bonds of macromolecules [126] to achieve high selectivity at mild operating conditions that can be under continuous reaction conditions. Enzymatic hydrolysis is widely used to produce ethanol from lignocellulosic materials, in which lignocellulose undergoes saccharification to form soluble monosaccharides with the help of cellulases. Enzymatic hydrolysis involves the following key steps [127, 128]: (i) enzymes transfer from aqueous phase to substrate surface; (i) enzymes adsorb onto substrate to form enzyme-substrate complexes, (iii) substrate undergoes hydrolysis, (iv) hydrolysis products transfer from substrate surface to aqueous phase. For the case of cellulose, cellodextrin , and cellobiose are further hydrolyzed into glucose in the aqueous phase. Enzymatic hydrolysis rates are sensitive to substrate type, substrate chemical structure, enzyme type, enzyme loading and operating conditions such as pH, temperature, substrate concentration, product concentration and oxygen content [128].
Enzymatic hydrolysis shows high conversion rates for specific substrates, high selectivity for product compounds and little undesirable by-product formation, so that it generates low amounts of aqueous waste [126, 128, 129]. Mild reaction conditions make enzymatic hydrolysis possible to be performed in commonly available equipment, thereby reducing equipment costs. However, reaction rates obtainable with enzymatic hydrolysis are relatively low (hours to days) compared with synthetic reactions and along with their low catalytic activity , there are few types hydrolytic enzymes making their cost high and their recycle necessary which is difficult for general OSW . Presently, enzymatic hydrolysis is limited to specific feedstocks such as lignocellulosic-based waste or polyesters (e.g., PET, PLA).
5 Advanced Technologies
5.1 Chemolysis
Chemolysis, which is also referred to as solvolysis or chemical depolymerization, is a recovery method following the principles of sustainable development [130]. Chemolysis is a process in which OSW can be treated to form chemical compounds or OSW can be depolymerized into monomeric compounds via selective breakage of C–O and C–N bonds [131]. Chemolysis is presently applicable to natural polymers (cellulose, chitin), petro-based polyesters, polyamides, polycarbonates, polylactides, polyethylenes terephthalates, polyurethanes, and nylons [131, 132]. Chemolysis processes can placed into categories of (i) hydrolysis, (ii) alcoholysis (e.g., methanolysis, glycolysis) or (iii) aminolysis as appropriate to the required degradation processes and desired chemical products [132]. Hydrolysis depolymerizes macromolecules in the aqueous phase using catalysts. Alcoholysis degrades polymers into suitable monomers in alcohol medium [133]. For example, when methanol is used as solvent, the process is called methanolysis; when polyols such as ethylene glycol , glycerol are used, the process is called glycolysis. Aminolysis uses nitrogen-containing solvents (e.g., ammonia, ammonium formate) to catalyze the depolymerization of polymers to form nitrogen-containing compounds [134]. Chemolysis is affected by substrate type and substrate chemical structure, reaction chemistry and reaction conditions [20, 135, 136]. In chemolysis processes, the type of catalyst is important for achieving desired product selectivity , for reducing reaction time and reaction temperatures and for improving reaction efficiency [20, 136]. Comparing with traditional thermochemical recovery processes, chemolysis has advantages of high selectivity , high product value, and high recovery rate [132]. Compared with traditional biochemical recovery process, chemolysis has advantages of high efficiency, high recovery rate and high selectivity . However, low substrate concentration is necessary to reduce side reactions that may occur during chemolysis processes, because byproducts complicate product recovery and solvent recycle. Therefore, classification of OSW prior to application of chemolysis is required. Development of robust chemolysis processes is required for future applications to paraffin waxes and thermoplastic polymers.
5.2 Mechanochemical Treatment
Mechanochemical treatment is a method that can convert OSW into small molecular compounds though coupling mechanical (shearing, grinding, compression, impaction) and chemical reactions that can be promoted by homogeneous or heterogeneous catalysts [137]. During the mechanochemical treatment process, mechanical stress changes the substrate physical structure by breaking weak chemical bonds, lowering crystallinity, and increasing specific surface area while chemical reactions occur simultaneously [138]. In addition, oxygen atmospheres can assist mechanochemical conversion reactions by promoting the generation of free radicals that accelerate degradation [139]. Therefore, mechanochemical treatment is applicable to the degradation of some types of OSW [137]. Mechanochemical treatment does not necessarily require solvent, which allows reduction of reagents and solvents and avoids post-processing separation or solvent recycle steps [138]. Compared with traditional recovery processes, mechanochemical treatment has advantages of simple processing steps, mild reaction conditions, short processing time and ecological safety [140]. Mechanochemistry has been regarded by the International Union of Pure and Applied Chemistry (IUPAC) as one of the ten world-changing technologies [141]. Although mechanochemistry has high potential for OSW valorization, energy consumption limits its application for large-scale industrialization. Thus, protocols that are both energy efficient and selective will be important for future development of mechanochemical treatment as a recycling technology.
5.3 Photodegradation
Photodegradation, which is also referred to as photooxidative degradation, is the process of decomposing polymers by absorbing energy from light to generate free radicals in the presence of oxygen [142]. Generally, light sources that promote photodegradation have near-ultraviolet wavelengths in the range of 290–400 nm [132, 139]. In photodegradation processes, polymer decomposition reactions include chain scission, crosslinking and secondary oxidative reactions, which are promoted via generation and transfer of free radicals [143]. There are two methods for generating free radicals in photodegradation: (i) irradiation of samples with ultraviolet light directly without catalyst and (ii) irradiation of samples with ultraviolet light with catalyst . Compared with other degradation technologies, photodegradation has the unique advantage of being spatiotemporally local, so that reactions can be controlled in a facile, green, and independent way [144]. Photodegradation can make use of sunlight in nature to degrade plastics for reducing time required for subsequent biodegradation or to completely degrade plastics [46]. On the other hand, photodegradation lacks product control and typically has low degradation rates along with low catalyst stability, making development of effective photodegradation systems a hot research topic.
