Abstract
Incineration is widely adopted in municipal solid waste management, which produces large amounts of municipal solid waste incineration (MSWI) fly ash. The harmless treatment of MSWI fly ash requires the appropriate disposal of heavy metals and dioxins that are enriched in fly ash. This review summarizes recently developed harmless disposal methods for MSWI fly ash including solidification/stabilization, thermal treatment, and separation/extraction. In addition, we discuss heavy metal and dioxin fixation, and the removal capacity of fly ash via solidification/stabilization (including cement solidification, chemical stabilization, hydrothermal processes, and mechano-chemical methods), thermal treatment (including sintering, fuel-burning, or electric melting/vitrification), and separation/extraction (including water-washing, chemical reagent leaching, biological leaching, electrodialysis separation, chemical reagent extraction, and nanomaterials extraction). The advantages and disadvantages of different harmless treatment methods are compared and future research prospects and suggestions are summarized. This review provides general guidelines for the harmless treatment of MSWI fly ash in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in population and the progress towards urbanization and industrialization in modern society leads to the generation of large amounts of solid waste, which places a significant burden on the sustainable development of the economy and society [1, 2]. As an important aspect of solid waste management, the amount of generated municipal solid waste (MSW) is also increasing. In China, the harmless treatment of MSW is constantly advancing in addition to the increase of treatment capacity, as shown in Fig. 1. According to the relevant data from the National Bureau of Statistics [3], the harmless treatment of MSW in China reached 248.1 million tons in 2017. These treatment procedures include sanitary landfills and incineration. The sanitary landfill method is a relatively common means of handling MSW due to its convenient implementation [4]. However, MSW produces greenhouse gases such as CH4 and CO2 in long-term landfills, and the leachate produced also contains a large amount of chemical oxygen demand (COD) and toxic heavy metal components that contaminate soil and groundwater [5,6,7,8]. The landfill approach also occupies land area and requires a long time for disposal. It is not applicable to small countries and regions with limited land resources such as Japan, Korea, and Singapore. As an alternative disposal method, incineration can be used for the rapid treatment of MSW. This approach requires a relatively small land area, and greatly reduces the volume and quality of MSW (up to 90% and 70% respectively) [9]. In addition, energy can be recovered in the form of electricity and heat [10,11,12]. Compared to sanitary landfills, incineration is more valued in developed countries and regions because they tend to have larger populations and land prices are higher, which highlights the advantages of incineration for the treatment of MSW [13].
However, the production of combustion residuals has continued to increase with the adoption of municipal solid waste incineration (MSWI). Incineration residues contain bottom ash and fly ash. According to the definition by the International Ash Working Group (IAWG) [14], fly ash specifically refers to ash that is discharged from the combustion chamber without the addition of any type of sorbents, such as the ash from a superheater, economizer, and air preheater. In the study of MSWI, fly ash refers to the air pollution control (APC) residues that include the ash trapped by electrostatic precipitators and bag filters that are installed at the rear of the incinerator [15]. It is estimated that the amount of fly ash produced accounts for approximately 3–10% of incineration waste. As such, the amount of fly ash that was disposed in China in 2017 was approximately 7.07 million tons according to this country’s MSWI, which is at 108.77 million tons (Fig. 1).
Heavy metals and dioxins are the two main types of pollutants in MSWI fly ash. Heavy metals refer to metallic elements that exist in nature and have a density greater than 5 g/cm3 [16]. These elements cannot be degraded in the environment, and are easily enriched in living organisms. They can be enriched in human beings along the food chain, thus causing serious harm [17]. Generally, the heavy metals in fly ash mainly include Zn, Pb, Hg, Cu, Cr, Cd, and Ni, and Zn and Pb constitute the highest content [18]. Dioxins include compounds such as polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF) and dioxin-like hydrocarbons that are highly toxic, persistent, and bio-accumulative organic compounds. Dioxins easily accumulate in living organisms and have a persistent negative impact on the environment and human health [19,20,21].
Numerous countries have introduced policies for the control and management of MSWI fly ash [22]. Waste managements of MSWI fly ash in various countries are concluded in Table 1. There are four types of MSWI fly ash disposal strategies include secured landfill, temporarily storage until better options are available, solidification and stabilization before landfill and resource utilization before landfill. Meanwhile, the Chinese government has also included MSWI fly ash in the National Hazardous Waste List (2016 edition), which treats fly ash as hazardous waste and therefore, cannot be directly landfilled. As a result, MSWI fly ash must be harmlessly disposed to reduce the negative impact of heavy metals and dioxins on the environment, which facilitates the disposal of MSW over a long period.
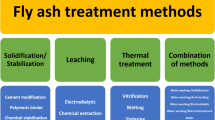
At present, there are numerous studies on the stabilization/removal of heavy metals and dioxins from MSWI fly ash. These harmless treatments can be classified into solidification/stabilization, thermal treatment, and separation/extraction. Cement solidification refers to the use of fly ash as an additive to the concrete production process. The harmful heavy metal components in fly ash are fixed in the concrete product during the production process [23, 24]. This method not only realizes the production of cement, but also facilitates the harmless treatment of fly ash. Chemical stabilization uses different inorganic chemicals [25, 26] or organic chelating reagents [27, 28] to reduce the leaching of heavy metals in fly ash to facilitate subsequent disposal. The disposal of fly ash by heat treatment consumes external energy to create a high-temperature atmosphere. The organic matter in the fly ash is burnt, and the inorganic substance forms harmless slag, in which the heavy metals are confined [29,30,31]. The thermal treatment can be divided into sintering [32] and melting/vitrification [33], depending on the processing temperature. In addition, it can be divided into fossil fuel melting [34, 35] and electric melting [36,37,38], depending on the different energy sources. The separation/extraction method initially separates the heavy metals from fly ash using a chemical reagent, biological reagent, or electrodialysis method [39], then extracts heavy metals from the leaching solution for metal recovery [40]. To date, there are many published reports on different methods for the disposal of MSWI fly ash, and new findings are constantly being reported. The current research focuses on the harmless treatment of fly ash, and relevant comparisons between different treatment methods are considered. This article summarizes state-of-the-art research on the harmless disposal of MSWI fly ash, and performs a comparison between different methods in relation to heavy metals and dioxins solidification/removal efficiency, and analyses their advantages and disadvantages. This review is expected to provide guidelines for future research, and to contribute to the sustainable development of treatments for municipal solid waste incineration.
Characteristics of municipal solid waste incineration fly ash
MSWI Fly ash is a fine-powder particle with a grey or dark grey coloration. Under a microscope, fly ash particles have different shapes including needle, strip and spherical. They also possess uneven particle size, high porosity, and a large specific surface area [41]. Many studies have shown that the physical and chemical characteristics of fly ash depend on different incineration technologies, operating conditions, and the property of MSW [42]. Therefore, it is necessary to summarize the basic characteristics of MSWI fly ash according to the aforementioned influencing factors, so that the subsequent harmless disposal of fly ash is facilitated.
Chemical characteristics
Table 2 summarizes the major chemical element components in MSWI fly ash from different countries. It can be concluded that the chemical composition of fly ash from different countries is similar, with major components that include the metallic elements Ca, Si, Al, Fe, Na, and non-metallic elements S and Cl. Among all the elements, Ca represents the main component. The content of Ca in fly ash from Japan and Korea is as high as 40% or more. Most of the Ca arises from the process of flue gas treatment. During this treatment, a large amount of Ca(OH)2 is sprayed to remove SO2 from the flue gas using a dry or semi-dry scrubber, is subsequently trapped by the precipitator, and remained in the fly ash. Compared to other countries, fly ash from China contains the highest Si component of 11.18%. This may be because MSW in China also contains more incombustible dust [43]. In addition, China's fly ash also has relatively high Al content (from 1.70 to 17.80%), thus creating a CaO–SiO2–Al2O3 system, which can be further modified to facilitate resource utilization.
The type of technology used by the incinerator also affects the chemical components of the fly ash. Table 3 shows the main chemical composition of waste incineration fly ash generated from grate furnace incineration and fluidized bed incineration.
It is evident that the elements of Si (approximately 10%) and Al (approximately 7%) in the fluidized bed fly ash are significantly higher than the Si (approximately 2%) and Al (approximately 1%) from the grate furnace. It was concluded that the reason is that coal is usually incorporated with MSW to enhance combustion in fluidized bed incineration technology, and the fly ash may contain Si and Al derived from coal ash [50]. The fly ash from the grate furnace incineration contains more Cl (approximately 21%), Na (approximately 6%), and K (approximately 6%), indicating that the fly ash from the grate furnace is more likely to enrich a large amount of salts, e.g., NaCl and KCl. These salts may be derived from the incineration of kitchen waste.
Heavy metals and their leaching properties
Heavy metals refer to metallic elements whose densities are over 5 g/cm3. In the process of MSWI, heavy metals in MSW would first evaporate in the incinerator and then condense accompanied by the flow of flue gas and finally enrich on the surface of fly ash in the APC system. These heavy metals cannot be degraded in the environment. If these MSWI fly ash ware not dispose properly, the heavy metals would penetrate into the soil and water. Through the food chain, these heavy metals eventually enriched in human bodies and cause serious harm to human health [17].
Table 4 presents a summary of several major heavy metals in MSWI fly ash from different countries including As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn. Compared to other countries, China has more heavy metal content in MSWI fly ash, which is associated with high potential environmental pressure among the investigation countries. Zn and Pb elements are often the most abundant constituents in fly ash, and was not correlated to any country. Considering China as an example, the highest Zn and Pb content can reach 19,880 mg/kg and 4769 mg/kg, respectively.
The heavy metals in fly ash depend on the chemical reactions between the heavy metals and non-metallic elements during the incineration of MSW. Some heavy metals are volatile. The Cl in MSW significantly promotes the volatilization of these heavy metals and forms heavy metal chlorides. These metal chlorides condense in the fly ash in an APC system, resulting in the enrichment of heavy metals in the fly ash [56]. Therefore, most of the heavy metals in fly ash are volatile heavy metals. Non-volatile heavy metals may be enriched with the flow of flue gas during incineration. The content of these heavy metals in fly ash is relatively low compared to volatile heavy metals. The factor that determines the content of heavy metals in fly ash is their boiling point or that of heavy metal chlorides, and the heavy metals or heavy metal chloride with low boiling points are easily enriched in MSWI fly ash [57].
The main potential environmental risk during the disposal and utilization of MSWI is the leaching of heavy metals. Most heavy metals in fly ash are chlorides and are soluble in water, making it easy to leach and contaminate soil and water [58]. There are many factors that affect the leaching characteristics of heavy metals, including the characteristics of ash, liquid/solid (L/S) ratio, and the pH value. For instance, fly ash with smaller particles can promote the leaching of heavy metals [59]. However, heavy metals are easily enriched on small particles. In addition, fly ash with smaller particles has a larger specific surface area, resulting in faster leaching kinetics [60]. The leaching of heavy metals is also closely related to their solubility. A higher liquid–solid ratio contributes to the dissolution of heavy metals and promotes the release of toxic elements [61]. Moreover, the solubility of heavy metals is also affected by the pH of the solution. Given that most heavy metals are present in the form of cations (such as Cu, Zn, Pb, Cd), their leaching pattern rises with a decrease in pH [62]. The leaching of As occurs readily for strong acid (pH < 2) and strong alkaline (pH > 12) conditions, thereby exhibiting an amphoteric characteristic. Se and Ba exhibit an oxyanionic leaching behavior, and have good leaching characteristics under alkaline conditions [63].
Therefore, the leaching properties of heavy metals in MSWI fly ash are highly dependent on their leaching methods. Relevant leaching tests on MSWI heavy metals by different leaching methods are summarized in Table 5. The leaching methods discussed include Korean standard leaching test (KSLT), Japanese test for leaching (JTL-13), horizontal vibration extraction procedure (HVEP), and toxicity characteristic leaching procedure (TCLP). Compared to other three methods, the TCLP method can be carried out at a relatively larger fly ash particle size (< 9.5 mm), but these particles have to be leached in acid condition (pH 2.88–4.93) with a high L/S ratio (L/S = 20). The KSLT method, JTL-13 method and HVEP method have the same L/S ratio at 10 mL/g. These methods aim to simulate the leaching characteristics in the natural environment under the weak acid condition.
The determination of the heavy metal (As, Cd, Cr, Cu, Hg, Ni, Pb and Zn) leaching characteristics of MSWI fly ash shows that Cu, Ni and As are low leached heavy metal, while the Pb and Cd are highly leached heavy metal elements. Despite different original Pb and Cd contents in fly ash, the leaching of Cd by KSLT and TCLP method and the leaching of Pb by KSLT, JTL-13 and HVEP method are all exceed the regulation level (See in Table 4). Heavy metal Zn and Hg may also exceed the limit value according to different fly ash sample. Hence, relevant studies on the harmless treatment of fly ash should focus on reducing the leaching of Pb and Cd, and Zn and Hg also need to be paid attention.
