Abstract
In this work, cellulose was extracted from giant reed (GR) via alkaline treatment and two different delignification processes (acidified NaClO2 and totally chlorine-free (TCF). After that, microcrystalline cellulose (MCCs) particles were successfully isolated from two prepared cellulose samples using HCl acid hydrolysis. The different properties of each sample were studied with various characterization tools such as infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetry (TGA), and differential scanning calorimeter (DSC). The FTIR spectra confirmed the removal of hemicellulose and lignin of the giant reed through the applied extraction processes. The XRD spectra exhibited that both MCCs belong to cellulose I type and showed higher crystallinity index compared to pure cellulose samples. DSC and TGA/DTG results showed that MCC samples have a higher thermal stability. Moreover, the use of a totally chlorine-free process allowed obtaining MCC with higher crystallinity and thermal stability. Consequently, giant reed can be considered as a potential source to extract MCC for which the green totally chlorine-free is a prominent process to produce MCC from cellulosic substrate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent few decades, the employment of micro/nanoparticles of cellulose in several applications has attracted much attention from researchers due to their important physicochemical features such as good thermal stability, great tensile strength, biodegradability, biocompatibility, low density, and low environmental influence (Trache 2017; Trache et al. 2020b; Fodil Cherif et al. 2020). The characteristics of these micro and nanoscale materials open new ways for their utilization in many industrial fields (Capron et al. 2017; Hussin et al. 2019; Trache et al. 2014, 2017; Trache et al. 2020a).
Microcrystalline cellulose (MCC) is considered as an effective micro-sized biomaterial that is largely used in pharmaceutical, food packaging and beverage, cosmetic, biocomposite reinforcements, and other applications. MCC is a purified, odorless, crystalline, and partially disintegrated cellulose (Trache et al. 2016; Tarchoun et al. 2019b). The variation of MCC structure and features can be attributed to the natural cellulosic properties, the exploited source in addition to the different conditions used for its isolation. MCC is a snowy powder that can be extracted from various lignocellulosic biomass using different chemical, physical and biological procedures, and a combination of two or more of these processes (Ilyas et al. 2018; Ren et al. 2019; Tarchoun et al. 2019a; Trache et al. 2016; Zeni et al. 2015). Besides, for the production of MCC from cellulosic fibers, the acid hydrolysis approach stays the most utilized process for its several benefits such as effectiveness, low cost, and short time of reaction. Over the acid hydrolysis method, H3O+ ions provoke the rupture of glycosidic linkages, where the cellulose amorphous regions are easily hydrolyzed, while the crystalline parts are more resistant to the attack of acids (Tarchoun et al. 2019).
Many researchers are currently seeking for new non-wood fibers as alternative sources of cotton and wood for the isolation of cellulose and their derivatives (Hussin et al. 2019). MCC extraction from different lignocellulosic sources such as Alfa grass (Trache et al. 2014), jute (Jahan et al. 2011), oil palm (Haafiz et al. 2013; Hussin et al. 2018; Owolabi et al. 2017; Ramli et al. 2015; Xiang et al. 2016; Yiin et al. 2019), corncob (Azubuike and Okhamafe 2012), posidonia oceanic brow algae (Tarchoun et al. 2019a), among others, has been reported.
Giant reed (Arundo Donax) is one of abundant aquatic plants that mainly grows in hot and tropical regions such as canals, rivers around marshes, and other humid lands. Giant reed is an interesting fast growing annual herb owing to its highly cellulose content (31–42%). It can be considered as powerful candidate for many fields (Lamb and Dixon 1997; Somerville et al. 2004). Thus, it is expected to be a potential source to produce MCC. Therefore, the aim of this study is to improve the efficient procedure for MCC isolation from Algerian giant reed through a series of chemical treatments like a delignification with two different methods (acidified NaClO2 and totally chlorine-free TCF), alkaline treatment, and then the acid hydrolysis. The produced samples are deeply characterized using FTIR, XRD, SEM, and TGA to inspect the influence of the used delignification method on the chemical structure, crystallinity, morphology, and thermal stability properties of the obtained MCC.