5.4 Other Advanced Technologies
There are several other advanced technologies applicable to conversion of OSW that should be mentioned: (i) microbial fuel cells, (ii) ozonation, and (iii) Fenton oxidation . Microbial fuel cells are bio-electrochemical processes that can produce electricity through oxidizing organic compounds into adenosine triphosphate (ATP) in a series of continuous biochemical reactions [145, 146]. Ozonation and Fenton oxidation (H2O2 with ferrous iron) generate oxygen species to degrade OSW into chemical compounds [132, 147,148,149]. Although the number of research reports is still relatively few, the unique attributes of these methods are bound to spark the curiosity of scientists and engineers interested in developing new methods.
6 Life Cycle Assessment
To evaluate environmental impact, economic costs, and energy benefits in the scale-up of recycling technologies, life cycle assessment (LCA) models are used to analyze incineration, gasification, pyrolysis, anaerobic digestion and composting with energy recovery. In LCA , all material and energy inputs and outputs (e.g., emissions to air, water, land and products like power, heat, chemicals, biofuels, and fertilizers) are identified and quantified [150]. The quality of the database used in LCA is important, which can govern the impact and validity of the model’s output, and can even bias the conclusions [151]. Currently, databases used for LCA include Ecoinvent, GaBi, Easewaste, Easetech, Triangle Institute (RTI) and GEMIS [152]. Among them, Ecoinvent database is widely used and contains more than more than 2500 processes. In addition to databases, software is also essential for LCA . More than 20 kinds of LCA software have been used to conduct studies, including SimaPro, GaBi, OpenLCA, and Easewaste. SimaPro is one of the most popular LCA software platforms, as it has been employed in more than 30% of the surveyed studies [152]. These LCA software tools allow estimation of mass and energy flows and contain modules to include different waste treatment processes. For waste to energy, the inputs and outputs of OSW and energy are used to estimate the cost of energy and related processes, raw materials, pumps, pipes, transportation, and construction of the processing plants. Emissions from processes are also accounted for in the LCA analysis software. Table 1.3 shows some LCA results on the energy generation potential and greenhouse gas (GHG) emissions due to scale-up.
7 Conclusions and Outlook
Large amounts of organic solid waste (OSW) are generated every year which will lead to serious environmental and energy consequences if not properly recycled. Therefore, taking Reduce, Recycle and Reuse (3R’s) as important criteria, conversion of OSW into raw materials for energy and chemicals will not only reduce world dependence on fossil fuels, but also provide new avenues to eco-friendly restoration of the Earth. This chapter has provided a brief overview of the types and characteristics of substrates of OSW and introduced relevant technologies including thermochemical (e.g., incineration, gasification, pyrolysis, and hydrothermal treating), bio-chemical (e.g., anaerobic fermentation, aerobic fermentation, and enzymatic hydrolysis) and several advanced methods (e.g., chemolysis, mechanochemical degradation, photodegradation, microbial fuel cell, and Fenton oxidation ) for processing of OSW .
Recovery of OSW still faces many challenges: types of raw materials, collection and transportation, land use, environmental and economic impact. In terms of types of raw materials, the physical properties, chemical composition, and prices vary greatly according to the source, type, and collection location of the raw materials. It is difficult to establish a reproducible supply framework that has specific transformation methods for different types of raw materials for biological control or biofuels with such a wide variation of material feedstocks. One possible solution is improved consumer segregation or classification of materials that is being practiced at some level in virtually every country. In terms of collection and transportation of OSW , combined recovery technologies that require large amounts of OSW typically need to be far away from the source in current methodology. The cost for collecting and transporting large amounts of OSW is still high and challenging, such that local type of processing systems need to be extensively developed. Furthermore, there are increasing restrictions on land use: large areas of land would be required to completely replace chemicals and petroleum-derived fuels with OSW . In terms of sustainability aspects of each technology, appropriate monitoring and life cycle assessments must be modeled to investigate detailed social, environmental, and economic impact of OSW recovery methods. Finally, in developing new methods and technologies for OSW , it is necessary to assess environment risks to society, nature and the Earth.
References
McNabb DE. The population growth barrier. In: Global pathways to water sustainability. Palgrave Macmillan, Champions; 2019. https://doi.org/10.1007/978-3-030-04085-7_5.
Hedblom M, Knez I, Ode Sang Å, Gunnarsson B. Evaluation of natural sounds in urban greenery: potential impact for urban nature preservation. R Soc Open Sci. 2017;4:170037. https://doi.org/10.1098/rsos.170037.
Jean F, Agnes BQ, Lionel F. The great shift: macroeconomic projections for the world economy at the 2050 Horizon (February 10, 2012). CEPII Working Paper No. (2012), 3: Available at SSRN: https://ssrn.com/abstract=2004332
De Medina-Salas L, Castillo-González E, Giraldi-Díaz MR, Jamed-Boza LO. Valorisation of the organic fraction of municipal solid waste. Waste Manag Res. 2019;37(1):59–73. https://doi.org/10.1177/0734242X18812651.
Kaza S, Yao LC, Bhada-Tata P, Van Woerden F. What a waste 2.0: a global snapshot of solid waste management to 2050. Urban development, 2018. World Bank. https://openknowledge.worldbank.org/handle/10986/30317
Kumar A, Samadder SR. A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manage. 2017;69:407–22. https://doi.org/10.1016/j.wasman.2017.08.046.
Wainaina S, Awasthi MK, Sarsaiya S, Chen H, Singh E, Kumar A, Ravindran B, Awasthi SK, Liu T, Duan Y, Kumar S, Zhang Z, Taherzadeh MJ. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour Technol. 2020;301:122778. https://doi.org/10.1016/j.biortech.2020.122778.
Ortiz FJG, Kruse A, Ramos F, Ollero P. Integral energy valorization of municipal solid waste reject fraction to biofuels. Energy Convers Manag. 2019;180:1167–84. https://doi.org/10.1016/j.enconman.2018.10.085.
Dixon N, Jones DRV. Engineering properties of municipal solid waste. Geotext Geomembr. 2005;23(3):205–33. https://doi.org/10.1016/j.geotexmem.2004.11.002.