Dioxins
During MSWI incineration processes, the formation of dioxins (or PCDD/Fs) is produced via three main mechanisms: (1) Presynthesis. PCDD/Fs already exist in feeding material and survive the incineration process. (2) Precursor synthesis. Precursors including polyhalogenated phenols, ortho-halogenated phenols, and chlorinated aromatic compounds are thermally destroyed and rearranged to form PCDD/Fs. (3) De novo synthesis. The de novo process is defined as a pathway by which PCDD/Fs are formed in the absence of aromatic precursors. The decomposition of macromolecular carbon structures of MSW leads to the formation of aromatic precursors, thereby forming dioxins via mechanism (2) [64, 65]. Various kinds of waste incinerators, especially MSW incinerators have been identified as major dioxin contributors given that incineration has become the mainstream technology for waste treatment in many countries. To control the emission of dioxins, the design of waste incinerators follows the “3 T” principle, namely; high temperature (Temperature > 80 °C), high turbulence, and long residence time (Time > 2 s). In addition, activated carbon is spayed in the incineration flue gas to absorbs dioxins. After absorption, these activated carbons are trapped by APC equipment and transferred to the fly ash. It is estimated that approximately 65.3% of dioxins produced by incinerators in China are discharged into the environment in the form of fly ash. As the number of incineration facilities continues to increase, the pollution caused by dioxin will become increasingly significant [66].
The International Toxic Equivalent Factor (I-TEF) was adopted to evaluate the toxicity of different dioxin molecules. The higher the value, the stronger toxicity the dioxin molecules have. Given that 2,3,7,8-TCDD is the most toxic dioxin congener, it was assigned a toxicity rating of 1.0 by convention. The TEFs for the other 2,3,7,8-positional congeners were determined based on the ratio of the toxicity of each individual congener to that of 2,3,7,8-TCDD. The toxicity of dioxins with any mixture of PCDDs and PCDFs can then be expressed using Eq. (1).
where Ci is the i-th concentration of the toxic dioxins congener, ng/g, and(I-TIF)i is the i-th international toxicity equivalent factor of the toxic dioxins. The toxic equivalent quantity(TEQ) of the dioxins was obtained by the addition of individual TEQs [67,68,69].
Table 6 summarizes the amount of dioxin, and their TEQ in different MSWI fly ash samples from different countries. The total content of PCDD/Fs in MSWI fly ash is between 23 and 294 ng/g, and the toxicity equivalent is between 0.71 and 6.71 ng TEQ/ng. The level of PCDD/Fs in the fly ash varies with the type of incinerator, the capacity of the incinerator, and the APC equipment at the end of the incinerator. The results of research on 15 large MSW incinerators in China [21] suggest that most of the incineration types are grate furnaces and fluidized bed furnaces. The treatment capacity of MSW ranges from 300 to 800 tons/day. The corresponding APC measures include semi-dry a scrubber, activated carbon injection and a baghouse filter. The PCDD/Fs content in the fly ash ranged from 2.8 to 190 ng/g, and the toxicity equivalent was 0.034–2.5 ng TEQ/g. The content of dioxin in fly ash is the same as that of the fly ash generated from a secondary copper and secondary zinc metallurgical plant [74]. Several studies have shown that if fly ash is directly dumped in a landfill without treatment, dioxin components will continue to leach and contaminate soil and water sources [75, 76]. In general, MSWI plants produce fly ash with high levels of dioxins, which is highly toxic. As such, appropriate and harmless disposal is required.
Harmless method to dispose of municipal solid waste incineration fly ash
MSWI fly ash is rich in toxic heavy metals and dioxins. The harmless treatment of MSWI fly ash requires the reduction of leaching toxicity of heavy metals and the reduction of dioxin content. This section summarizes the various approaches for disposing of fly ash that have been developed in recent years, including solidification/stabilization, heat treatment, and separation/extraction.
Solidification/stabilization
Cement solidification
As an important harmless treatment method, cement solidification has a relatively low disposal cost and its solidified product has long-term physical, chemical, and biological stability [77], which plays a very important role in the disposal of hazardous waste [78,79,80,81]. As a procedure for treating hazardous waste, the disposal of MSWI fly ash by solidification has also undergone long-term research.
Principle of cement solidification method
The hydration reaction between cement and water is the key mechanism for cement formation and solidification of MSWI fly ash. The hydration reaction can be divided into two steps. The first step is to form primary cementitious products such as hydrated calcium silicates, hydrated calcium aluminates, and hydrated lime. The second step is the continued decomposition of alkaline calcium hydroxide to produce OH− and Ca2+, which continue to react with hydrated calcium silicate and hydrated calcium aluminate to form the insoluble compounds C–S–H and C–A–H gel [82]. Given that the main chemical composition of fly ash (e.g., CaO, SiO2, and Al2O3) is consistent with the chemical composition of cement raw materials, fly ash can be used as an additive during the production of cement [83]. When fly ash and cement are mixed in a certain proportion and hydrated, the heavy metals and dioxins are encapsulated in C–S–H gel or C–A–H gel, thus leaching of these two hazardous byproducts is prevented from solidified products [84].
Heavy metal leaching characteristics of cement solidified products
It has been demonstrated in numerous studies that cement solidification can effectively fix the heavy metals in MSWI fly ash [85,86,87]. However, the heavy metals in cement products are not stable in the long term. On one hand, the increase in the amount of fly ash increases the content of heavy metals in the cement product, which also improves the leaching ability of heavy metals in the solidified product [24]. On the other hand, the leaching characteristics of heavy metals in the cement product are also affected by the exposed environment. Lu et al. [88] conducted studies on the leaching characteristics of heavy metals in cement products under different simulated environments including seawater, underground water, and acid rain. The result indicated that Cu, Cd, Pb, Zn, Mn, Sb, and Ti did not leach under these environmental conditions, whereas Cr, Ni, As, and V leached under the simulated acid rain and seawater environment, and the amount of heavy metal leached was inversely proportional to the particle size of the cement product. Cr is the most unstable element and can be easily leached especially in acid environments. This is consistent with the results obtained in studies by Yu et al. [24] and Li et al. [89].
When considering the long-term leaching characteristics of solidified products, aging or carbonation will have an effect on heavy metals. Supercritical carbonation can accelerate the study of the aging characteristics of solidified products [90]. Zha et al. [91] used a CO2 closed-cycle system [92] to study the heavy metal leaching characteristics of supercritical carbonated cement product, and found that supercritical carbonation promoted the leaching of Cu and Pb.
In addition, not all heavy metal elements such as Hg can be fixed by cement solidification. Du et al. [93] demonstrated that the Hg released by the solidified product reaches 8.51 ng/g–18.48 ng/g, which is in accordance with raw fly ash. In addition, the rise of temperature and water content promote the release of Hg.
Dioxins leaching characteristics of cement solidified product
The cement solidification process encapsulates the dioxin from the fly ash in the cement C–S–H gel or C–A–H gel, thus reducing leaching. However, it has been shown in several studies [94, 95] that the solidification product is stable in an acid environment. When humid acid is used as the leaching agent, the dioxin leached from the fly ash is reduced by 98%. Most of these leached dioxins are high chlorine-containing such as HpCDD/F and COOD/F. However, when using n-Hexane as the leaching agent, the dioxins that are leached from the fly ash is significantly increased, including not only high chlorine-containing dioxins, but also low chlorine-containing dioxins. This result indicates that under the influence of polar leaching agents (such as rainwater and natural organic solutions), cement solidification can effectively fix dioxin, whereas, in the case of non-polar leaching agents, cement solidification has the opposite effect.
However, the solidification process does not lead to the degradation or destruction of these dioxins. As the solidified product is impregnated and aging occurs in the environment, the solidified dioxins are re-dissolved to contaminate the solid. Wang et al. [96] investigated soils in which fly ash solidified products enter the landfill, and noted that although high concentrations of dioxins from fly ash solidification product leachate were not detected, the dioxin concentration in the soil near the landfill was 2.8 ng I-TEQ/g, which was 2.8 times higher than the standard value.
Effect of cement solidification of fly ash on the mechanical strength of cement
Although the cement solidification of fly ash can reduce the leaching of heavy metals and dioxins, the addition of fly ash has a negative impact on the strength of cement. On one hand, excessive fly ash addition leads to a decrease of C–S–H or C–A–H gel content in the cement, resulting in a reduced cement strength. On the other hand, MSWI fly ash contains a large amount of Cl. Cl can react with the aluminate in cement raw material to form Friedel’s salt, which affects the hydration process of the cement and hinders its formation [52]. Several works indicate that the addition of fly ash causes a decrease in the mechanical strength of cement products. A study by Yu et al. [24] showed that the addition of fly ash caused a significant decrease in the compressive strength of cement product from 51.5 MPa without fly ash to 22.6 MPa for the addition of 25% fly ash, and to 19.2 MPa for the addition of 40% fly ash, with a total reduction of compressive strength by 62%. The studies by Yang et al. [97] and Bie et al. [98] also support this view. In addition, the aging and carbonation of solidification products resulted in an increasing amount of calcium carbonate in the voids of the cement. The generated calcium carbonate exceeded the limit of the pores, which caused additional internal pressure and micro-cracks, thereby reducing the mechanical strength of the cement product [91]. It is evident that the cement solidified product of MSWI fly ash can only be used as a low-strength road basement, and base construction material [99].
Novel materials for solidification of MSWI fly ash
In recent years, research on the solidification of MSWI fly ash has focused on new solidified materials. Portland cement is the conventional solidification material for treating fly ash. The new materials include magnesium potassium phosphate cement, intermediate-calcium based cementitious material, and blast furnace slag are currently under investigation for the solidification of fly ash, with good results.
Potassium magnesium phosphate cement is chemically bonded ceramic materials with excellent properties such as rapid setting, high early strength, and high adhesive properties [100]. It has been experimentally demonstrated [101] that the mechanical strength of solidified cement meets the cement standard for construction when the amount of fly ash is less than 20%, and the leaching of Cd and Pb also meets the required standard.
Intermediate calcium-based cementitious material (ICCM) has 30–40 wt.% CaO. The other component has a CaO/SiO2 ratio close to 1 or (CaO + MgO)/(SiO2 + Al2O3) ratio ranging from 0.75 to 0.90. The hydration products of ICCM are ettringite, calcium hydroxide and C–S–H gel. The co-effect of these products makes the paste compactable and hard [102]. It has been shown that the optimum solidification condition depends on the (CaO + MgO)/(SiO2 + Al2O3) ratio and the addition amount of MSWI fly ash [103]. When fly ash is added at 10 wt.%, the best resulting (CaO + MgO)/(SiO2 + Al2O3) ratio is 0.76–0.88. The compressive strength of the solidified ICCM product can reach 42.5R in normal Portland cement. The concentrate of Pb, Zn, Cu, and Cr in leachate all meet the national standard (GB8978-1996). Moreover, Cl in fly ash is also well-solidified.
MSWI fly ash can be solidified using ground granulated blast furnace slag (BFS) to produce alkali-activated slag (AAS) cement. The AAS cement presents is present in the form of C–S–H gel and C–A–S–H gel. Research results have shown [104] that the reduction of the compressive strength of AAS cement is inevitable when fly ash is added. The highest compressive strength reaches 64.37 MPa for the addition of 30 wt.% fly ash, and a curing time of 28 days. However, relevant research on the heavy metal leaching characteristic of AAS cement is insufficient and needs to be improved.
Chemical stabilization
The chemical stabilization is based on the chemical reaction between heavy metals in MSWI fly ash and chemical reagents to reduce the leaching of heavy metal ions, thereby achieving stabilization of heavy metals. The chemical reagents employed can be classified as inorganic and organic reagents. Several types of different reagents are selected, and this will be discussed in the following section, as well as the stabilizing effects of these reagents on heavy metals.
Inorganic chemical reagents
Phosphates The addition of fly ash to the phosphate solution combines the heavy metals in the fly ash with the phosphate radical to form insoluble materials, which can reduce the leaching of heavy metals [105]. Previous studies have shown that phosphate can effectively immobilize the heavy metals Zn, Pb, and Cu [106, 107]. Phosphates can be sourced from biological material such as fishbone [46, 108], which is rich in hydroxyapatite. Wang et al. [109] used phosphoric acid to stabilize heavy metals in fly ash. It was determined that under acidic conditions, a reduction of leaching was observed only for the heavy metals Zn, Cd, and Cr, whereas the other heavy metal showed an enhancement.
Silicates Silicates are another type of inorganic chemical reagent. The stabilization principle of silicates is similar to that of cement solidification, in that C–S–H gel is produced to encapsulate the heavy metals. Silica fume is a pozzolanic material with a high specific surface area and has good potential for the fixing of heavy metals [110]. A study by Li et al. [25] showed that silica fumes exhibit good stabilization of the heavy metals Cu, Pb, and Zn.
Sulfides Similar to phosphates, the addition of sulfides immobilizes heavy metals to form low soluble heavy metal sulfides [111]. Sulfides have good fixation for Pb and Cd. The research conducted by Zhao et al. [112] proved that the amount of Na2S added reaches 1.8 wt.% and 5 wt.% of the MSWI fly ash when the concentration of Pb and Cd in leachate reached 7.26 mg/L and 0.10 mg/L, respectively. This meets the leaching toxicity standard. The drawback of this method is that heavy metal sulfides can be converted into H2S under acidic conditions. Sun et al. [26] developed a new and inexpensive polysulfide to achieve long-term stability of Pb in fly ash, without producing H2S gas.
Iron oxides FeSO4 is an iron oxide that can effectively stabilize heavy metals in fly ash. The Ferrox-process developed by Lundtorp et al. [113] fixes heavy metals with FeSO4. In the Ferrox-process, the MSWI fly ash is suspended in a FeSO4 solution and the suspension is subsequently oxidized to form iron oxides on the fly ash. The salts are then washed out and the heavy metals in the solids are retained, thereby leading to a reduction of the leaching of the salts and metals from the treated fly ash. The results indicate that the fly ash treated using this method leads to a large amount of salt in the leachate, and the leaching amount of Pb, Cd, Zn and Cu is effectively controlled. However, it does not have a significant impact on the leaching of Cr and Pb.