2 Material and Methods
2.1 Materials
Giant reed fibers utilized in this work were gathered from the Oum El Bouaghi area, in Algeria. These fibers were cleaned with distilled water then dried in an oven at 110 °C for 24 h. After that, the giant reed fibers milled and sifted into particle size of 35 mesh, then preserved in a vacuum desiccator. Ethanol (96%), toluene (99.5%), hydrogen peroxide (H2O2, 30%), acetic acid glacial (CH3COOH, 96%), nitric acid (HNO3, 67%), and hydrochloric acid (HCl, 37%) were purchased from VWR Prolabo. Sodium hydroxide (NaOH) pellet and sodium chlorite (NaClO2) were supplied by Sigma Aldrich. All reactants are utilized without any prior purification.
2.2 Methods
2.2.1 Preparation of Giant Reed Cellulose Samples
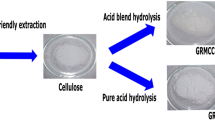
In order to isolate cellulose, many treatments were applied to giant reed fibers. The natural fibers (15 g) are treated with 220 mL of toluene/ethanol (2/1) mixture using a Soxhlet extractor at a fixed temperature during 6 h to remove organic solvent extracts (Tappi 1997). Then, the obtained fibers are treated with 200 mL of distilled water in an oil bath at 105 °C for 5 h to remove sugars, coloring matter ad starch (Kilic and Niemz 2012). After that, the delignification of fibers has been carried out with two different delignification processes using either acidified NaClO2 process or the totally chlorine-free beaching with hydrogen peroxide, as schematized in Fig. 1.
The acidified sodium chlorite process is employed in accordance with the method described by Ilyas et al. (Ilyas et al. 2018). Briefly, 10 g of the giant reed fibers are placed in a 500 mL round bottom flask containing 325 mL of distilled water. 4 mL of acetic acid and 5 g of sodium chlorite are added to the mixture (once every one hour) for 7 h, and the mixture temperature was adjusted at 70 °C. The obtained holocellulose (holocellulose-giant reed-NaClO2) is washed with distilled water during the filtration and then dried in oven at 110 °C.
The TCF process, however, is employed with three successive steps. The first step is performed following Kuznetsov et al. method with slight modification (Kuznetsov et al. 2017). 10 g of the giant reed fiber with no extractives is soaked in a 250 mL round bottom flask containing 150 mL of hydrogen peroxide (6%)/acetic acid (25%) mixture at 100 °C for 5 h. Then, the mixture is filtered and rinsed with distilled water. In the second stage, this residue is placed in a 250 mL round bottom containing 150 mL of nitric acid (20%)/acetic acid (25%) mixture at 120 °C for 20 min. The obtained product is filtered and washed with distilled water (Rosa et al. 2012). In the final step, the residue is treated with 200 mL of hydrogen peroxide (5%)/sodium hydroxide (4%) mixture at 50 °C for 90 min. The obtained holocellulose, named holocellulose-giant reed-TCF, is filtered, washed with distilled water, and dried in oven at 105 °C overnight (Sun et al. 2004).
The both samples of holocellulose are treated with sodium chloride in order to remove the residual lignin and hemicellulose to isolate pure samples of cellulose. The process is performed following the method of Jiang et al. (2015). The samples are mixed with NaOH (5%) solution with a ratio of (1/20) during 24 h under magnetic stirring at room temperature, which is then increased to 90 °C for 2 h. The isolated giant reed cellulose samples are filtered, washed with distilled water and diluted acetic acid, and then dried at 90 °C during 48 h.
2.2.2 Preparation of Microcrystalline Cellulose
To isolate giant reed MCCs, the hydrolysis acid of giant reed cellulose samples is performed according to the process reported in the literature with small modification (Trache et al. 2014). Both cellulose samples are hydrolyzed using 2.5 M of hydrochloric HCl acid at 100 °C under reflux for 30 min with the ratio of 1/20 (g/Ml) fiber to liquor. After that, large amount of cold distilled water was added to the mixture to stop the reaction. The resultant cellulose suspensions were then rinsed with distilled water and NaOH (0.5 M) solution to reach a neutral pH. Finally, the generated MCC was dried in oven at 60 °C for 24 h until constant weight was reached. The obtained solids were snowy-white in appearance. The different stages of giant reed MCCs extraction are presented in Fig. 1.
2.3 Characterization
2.3.1 Chemical Composition
The chemical composition of giant reed fibers was obtained according to Technical Association of the Pulp and Paper Industry (TAPPI) standard methods and previous investigations (Reddy et al. 2016; Hu et al. 2014). The ash content is evaluated with the standard T 211 om-07, the toluene/ethanol solvent extractives content is evaluated using T 204 cm-07, the hot water extractives are quantified with the standard T-257, the lignin content is quantified using the standard T-222 cm-06, and then, the α-cellulose content is obtained with the standard T 203 cm-99. The holocellulose content was obtained using the method reported in the work of Wise et al. (1946), whereas the hemicellulose content was measured as the difference between holocellulose and α-cellulose contents. The measurements were performed in triplicate.