Stehlík P. Contribution to advances in waste-to-energy technologies. J Clean Prod. 2009;17(10):919–31. https://doi.org/10.1016/j.jclepro.2009.02.011.
Kothari R, Tyagi VV, Pathak A. Waste-to-energy: a way from renewable energy sources to sustainable development. Renew Sust Energ Rev. 2010;14(9):3164–70. https://doi.org/10.1016/j.rser.2010.05.005.
Sharma S, Basu S, Shetti NP, Kamali M, Walvekar P, Aminabhavi TM. Waste-to-energy nexus: a sustainable development. Environ Pollut. 2020;115501 https://doi.org/10.1016/j.envpol.2020.115501.
Assi A, Bilo F, Zanoletti A, Pontiet J, Valsesia A, Spina R, Zacco A, Bontempi E. Zero-waste approach in municipal solid waste incineration: reuse of bottom ash to stabilize fly ash. J Clean Prod. 2020;245:118779. https://doi.org/10.1016/j.jclepro.2019.118779.
Morris M, Waldheim L. Energy recovery from solid waste fuels using advanced gasification technology. Waste Manag. 1998;18(6–8):557–64. https://doi.org/10.1016/S0956-053X(98)00146-9.
Chen D, Yin L, Wang H, He P. Pyrolysis technologies for municipal solid waste: a review. Waste Manag. 2014;34(12):2466–86. https://doi.org/10.1016/j.wasman.2014.08.004.
Zhan L, Jiang L, Zhang Y, Gao B, Xu Z. Reduction, detoxification and recycling of solid waste by hydrothermal technology: a review. Chem Eng J. 2020;390:124651. https://doi.org/10.1016/j.cej.2020.124651.
Zamri M, Hasmady S, Akhiar A, Ideris F, Shamsuddin AH, Mofijur M, Fattah IMR, Mahlia TMI. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew Sust Energ Rev. 2021;137:110637. https://doi.org/10.1016/j.rser.2020.110637.
Jensen JW, Felby C, Jørgensen H, Nørholm ND, Rønsch G. Enzymatic processing of municipal solid waste. Waste Manag. 2010;30(12):2497–503. https://doi.org/10.1016/j.wasman.2010.07.009.
Kumar S, Panda AK, Singh RK. A review on tertiary recycling of high-density polyethylene to fuel. Resource Conserv Recycl. 2011;55(11):893–910. https://doi.org/10.1016/j.resconrec.2011.05.005.
Ragaert K, Delva L, Van Geem K, Laurenti E, Montoneri E, Arques A, Carlos L. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017;69:24–58. https://doi.org/10.1016/j.wasman.2017.07.044.
Avetta P, Bella F, Prevot AB, Laurenti E, Montoneri E, Arques A, Carlos L. Waste cleaning waste: photodegradation of monochlorophenols in the presence of waste-derived photosensitizer. ACS Sustain Chem Eng. 2013;1(12):1545–50. https://doi.org/10.1021/sc400294z.
Lee Y, Nirmalakhandan N. Electricity production in membrane-less microbial fuel cell fed with livestock organic solid waste. Bioresour Technol. 2011;102(10):5831–5. https://doi.org/10.1016/j.biortech.2011.02.090.
Singh RP, Tyagi VV, Allen T, Ibrahim MH, Kothari R. An overview for exploring the possibilities of energy generation from municipal solid waste (MSW) in Indian scenario. Renew Sust Energ Rev. 2011;15(9):4797–808. https://doi.org/10.1016/j.rser.2011.07.071.
Baawain M, Al-Mamun A, Omidvarborna H, Al-Amri W. Ultimate composition analysis of municipal solid waste in Muscat. J Clean Prod. 2017;148:355–62. https://doi.org/10.1016/j.jclepro.2017.02.013.
Colon J, Cadena E, Colazo AB, Quiros R, Sanchez A, Font X, Artola A. Toward the implementation of new regional biowaste management plans: environmental assessment of different waste management scenarios in Catalonia. Resources Conserv Recycl. 2015;95:143–55. https://doi.org/10.1016/j.resconrec.2014.12.012.
Mukherjee C, Denney J, Mbonimpa EG, Slagley J, Bhowmik R. A review on municipal solid waste-to-energy trends in the USA. Renew Sust Energ Rev. 2020;119:109512. https://doi.org/10.1016/j.rser.2019.109512.
Mou Z, Scheutz C, Kjedsen P. Evaluating the biochemical methane potential (BMP) of low-organic waste at Danish landfills. Waste Manag. 2014;34:2251–9. https://doi.org/10.1016/j.wasman.2014.06.025.
Hao Z, Yang B, Jahng D. Combustion characteristics of biodried sewage sludge. Waste Manage. 2018;72:296–305. https://doi.org/10.1016/j.wasman.2017.11.008.
Zhang Q, Hu J, Lee D-J, Chang Y, Lee Y-J. Sludge treatment: current research trends. Bioresour Technol. 2017;243:1159–72. https://doi.org/10.1016/j.biortech.2017.07.070.
Wu RM, Lee DJ. Hydrodynamic drag force exerted on a moving floc and its implication to free-settling tests. Water Res. 1998;32:760–8. https://doi.org/10.1016/S0043-1354(97)00320-5.
Gottumukkala L D, Haigh K, Collard F X, Rensburg Van R E, Görgens J. Opportunities and prospects of biorefinery-based valorisation of pulp and paper sludge. Bioresour Technol (2016) 215: 37–49. doi: https://doi.org/10.1016/j.biortech.2016.04.015
Xu G, Yang X, Spinosa L. Development of sludge-based adsorbents: preparation, characterization, utilization and its feasibility assessment. J Environ Manage. 2015;151:221–32. https://doi.org/10.1016/j.jenvman.2014.08.001.
Xie C, Liu J, Zhang X, Xie W, Sun J, Chang K, Kuo J, Xie W, Liu C, Sun S. Co-combustion thermal conversion characteristics of textile dyeing sludge and pomelo peel using TGA and artificial neural networks. Appl Energy. 2018;212:786–95. https://doi.org/10.1016/j.apenergy.2017.12.084.