Organic chemical reagent
Numerous organic chemical reagents that can achieve chemical stabilization of heavy metals have been investigated. In previous reports [52, 114, 115], thiodiglycolic acid (TGA), pyrrolidines, imines, carbamates, and thiols have been shown to effectively stabilize heavy metals in MSWI fly ash. In addition, new chemical reagents are also being developed. The following section will introduce several new types of organic chemicals for chemical stabilization of heavy metals.
Thiourea The thiourea functional group is a sulfur-containing complexing ligand that can stabilize heavy metals [116]. A new water-soluble thiourea-formaldehyde resin was developed to verify its stabilization efficiency for MSWI fly ash [117]. When the resin addition was 3 wt.% of the fly ash, the concentration of the heavy metals Cr, Pb and Cd in leachate was reduced by 96.7%, 92.4%, and 85.8%, respectively, and meets the national standard. However, this study also demonstrates that when the resin is used to stabilize fly ash, it may be unstable and harmful to the environment. Therefore, the stability and toxicity of the thiourea-formaldehyde resin need to be evaluated in subsequent industrial-scale applications.
Dithiocarbamates Dithiocarbamate ions can react with a metal ion to form the non-polar compound, dithiocarbamate metal (DCM), to reduce the leaching of heavy metals [118]. Wang et al. [109] used sodium diethyldithiocarbamate (SDD) as a chelating agent and citric acid as a leaching agent to study the leaching characteristics of the heavy metals Pb, Zn, Cr, Cu and Ni in fly ash. The results showed that SDD could effectively stabilize Pb, Cd, and Cu. However, in an acidic environment, it could promote the leaching of Zn, Cr, Ni, and Pb.
Sixthio guanidine acid and etrathio bicarbamic acid The chelating groups in sixthio guanidine acid (SGA) and etrathio bicarbamic acid (TBA) demonstrate excellent ability to stabilize heavy metals [119]. The study by Wang et al. [120] proved that for SGA and TBA, the hydrosulfide groups are more capable of binding to heavy metals compared to SDD or Na2S. They can effectively limit the leaching of Pb, Zn, Cr, Cu, Ni, and Cd. However, under an acidic environment, the leaching of heavy metals is accelerated.
Effect of chemical stabilization on different heavy metals
Tables 7 and 8 summarize the effects of different chemical reagents on the leaching characteristics of heavy metals from stabilized MSWI fly ash under neutral and acid conditions, respectively. Comparing the leaching ability of different heavy metals from Tables 7 and 8, it can be determined that the leaching characteristics of different heavy metals stabilized by different chemical reagents exhibit different properties. Under acidic conditions, the leaching abilities of most heavy metals are improved. This indicates that after chemical stabilization treatment, the risk of MSWI fly ash heavy metal re-dissolution increases in an acidic environment.
The Cd in the fly ash is an element that is relatively easy to stabilize, and most chemical reagents can stabilize it, such as the formation of stable Cd3(PO4)2 treated with phosphoric acid [121], and the formation of stable chelating products due to an organic chelating reagent [27]. Only the fly ash fixed by two organic chelating agents, SGA and TBA, resulted in the increased dissolution of Cd under an acidic environment.
The Cr in fly ash is an element that is difficult to fix by chemical stabilization. Only thiourea reagent can fix Cr, and this element is stable in the product only under a neutral environment. The form of Cr in fly ash is mainly Cr3+ and Cr6+. At low pH, Cr3+ can be oxidized to Cr6+, which exists in the form of highly soluble HCrO4− [122]. The improvement in the leaching ability of Cr stabilized by an organic reagent is due to the insufficient oxyanions HCrO4− trapping ability of the reagent [107].
Cu in fly ash is also easy to fix, but it can be leached in an acidic environment. This is because the stabilized Cu is partially present in the form of Cu(OH)2 (especially when an inorganic chemical reagent is used), which is easily dissolved under acidic conditions [123].
To fix Ni in the fly ash using the chemical stabilization method, the stabilized product should be placed in a neutral environment. The dissolution of Ni then increases again [89] no matter the kind of chemical reagent used.
Pb in fly ash is also an element that is easy to fix. Most chemical stabilizers can stabilize Pb. However, similar to Cu and Ni, when the stabilized product is placed in an acidic environment, Pb is easily leached. For instance, Pb in fly ash will be converted to Pb3(PO4)2 and Pb5(PO4)3OH via phosphate stabilization. These products are more easily leached under acidic conditions [124]. Similar results can be observed after the treatment of an organic stabilizer [27].
Similar to Ni, the stabilized product of Zn from fly ash remains stable under a neutral environment. Zn can be converted into Zn3(PO4)2 and Zn3(PO4)3Cl after treatment with phosphoric acid, thus achieving the stabilization of Zn [125]. The organic chemical reagent can change the structures of Zn-bearing minerals, which are altered during the stabilization process, leading to more dissolution under acidic conditions [126].
Collaboration of cement solidification and chemical stabilization
To dispose of MSWI fly ash, at effective method is to combine cement solidification and chemical stabilization. Ma et al. [127] selected 4 different chemical chelating agents (dithiocarbamate, dithiocarbamic acid dipotassium salt, amino dithiocarbamate chelating resin, and thiourea). The chelating agent solution was mixed with a certain proportion of fly ash, cement raw material, and water to achieve solidification and stabilization of the fly ash. It was determined that dithiocarbamate had the best performance in terms of the stabilization of fly ash heavy metals, but the stabilized heavy metals were easily leached in an acid solution. When chelating stabilization was combined with cement solidification, the leaching concentration of the heavy metals continued to reduce. The amount of the heavy metals Cd, Pb and Ni that was leached decreased from 0.21 mg/L, 0.046 mg/L, and 0.444 mg/L respectively without cement solidification, to 0.095 mg/L, not detected and 0.285 mg/L respectively for cement solidification. Nevertheless, the addition of fly ash leads to a decrease in the compressive strength of the cement. This is consistent with the treatment of fly ash by solidification only.
Hydrothermal treatment
Hydrothermal treatment is a method for large-scale stabilization of MWSI fly ash at a relatively low treating temperature (150–200 °C) [128]. Under hydrothermal conditions, the movement of water molecules is accelerated, thus increasing the ionization product constant and diffusion coefficient, thereby promoting the chemical reaction. The hydrothermal treatment technology synthesizes aluminosilicate minerals to stabilize heavy metals in minerals under alkaline conditions (NaOH or KOH) using Al or Si sources in fly ash or by adding external Al and Si.
In the synthesis of aluminosilicate minerals in an experiment conducted by Bayuseno et al. [129], it was determined that a significant amount of Al-substituted 11 Å tobermorite (Ca5(Si,Al)6(OH)2O16·4H2O) and katoite (Ca3Al2(SiO4)(OH)8) in addition to minor amounts of zeolites were formed under experimental conditions with 0.5 M NaOH at a temperature of 180 °C for 48 h. The hydrothermal product exhibited excellent stability for heavy metals and there was a reduction of their release relative to the untreated parent materials, even under acidic conditions.
To reduce energy consumption, the dissolution of Al and Si is accelerated, the crystallization period is reduced, and microwave technology is applied for hydrothermal treatment. Gong et al. [130] determined that during microwave-assisted hydrothermal treatment, the leaching of Pb, Cu, and Zn in fly ash decreased while the leaching of Cr was improved. This is probably because the Cr in fly ash is converted from Cr3+ to Cr6+, forming HCrO4−, which is highly soluble in leachate. Qu et al. [49] conducted research on microwave-assisted hydrothermal treatment of MSWI fly ash. Three process additives of NaOH, Na2HPO4, and H2O were compared for the stabilization of heavy metals. The result indicated that Na2HPO4 is an efficient additive in the microwave-assisted hydrothermal process for the stabilization of heavy metals in fly ash. The leaching of Cd, Cr, Cu, Ni, Pb, and Zn all met the national standard. Several types of zeolites were formed during the disposal process. Compared to conventional hydrothermal treatment, microwave-assisted hydrothermal treatment offers a promising technology for the management of MSWI fly ash with high efficiency and energy savings.
Hydrothermal methods can also promote the decomposition of dioxins. Based on hydrothermal research [131], it has been shown that the toxic equivalent of dioxins in fly ash reaches the lowest value with the addition of 0.1 wt.% carbohydrazide to fly ash for an operating temperature of 533 K. Hu et al. [132] used a mixture of iron and ferrous sulfate to enhance the decomposition of dioxins. The results revealed that temperature is the most important factor that affects the degradation of dioxins. A higher operating temperature leads to further degradation. Washing-pretreatment and the addition of iron and ferrous sulfate can also facilitate the decomposition of dioxins. The effect of Fe addition on the decomposition of dioxins is weaker than that of temperature.
Mechanochemical method
The principle of the mechano-chemical method is to induce changes in the physical and chemical properties of solid particles using various mechanical forces, such as collision, compression, shear, and friction, thereby activating or accelerating the reaction between solid particles [133, 134].
Mechano-chemical methods can achieve the fixation of heavy metals in MSWI fly ash using ball milling [135]. Nomura et al. [136] utilized CaO as an additive to mechanically convert Pb from fly ash to a readily soluble PbCl2 form to a stable insoluble Pb3O4, effectively reducing the leaching of Pb by 92.8%. The study by Li et al. [137] also confirmed that the mechanochemical method has excellent stabilization of Pb. The research conducted by Chen et al. [138] demonstrated that the mechanochemical method can effectively fix Cu, Pb, and Cr in fly ash, but has little effect on the stability of Cd, Zn and Ni. To be specific, the leaching ability of Cd and Zn does not change significantly, and the leaching ability of Ni is enhanced.
Related studies have shown that mechanochemical methods can effectively reduce dioxin in MSWI fly ash [139]. To promote the degradation of dioxin, CaO, SiO2, and iron powder. are often used as additives during the disposal of fly ash. Lu et al. [140] studied the mechano-chemical mechanism of the degradation of 2,4,6-trichlorophenol via ball milling by adding CaO and SiO2 to 2,4,6-trichlorophenol. The reaction mechanism of a four-step pathway was obtained, which provided a basis for the study of the degradation mechanism of dioxins. Zhang et al. [141] investigated the mechano-chemical degradation of dioxins in pentachloronitrobenzene (PCNB) using iron powder as an additive. The results revealed that the TEQ of dioxins in PCNB decreased from 1146 ng-TEQ/kg to 0.04 ng-TEQ/kg. Chen et al. [142, 143] developed a new additive SiO2-Al to decompose dioxins. The mechano-chemical degradation of dioxins resulted in a decrease from 6.75 ng-TEQ/g to 0.64 ng-TEQ/g. In addition, the detailed degradation path of the mechano-chemically treated dioxins and the mechanism of action of the additives were revealed.
Thermal treatment
The thermal method to treat MSWI fly ash involves exposing the fly ash to a high temperature to transform it into a stable environmentally-friendly substance. During the heat treatment process, organic matter is decomposed, burned, and vaporized, whereas inorganic matter is transformed into a stable glassy slag. After heat treatment, organic pollutants such as dioxins in the fly ash are destroyed, whereas involatile inorganic heavy metals are sealed in a glassy slag that is safe to put in a landfill. Volatile inorganic heavy metals end up in secondary fly ash and are discharged. The density of fly ash significantly increases after thermal treatment, and the volume reduction can be 50% or more. In addition, the metals in the ash can be recovered, and the stable slag can be used as a road base material or as other raw materials [144, 145]. Thermal treatment methods for fly ash can be divided into sintering and melting/vitrification [146].
Sintering
The theory of sintering began in the middle of the twentieth century and was introduced in the work of Frenkel [32]. Sintering is a mainstream thermal treatment method, and the temperature of this procedure is generally between 900 and 1200 °C. It is viable to produce glass or glass–ceramics from fly ash based on this approach. Sintering involves heating the fly ash to the point at which particle bonding occurs, and chemical phases in the fly ash are reorganize. The fly ash then aggregates from small particles into a coherent mass [147]. The sintering product has reduced porosity and high strength. The heavy metals in the sintering product are difficult to leach. Karamanov et al. [148] proposed that the sintering process also causes crystallization of the MSWI fly ash, which has a negative effect on densification and creates small pores in the sintering products. A higher heating rate leads to a superior sintering product with denser crystallization and improved mechanical properties.
Effect of sintering on solidification of heavy metals
Heavy metals in MSWI fly ash can be solidified using the sintering method. Based on experimental research [149], it has been shown that the sintered heavy metals Zn, Cr, Pb, and Cu are mainly presented in an oxidized form. The leaching concentration of most heavy metals, except Pb, achieves the national standard. After 30 days aging of the sintering products, the leaching concentration of heavy metals did not increase significantly. This result is indicative of the excellent ability of sintering to solidified heavy metals in fly ash.
The operational conditions such as the temperature and sintering time have an influence on the solidification of heavy metals. Research conducted by Li et al. [29] established an overall pollution toxicity index (OPTI) for the evaluation of heavy metal pollution. The results revealed that a higher sintering temperature led to more volatilization of heavy metals, and the lowest value of the OPTI was at 1040 °C. A long sintering time might also result in more volatilization, leading to higher OPTI values. Thus, higher integrated control efficiency conditions of heavy metals were achieved at lower temperatures and shorter times.