2.3.2 FTIR Analysis
FTIR spectroscopic analysis was carried out to study the modifications of functional groups and chemical transformation in the structure of the different specimens. The dried samples (0.1–2 mg) were pelletized with KBr (100 mg) and were analyzed with Parkin Elmer FTIR spectrometer. The spectra were recorded in transmittance band mode, in the wavenumber region of 400–4000 cm−1 by averaging of 64 scans at resolution of 4 cm−1. Before each measurement, background spectra were obtained at room temperature and then subtracted automatically from the sample spectrum.
2.3.3 X-ray Diffraction Analysis
X-ray diffraction analysis is performed to investigate the structure and to gather the diffractograms of the different cellulose and microcrystalline cellulose samples. The results were collected using a PANalytical X’Pent PRO Multi-purpose diffractometer with Cu Kα radiation at a generator voltage of 45 kV and current of 40 mA. An X’celerator detector was employed to record the data over a 2θ angular range of 5–50° with a step size of 0.017°/2θ and a count time of 50.1650 s at each step. The crystalline indexes of raw giant reed and all cellulose samples and MCCs were obtained from different diffractograms, according to Segal’s method (Segal et al. 1959).
where CrI is the crystallinity index, I200 is the maximum intensity of the 200 peak at 2θ = 22° and Iam is the intensity at 2θ = 18°.
2.3.4 Scanning Electron Microscopy (SEM)
The morphologies of all cellulose samples and MCCs were studied with FEI Quanta 250 scanning electron microscope tool at a 10 mm working distance and 10 kV accelerating voltage. Dry powders of different cellulosic samples are placed on double-sided conductive adhesive tape. Then, they were analyzed by using the secondary electrons (SE) for morphology. The particle size of the samples was measured using Image J software (Ilyas et al. 2018).
2.3.5 Thermal Analysis
The thermal behavior of cellulose samples and MCCs was examined using a thermogravimetric analysis (TGA). The TGA experiments were conducted using a TGA Q500 V20.13 Build 39. A sample mass of 4 mg was used for the different analyses, which are conducted in the temperature range of 50–500 °C at a heating rate of 10 °C min−1. The different analyses were carried out under inert nitrogen gas atmosphere.
3 Results and Discussion
3.1 Chemical Composition
The chemical composition of raw giant reed fibers is shown in Table 1. It can be seen that the raw fibers are composed of 35.4% cellulose, 21.29% of hemicellulose, and 21.2%, lignin. The hot water extractives content is about 7.2%, that of toluene/ethanol extractives is 8.82%, whereas the ash content is 3.16%. This composition of the utilized giant reed is in agreement with that reported in the literature (Tarchoun et al. 2019).
3.2 FTIR Analysis
FTIR spectra of the untreated and the treated samples are presented in Fig. 2. The spectra showed that practically all fibers have similar patterns, indicating that the different employed processes did not have any unfavorable influence on the chemical functionality of cellulose structure. Moreover, the absorption peaks approximately of 3450 cm−1 are assigned to –OH group linkages, for which the intensity decreases after delignification and alkaline treatments owing to the formation of Na-cellulose bonds that minimizes the intra/intermolecular hydrogen bonds in relation to –OH groups (Hussin et al. 2019; Kalita et al. 2013; Zhao et al. 2018). However, the intensity of these peaks raised after acid hydrolysis in both giant reed MCC spectra, which is referred to the highly exposed cellulose area created by the scission of cellulose chains. In addition, the appearance of peaks at 2915 cm−1 is attributed to C–H symmetric stretching vibrations of the cellulose structure. The peak at 2860 cm−1 corresponded to the asymmetric and symmetric stretching vibration of the CH2 groups of hemicellulose in giant reed fibers. These latter decreased after the treatment carried out with either NaClO2 or totally chlorine-free. The totally chlorine-free (TCF) with hydrogen peroxide process seems to be more efficient compared to the acidified NaClO2. Besides, the acidified sodium chlorite treatment initially dissolves the lignin without taking into account the removal of hemicellulose (Reddy et al. 2018; Liu et al. 2019), while the totally chlorine-free process yields to a better and effective delignification. The hydroperoxide anions (HOO−) in the TCH treatment eliminate the lignin