Zhang H, Gao Z, Liu Y, Ran C, Mao X, Kang Q, Ao W, Fu J, Li J, Liu G, Dai J. Microwave-assisted pyrolysis of textile dyeing sludge, and migration and distribution of heavy metals. J Hazard Mater. 2018;355:128–35. https://doi.org/10.1016/j.jhazmat.2018.04.080.
Al-Salem SM, Lettieri P, Baeyens J. Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag. 2009;29(10):2625–43. https://doi.org/10.1016/j.wasman.2009.06.004.
Al-Salem SM, Lettieri P, Baeyens J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: from re-use to energy and chemicals. Prog Energy Combust Sci. 2010;36(1):103–29. https://doi.org/10.1016/j.pecs.2009.09.001.
Liu H, Wang Y, Zhao S, Hu H, Cao C, Li A, Yu Y, Yao H. Review on the current status of the co-combustion technology of Organic Solid Waste (OSW) and coal in China. Energy Fuel. 2020;34(12):15448–87. https://doi.org/10.1021/acs.energyfuels.0c02177.
Okan M, Aydin HM, Barsbay M. Current approaches to waste polymer utilization and minimization: a review. J Chem Technol Biotechnol. 2019;94(1):8–21. https://doi.org/10.1002/jctb.5778.
Coates GW, Getzler YDYL. Chemical recycling to monomer for an ideal, circular polymer economy. Nat Rev Mater. 2020;5(7):501–16. https://doi.org/10.1038/s41578-020-0190-4.
Silvarrey LSD, Phan AN. Kinetic study of municipal plastic waste. Int J Hydrog Energy. 2016;41(37):16352–64. https://doi.org/10.1016/j.ijhydene.2016.05.202.
Miskolczi N, Bartha L, Deak G, Jover B. Thermal degradation of municipal plastic waste for production of fuel-like hydrocarbons. Polym Degrad Stab. 2004;86(2):357–66. https://doi.org/10.1016/j.polymdegradstab.2004.04.025.
Quecholac-Piña X, García-Rivera MA, Espinosa-Valdemar RM, Vázquez-Morillas A, Beltrán-Villavicencio M, de la Luz Cisneros-Ramos A. Biodegradation of compostable and oxodegradable plastic films by backyard composting and bioaugmentation. Environ Sci Pollut Res. 2017;24(33):25725–30. https://doi.org/10.1007/s11356-016-6553-0.
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. https://doi.org/10.1126/sciadv.1700782.
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–71. https://doi.org/10.1126/science.1260352.
Zhao YB, Lv XD, Ni HG. Solvent-based separation and recycling of waste plastics: A review. Chemosphere. 2018;209:707–20. https://doi.org/10.1016/j.chemosphere.2018.06.095.
Jiao X, Zheng K, Chen Q, Li X, Li Y, Shao W, Xu J, Zhu J, Pan Y, Sun Y, Xie Y. Photocatalytic conversion of waste plastics into C2 fuels under simulated natural environment conditions. Angew Chem Int Ed. 2020;59(36):15497–501. https://doi.org/10.1002/anie.201915766.
Barnes SJ. Understanding plastics pollution: the role of economic development and technological research. Environ Pollut. 2019;249:812–21. https://doi.org/10.1016/j.envpol.2019.03.108.
Larsen MB, Schultz L, Glarborg P, Skaarup-Jensen L, Dam-Johansen K, Frandsen F, Henriksen U. Devolatilization characteristics of large particles of tyre rubber under combustion conditions. Fuel. 2006;85(10–11):1335–45. https://doi.org/10.1016/j.fuel.2005.12.014.
Fazli A, Rodrigue D. Waste rubber recycling: a review on the evolution and properties of thermoplastic elastomers. Materials. 2020;13(3):782. https://doi.org/10.3390/ma13030782.
Tang X, Chen Z, Liu J, Chen Z, Xie W, Evrendilek F, Buyukada M. Dynamic pyrolysis behaviors, products, and mechanisms of waste rubber and polyurethane bicycle tires. J Hazard Mater. 2021;402:123516. https://doi.org/10.1016/j.jhazmat.2020.123516.
Chen R, Li Q, Zhang Y, Xu X, Zhang D. Pyrolysis kinetics and mechanism of typical industrial non-tyre rubber wastes by peak-differentiating analysis and multi kinetics methods. Fuel. 2019;235:1224–37. https://doi.org/10.1016/j.fuel.2018.08.121.
Thomas BS, Gupta RC. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew Sust Energ Rev. 2016;54:1323–33. https://doi.org/10.1016/j.rser.2015.10.092.
Ramarad S, Khalid M, Ratnam CT, Chuah AL, Rashmi W. Waste tire rubber in polymer blends: a review on the evolution, properties and future. Prog Mater Sci. 2015;72:100–40. https://doi.org/10.1016/j.pmatsci.2015.02.004.
Miranda M, Pinto F, Gulyurtlu I, Cabrita I. Pyrolysis of rubber tyre wastes: a kinetic study. Fuel. 2013;103:542–52. https://doi.org/10.1016/j.fuel.2012.06.114.
Chen W, Lin B, Lin Y, Chu Y, Ubando AT, Show PL, Ong HC, Chang J, Ho S, Culaba AB, Petrissans A, Petrissans M. Progress in biomass torrefaction: Principles, applications and challenges. Prog Energy Combust Sci. 2021;82:100887. https://doi.org/10.1016/j.pecs.2020.100887.
Nowak DJ, Greenfield EJ, Ash RM. Annual biomass loss and potential value of urban tree waste in the United States. Urban For Urban Green. 2019;46:126469. https://doi.org/10.1016/j.ufug.2019.126469.
Sayed ET, Wilberforce T, Elsaid K, Rabaia MKH, Abdelkareem MA, Chae KJ, Olabi AG. A critical review on environmental impacts of renewable energy systems and mitigation strategies: wind, hydro, biomass and geothermal. Sci Total Environ. 2020:144505. https://doi.org/10.1016/j.scitotenv.2020.144505.
Arevalo-Gallegos A, Ahmad Z, Asgher M, Parra-Saldivar R, Iqbal HMN. Lignocellulose: a sustainable material to produce value-added products with a zero waste approach-a review. Int J Biol Macromol. 2017;99:308–18. https://doi.org/10.1016/j.ijbiomac.2017.02.097.