In addition, the pre-washing of MSWI fly ash can affect the leaching ability of sintered fly ash. Liu et al. [147] compared the sintering results for raw fly ash (RFA) and washed-pretreated fly ash (WFA) and discovered that washing pretreatment reduces the leaching rate of Cd, Pb, and Ni, but increases the leaching rate of Cr. The heavy metal leaching concentration of sintering products from RFA and WFA both meet the Chinese regulatory level (GB5085.3-1996).
Effect of sintering on dioxins in fly ash
In a study [150] on the decomposition of dioxins in fly ash via sintering suggested that most of the dioxins and their homologs in fly ash are destroyed during the sintering process. However, new dioxins homologs with lower chlorination and moderate chlorination are synthesized. These toxic homologs are present in the sintering flue gas. As a result, sintering can reduce the dioxin content of fly ash; however, it leads to more dioxins in the flue gas. The sintering treatment of fly ash generates dioxins and has the potential harm to the environment.
Melting/vitrification
Compared to the sintering process, melting/vitrification of fly ash requires a higher processing temperature (usually 1100–1500 °C). The melting/vitrification technology was derived from the metallurgical industry. It generally utilizes fossil fuel or electricity for the melting/vitrification of materials [151, 152]. During the melting process, the organic part of the MSWI fly ash is decomposed, whereas the inorganic part is transformed into a glass slag via crystal phase transformation. The difference between melting and vitrification is that the latter requires glass-forming additives or other solid waste to form a homogeneous liquid phase material that is subsequently cooled down to form an amorphous, homogenous single-phase glass [146]. These vitreous products have excellent potential for resource utilization, and this treatment is a promising approach for the harmless disposal of MSWI fly ash.
The principle of melting/vitrification
The study of fly ash melting reveals that the melting process is an endothermic reaction that involves the three main processes of dehydration, polymorphic transition and fusion [33], and occurs in the temperature range of 100–200 °C, 480–670 °C and 1101–1244 °C, respectively (as shown in Fig. 2). Figure 3 illustrates the differential thermal analysis (DTA) and differential scanning calorimetry (DSC) curves for a fly ash sample under an oxygen atmosphere at 10 ℃/min. Three absorption peaks are observed in Fig. 3, which represent the process of dehydration, polymorphic transition, and fusion.
The mechanism of the melting of MSWI fly ash involves several steps [33]. When more than one crystal state of the same substance is found, classic thermodynamic considerations show that the polymorph with the lowest free enthalpy is most stable. The crystal becomes mechanically unstable or unrealizable when the temperature is increased, which leads to a polymorphic transformation and equilibrium change from one phase to another.
During melting treatment, the heat and temperature required for melting mainly depend on the composition of the fly ash. The interaction between its internal components also affects the melting temperature. It has been experimentally shown that the addition of SiO2 increases the viscosity of the liquid slag, thus leading to the increase of the melting temperature [33]. When the fly ash has relatively low alkalinity, CaO can be used to reduce the viscosity of liquid slag, thereby lowering the melting temperature and accelerating the melting and crystallization processes. In a study on the influence of CaO and Fe2O3 on the melting temperature of MSWI fly ash [153], it was determined that as the proportion of CaO/Fe2O3 increased, the melting temperature of the fly ash initially increased, then slowly increased thereafter. For other types of elements, Wang et al. [154] demonstrated that both V and Ni can increase the melting temperature. The study by Zhang et al. [155] proved that P can lower the melting temperature only in fly with high Al2O3 content. It can be concluded that for MSWI fly ash, acidic oxides (such as SiO2, P2O5, and TiO2) generally increase the melting temperature, whereas basic oxides (such as Na2O, K2O, MgO, CaO, and MnO2) generally lower the melting temperature [156]. The amphoteric oxides of Al2O3 and Fe2O3, Al2O3 exhibit the characteristics of an acidic oxide with respect to increasing the melting temperature, and Fe2O3 exhibits the characteristics of a basic oxide in that the melting temperature is reduced.
Fuel-burning melting/vitrification
Research on the utilization of fuel to achieve melting/vitrification of fly ash has been conducted over two decades. The fuel utilized in this research includes natural gas, fuel oil and coke [152]. Sakai et al. [157] summarized the properties of the fuel-burning melting/vitrification furnace and system, including the surface melting system, swirling-flow melting system, coke-bed melting furnace, rotary kiln melting furnace, and the internal melting furnace.
The high-temperature environment created by the melt treatment is beneficial to the decomposition of dioxins in fly ash, Sakai et al. [157] established that dioxins in fly ash can be completely destroyed at 1400 °C, with a decrease in the concentration of PCDD/Fs from 320 ng/g to 0.012 ng/g, and a corresponding decrease in the toxicity equivalent from 3.7 to 0.0064 ng-TEQ/g. Wang et al. [34] built a pilot-scale melting furnace that used a spraying tower to rapidly reduce the temperature of flue gas (below 200 °C) to avoid subsequent dioxin synthesis. The testing results revealed that the dioxin toxicity equivalent of the flue gas generated by the fly ash melting process is 0.053 ng-TEQ/m3, which meets the national standard requirements.
The involatile heavy metals in fly ash are transformed and solidified in the dense lattice structure of melted slag, thereby reducing their leaching rate. However, the volatile heavy metals in fly ash exist in the secondary fly ash during the melting process. The volatilization of these heavy metals is related to the high content of Cl in the raw fly ash. The melting process in the surface melting furnace at 1260–1350 °C proves that although the leaching of heavy metals meets the national standard, a relatively high concentration of heavy metals is detected in the secondary fly ash [34]. The residual levels of stabilized heavy metals are in the order of Cr > Zn > Cu > Cd > Pb in melted slag. The main components of secondary fly ash collected from fabric filter bags were metal salts such as NaCl and KCl. Similar results were obtained by Wang et al. [31] for a pilot-scale swirling melting system at the melting temperature of 1250–1400 °C. This indicates that Cr and Ni can be better solidified in melting slag, whereas the highly volatile heavy metals such as Hg, Cd, Pb, and Zn are difficult to stabilize. In addition, the study on co-melting and vitrification treatment with other hazardous waste such as electroplating sludge, suggest that the Cl present in fly ash during the melting process greatly promotes the volatilization of heavy metals, resulting in the volatilization of 96% of Pb, 79% of Cd and 81% of Cr [158].
In the study by Okada et al. [159], it was determined that the volatilization of Cl in a fly ash melting furnace is directly related to the molar ratio of Cl/(Na + K) in the raw fly ash sample. A higher Cl/(Na + K) molar ratio promoted the volatilization of Cl, leading to the detection of more PbCl2 in the secondary fly ash. They then introduced ash circulation to return the melting furnace fly ash to the melting furnace to convert the ash to slag [35]. This study suggested that ash circulation promotes the formation of HCl gas, which leads to a high concentration of Pb in the secondary fly ash. In addition, the lead extraction from the melting furnace fly ash into NaOH solution was also enhanced by ash circulation, and the recovery was over 90%. As such, a new solution for the recycling of heavy metals from MSWI fly ash was presented.
Oxygen-fuel melting/vitrification is a new technology for the disposal of MSWI fly ash. On one hand, it enhances the combustion process and increases the flame temperature, which facilitates melting. On the other hand, the nitrogen content in the flue gas is low after combustion. As such, the heat loss due to the emission of nitrogen in flue gas is less, which greatly conserves energy during the melting process. Moreover, the flue gas contains a large amount of CO2 after oxygen-fuel combustion, and CO2 is believed to be effective in immobilizing heavy metals from fly ash via carbonation [160, 161]. Therefore, oxygen-fuel melting/vitrification can also reduce the leaching of fly ash heavy metals [162], which is a promising approach and worthy of further investigation.
Electric melting/vitrification
The electric melting/vitrification treatments for fly ash were summarized by Sakai et al. and utilize the electric-arc melting furnace, electric resistance melting furnace, plasma melting furnace, and the induction melting furnace [157]. Compared to fuel-burning methods, the electric melting/vitrification of fly ash requires electricity, which is highly suitable for coal-fired power plants where a large amount of electricity is generated.
Plasma is currently the primary area of interest among electric melting/vitrification methods. The high-energy–density and high-temperature of a plasma flame can promote the melting/vitrification reactions and reduce the residence time of MSWI fly ash. Related research [37, 38, 163, 164] has shown that after plasma melting/vitrification treatment, the volume reduction of MSWI fly ash is between 60% and 82.2%, and the mass reduction is between 10% and 56.7%. The analysis of melting products [36, 38, 164] revealed that the density is mostly concentrated between 2.75 and 2.9 g/cm3, and the hardness is in the range of 6–7 GPa. The microstructures of most slags are completely uniform, dense, glassy or contain a glass grid, but the low-temperature process during the plasma melting/vitrification and the addictive CaO in the MSWI fly ash affects the microstructure of slag and reduces its hardness to some extent [164].
In the comparison of migration and the leaching characteristics of heavy metals before and after plasma melting/vitrification [36,37,38, 163, 165], it is determined that most heavy metals sealed in vitreous slag are Cr, and Ni, which are involatile heavy metals. In comparison, Cd and Pb exhibit a strong volatile property. The leaching of other heavy metals in melting/vitrification slag such as Zn and Cu is significantly lower than that of the original fly ash, which proves that vitreous slag can seal the heavy metals from fly ash. Zhao et al. [37] identified the different heavy metal compositions in vitreous slag using different cooling methods such as water-cooling, air-cooling, and composite-cooling. The concentration of Zn, Cd, and Pb in air-cooled slag is significantly higher than that of the other two methods, while the concentration of As and Hg are opposite. The concentration of Cr metal is almost the same for the three methods. The results show that the Zn, Cd and Pb compounds in slag can dissolve in water, thereby reducing the solidification of these heavy metals. As and Hg are highly volatile metals but are insoluble in water; therefore, a portion of these metals may diffuse into the air during air cooling, and their content is consequently lower compared to water-cooling.
Plasma melting/vitrification also plays a significant role in the decomposition of dioxins [38, 165]. It has been experimentally shown that when fly ash passes through the electron or ion stream generated by plasma, significant mechanical damage occurs on the surface of these particles, and the dioxin compounds they contain are effectively destroyed [166]. The degradation of the dioxins is affected by its concentration in the fly ash. A higher concentration leads to more decomposition. The highest destruction rate of 2,3,7,8-T4CDD was 81% of its original amount. It was also determined in a study that dioxins compounds with a higher TEQ value were more easily destroyed in comparison to several dioxin congeners. This result indicates that plasma technology can be used as an effective method to eliminate highly toxic substances such as dioxins. Ren et al. [167] developed a vortex-shaped gliding arc plasma (VGAP), which has several advantages including a larger plasma area, longer reaction time, and wider flow adjustment range. The results indicate that the degradation rate of dioxins via VGAP is 54.9–66.8%, and the corresponding TEQ value is reduced by 60.7–70.1%. During processing, some of the high chloride PCDD/Fs homologs in raw fly ash were converted to low chloride homologs. Therefore, dechlorination is considered to be the primary mechanism for plasma degradation of dioxins.
Separation/extraction
Contrary to the principle of the solidification/stabilizing method, the separation/extraction method involves a series of separation methods (e.g., washing, electrochemical separation, and chemical/biological reagent leaching) to separate heavy metals from MSWI fly ash [168]. The heavy metals in the heavy metal-rich leachate are then extracted to facilitate recovery.
Water-washing
Compared to other harmless treatment methods, the water-washing method is similar to a pretreatment method for the removal of soluble, harmful, and toxic substances from fly ash, thereby facilitating the subsequent disposal of MSWI fly ash [147]. Given that fly ash contains approximately 6 wt.% of Cl, this causes difficulty in the utilization of construction materials [99, 169, 170]. During thermal treatment, the Cl in fly ash forms volatile heavy metal chlorides, which hinders the fixation of heavy metals in the melting product [171]. Therefore, it is necessary to perform dechlorination treatment of fly ash prior to the aforementioned harmless disposal method.
Cl in fly ash is mostly in the form of soluble chlorides such as NaCl, MgCl2, and CaCl2. These chlorides are readily soluble in water and can be removed by washing with this liquid. The factors that influence the effect of chlorine removal by water-washing include the liquid–solid ratio (L/S) and the washing time. Jiang et al. [172] determined that the removal rate of Cl in fly ash reached 72.8% when L/S = 10. A study by Yang et al. [173] demonstrated that 70% of the chloride in the fly ash could be removed using the water washing method. The most economical washing condition was achieved when the washing time was 5 min. and L/S = 3. However, in the study by Wang et al. [170], the best washing condition was L/S = 10 and 2 h of washing time, which yielded a chlorine removal rate of 75.33%. Yang et al. [174] showed that the best washing condition was achieved for L/S = 25. The best washing conditions vary in the literature, suggesting that the optimal dechlorination operating conditions of fly ash are closely related to the characteristics of the fly ash itself.
The removal of Cl is accompanied by the removal of Na, Ca, K, and sulfide during the washing process [172, 173]. This is sometimes associated with negative effects such as the lack of Ca in cement solidification when the water pretreated fly ash is added to the cement. Therefore, Chen et al. [175] developed a multi-step, cyclic water-washing method to enhance the removal of chlorine in fly ash without affecting other soluble substances. The result facilitates subsequent cement solidification. In the study, CaCl2 was used as an additive. The multi-step recirculating water treatment was successful in removing most of the chlorine salt in the fly ash except CaCl2, and also reduced the total content of Cl.