chromophore groups by attacking ethylenic and carbonyl groups, whereas the other radical species such as (OH−) dissolve the hemicellulose, giving rise to purified cellulose (Ditzel et al. 2017). In addition, the bond at about 1736 cm−1 is associated to the C–O bonds of uronic acids and acetyl ester groups of hemicellulose or the carboxylic groups of the ferulic and p-coumeric acids of lignin and/or hemicellulose. This bond is present for raw giant reed fibers; however, it is disappeared in spectra of giant reed cellulose and giant reed MCC fibers. This finding proved that the residual amount of non-cellulosic components hemicellulose and/or lignin is dissolved with alkaline treatment and the two different used delignification processes (Bian et al. 2012). After delignification, the absence of characteristic peaks absorption at about 1509 and 1250 cm−1 is linked to C = C of aromatic vibration and C–O–C of aryl-alkyl-ether in lignin, respectively, indicating the removal of lignin for both treatments. Meanwhile, the peak at approximately 1646 cm−1 is related to the cellulose water interaction in those fibers. The peak is enlarged after hydrochloric acid hydrolysis indicating that the giant reed MCC has higher hygroscopic property. In addition, the peak at around 1420 cm−1 is linked to CH2 symmetric stretching and related to the crystallinity of cellulose. The peak is increased after acid hydrolysis in MCC spectra raveling the hydrolysis of mostly cellulose amorphous parts. Moreover, the peaks at about 898 and 1036 cm−1 are corresponding to the β-glycosidic bond stretch, C–O–C pyranose ring skeletal stretch, and C–H asymmetric deformation (Adel and El-Shinnawy 2012; Rosa et al. 2012). These peaks are augmented in giant reed MCC spectra, confirming the breaking of cellulose chains. From the literature and the obtained FTIR spectra, it can be pointed out that the different produced cellulose samples are successfully synthetized using different delignification processes (Trache 2016; Trache et al. 2013, 2014). On the other hand, the prepared MCCs from giant reed using hydrochloric acid hydrolysis present typical chemical composition than that obtained in the literature (Trache et al. 2014).
3.3 XRD Analysis
The crystalline structure and the crystallinity index of the raw giant reed, cellulose samples, and giant reed MCCs are investigated with X-ray diffraction analysis. The XRD diffractograms of raw giant reed fibers, giant reed cellulose, and giant reed MCC are presented in Fig. 3. From the obtained results, all samples exhibited similar peaks of cellulose I at about 15.04°, 16.39°, 22.43°, and 34.53°, which corresponded to 1ī0, 110, 200, and 004 planes, respectively. It is essential to point out that the different obtained patterns showed the non-appearance of cellulose II because there is no doublet in the main peak situated at 22.43° (Hu et al. 2014; Kian et al. 2017). In addition, it is obvious that the peak at about 22.43° becomes tight and sharper, designating the crystallinity augmentation of microcrystalline cellulose compared to the cellulose and raw fibers, respectively. This finding indicates that the different used treatments had no influence on the chemical structure of raw fibers.
The crystallinity indexes of the different samples are measured and given in Table 2. The raw giant reed fibers consist of amorphous non-cellulosic components with lower crystallinity index of 52.36% compared to the pure cellulose samples and MCCs (Kumar et al. 2013). However, the giant reed MCC prepared with TCF exhibited higher crystallinity index compared to that obtained with acidified NaClO2. During acid hydrolysis of cellulose derived from the TCF process, a higher amount of protons (H+) from HCl opens the fiber structure to simplify the access to cellulose microstructure, distracts the farthest amorphous cellulosic regions, and then breaks the β1,4-glycosidic bonds, conducting to higher crystalline MCC. However, in the case of the acidified NaClO2, the obtained cellulose contains a small amount of hemicelluloses, which allows the acid hydrolysis to selectively dissolve hemicellulose than the amorphous parts of cellulose (Adel and El-Shinnawy 2012; Haafiz et al. 2013; Ramli et al. 2015; Trache et al. 2013; Wyman et al. 2004; Kishani et al. 2018). Hence, the increase of crystallinity value of MCC obtained with totally chlorine-free delignification will certainly offer better physical and mechanical properties for applications in several fields.