Vassilev SV, Baxter D, Andersen LK, Vassileva CG. An overview of the chemical composition of biomass. Fuel. 2010;89(5):913–33. https://doi.org/10.1016/j.fuel.2009.10.022.
Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci. 2018;115(25):6506–11. https://doi.org/10.1073/pnas.1711842115.
Yang H, Yan R, Chen H, Zheng C, Lee D, Liang D. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuel. 2006;20(1):388–93. https://doi.org/10.1021/ef0580117.
Brinchi L, Cotana F, Fortunati E, Kenny JM. Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym. 2013;94(1):154–69. https://doi.org/10.1016/j.carbpol.2013.01.033.
Luo Y, Li Z, Li X, Liu X, Fan J, Clark JH, Hu C. The production of furfural directly from hemicellulose in lignocellulosic biomass: a review. Catal Today. 2019;319:14–24. https://doi.org/10.1016/j.cattod.2018.06.042.
Gani A, Naruse I. Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew Energy. 2007;32(4):649–61. https://doi.org/10.1016/j.renene.2006.02.017.
Hu J, Zhang Q, Lee DJ. Kraft lignin biorefinery: a perspective. Bioresour Technol. 2018;247:1181–3. https://doi.org/10.1016/j.biortech.2017.08.169.
Haq I, Qaisar K, Nawaz A, Akram F, Mukhtar H, Zohu X, Xu Y, Mumtaz MW, Rashid U, Ghani WAWAK, Choong TSY. Advances in valorization of lignocellulosic biomass towards energy generation. Catalysts. 2021;11(3):309. https://doi.org/10.3390/catal11030309.
Ji H, Dong C, Yang G, Pang Z. Valorization of lignocellulosic biomass toward multipurpose fractionation: furfural, phenolic compounds, and ethanol. ACS Sustain Chem Eng. 2018;6(11):15306–15. https://doi.org/10.1021/acssuschemeng.8b03766.
Tuck CO, Pérez E, Horváth IT, Sheldon RA, Poliakoff M. Valorization of biomass: deriving more value from waste. Science. 2012;337(6095):695–9. https://doi.org/10.1126/science.1218930.
Esteves EMM, Herrera AMN, Esteves VPP, Morgado CDRV. Life cycle assessment of manure biogas production: a review. J Clean Prod. 2019;219:411–23. https://doi.org/10.1016/j.jclepro.2019.02.091.
Berendes DM, Yang PJ, Lai A, Hu D, Brown J. Estimation of global recoverable human and animal faecal biomass. Nat Sustain. 2018;1(11):679–85. https://doi.org/10.1038/s41893-018-0167-0.
Mihelcic JR, Fry LM, Shaw R. Global potential of phosphorus recovery from human urine and feces. Chemosphere. 2011;84(6):832–9. https://doi.org/10.1016/j.chemosphere.2011.02.046.
Hamoda MF, Abu Qdais HA, Newham J. Evaluation of municipal solid waste composting kinetics. Resources Conserv Recycl. 1998;23:209–23. https://doi.org/10.1016/S0921-3449(98)00021-4.
Vermeulen LC, Benders J, Medema G, Hofstra N. Global Cryptosporidium loads from livestock manure. Environ Sci Technol. 2017;51(15):8663–71. https://doi.org/10.1021/acs.est.7b00452.
Zhang L, Xu CC, Champagne P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers Manag. 2010;51(5):969–82. https://doi.org/10.1016/j.enconman.2009.11.038.
Xu L, Shi C, He Z, Zhang H, Chen M, Fang Z, Zhang Y. Recent advances of producing biobased N-containing compounds via thermo-chemical conversion with ammonia process. Energy Fuel. 2020;34(9):10441–58. https://doi.org/10.1021/acs.energyfuels.0c01993.
Lopez G, Artetxe M, Amutio M, Bilbao J, Olazar M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. a review. Renew Sust Energ Rev. 2017;73:346–68. https://doi.org/10.1016/j.rser.2017.01.142.
Sabbas T, Polettini A, Pomi R, Astrup T, Hjelmar O, Mostbauer P, Cappai G, Magel G, Salhofer S, Speiser C, Heuss-Assbichler S, Klein R, Lechner P. Management of municipal solid waste incineration residues. Waste Manag. 2003;23(1):61–88. https://doi.org/10.1016/S0956-053X(02)00161-7.
Lu J, Zhang S, Hai J, Lei M. Status and perspectives of municipal solid waste incineration in China: a comparison with developed regions. Waste Manag. 2017;69:170–86. https://doi.org/10.1016/j.wasman.2017.04.014.
Wang P, Hu Y, Cheng H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ Pollut. 2019;252:461–75. https://doi.org/10.1016/j.envpol.2019.04.082.
Psaltis P, Komilis D. Environmental and economic assessment of the use of biodrying before thermal treatment of municipal solid waste. Waste Manag. 2019;83:95–103. https://doi.org/10.1016/j.wasman.2018.11.007.
Makarichi L, Jutidamrongphan W, Techato K. The evolution of waste-to-energy incineration: a review. Renew Sust Energ Rev. 2018;91:812–21. https://doi.org/10.1016/j.rser.2018.04.088.
Panepinto D, Zanetti MC. Municipal solid waste incineration plant: a multi-step approach to the evaluation of an energy-recovery configuration. Waste Manag. 2018;73:332–41. https://doi.org/10.1016/j.wasman.2017.07.036.
Nyashina GS, Vershinina KY, Shlegel NE, Strizhak PA. Effective incineration of fuel-waste slurries from several related industries. Environ Res. 2019;176:108559. https://doi.org/10.1016/j.envres.2019.108559.
Zhang Y, Ma Z, Fang Z, Qian Y, Zhong P, Yan J. Review of harmless treatment of municipal solid waste incineration fly ash. Waste Disposal Sustain Energy. 2020;2(1):1–25. https://doi.org/10.1007/s42768-020-00033-0.