In general, the short-term washing process of fly ash does not cause the dissolution of heavy metals [176]. As the washing process is prolonged, part of the heavy metals may be dissolved in the washing effluent. It has been experimentally shown that the concentration of Co, Cr, Fe, Ni, and Cu in the washing effluent is controlled by the reaction kinetics, and increases with the progression of washing [173]. However, the extraction behavior of Pb and Zn is dominated mainly by the carbonation effect, and their concentration decreases with the washing time. The extracting behavior of Ba and Mn are controlled by both reaction kinetics and the carbonation effect. Their concentration in the water effluent initially increased then subsequently declined. The concentration of Cd and As do not change with the leaching time.
In addition, during the washing process of the fly ash, the form of some heavy metals also changes, which influences their stabilization in the subsequent harmless treatment process. Chiang et al. [177] investigated the effect of water washing on the volatilization of heavy metals in fly ash during the melting process. An experiment was conducted at 1450 ℃ and the results revealed that the melting product contains higher Pb, Cu and lower Zn, Cd, suggesting that the washing process changed the form of Pb and Cu and promotes the solidification of these two metals, but did not affect Zn and Cd. Similar results were obtained by Jiang et al. [172], and it was shown that for a melting process below 1350 ℃, the washing process only promotes the fixation of Cu and Pb, but has no effect on Cd and Cr.
Chemical reagent leaching
The chemical leaching method utilizes a chemical agent to react with heavy metals in fly ash to promote the leaching of heavy metals into the solution. The solution is then treated by centrifugation to obtain fly ash with a low heavy metal content and a leaching solution enriched with heavy metals.
Commonly used chemical agents for the leaching of fly ash heavy metals include acids, bases, salts, and organic chelating agents. Several studies have shown that the leaching characteristics of heavy metals in fly ash are improved under acidic conditions [178], especially for heavy metals in the form of cations such as Cu, Zn, Cd, and Pb [179]. The acid used in the chemical leaching study includes inorganic acids (e.g., HCl, H2SO4, and HNO3) and organic acids (e.g., formic acid, acetic acid, lactic acid, and oxalic acid). The results of several investigations reveal that the leaching effect of organic acids on heavy metals is not as effective as that of inorganic acids [180]. Different inorganic acids have different extraction abilities for heavy metals. For instance, Tang et al. [62] compared the leaching characteristics of heavy metals treated using different inorganic acids (HCl, H2SO4, and HNO3) and found that HCl had the best effect on the leaching of Cu and Zn. Zhang et al. [181, 182] showed that the extraction effect of H2SO4 on different heavy metals in fly ash followed the order Cd > Cu > Pb > Zn, whereas the extraction effect of HNO3 followed the order Pb > Cd > Pu > Zn.
Heavy metals in the form of oxyanions in fly ash such as Cr and Mo are easily removed in an alkaline environment [179]. The alkaline used for leaching were NaOH, KOH, and NH4OH. Kang et al. [183] used alkaline leaching solution with 0.5 mol/L NaOH, KOH and NH4OH to leach heavy metals in fly ash. It was determined that the NH4OH solution achieved the highest extraction rate of heavy metals in fly ash, especially in Cu and Zn, while most of the Pb was leached in KOH solution.
Salts can also be used as extractants to separate heavy metals from fly ash. Lassesson et al. [184] studied the separation of Cu from fly ash using NH4NO3, and found that the leaching characteristics of Cu depended on its composition in the fly ash. The Cu that is leached by NH4NO3 is mainly in the form of copper sulfate, copper hydroxide, and copper chloride, while the form of Cu in the remaining precipitate is mainly divalent copper phosphate silicate, divalent copper oxide, and monovalent copper sulfide or chloride, indicating that these forms of Cu are difficult to leach.
The organic chelating agent ethylenediaminetetraacetic acid (EDTA) is an excellent heavy metal extractor. Yin et al. [185] used EDTA to separate heavy metals from fly ash collected using an electrostatic precipitator (ESP) and bag filter (BF). The results showed that heavy metals in ESP fly ash are more easily separated via EDTA. EDTA has a better removal effect on Cd, Cu, Pb and Zn in fly ash from ESP, and Cr, Pb and Ni in the fly ash from BF. The EDTA leaching of heavy metals in fly ash is affected by the dosage, leaching time and, geochemical properties of fly ash. For example, the leaching of As, Cu, and Zn in EPS is affected by the dosage of EDTA, whereas the leaching of Pb decreases with the increase of the leaching time.
Biological reagent leaching
The biological reagent used for heavy metal extraction is an acid-resisting and heavy metal-resisting strain. After culturing, it can extract heavy metals from fly ash under the appropriate conditions. Several strains can facilitate heavy metal extraction including autotrophic thiobacillus species [186] that are acid-resisting and can obtain energy from Fe2+ and S2− in solution, and heterotrophic Aspergillus niger [187]. The latter has good potential for generating a variety of organic acids that are effective for metal solubilization. The effectiveness of organic acid was enhanced when sulfuric acid was added to the medium.
In the study of bioleaching of fly ash with thiobacillus species, the amount of fly ash, the pH value and the sulfide content all affect the efficiency of this process when it is used to extract heavy metals. Research conducted by Krebs et al. [188] revealed that the best bioleaching effect was achieved in the presence of up to 8% fly ash, and a pH value less than 4. Under this condition, the leaching rate of Cd, Cu, and Zn all exceeded 80% with Al at approximately 60%, and Fe and Ni at approximately 30%. To reduce the fly ash processing time, thiobacillus strains were co-cultured with sewage sludge as inoculum, thus reducing the treatment period from 110 to 70 days.
In the study of bioleaching of fly ash by Aspergillus niger, Xu et al. [189] discovered that the precipitation of calcium oxalate affects bioleaching via the weakening of molecule combination in fly ash, thus facilitating the release of heavy metals. Wang et al. [190] compared the bioleaching characteristics of heavy metals in fly ash with and without washing. It was determined that the chloride component in the fly ash washing process was dissolved via washing, which resulted in the creation of fine fly ash particles. This promotes the bioleaching of heavy metals and reduces the required time for this process. The experimental results revealed that the washing process reduces the treatment period from 30 to 20 days, and the leaching rate of the heavy metals is 96% Cd, 91% Mn, 73% Pb, 68% Zn, 35% Cr, and 30% Fe.
New alkali-resistant strains are also under investigation. In Ramanathan et al.’s work [191], Alkalibacterium sp. TRTYP6 is cultured to bio-leach heavy metals from fly ash. The results indicate that Alkalibacterium sp. TRTYP6 can achieve 52% Cu extraction from fly ash for 20% w/v fly ash addition and growth in the pH range 8–12.5.
Considering chemical leaching and bioleaching, Funari et al.’s study [192] showed that both methods could effectively leach Mg and Zn (leaching rate > 90%), Al and Mn (leaching rate > 85%), Cr (> 65%), Ga (> 60%), Ce (> 50%) from fly ash. The use of chemical reagents led to more effective leaching of Cu, Fe, and Ni, whereas biological reagents promoted the leaching of Nd, Pb, and Co. Compared to chemical leaching, bioleaching can reduce the H2SO4 dosage by half, but requires more processing time (25 days in this experiment).
Electrodialytic treatment
The principle of electrodialytic separation (EDS) or electrodialytic remediation (EDR) involves suspending fly ash in a solution placed in an electrodialysis chamber. An electric field is applied across the cell, causing the metal ions to migrate towards the electrodes according to their charges. Selective ion-exchange membranes placed between two compartments prevent the metal ions from reaching the electrodes. Instead, they are accumulated in “concentration” compartments and are subsequently removed [193].
The presence of heavy metal ions in fly ash can affect their separation via EDS. Research [194] has shown that EDS can effectively remove heavy metals such as Cu, Hg, and Zn, but have little effect on Cd, Cr, and Pb. In fly ash, the heavy metal Cr is the most difficult to remove via EDS. This is probably because of speciation and the formation of uncharged species.
Changing the form of heavy metal ions in fly ash can improve their removal efficiency via the EDS method. Relative methods involve water-washing of fly ash, reduction of the pH value or changing the redox condition of the electrodialytic solution. Chen et al. [195] determined that the acid environment during the EDS process resulted in an increase in the leaching of Cd and Zn from fly ash, and the decrease of the leaching of Cr. During this process, 58.6% of Zn and 5.5% of Pb were extracted. The researchers then developed three electrodialytic cells to gradually increase the pH of the electrodialysis chamber solution to obtain the maximum leaching rate of Cr (27.5%) [196].
Heavy metal extraction
The chemical leaching, bioleaching, and electrodialysis leaching discussed in the previous section all generate a large amount of heavy metal-rich solution. These solutions must undergo extraction to achieve heavy metal enrichment and recovery.
Leachate is a solution containing a variety of salts including heavy metal salts. It is feasible to remove water from this solution by evaporation to achieve the enrichment of heavy metal salts. In the field of desalination, multi-effect distillation [197], dew-vaporation [198], and submerged combustion evaporation [199] can be used to evaporate water to produce salts. However, the salts produced by these methods are rich in a variety of heavy metals, and their composition is complicated. Appropriate treatment is required to realize the harmless disposal of these salts.
Heavy metals in a solution can be extracted using organic extractants to form precipitates prior to removal. Among them, LIX860N-I and Cyanex have good effects on the precipitation of Cu and Zn. Research conducted by Tang et al. [200] proved that LIX860N-I and Cyanex can achieve 95% Cu recovery and 61% Zn recovery, respectively. For different Cyanex extractants [201], Cyanex572 has the best performance for the extraction of Zn, and the Zn recovery process is relatively simple and produces less pollution. However, organic extractants are selective for the extraction of heavy metals, and the extractant itself can also cause potential pollution to the environment.
Nanomaterials are used as adsorbents for heavy metals in solution due to their excellent adsorption capacity, mild stability, and environmental-friendly performance [202]. Novel nanomaterials that have been adopted to absorb heavy metals in solution include metal–organic frameworks (MOFs) [203], nano-zero-valent iron (nZVI) [204], two-dimensional transition metal carbonitrides (MXenes) [205], and graphitic carbon nitride (g-C3N4) [206]. These nanomaterials have excellent ability to adsorb heavy metals such as As, Cr, Pb, Hg, Cd, and Ni. However, they are easily aggregated in practical applications, and the surface properties of some nanomaterials are unstable and easily oxidized, which reduces their sorption capacity. In addition, nanomaterials have low selectivity for metal ions under complicated conditions. Therefore, future research should focus on overcoming these challenges to achieve superior ability to absorb and remove heavy metals in solution.
Comparison of different disposal methods
After an extensive review of the literature on the harmless treatment of MSWI fly ash, and summary of a wide variety of treatment methods, it is concluded that solidification/stabilization, thermal treatment, and separation/extraction methods are the mainstream harmless treatments at present. These methods are dedicated to the effective removal of two major pollutants, heavy metals, and dioxins, in fly ash. A detailed comparison of heavy metal and dioxin stabilization/removal including advantages and disadvantages of different harmless disposal methods for MSWI fly ash are summarized in Table 9.
By comparing different treatments for solidification/removal of heavy metals in fly ash, it can be determined that the solidification/stabilization method can effectively fix heavy metals. However, with the aging of solidification/stabilization products via continuous absorption of CO2 from the environment, these heavy metals are easily leached, especially under acidic conditions. This indicates that the solidification/stabilization of heavy metals is not permanent, and an assessment of the long-term environmental impact of this process is required. During thermal treatment, given that fly ash contains a considerable amount of Cl element, this procedure may cause volatilization of heavy metals such as Zn, Pb, and Cd. Therefore, the water-washing pretreatment is adopted to remove Cl from fly ash prior to thermal treatment. This can reduce the amount of heavy metals in secondary fly ash. Separation/extraction can facilitate leaching and separation of heavy metals from fly ash, which greatly reduces the content of heavy metals. In addition, the extracted heavy metals can be recycled. However, the reagents are selective, and all the heavy metals cannot be extracted using a single reagent. At present, this method is mostly used at the laboratory scale, and further research is required for future development.
By comparing different treatments for the solidification/removal of dioxins in fly ash, similar to the heavy metals, the solidification method cannot achieve a long-term solidification of dioxins. With the progression of time, the solidified product ages and the dioxins that are released back into the environment and cause pollution. Although, hydrothermal treatment and mechano-chemical methods can destroy dioxins in fly ash, in thermal treatment, the temperature is not high enough for sintering to completely destroy the dioxins in the fly ash, and these dioxins can be transferred to flue gas. However, the temperature used in the melting/vitrification method is sufficiently high to destroy dioxins. Related studies on the effects of chemical stabilization and separation/extraction methods to decompose dioxins in fly ash are insufficient and require further research.
Given that the component of fly ash is similar to that of cement raw materials, solidified raw material is cheap and easy to obtain, and the process is relatively simple, the cement solidification method for the treatment of MSWI fly ash has been widely adopted. However, this approach has certain limitations such as the volume expansion of the fly ash after solidification, the decrease of the mechanical strength of the solidified product, and long-term instability of heavy metals and dioxins, which limit the application of cement products. In recent years, research on new types of cement solidification materials has become more widespread, with the objective of overcoming the aforementioned difficulties related to cement solidification.