3.4 Morphology
The morphological structure of giant reed cellulose samples and MCCs extracted with different delignification processes (acidified NaClO2 and totally chlorine-free) is examined with SEM. The obtained SEM micrographs are presented in Fig. 4.
From cellulose images (a and c samples), the cellulose fibers showed clean smooth surface, long, individualized filaments owing to the removal of non-cellulosic components during the used pretreatments, delignification processes, and alkaline treatment (Xiang et al. 2016). These findings are in accordance with FTIR results, which evidencing the elimination of lignin and hemicellulose after the different treatments. The diameter of the analyzed samples was calculated by Image J processing software (IJ 1.46) from the SEM images. More than 30 fibers have been employed to certify the reproducibility of the obtained data. Moreover, the giant reed cellulose produced with TCF process showed lower diameter than that obtained with the acidified NaClO2 process (Table 2). It can be attributed to the effect of nitric and acetic acid mixture used in the procedure of TCF at high temperature and to the impact of oxidation with hydrogen peroxide under alkaline and acidified conditions, leading to more degradation of cellulose amorphous parts (Francis et al. 1998; García Hortal and Vidal Lluciá 1984; Ramos et al. 2008; Sun et al. 2004). It is clear that MCCs present irregular shapes with rough surfaces, which can be assigned to the degradation of the structure of cellulose fibers due to HCl acid hydrolysis. Short length particles have been obtained after hydrolysis because of the cleavage of the amorphous parts linkages of cellulose (Trache et al. 2014). This finding is confirmed by XRD results. Otherwise, from SEM images, it is observable that the MCCs length is lower than their corresponding diameters. It is obvious that the isolated MCCs show some quantity of aggregates owing to the strong cohesion of hydrogen bonding between microfibers.
3.5 Thermal Stability
The TGA analyses are performed to study the thermal stability of the produced cellulose samples and to estimate the efficiency of the material and its application in different fields. The thermal properties of cellulose and cellulose microcrystalline samples are displayed in Table 2.
The TGA and DTG curves of the produced giant reed cellulose (a) and MCCs (b) are depicted in Fig. 5. As can be seen, all fibers showed a weight loss within temperature range of 280–370 °C because of the degradation of cellulose caused by the decarboxylation, depolymerization, and the decomposition of glycosidic units followed by the formation of a charred residue (Adel and El-Shinnawy 2012; Trache et al. 2016). It can be also observed that the MCCs illustrated the better thermal stability with higher temperature of decomposition compared to their corresponding pristine cellulose. The giant reed cellulose and MCC samples isolated with acidified NaClO2 treatment displayed lower thermal stability in comparison with giant reed MCC isolated using the TCF delignification treatment. This lower temperature of decomposition is referred to the residual amount of amorphous hemicellulose, which degrades at lower temperature, presenting negative influence on the stability of cellulose fibers. The isolated giant reed MCC produced with totally chlorine-free treatment and acid hydrolysis process presents better thermal stability, which makes it a potential candidate to produce high-value products.
4 Conclusion
The present study is carried out to investigate the effect of different delignification media (acidified NaClO2 and the green media TCF), alkaline treatment, and HCl acid hydrolysis to produce giant reed MCC. The FTIR examination demonstrated that the employed delignification processes have significant impact on the isolation of cellulose without any effect on its chemical structure. The eco-friendly process (TCF) provided higher cellulose purity compared to the acidified sodium chlorite. The XRD results revealed that the isolated MCCs presented typical cellulose type I with high crystallinity index values of 75–77%. The thermal stability of giant reed MCC produced using TCG is better than that of MCC prepared with acidified NaClO2. Consequently, the obtained MCC using TCF can be employed in several promising applications.