Eriksson O, Finnveden G. Energy recovery from waste incineration—the importance of technology data and system boundaries on CO2 emissions. Energies. 2017;10(4):539. https://doi.org/10.3390/en10040539.
Martínez JD, Puy N, Murillo R, Garcia T, Victoria Navarro M, Mastral MA. Waste tyre pyrolysis—a review. Renew Sust Energ Rev. 2013;23:179–213. https://doi.org/10.1016/j.rser.2013.02.038.
Sharuddin SDA, Abnisa F, Daud WMAW, Aroua MK. A review on pyrolysis of plastic wastes. Energy Convers Manag. 2016;115:308–26. https://doi.org/10.1016/j.enconman.2016.02.037.
Kan T, Strezov V, Evans TJ. Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sust Energ Rev. 2016;57:1126–40. https://doi.org/10.1016/j.rser.2015.12.185.
Liu C, Wang H, Karim AM, Sun J, Wang Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem Soc Rev. 2014;43(22):7594–623. https://doi.org/10.1039/C3CS60414D.
Kumar R, Strezov V, Weldekidan H, He J, Singh S, Kan T, Dastjerdi B. Lignocellulose biomass pyrolysis for bio-oil production: a review of biomass pre-treatment methods for production of drop-in fuels. Renew Sust Energ Rev. 2020;123:109763. https://doi.org/10.1016/j.rser.2020.109763.
Perkins G, Bhaskar T, Konarova M. Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass. Renew Sust Energ Rev. 2018;90:292–315. https://doi.org/10.1016/j.rser.2018.03.048.
Kumar V, Nanda M. Biomass pyrolysis-current status and future directions. Energy Sources Part A: Recov Util Environ Effects. 2016;38(19):2914–21. https://doi.org/10.1080/15567036.2015.1098751.
Dickerson T, Soria J. Catalytic fast pyrolysis: a review. Energies. 2013;6(1):514–38. https://doi.org/10.3390/en6010514.
Venderbosch RH. A critical view on catalytic pyrolysis of biomass. ChemSusChem. 2015;8(8):1306–16. https://doi.org/10.1002/cssc.201500115.
Li B, Ou L, Dang Q, Meyer P, Jones S, Brown R, Wright M. Techno-economic and uncertainty analysis of in situ and ex situ fast pyrolysis for biofuel production. Bioresour Technol. 2015;196:49–56. https://doi.org/10.1016/j.biortech.2015.07.073.
Wan S, Wang Y. A review on ex situ catalytic fast pyrolysis of biomass. Front Chem Sci Eng. 2014;8(3):280–94. https://doi.org/10.1007/s11705-014-1436-8.
Cai R, Pei X, Pan H, Wan K, Chen H, Zhang Z, Zhang Y. Biomass catalytic pyrolysis over zeolite catalysts with an emphasis on porosity and acidity: a state-of-the-art review. Energy Fuel. 2020;34(10):11771–90. https://doi.org/10.1021/acs.energyfuels.0c02147.
Hameed S, Sharma A, Pareek V, Wu H, Yu Y. A review on biomass pyrolysis models: kinetic, network and mechanistic models. Biomass Bioenergy. 2019;123:104–22. https://doi.org/10.1016/j.biombioe.2019.02.008.
Sansaniwal SK, Pal K, Rosen MA, Tyagi SK. Recent advances in the development of biomass gasification technology: a comprehensive review. Renew Sust Energ Rev. 2017;72:363–84. https://doi.org/10.1016/j.rser.2017.01.038.
Molino A, Chianese S, Musmarra D. Biomass gasification technology: the state of the art overview. J Energy Chem. 2016;25(1):10–25. https://doi.org/10.1016/j.jechem.2015.11.005.
Belgiorno V, De Feo G, Della Rocca C, Napoli RMA. Energy from gasification of solid wastes. Waste Manag. 2003;23(1):1–15. https://doi.org/10.1016/S0956-053X(02)00149-6.
Wilhelm DJ, Simbeck DR, Karp AD, Dickenson RL. Syngas production for gas-to-liquids applications: technologies, issues and outlook. Fuel Process Technol. 2001;71(1–3):139–48. https://doi.org/10.1016/S0378-3820(01)00140-0.
Werle S, Wilk RK. A review of methods for the thermal utilization of sewage sludge: the Polish perspective. Renew Energy. 2010;35(9):1914–9. https://doi.org/10.1016/j.renene.2010.01.019.
Schmieder H, Abeln J, Boukis N, Dinjus E, Kruse A, Kluth M, Petrich G, Sadri E, Schacht M. Hydrothermal gasification of biomass and organic wastes. J Supercrit Fluids. 2000;17(2):145–53. https://doi.org/10.1016/S0896-8446(99)00051-0.
Guo L, Jin H, Lu Y. Supercritical water gasification research and development in China. J Supercrit Fluids. 2015;96:144–50. https://doi.org/10.1016/j.supflu.2014.09.023.
Nanda S, Gong M, Hunter HN, Dalai AK, Gokalp I, Kozinski JA. An assessment of pinecone gasification in subcritical, near-critical and supercritical water. Fuel Process Technol. 2017;168:84–96. https://doi.org/10.1016/j.fuproc.2017.08.017.
Wang D, Yuan W, Ji W. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl Energy. 2011;88(5):1656–63. https://doi.org/10.1016/j.apenergy.2010.11.041.
Xiang X, Gong G, Wang C, Cai N, Zhou X, Li Y. Exergy analysis of updraft and downdraft fixed bed gasification of village-level solid waste. Int J Hydrog Energy. 2021;46(1):221–33. https://doi.org/10.1016/j.ijhydene.2020.09.247.
Minowa T, Murakami M, Dote Y, Ogi T, Yokoyama SY. Oil production from garbage by thermochemical liquefaction. Biomass Bioenergy. 1995;8(2):117–20. https://doi.org/10.1016/0961-9534(95)00017-2.
Gollakota ARK, Kishore N, Gu S. A review on hydrothermal liquefaction of biomass. Renew Sust Energ Rev. 2018;81:1378–92. https://doi.org/10.1016/j.rser.2017.05.178.