The different chemical agents have a significant influence on heavy metal stabilization in MSWI fly ash. Similar to the cement solidification method, chemically stabilized heavy metals are unstable in an acidic environment. To improve the stability of heavy metals, the combination of chemical stabilization and cement solidification has proven to be reliable. In addition, the hydrothermal method, which is the combination of chemical stabilization and thermal treatment, and the mechano-chemical method, results in the reduction of the leaching of heavy metals, and can achieve the degradation of dioxins, which has great potential.
Compared to the sintering method, the melting/vitrification method has a superior for the removal of dioxins, which is the primary objective in current thermal treatment research. The limitation with respect to the development of the melting/vitrification method for the disposal of MSWI fly ash is mainly the volatilization of heavy metals and the consumption of large amounts of energy for melting. Pretreatment of fly ash using the water-washing method can effectively reduce the volatilization of heavy metals during the melting process. The fly ash circulation process proposed by Okada et al. [35, 159] exploits the volatility of heavy metals in fly ash, and the realization of the enrichment of these metal in secondary fly ash, leading to a new approach for recycling. Oxygen-fuel melting can achieve higher melting temperatures by consuming less fossil fuel, resulting in a new approach for energy conservation during the melting process.
The washing method is usually combined with other methods to realize the harmless treatment of MSWI fly ash. For example, this method is utilized with cement solidification and thermal treatment to improve the stability of solidification product and to reduce the volatilization of heavy metals, respectively. The biological leaching method requires a long processing time, and the amount of fly ash disposed is strictly controlled. Currently, research is only being conducted at the laboratory scale. Chemical leaching and electrodialysis separation produce a large amount of salty wastewater, which requires subsequent extraction for the recovery of heavy metals. However, the extractant is selective for heavy metals, and it is difficult to extract them. In addition, the extractant poses a potential risk to the environment; therefore, further research is required to identify more general and environmentally friendly heavy metal extractants.
Conclusions
The development of society and the improvement of living standards have resulted in the increasing production of MSW. To address the problem of harmless disposal of MSW, the incineration method has been widely adopted because of its fast processing time and excellent volume reduction of MSW. However, incineration generates MSWI fly ash that is rich in heavy metals and dioxins, and therefore requires appropriate disposal properly. Harmless disposal of MSWI fly ash includes solidification/stabilization, thermal treatment, and the separation/extraction methods. It can be concluded based on a review of recent literature that the solidification/stabilization method is capable of fixing the heavy metal and dioxin components in fly ash, but the solidification/stabilization effect is poor under acidic conditions or after a long period of aging. The melting/vitrification method, as one of the thermal treatment approaches, has a significant effect on the decomposition of dioxins, but most heavy metals cannot be fixed in the melting product. As such, the water-washing pretreatment of raw fly ash, or fly ash circulation is required to enrich heavy metal in the secondary fly ash for further utilization. The separation/extraction method can selectively recover heavy metal elements, but a large amount of salty wastewater is generated after the treatment, which increases the difficulty of subsequent harmless disposal. This method is still in the laboratory scale and further research is required. Therefore, it is necessary to comprehensively consider the advantages and disadvantages of different treatment methods, and to identify appropriate methods to achieve harmless disposal of MSWI fly ash.
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
References
Das S, Lee S, Kumar P, et al. Solid waste management: scope and the challenge of sustainability. J Clean Prod. 2019;228:658–78.
Pujara Y, Pathak P, Sharma A, et al. Review on Indian Municipal Solid Waste Management practices for reduction of environmental impacts to achieve sustainable development goals. J Environ Manag. 2019;248:1–14.
National Bureau of Statistics of China. 2017. https://data.stats.gov.cn/easyquery.htm?cn=C01. Accessed 20 Oct 2019.
Madon I, Drev D, Likar J. Long-term risk assessments comparing environmental performance of different types of sanitary landfills. Waste Manag. 2019;96:96–107.
Aracil C, Pedro H, Diego F, et al. Implementation of waste-to-energy options in landfill-dominated countries: Economic evaluation and GHG impact. Waste Manag. 2018;76:443–56.
Souza WDDM, Rodrigue WS, Filho MMSL, et al. Heavy metals uptake on Malpighia emarginata D.C. seed fiber microparticles: physicochemical characterization, modeling and application in landfill leachate. Waste Manag. 2018;78:356–65.
Liu S, Xi B, Qiu Z, et al. Succession and diversity of microbial communities in landfills with depths and ages and its association with dissolved organic matter and heavy metals. Sci Total Environ. 2019;651:909–16.
Kumar SS, Kumar V, Kumar R, et al. Ferrous sulfate as an in-situ anodic coagulant for enhanced bioelectricity generation and COD removal from landfill leachate. Energy. 2019;176:570–81.
Fellner J, Lederer J, Purgar A, et al. Evaluation of resource recovery from waste incineration residues–the case of zinc. Waste Manag. 2015;37(3):95–103.
Makarichi L, Jutidamrongphan W, Techato K. The evolution of waste-to-energy incineration: a review. Renew Sustain Energy Rev. 2018;91:812–21.
Ettouney RS, El-Rifai MA, El-Behairy SA. Control of thermally integrated incineration–waste heat recovery systems, a case study. Appl Therm Eng. 2005;25(8):1195–205.
Allegrini E, Vadenbo C, Boldrin A, et al. Life cycle assessment of resource recovery from municipal solid waste incineration bottom ash. J Environ Manag. 2015;151:132–43.
Song J, Sun Y, Jin L. PESTEL analysis of the development of the waste-to-energy incineration industry in China. Renew Sustain Energy Rev. 2017;80:276–89.
IAWG (International ash working group). Municipal solid waste incinerator residues. Stud Environ Sci. 1997;67–68.
Boom AD, Degrez M. Belgian MSWI fly ashes and APC residues: a characterisation study. Waste Manag. 2012;32(6):1163–70.
Tchounwou PB, Yedjou CG, Patlolla AK, et al. Molecular, clinical and environmental toxicology: heavy metal toxicity and the. Environment. 2012;3:133–64.
Fei J, Min X, Wang Z, et al. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: a case study from South China. Environ Sci Pollut Res. 2017;24(35):27573–86.
Pan Y, Wu Z, Zhou J, et al. Chemical characteristics and risk assessment of typical municipal solid waste incineration (MSWI) fly ash in China. J Hazard Mater. 2013;261(20):269–76.
Milbrath MO, Wenger Y, Chang CW, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117(3):417–25.
Sun J, Hu J, Zhu G, et al. PCDD/Fs distribution characteristics and health risk assessment in fly ash discharged from MSWIs in China. Ecotoxicol Environ Saf. 2017;139:83–8.
Pan Y, Yang L, Zhou J, et al. Characteristics of dioxins content in fly ash from municipal solid waste incinerators in China. Chemosphere. 2013;92(7):765–71.
Quina MJ, Bordado JC, Quinta-Ferreira RM. Treatment and use of air pollution control residues from MSW incineration: an overview. Waste Manag. 2008;28(11):2097–121.
Sakai E, Miyahara S, Ohsawa S, et al. Hydration of fly ash cement. Cem Concr Res. 2005;35(6):1135–40.
Yu Q, Nagataki S, Lin J, et al. The leachability of heavy metals in hardened fly ash cement and cement-solidified fly ash. Cem Concr Res. 2005;35(6):1056–63.
Li X, Chen Q, Zhou Y, et al. Stabilization of heavy metals in MSWI fly ash using silica fume. Waste Manag. 2014;34(12):2494–504.
Sun Y, Watanabe N, Qiao W, et al. Polysulfide as a novel chemical agent to solidify/stabilize lead in fly ash from municipal solid waste incineration. Chemosphere. 2010;81(1):120–6.
Jiang J, Wang J, Xu X, et al. Heavy metal stabilization in municipal solid waste incineration flyash using heavy metal chelating agents. J Hazard Mater. 2004;113(1):141–6.
Sukandar, Padmi T, Tanaka M et al. Chemical stabilization of medical waste fly ash using chelating agent and phosphates: heavy metals and ecotoxicity evaluation. Waste Manage. 2009;29(7):2065–70.
Li R, Li Y, Yang T, et al. A new integrated evaluation method of heavy metals pollution control during melting and sintering of MSWI fly ash. J Hazard Mater. 2015;289:197–203.
Sobiecka E. Thermal and physicochemical technologies used in hospital incineration fly ash utilization before landfill in Poland. J Chem Technol Biotechnol. 2016;91(9):2457–61.
Wang X, Jin B, Xu B, et al. Melting characteristics during the vitrification of MSW incinerator fly ash by swirling melting treatment. J Mater Cycles Waste Manag. 2017;19(1):483–95.
Ristić M, Milosević SD. Frenkel’s theory of sintering. Sci Sintering. 2006;38:7–11.
Li R, Wang L, Yang T, et al. Investigation of MSWI fly ash melting characteristic by DSC–DTA. Waste Manag. 2007;27(10):1383–92.
Wang Q, Tian S, Wang Q, et al. Melting characteristics during the vitrification of MSWI fly ash with a pilot-scale diesel oil furnace. J Hazard Mater. 2008;160(2):376–81.
Okada T, Suzuki M. Effect of ash circulation in gasification melting system on concentration and leachability of lead in melting furnace fly ash. J Environ Manag. 2013;130:347–53.
Wang Q, Yan J, Tu X, et al. Thermal treatment of municipal solid waste incinerator fly ash using DC double arc argon plasma. Fuel. 2009;88(5):955–8.
Zhao P, Ni G, Jiang Y, et al. Destruction of inorganic municipal solid waste incinerator fly ash in a DC arc plasma furnace. J Hazard Mater. 2010;181(1):580–5.
Wang Q, Yan J, Chi Y, et al. Application of thermal plasma to vitrify fly ash from municipal solid waste incinerators. Chemosphere. 2010;78(5):626–30.
Tang J, Petranikova M, Ekberg C, et al. Mixer-settler system for the recovery of copper and zinc from MSWI fly ash leachates: an evaluation of a hydrometallurgical process. J Clean Prod. 2017;148:595–605.
Tang J, Steenari BM. Solvent extraction separation of copper and zinc from MSWI fly ash leachates. Waste Manag. 2015;44:147–54.
Li M, Xiang J, Hu S, et al. Characterization of solid residues from municipal solid waste incinerator. Fuel. 2004;83(10):1397–405.
Rendek E, Ducom G, Germain P. Influence of waste input and combustion technology on MSWI bottom ash quality. Waste Manag. 2007;27(10):1403–7.
Wang Y, Zhang X, Liao W, et al. Investigating impact of waste reuse on the sustainability of municipal solid waste (MSW) incineration industry using emergy approach: a case study from Sichuan province. China. Waste Manage. 2018;77:252–67.
Park K, Hyun J, Maken S, et al. Vitrification of municipal solid waste incinerator fly ash using brown’s gas. Energy Fuels. 2005;19(1):258–62.
Qiu Q, Jiang X, Lv G, et al. Adsorption of heavy metal ions using zeolite materials of municipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment. Powder Technol. 2018;335:156–63.
Mu Y, Saffarzadeh A, Shimaoka T. Influence of ignition of waste fishbone on enhancing heavy metal stabilization in municipal solid waste incineration (MSWI) fly ash. J Clean Prod. 2018;189:396–405.
Huber F, Blasenbauer D, Mallow O, et al. Thermal co-treatment of combustible hazardous waste and waste incineration fly ash in a rotary kiln. Waste Manag. 2016;58(58):181–90.
Kalmykova Y, Karlfeldt FK. Phosphorus recovery from municipal solid waste incineration fly ash. Waste Manag. 2013;33(6):1403–10.
Qiu Q, Jiang X, Chen Z, et al. Microwave-assisted hydrothermal treatment with soluble phosphate added for heavy metals solidification in MSWI fly ash. Energy Fuels. 2017;31(5):5222–32.
Bhatt AH, Priyadarshini S, Mohanakrishnan AA, et al. Physical, chemical, and geotechnical properties of coal fly ash: a global review. Case Stud Constr Mater. 2019;11:1–11.
Shim YS, Rhee SW, Lee WK. Comparison of leaching characteristics of heavy metals from bottom and fly ashes in Korea and Japan. Waste Manag. 2005;25(5):473–80.
Gao X, Wang W, Ye T, et al. Utilization of washed MSWI fly ash as partial cement substitute with the addition of dithiocarbamic chelate. J Environ Manag. 2008;88(2):293–9.
Zhang H, Zhao Y, Qi J. Characterization of heavy metals in fly ash from municipal solid waste incinerators in Shanghai. Procces Saf Envrion. 2010;88(2):114–24.
Wang KS, Chiang KY, Lin KL, et al. Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy. 2001;62(2):73–81.
Wan X, Wang W, Ye T, et al. A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure. J Hazard Mater. 2006;134(1):197–201.
Li Q, Meng A, Jia J, et al. Investigation of heavy metal partitioning influenced by flue gas moisture and chlorine content during waste incineration. J Environ Sci China. 2010;22(5):760–8.
Kurashima K, Matsuda K, Kumagai S, et al. A combined kinetic and thermodynamic approach for interpreting the complex interactions during chloride volatilization of heavy metals in municipal solid waste fly ash. Waste Manag. 2019;87:204–17.
Weibel G, Eggenberger U, Kulik D, et al. Extraction of heavy metals from MSWI fly ash using hydrochloric acid and sodium chloride solution. Waste Manag. 2018;76:457–71.