References
Adel AM, El-Shinnawy NA (2012) Hypolipidemic applications of microcrystalline cellulose composite synthesized from different agricultural residues. Int J Biol Macromol 51(5):1091–1102
Azubuike CP, Okhamafe AO (2012) Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int J Recycl Org Waste Agric 1(1):9
Bian J, Peng F, Peng XP, Peng P, Xu F, Sun RC (2012) Acetic acid enhanced purification of crude cellulose from sugarcane bagasse: structural and morphological characterization. BioResources 7(4):4626–4639
Capron I, Rojas OJ, Bordes R (2017) Behavior of nanocelluloses at interfaces. J Curr Opin Colloid Interface Sci 29:83–95
Ditzel FI, Prestes E, Carvalho BM, Demiate IM, Pinheiro LA (2017) Nanocrystalline cellulose extracted from pine wood and corncob. Carbohyd Polym 157:1577–1585
Fodil Cherif M, Trache D, Brosse N et al (2020) Comparison of the physicochemical properties and thermal stability of Organosolv and Kraft Lignins from hardwood and softwood biomass for their potential valorization. Waste Biomass Valor. https://doi.org/10.1007/s12649-020-00955-0
Francis RC, Rodriguez SK, Bose SK, Granzow SG, Evans TD (1998) The critical role of transition metals in high-temperature peroxide (PO) bleaching. Annual Meeting-Technical Section Canadian Pulp and Paper Association, vol 84, pp A49–A56
Haafiz MKM, Eichhorn SJ, Hassan A, Jawaid M (2013) Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohyd Polym 93(2):628–634
Hortal G, Antonio J, Lluciá TV (1984) Blanqueo de pastas en la industria papelera: UPC, ETSII de Terrassa
Hu Y, Tang L, Lu Q, Wang S, Chen X, Huang B (2014) Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 21(3):1611–1618
Hussin MH, Husin NA, Bello I, Othman N, Abu Bakar M, Haafiz MKM (2018) Isolation of Microcrystalline Cellulose (MCC) from oil palm frond as potential natural filler for PVA-LiClO4. Polymer Electrolyte. Int J Electrochem Sci 13:3356–3371
Hussin MH, Trache D, Chuin CTH, Fazita MRN, Haafiz MKM, Hossain MdS (2019) Extraction of cellulose nanofibers and their eco-friendly polymer composites. In: Sustainable polymer composites and nanocomposites. Springer, pp 653–691
Ilyas RA, Sapuan SM, Ishak MR (2018) Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohyd Polym 181:1038–1051
Jahan MS, Saeed A, He Z, Ni Y (2011) Jute as raw material for the preparation of microcrystalline cellulose. J Cellulose 18(2):451–459
Jiang F, Hsieh YL (2015) Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohyd Polym 122:60–68
Kalita RD, Nath Y, Ochubiojo ME, Buragohain AK (2013) Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids Surf, B 108:85–89
Kian LK, Jawaid M, Ariffin H, Alothman OY (2017) Isolation and characterization of microcrystalline cellulose from roselle fibers. Int J Biol Macromol 103:931–940. https://doi.org/10.1016/j.ijbiomac.2017.05.135
Kilic Aand Niemz P (2012) Extractives in some tropical woods. Eur J Wood Wood Prod 70(1–3):79–83
Kishani S, Vilaplana F, Xu W, Xu C, Wågberg L (2018) Solubility of softwood hemicelluloses. Biomacromol 19(4):1245–1255
Kumar R, Hu F, Hubbell CA, Ragauskas AJ, Wyman CE (2013) Comparison of laboratory delignification methods, their selectivity, and impacts on physiochemical characteristics of cellulosic biomass. Biores Technol 130:372–381
Kuznetsov BN, Sudakova IG, Garyntseva NV, Djakovitch L, Pinel C (2017) Kinetic studies and optimization of abies wood fractionation by hydrogen peroxide under mild conditions with TiO2 catalyst. React Kinet Mech Catal 120(1):81–94
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Biol 48(1):251–275
Liu Y, Nie Y, Lu X, Zhang X, He H, Pan F, Zhou L, Liu X, Ji X, Zhang S (2019) Cascade utilization of lignocellulosic biomass to high-value products. Green Chem 21(13):3499–3535
Owolabi AF, Haafiz MKM, Hossain MS, Hussin MH, Fazita NMR (2017) Influence of alkaline hydrogen peroxide pre-hydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int J Biol Macromol 95:1228–1234
Ramli R, Junadi N, Beg M, DH and Yunus RM (2015) Microcrystalline cellulose (MCC) from oil palm empty fruit bunch (EFB) fiber via simultaneous ultrasonic and alkali treatment. Chem Mol Nuclear Mater Metall Eng 9(1):8–11
Ramos E, Calatrava SF, Jiménez L (2008) Bleaching with hydrogen peroxide. A review. Afinidad 65(537)