Dimitriadis A, Bezergianni S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: a state of the art review. Renew Sust Energ Rev. 2017;68:113–25. https://doi.org/10.1016/j.rser.2016.09.120.
Meegoda JN, Li B, Patel K, Wang LB. A review of the processes, parameters, and optimization of anaerobic digestion. Int J Environ Res Public Health. 2018;15(10):2224. https://doi.org/10.3390/ijerph15102224.
Jenicek P, Koubova J, Bindzar J, Zabranska J. Advantages of anaerobic digestion of sludge in microaerobic conditions. Water Sci Technol. 2010;62(2):427–34. https://doi.org/10.2166/wst.2010.305.
Angelidaki I, Ellegaard L, Ahring BK. Applications of the anaerobic digestion process. Biomethanation II. 2003:1–33. https://doi.org/10.1007/3-540-45838-7_1.
Neves NG, Berni M, Dragone G, Mussatto SI, Carneiro FT. Anaerobic digestion process: technological aspects and recent developments. Int J Environ Sci Technol. 2018;15(9):2033–46. https://doi.org/10.1007/s13762-018-1682-2.
Mao C, Feng Y, Wang X, Ren G. Review on research achievements of biogas from anaerobic digestion. Renew Sust Energ Rev. 2015;45:540–55. https://doi.org/10.1016/j.rser.2015.02.032.
Tran HT, Lin C, Bui XT, Ngo HH, Cheruiyot NK, Hoang HG, Vu CT. Aerobic composting remediation of petroleum hydrocarbon-contaminated soil. Curr Future Perspect Sci Total Environ. 2021;753:142250. https://doi.org/10.1016/j.scitotenv.2020.142250.
Gómez RB, Lima FV, Ferrer AS. The use of respiration indices in the composting process: a review. Waste Manag Res. 2006;24(1):37–47. https://doi.org/10.1177/0734242X06062385.
Himanen M, Hänninen K. Composting of bio-waste, aerobic and anaerobic sludges – effect of feedstock on the process and quality of compost. Bioresour Technol. 2011;102(3):2842–52. https://doi.org/10.1016/j.biortech.2010.10.059.
Sánchez ÓJ, Ospina DA, Montoya S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017;69:136–53. https://doi.org/10.1016/j.wasman.2017.08.012.
Singh S, Nain L. Microorganisms in the conversion of agricultural wastes to compost. Process Indian Natl Sci Acad. 2014;80(2):473–81. https://doi.org/10.16943/ptinsa/2014/v80i2/7.
Azim K, Soudi B, Boukhari S, Perissol C, Roussos S, Thami AI. Composting parameters and compost quality: a literature review. Org Agric. 2018;8(2):141–58. https://doi.org/10.1007/s13165-017-0180-z.
Hungría J, Gutiérrez MC, Siles JA, Martin MA. Advantages and drawbacks of OFMSW and winery waste co-composting at pilot scale. J Clean Prod. 2017;164:1050–7. https://doi.org/10.1016/j.jclepro.2017.07.029.
Houfani AA, Anders N, Spiess AC, Baldrian P, Benallaoua S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars – a review. Biomass Bioenergy. 2020;134:105481. https://doi.org/10.1016/j.biombioe.2020.105481.
Radenkovs V, Juhnevica-Radenkova K, Górnaś P, Seglina D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci Technol. 2018;77:64–76. https://doi.org/10.1016/j.tifs.2018.05.013.
Arfi Y, Shamshoum M, Rogachev I, Peleg Y, Bayer EA. Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation. Proc Natl Acad Sci. 2014;111(25):9109–14. https://doi.org/10.1073/pnas.1404148111.
Levine SE, Fox JM, Blanch HW, Clark DS. A mechanistic model of the enzymatic hydrolysis of cellulose. Biotechnol Bioeng. 2010;107(1):37–51. https://doi.org/10.1002/bit.22789.
Modenbach AA, Nokes SE. Enzymatic hydrolysis of biomass at high-solids loadings – a review. Biomass Bioenergy. 2013;56:526–44. https://doi.org/10.1016/j.biombioe.2013.05.031.
Yu Y, Lou X, Wu H. Some recent advances in hydrolysis of biomass in hot-compressed water and its comparisons with other hydrolysis methods. Energy Fuel. 2008;22(1):46–60. https://doi.org/10.1021/ef700292p.
Kumar S, Panda AK, Singh RK. A review on tertiary recycling of high-density polyethylene to fuel. Resour Conserv Recycl. 2011;55(11):893–910. https://doi.org/10.1016/j.resconrec.2011.05.005.
Oliveux G, Dandy LO, Leeke GA. Current status of recycling of fibre reinforced polymers: review of technologies, reuse and resulting properties. Prog Mater Sci. 2015;72:61–99. https://doi.org/10.1016/j.pmatsci.2015.01.004.
Zhang F, Zhao Y, Wang D, Yan M, Zhang J, Zhang P, Ding T, Chen L, Chen C. Current technologies for plastic waste treatment: a review. J Clean Prod. 2020;124523 https://doi.org/10.1016/j.jclepro.2020.124523.
Pardal F, Tersac G. Comparative reactivity of glycols in PET glycolysis. Polym Degrad Stab. 2006;91(11):2567–78. https://doi.org/10.1016/j.polymdegradstab.2006.05.016.
Demarteau J, Olazabal I, Jehanno C, Sardon H. Aminolytic upcycling of poly (ethylene terephthalate) wastes using a thermally-stable organocatalyst. Polym Chem. 2020;11(30):4875–82. https://doi.org/10.1039/D0PY00067A.
Singh N, Hui D, Singh R, Ahuja IPS, Feo L, Fraternali F. Recycling of plastic solid waste: a state of art review and future applications. Compos Part B. 2017;115:409–22. https://doi.org/10.1016/j.compositesb.2016.09.013.
Payne J, McKeown P, Jones MD. A circular economy approach to plastic waste. Polym Degrad Stab. 2019;165:170–81. https://doi.org/10.1016/j.polymdegradstab.2019.05.014.