Xia Y, He P, Shao L, et al. Metal distribution characteristic of MSWI bottom ash in view of metal recovery. J Environ Sci China. 2017;52(2):178–89.
Luo H, Wu Y, Zhao A, et al. Hydrothermally synthesized porous materials from municipal solid waste incineration bottom ash and their interfacial interactions with chloroaromatic compounds. J Cleaner Prod. 2017;162:411–9.
Mizutani S, Yoshida T, Sakai S, et al. Release of metals from MSW I fly ash and availability in alkali condition. Waste Manag. 1996;16:537–44.
Tang J, Steenari B. Leaching optimization of municipal solid waste incineration ash for resource recovery: a case study of Cu. Zn Pb and Cd. Waste Manag. 2016;48(48):315–22.
Zhang Y, Cetin B, Likos WJ, et al. Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel. 2016;184:815–25.
Trinh MM, Chang MB. Review on occurrence and behavior of PCDD/Fs and dl-PCBs in atmosphere of East Asia. Atmos Environ. 2018;180:23–36.
Cieplik MK, Vincent DJ, Jelena B, et al. Formation of dioxins from combustion micropollutants over MSWI fly ash. Environ Sci Technol. 2006;40(4):1263–9.
Tang Z, Huang Q, Yang Y. PCDD/Fs in fly ash from waste incineration in china: a need for effective risk management. Environ Sci Technol. 2013;47(11):5520–1.
Song S, Zhou X, Guo C, et al. Emission characteristics of polychlorinated, polybrominated and mixed polybrominated/chlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs, PBDD/Fs, and PBCDD/Fs) from waste incineration and metallurgical processes in China. Ecotoxicol Environ Saf. 2019;184:109608.
Barghi M, Choi S, Kwon H, et al. Influence of non-detect data-handling on toxic equivalency quantities of PCDD/Fs and dioxin-like PCBs: a case study of major fish species purchased in Korea. Environ Pollut. 2016;214:532–8.
Liu Y, Liu Y. Novel incineration technology integrated with drying, pyrolysis, gasification, and combustion of MSW and ashes vitrification. Environ Sci Technol. 2005;39(10):3855–63.
Song GJ, Kim SH, Seo YC, et al. Dechlorination and destruction of PCDDs/PCDFs in fly ashes from municipal solid waste incinerators by low temperature thermal treatment. Chemosphere. 2008;71(2):248–57.
Chang Y, Fan W, Dai W, et al. Characteristics of PCDD/F content in fly ash discharged from municipal solid waste incinerators. J Hazard Mater. 2011;192(2):521–9.
Yasuhara AT. Katami Leaching behavior of polychlorinated dibenzo-p-dioxins and furans from the fly ash and bottom ash of a municipal solid waste incinerator. Waste Manag. 2007;27(3):439–47.
Lundin L, Marklund S. Thermal degradation of PCDD/F, PCB and HCB in municipal solid waste ash. Chemosphere. 2007;67(3):474–81.
Liu G, Jiang X, Wang M, et al. Comparison of PCDD/F levels and profiles in fly ash samples from multiple industrial thermal sources. Chemosphere. 2015;133(1):68–74.
Ham SY, KimYJ Lee DH. Leaching characteristics of PCDDs/DFs and dioxin-like PCBs from landfills containing municipal solid waste and incineration residues. Chemosphere. 2008;70(9):1685–93.
Choi K, Lee D. PCDD/DF in leachates from Korean MSW landfills. Chemosphere. 2006;63(8):1353–60.
Spence R. Designing of cement-based formula for solidification/stabilization of hazardous, radioactive, and mixed wastes. Crit Rev Environ Sci Technol. 2004;34(4):391–417.
Koo D, Sung H, Kim S, et al. Characteristics of cement solidification of metal hydroxide waste. Nucl Eng Technol. 2017;49(1):165–71.
Zhang R, Ren HQ, Ding LL, et al. Evaluating solidification characteristics of textile dyeing sludge with addition of portland cement and attapulgite. Appl Mech Mater. 2015;768:375–84.
Bayar S, Talinli I. Solidification/stabilization of hazardous waste sludge obtained from a chemical industry. Clean Technol Environ Policy. 2013;15(1):157–65.
Wang Z, Song Y. Adsorption properties of CFBC ash-cement pastes as compared with PCC fly ash-cement pastes. Int J Coal Sci Technol. 2016;3(1):62–7.
Petersen T, Valdenaire P, Pellenq R, et al. A reaction model for cement solidification: evolving the C–S–H packing density at the micrometer-scale. J Mech Phys Solids. 2018;118:58–73.
Nabajyoti S, Shigeru K, Toshinori K. Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Manag. 2007;27(9):1178–89.
Saeed KA, Kassim KA, Eisazadeh A. Interferences of cement based-solidification/stabilization and heavy metals: A review. Electron J Geotech Eng. 2012;17:2555–65.
Zhang J, Liu J, Li C, et al. Comparison of the fixation effects of heavy metals by cement rotary kiln co-processing and cement based solidification/stabilization. J Hazard Mater. 2009;165(1):1179–85.
Valls S, Vàzquez E. Leaching properties of stabilised/solidified cement-admixtures-sewage sludges systems. Waste Manag. 2002;22(1):37–45.
Conner JR, Hoeffner SL. A critical review of stabilization/solidification technology. Crit Rev Environ Control. 1998;28(4):397–462.
Lu H, Wei F, Tang J, et al. Leaching of metals from cement under simulated environmental conditions. J Environ Manag. 2016;169:319–27.
Li W, Sun Y, Huang Y, et al. Evaluation of chemical speciation and environmental risk levels of heavy metals during varied acid corrosion conditions for raw and solidified/stabilized MSWI fly ash. Waste Manag. 2019;87:407–16.
Gerven TV, Baelen DV, Dutré V, et al. Influence of carbonation and carbonation methods on leaching of metals from mortars. Cem Concr Res. 2004;34(1):149–56.
Zha X, Ning J, Saafi M, et al. Effect of supercritical carbonation on the strength and heavy metal retention of cement-solidified fly ash. Cem Concr Res. 2019;120:36–45.
Zha X, Wang H, Xie P, et al. Leaching resistance of hazardous waste cement solidification after accelerated carbonation. Cem Concr Compos. 2016;72:125–32.
Wen D, Zhang CY, Kong XM, et al. Mercury release from fly ashes and hydrated fly ash cement pastes. Atmos Environ. 2018;178:11–8.
His H, Wang L, Yu T. Effects of injected activated carbon and solidification treatment on the leachability of polychlorinated dibenzo-p-dioxins and dibenzofurans from air pollution control residues of municipal waste incineration. Chemosphere. 2007;67(7):1394–402.
Hsi HC, Yu TH. Evaluation of the leachability of polychlorinated dibenzo–dioxins and dibenzofurans in raw and solidified air pollution control residues from municipal waste incinerators. Chemosphere. 2007;67(7):1434–43.
Wang MS, Wang LC, Chang-Chien GP. Distribution of polychlorinated dibenzo- p -dioxins and dibenzofurans in the landfill site for solidified monoliths of fly ash. J Hazard Mater. 2006;133(1):177–82.
Yang Z, Tian S, Liu L, et al. Application of washed MSWI fly ash in cement composites: long-term environmental impacts. Environ Sci Pollut Res. 2018;25(12):12127–38.
Bie R, Pei C, Song X, et al. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J Energy Inst. 2016;89(4):704–12.
Colangelo F, Cioffi R, Montagnaro F, et al. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012;32(6):1179–85.
Ding Z, Dong B, Xing F, et al. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram Int. 2012;38(8):6281–8.
Su Y, Yang J, Liu D, et al. Effects of municipal solid waste incineration fly ash on solidification/stabilization of Cd and Pb by magnesium potassium phosphate cement. J Environ Chem Eng. 2016;4(1):259–65.
Zhang N, Liu X, Sun H. Hydration characteristics of intermediate-calcium based cementitious materials from red mud and coal gangue. Chin J Mater Res. 2014;28(5):325–32.
Liu X, Zhao X, Yin H, et al. Intermediate-calcium based cementitious materials prepared by MSWI fly ash and other solid wastes: hydration characteristics and heavy metals solidification behavior. J Hazard Mater. 2018;349:262–71.
Huang K, Fan X, Gan M, et al. Use of municipal solid waste incinerator (MSWI) fly ash in alkali activated slag cement. In: Li B et al, editors. Characterization of minerals, metals, and materials. Cham: Springer; 2019. p 401–10.
Bournonville B, Nzihou A, Sharrock P, et al. Stabilisation of heavy metal containing dusts by reaction with phosphoric acid: study of the reactivity of fly ash. J Hazard Mater. 2004;116(1):65–74.
Piantone P, Bodenan F, Derie R, et al. Monitoring the stabilization of municipal solid waste incineration fly ash by phosphation: mineralogical and balance approach. Waste Manag. 2003;23(3):225–43.
Quina MJ, Bordado JC, Quintaferreira RM. Chemical stabilization of air pollution control residues from municipal solid waste incineration. J Hazard Mater. 2010;179(1):382–92.
Mu Y, Saffarzadeh A, Shimaoka T. Feasibility of using natural fishbone apatite on removal of Pb from municipal solid waste incineration (MSWI) fly ash. Proc Environ Sci. 2016;31:345–50.
Wang H, Fan X, Wang Y, et al. Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J Environ Manag. 2018;208:15–23.
Rodella N, Bosio A, Dalipi R, et al. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab J Chem. 2017;10(S2):3676–81.
Yuan W, Xu W, Wu Z, et al. Mechanochemical treatment of Cr(VI) contaminated soil using a sodium sulfide coupled solidification/stabilization process. Chemosphere. 2018;212:540–7.
Zhao Y, Song L, Li G. Chemical stabilization of MSW incinerator fly ashes. J Hazard Mater. 2002;95(1):47–63.
Lundtorp K, Jensen DL, SRensen MA, et al. Treatment of waste incinerator air-pollution-control residues with FeSO4: concept and product characterisation. Waste Manag Res. 2002;20(1):69–79.
Huang WJ, Lo JS. Synthesis and efficiency of a new chemical fixation agent for stabilizing MSWI fly ash. J Hazard Mater. 2004;112(1):79–86.
Ecke H, Sakanankura H, Matsuto T, et al. State-of-the-art treatment processes for municipal solid waste incineration residues in Japan. Waste Manag Res. 2010;18(1):41–51.
Çelik Z, Gülfen M, Aydın AO. Synthesis of a novel dithiooxamide–formaldehyde resin and its application to the adsorption and separation of silver ions. J Hazard Mater. 2010;174(1):556–62.
Liu S, Guo Y, Yang H, et al. Synthesis of a water-soluble thiourea-formaldehyde (WTF) resin and its application to immobilize the heavy metal in MSWI fly ash. J Environ Manag. 2016;182:328–34.
Sakanakura H. Formation and durability of dithiocarbamic metals in stabilized air pollution control residue from municipal solid waste incineration and melting processes. Environ Sci Technol. 2007;41(5):1717–22.
Wang FH, Zhao B, Zhang F, et al. A novel heavy metal chelating agent sixthio guanidine acid for in situ remediation of soils contaminated with multielements: its synthesis, solidification, biodegradability, and leachability. J Soils Sedime. 2016;16(2):371–81.
Wang F, Zhang F, Chen Y, et al. A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J Hazard Mater. 2015;300:451–8.
Eighmy TT, Crannell BS, Butler LG, et al. Heavy metal stabilization in municipal solid waste combustion dry scrubber residue using soluble phosphate. Environ Sci Technol. 1997;31(11):3330–8.
Hyks J, Astrup TF, Christensen TH. Long-term leaching from MSWI air-pollution-control residues: leaching characterization and modeling. J Hazard Mater. 2009;162(1):80–91.
Jiri H, Thomas A, Christensen TH. Influence of test conditions on solubility controlled leaching predictions from air-pollution-control residues. Waste Manag Res J Int Solid Wastes Public Clean Assoc ISWA. 2007;25(5):457–66.
Ma QY, Traina SJ, Logan T, et al. In situ lead immobilization by apatite. Environ Sci Technol. 1993;27(9):1803–10.
Crannell BS, Eighmy TT, Krzanowski JE, et al. Heavy metal stabilization in municipal solid waste combustion bottom ash using soluble phosphate. Waste Manag. 2000;20(2):135–48.
Hong K, Tokunaga S, Kajiuchi T. Extraction of heavy metals from MSW incinerator fly ashes by chelating agents. J Hazard Mater. 2000;75(1):57–73.
Ma W, Chen D, Pan M, et al. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: a comparative study. J Environ Manag. 2019;247:169–77.
Jing Z, Ran X, Jin F, et al. Hydrothermal solidification of municipal solid waste incineration bottom ash with slag addition. Waste Manag. 2010;30(8):1521–7.
Bayuseno AP, Schmahl WW, Mullejans T. Hydrothermal processing of MSWI fly ash-towards new stable minerals and fixation of heavy metals. J Hazard Mater. 2009;167(1):250–9.
Gong B, Deng Y, Yang Y, et al. Effects of microwave-assisted thermal treatment on the fate of heavy metals in municipal solid waste incineration fly ash. Energy Fuels. 2017;31(11):12446–54.
Xie JL, Hu Y, Chen D, et al. Hydrothermal treatment of MSWI fly ash for simultaneous dioxins decomposition and heavy metal stabilization. Front Environ Sci Eng China. 2010;4(1):108–15.
Hu Y, Zhang P, Chen D, et al. Hydrothermal treatment of municipal solid waste incineration fly ash for dioxin decomposition. J Hazard Mater. 2012;207:79–85.
Gilman JJ. Mechanochemistry. Science. 1996;274(5284):65.
Do J, Friscic T. Mechanochemistry: a force of synthesis. ACS Cent Sci. 2017;3(1):13–9.
Montinaro S, Concas A, Pisu M, et al. Immobilization of heavy metals in contaminated soils through ball milling with and without additives. Chem Eng J. 2008;142(3):271–84.
Nomura Y, Fujiwara K, Terada A, et al. Prevention of lead leaching from fly ashes by mechanochemical treatment. Waste Manag. 2010;30(7):1290–5.
Li M, Sun C, Gau S, et al. Effects of wet ball milling on lead stabilization and particle size variation in municipal solid waste incinerator fly ash. J Hazard Mater. 2010;174(1):586–91.
Chen Z, Lu S, Mao Q, et al. Suppressing heavy metal leaching through ball milling of fly ash. Energies. 2016;9(524):1–13.
Yoshiharu M, Norie T, Maki T, et al. Calcium-promoted catalytic degradation of PCDDs, PCDFs, and coplanar PCBs under a mild wet process. Environ Sci Technol. 2006;40(6):1849–54.
Lu S, Huang J, Zheng P, et al. Ball milling 2,4,6-trichlorophenol with calcium oxide: dechlorination experiment and mechanism considerations. Chem Eng J. 2012;195–196(7):62–8.
Wang Z, Huang J, Xu F, et al. Mechanochemical destruction of pentachloronitrobenzene with reactive iron powder. J Hazard Mater. 2011;198(2):275–81.
Chen Z, Tang M, Lu S, et al. Evolution of PCDD/F-signatures during mechanochemical degradation in municipal solid waste incineration filter ash. Chemosphere. 2018;208:176–84.
Chen Z, Mao Q, Lu S, et al. Dioxins degradation and reformation during mechanochemical treatment. Chemosphere. 2017;180:130–40.
Yang GCC, Chuang T, Huang C. Achieving zero waste of municipal incinerator fly ash by melting in electric arc furnaces while steelmaking. Waste Manag. 2017;62:160–8.
Yang J, Xiao B, Boccaccini AR. Preparation of low melting temperature glass–ceramics from municipal waste incineration fly ash. Fuel. 2009;88(7):1275–80.
Lindberg D, Molin C, Hupa M. Thermal treatment of solid residues from WtE units: a review. Waste Manag. 2015;37(3):82–94.
Liu Y, Zheng L, Li X, et al. SEM/EDS and XRD characterization of raw and washed MSWI fly ash sintered at different temperatures. J Hazard Mater. 2009;162(1):161–73.
Karamanov A, Aloisi M, Pelino M. Sintering behaviour of a glass obtained from MSWI ash. J Eur Ceram Soc. 2005;25(9):1531–40.
Chou S, Lo S, Hsieh C, et al. Sintering of MSWI fly ash by microwave energy. J Hazard Mater. 2009;163(1):357–62.
Min Y, Liu C, Shi P, et al. Effects of the addition of municipal solid waste incineration fly ash on the behavior of polychlorinated dibenzo-p-dioxins and furans in the iron ore sintering process. Waste Manag. 2018;77:287–93.
Bingham PA, Hand RJ. Vitrification of toxic wastes: a brief review. Br Ceram Trans. 2006;105(1):21–31.
Colombo P, Brusatin G, Bernardo E, et al. Inertization and reuse of waste materials by vitrification and fabrication of glass-based products. Curr Opin Solid State Mater Sci. 2003;7(3):225–39.
Shi WJ, Kong LX, Bai J, et al. Effect of CaO/Fe2O3 on fusion behaviors of coal ash at high temperatures. Fuel Process Technol. 2018;181:18–24.
Wang ZG, Kong LX, Bai J, et al. Effect of vanadium and nickel on iron-rich ash fusion characteristics. Fuel. 2019;246:491–9.
Qiang Z, Liu H, Qian Y, et al. The influence of phosphorus on ash fusion temperature of sludge and coal. Fuel Process Technol. 2013;110(110):218–26.
Liu Z, Zhang T, Zhang J, et al. Ash fusion characteristics of bamboo, wood and coal. Energy. 2018;161:517–22.
Sakai SI, Hiraoka M. Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag. 2000;20(2):249–58.
Yue Y, Zhang J, Sun F, et al. Heavy metal leaching and distribution in glass products from the co-melting treatment of electroplating sludge and MSWI fly ash. J Environ Manag. 2019;232:226–35.
Okada T, Tomikawa H. Leaching characteristics of lead from melting furnace fly ash generated by melting of incineration fly ash. J Environ Manag. 2012;110:207–14.
Rendek E, Ducom G, Germain P. Carbon dioxide sequestration in municipal solid waste incinerator (MSWI) bottom ash. J Hazard Mater. 2006;128(1):73–9.
Nilsson M, Andreas L, Lagerkvist A. Effect of accelerated carbonation and zero valent iron on metal leaching from bottom ash. Waste Manag. 2016;51(51):97–104.
Ni P, Xiong Z, Tian C, et al. Influence of carbonation under oxy-fuel combustion flue gas on the leachability of heavy metals in MSWI fly ash. Waste Manag. 2017;67:171–80.
Ma W, Fang Y, Chen D, et al. Volatilization and leaching behavior of heavy metals in MSW incineration fly ash in a DC arc plasma furnace. Fuel. 2017;210:145–53.
Karoly Z, Mohai I, Toth M, et al. Production of glass–ceramics from fly ash using arc plasma. J Eur Ceram Soc. 2007;27(2):1721–5.
Čarnogurská M, Lázár M, Puškár M, et al. Measurement and evaluation of properties of MSW fly ash treated by plasma. Measurement. 2015;62:155–61.
Zhou Y, Yan P, Cheng Z, et al. Application of non-thermal plasmas on toxic removal of dioxin-contained fly ash. Powder Technol. 2003;135:345–53.
Ren Y, Li X, Yu L, et al. Degradation of PCDD/Fs in Fly Ash by Vortex-shaped Gliding Arc Plasma. Plasma Chem Plasma Process. 2013;33(1):293–305.
Luo H, Cheng Y, He D, et al. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci Total Environ. 2019;668:90–103.
Ferreira C, RibeiroAB, Ottosen LM. Possible applications for municipal solid waste fly ash. J Hazard Mater. 2003;96(2):201–216.
Wang X, Li A, Zhang Z. The effects of water washing on cement-based stabilization of MWSI fly ash. Proc Environ Sci. 2016;31:440–6.
Nowak B, Pessl A, Aschenmrenner P, et al. Heavy metal removal from municipal solid waste fly ash by chlorination and thermal treatment. J Hazard Mater. 2010;179(1):323–31.
Jiang Y, Xi B, Li X, et al. Effect of water-extraction on characteristics of melting and solidification of fly ash from municipal solid waste incinerator. J Hazard Mater. 2009;161(2):871–7.
Yang Z, Tian S, Ji R, et al. Effect of water-washing on the co-removal of chlorine and heavy metals in air pollution control residue from MSW incineration. Waste Manag. 2017;68:221–31.
Yang R, Liao WP, Wu PH. Basic characteristics of leachate produced by various washing processes for MSWI ashes in Taiwan. J Environ Manag. 2012;104(16):67–76.
Chen X, Bi Y, Zhang H, et al. Chlorides removal and control through water-washing process on MSWI fly ash. Proc Environ Sci. 2016;31:560–6.
Bayuseno AP, Schmahl WW. Characterization of MSWI fly ash through mineralogy and water extraction. Resour Conserv Recycl. 2011;55(5):524–34.
Chiang KY, Hu YH. Water washing effects on metals emission reduction during municipal solid waste incinerator (MSWI) fly ash melting process. Waste Manag. 2010;30(5):831–8.
Huang K, Inoue K, Harada H, et al. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans Nonferr Met Soc China. 2011;21(6):1422–7.
Nordmark D, Lagerkvist A. Controlling the mobility of chromium and molybdenum in MSWI fly ash in a washing process. Waste Manage. 2018;76:727–33.
Fedje KK, Ekberg C, Skarnemark G, et al. Removal of hazardous metals from MSW fly ash—an evaluation of ash leaching methods. J Hazard Mater. 2010;173(1):310–7.
Zhang HY, Ma GX. Leaching of heavy metals from municipal solid waste incineration (MSWI) fly ash using sulfuric acid. Appl Mech Mater. 2012;249–250:922–6.
Zhang HY, Ma GX. Leaching of heavy metals from municipal solid waste incineration (MSWI) fly ash using nitric acid. Appl Mech Mater. 2012;249–250:918–21.
Kang D, Son J, Yoo Y, et al. Heavy-metal reduction and solidification in municipal solid waste incineration (MSWI) fly ash using water, NaOH, KOH, and NH4OH in combination with CO2 uptake procedure. Chem Eng J. 2020;380:1–11.
Henric L, Karin Karlfeldt F, Britt-Marie S. Leaching for recovery of copper from municipal solid waste incineration fly ash: influence of ash properties and metal speciation. Waste Manag Res. 2014;32(8):755–62.
Ke Y, Li P, Wei P, et al. Characteristics of heavy metals leaching from MSWI fly ashes in sequential scrubbing processes. J Mater Cycles Waste Manag. 2018;20(1):604–13.
Tomonori I, Akane N, Masafumi T, et al. Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere. 2005;60(8):1087–94.
Mulligan CN, Mahtab K, Gibbs BF. Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J Hazard Mater. 2004;110(1–3):77–84.
Krebs W, Bachofen R, Brandl H. Growth stimulation of sulfur oxidizing bacteria for optimization of metal leaching efficiency of fly ash from municipal solid waste incineration. Hydrometallurgy. 2001;59(2):283–90.
Xu TJ, Ramanathan T, Ting YP. Bioleaching of incineration fly ash by Aspergillus niger—precipitation of metallic salt crystals and morphological alteration of the fungus. Biotechnol Rep. 2014;3:8–14.
Wang Q, Yang J, Wang Q, et al. Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incinerator fly ash. J Hazard Mater. 2009;162(2):812–8.
Ramanathan T, Ting YP. Alkaline bioleaching of municipal solid waste incineration fly ash by autochthonous extremophiles. Chemosphere. 2016;160:54–61.
Funari V, Mäkinen J, Salminen L, et al. Metal removal from Municipal Solid Waste Incineration fly ash: a comparison between chemical leaching and bioleaching. Waste Manag. 2016;60:397–406.
Ferreira C, Jensen P, Ottosen L, et al. Removal of selected heavy metals from MSW fly ash by the electrodialytic process. Eng Geol. 2005;77(3):339–47.
Kirkelund GM, Jensen PE. Electrodialytic treatment of Greenlandic municipal solid waste incineration fly ash. Waste Manage. 2018;80:241–51.
Chen W, Kirkelund GM, Jensen PE, et al. Comparison of different MSWI fly ash treatment processes on the thermal behavior of As, Cr, Pb and Zn in the ash. Waste Manag. 2017;24(27):21591–600.
Chen W, Kirkelund GM, Jensen PE, et al. Electrodialytic extraction of Cr from water-washed MSWI fly ash by changing pH and redox conditions. Waste Manag. 2018;71:215–23.
Andrés-Mañas JA, Ruiz-Aguirre A, Acién FG, et al. Assessment of a pilot system for seawater desalination based on vacuum multi-effect membrane distillation with enhanced heat recovery. Desalination. 2018;443:110–21.
Hamieh BM, Beckman JR. Seawater desalination using Dewvaporation technique: theoretical development and design evolution. Desalination. 2006;195(1):1–13.
Yue D, Xu Y, Mahar R, et al. Laboratory-scale experiments applied to the design of a two-stage submerged combustion evaporation system. Waste Manag. 2007;27(5):704–10.
Tang J, Su M, Zhang H, et al. Assessment of copper and zinc recovery from MSWI fly ash in Guangzhou based on a hydrometallurgical process. Waste Manag. 2018;76:225–33.
Tang J, Yimen R, Petranikova M, et al. Comparative study of the application of traditional and novel extractants for the separation of metals from MSWI fly ash leachates. J Clean Prod. 2018;172:143–54.
Wu YW, Pang H, Liu Y, et al. Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut. 2019;246:608–20.
Xing L, Yang L, Zhang C, et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem Eng J. 2017;336:241–52.
Tang J, Su M, Wu Q, et al. Highly efficient recovery and clean-up of four heavy metals from MSWI fly ash by integrating leaching, selective extraction and adsorption. J Clean Prod. 2019;234:139–49.
Ng VMH, Hui H, Zhou K, et al. Correction: recent progress in layered transition metal carbides and/or nitrides (MXenes) and their composites: synthesis and applications. J Mater Chem A. 2017;5(18):3039–68.
Zou Y, Wang P, Wen Y, et al. Synergistic immobilization of UO22+ by novel graphitic carbon nitride @ layered double hydroxide nanocomposites from wastewater. Chem Eng J. 2017;330:573–84.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Ma, Z., Fang, Z. et al. Review of harmless treatment of municipal solid waste incineration fly ash. Waste Dispos. Sustain. Energy 2, 1–25 (2020). https://doi.org/10.1007/s42768-020-00033-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-020-00033-0