Gaudino EC, Cravotto G, Manzoli M, Tabasso S. Sono-and mechanochemical technologies in the catalytic conversion of biomass. Chem Soc Rev. 2021;50:1785–812. https://doi.org/10.1039/D0CS01152E.
Shen F, Xiong X, Fu J, Yang J, Qiu M, Qi X, Tsang DCW. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources. Renew Sust Energ Rev. 2020;130:109944. https://doi.org/10.1016/j.rser.2020.109944.
Singh B, Sharma N. Mechanistic implications of plastic degradation. Polym Degrad Stab. 2008;93(3):561–84. https://doi.org/10.1016/j.polymdegradstab.2007.11.008.
Bychkov A, Podgorbunskikh E, Bychkova E, Lomovsky O. Current achievements in the mechanically pretreated conversion of plant biomass. Biotechnol Bioeng. 2019;116(5):1231–44. https://doi.org/10.1002/bit.26925.
Ten G-BF. Chemical innovations that will change our world: IUPAC identifies emerging technologies in chemistry with potential to make our planet more sustainable. Chem Int. 2019;41(2):12–7. https://doi.org/10.1515/ci-2019-0203.
Ravelli D, Protti S, Fagnoni M. Carbon–carbon bond forming reactions via photogenerated intermediates. Chem Rev. 2016;116(17):9850–913. https://doi.org/10.1021/acs.chemrev.5b00662.
Bracco P, Costa L, Luda MP, Billingham N. A review of experimental studies of the role of free-radicals in polyethylene oxidation. Polym Degrad Stab. 2018;155:67–83. https://doi.org/10.1016/j.polymdegradstab.2018.07.011.
Chatani S, Kloxin CJ, Bowman CN. The power of light in polymer science: photochemical processes to manipulate polymer formation, structure, and properties. Polym Chem. 2014;5(7):2187–201. https://doi.org/10.1039/C3PY01334K.
Santoro C, Arbizzani C, Erable B, Ieropoulos I. Microbial fuel cells: From fundamentals to applications. A review. J Power Sources. 2017;356:225–44. https://doi.org/10.1016/j.jpowsour.2017.03.109.
Zhao Q, Yu H, Zhang W, Kabutey FT, Jiang J, Zhang Y, Wang K, Ding J. Microbial fuel cell with high content solid wastes as substrates: a review. Front Environ Sci Eng. 2017;11(2):13. https://doi.org/10.1007/s11783-017-0918-6.
Cesaro A, Belgiorno V. Sonolysis and ozonation as pretreatment for anaerobic digestion of solid organic waste. Ultrason Sonochem. 2013;20(3):931–6. https://doi.org/10.1016/j.ultsonch.2012.10.017.
Li J, Zhao L, Qin L, Tian X, Wang A, Zhou Y, Meng L, Chen Y. Removal of refractory organics in nanofiltration concentrates of municipal solid waste leachate treatment plants by combined Fenton oxidative-coagulation with photo–Fenton processes. Chemosphere. 2016;146:442–9. https://doi.org/10.1016/j.chemosphere.2015.12.069.
Liu P, Qian L, Wang H, Zhan X, Lu K, Gu C, Gao S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ Sci Technol. 2019;53(7):3579–88. https://doi.org/10.1021/acs.est.9b00493.
Khandelwal H, Dhar H, Thalla AK, Kumar S. Application of life cycle assessment in municipal solid waste management: a worldwide critical review. J Clean Prod. 2019;209:630–54. https://doi.org/10.1016/j.jclepro.2018.10.233.
Winkler J, Bilitewski B. Comparative evaluation of life cycle assessment models for solid waste management. Waste Manag. 2007;27:1021–31. https://doi.org/10.1016/j.wasman.2007.02.023.
Dastjerdi B, Strezov V, Ali Rajaeifar M, Kumar R, Behnia M. A systematic review on life cycle assessment of different waste to energy valorization technologies. J Clean Prod. 2021;290:125747. https://doi.org/10.1016/j.jclepro.2020.125747.
El Hanandeh A, El Zein A. Are the aims of increasing the share of green electricity generation and reducing GHG emissions always compatible? Renew Energy. 2011;36:3031–6. https://doi.org/10.1016/j.renene.2011.03.034.
Fernandez-Gonzalez JM, Grindlay AL, Serrano-Bernardo F, Rodriguez-Rojas MI, Zamorano M. Economic and environmental review of Waste-to-Energy systems for municipal solid waste management in medium and small munic-ipalities. Waste Manag. 2017;67:360–74. https://doi.org/10.1016/j.wasman.2017.05.003.
Leme MMV, Rocha MH, Lora EES, Venturini OJ, Lopes BM, Ferreira CH. Techno-economic analysis and environmental impact assessment of energy recovery from Municipal Solid Waste (MSW) in Brazil. Resource Conserv Recycl. 2014;87:8–20. https://doi.org/10.1016/j.resconrec.2014.03.003.
Di Maria F, Fantozzi F. Life cycle assessment of waste to energy micro-pyrolysis system: case study for an Italian town. Int J Energy Res. 2004;28:449–61. https://doi.org/10.1002/er.977.
Thyberg KL, Tonjes DJ. The environmental impacts of alternative food waste treatment technologies in the US. J Clean Prod. 2017;158:101–8. https://doi.org/10.1016/j.jclepro.2017.04.169.
Ebner J, Babbitt C, Winer M, Hilton B, Williamson A. Life cycle green-house gas (GHG) impacts of a novel process for converting food waste to ethanol and co-products. Apply Energy. 2014;130:86–93. https://doi.org/10.1016/j.apenergy.2014.04.099.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Xu, L., Zhou, X., Dong, C., Fang, Z., Smith, R.L. (2022). Sustainable Technologies for Recycling Organic Solid Wastes. In: Fang, Z., Smith Jr., R.L., Xu, L. (eds) Production of Biofuels and Chemicals from Sustainable Recycling of Organic Solid Waste. Biofuels and Biorefineries, vol 11. Springer, Singapore. https://doi.org/10.1007/978-981-16-6162-4_1
Download citation
DOI: https://doi.org/10.1007/978-981-16-6162-4_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6161-7
Online ISBN: 978-981-16-6162-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)