Reddy KO, Maheswari CU, Muzenda E, Shukla M, Rajulu AV (2016) Extraction and characterization of cellulose from pretreated ficus (peepal tree) leaf fibers. J Nat Fibers 13(1):54–64
Reddy KO, Maheswari CU, Dhlamini MS, Mothudi BM, Kommula VP, Zhang J, Zhang J, Rajulu AV (2018) Extraction and characterization of cellulose single fibers from native African Napier grass. Carbohyd Polym 188:85–91
Ren H, Shen J, Pei J, Wang Z, Peng Z, Fu S, Zheng Y (2019) Characteristic microcrystalline cellulose extracted by combined acid and enzyme hydrolysis of sweet sorghum. Cellulose 26(15):8367–8381
Rosa SML, Rehman N, de Miranda MIG, Nachtigall SMB, Bica CID (2012) Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohyd Polym 87(2):1131–1138
Segal LGJMA, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T (2004) Toward a systems approach to understanding plant cell walls. Science 306(5705):2206–2211
Sun JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab 84(2):331–339
Tappi S (1997) Solvent extractives of wood and pulp. TAPPI T 204
Tarchoun AF, Trache D, Klapötke TM, Chelouche S, Derradji M, Bessa W, Mezroua A (2019a) A promising energetic polymer from Posidonia Oceanica brown algae: synthesis, characterization, and kinetic modeling. Macromol Chem Phys 220(22):1900358
Tarchoun AF, Trache D, Klapötke TM, Derradji M, Bessa W (2019b) Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 26(13–14):7635–7651
Trache D, Khimeche K, Donnot A, Benelmir R (2013) FTIR spectroscopy and X-ray powder diffraction characterization of microcrystalline cellulose obtained from alfa fibers. 39th Edition of the Joint European Days on Equilibrium between Phases
Trache D, Donnot A, Khimeche K, Benelmir R, Brosse N (2014) Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohyd Polym 104:223–230
Trache D (2017) Microcrystalline cellulose and related polymer composites: synthesis, characterization and properties. In: Thakur VK, Thakur MK, Kessle MR (eds) Handbook of composites from renewable materials, structure and chemistry. Wiley, pp 61–92
Trache D, Hussin MH, Chuin CTH, Sabar S, Fazita MRN, Taiwo OFA, Hassan TM, Haafiz MKM (2016a) Microcrystalline cellulose: isolation, characterization and bio-composites application—a review. Int J Biol Macromol 93:789–804
Trache D, Khimeche K, Mezroua A, Benziane M (2016b) Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J Therm Anal Calorim 124(3):1485–1496
Trache D, Hussin MH, Haafiz MKM, Thakur VK (2017) Recent progress in cellulose nanocrystals: sources and production. Nanoscale 9(5):1763–1786
Trache D, Tarchoun AF, Derradji M, Hamidon TS, Masruchin N, Brosse N, Hussin MH (2020a) Nanocellulose: from fundamentals to advanced applications. Front Chem 8:392
Trache D, Tarchoun AF, Derradji M, Mehelli O, Hussin MH, Bessa W (2020b) Cellulose fibers and nanocrystals: preparation, characterization and surface modification. In: Kumar V, Guleria P, Dasgupta N, Ranjan S (eds) Functionalized nanomaterials I: fabrication. Taylor & Francis
Wise LE (1946) Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Paper Trade 122:35–43
Wyman CE, Decker SR, Himmel ME, Brady JW, Skopec CE, Viikari L (2004) Hydrolysis of cellulose and hemicellulose. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility. CRC Press, 1023–1062
Xiang LY, Mohammed MAP, Baharuddin AS (2016) Characterisation of microcrystalline cellulose from oil palm fibres for food applications. Carbohyd Polym 148:11–20
Yiin CL, Yusup SHS, Quitain AT, Chan YH, Loy ACM, Gwee YL (2019) Recovery of cellulose fibers from oil palm empty fruit bunch for pulp and paper using green delignification approach. Biores Technol 290:121797
Zeni M, Favero D, Pacheco K, Grisa A (2015) Preparation of microcellulose (Mcc) and nanocellulose (Ncc) from eucalyptus kraft ssp pulp. J Polym Sci 1:1–5
Zhao T, Chen Z, Lin X, Ren Z, Li B, Zhang Y (2018) Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohyd Polym 184:164–170
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Beroual, M. et al. (2021). Synthesis and Characterization of Microcrystalline Cellulose from Giant Reed Using Different Delignification Processes. In: Trache, D., Benaliouche, F., Mekki, A. (eds) Materials Research and Applications. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-15-9223-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-9223-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9222-5
Online ISBN: 978-981-15-9223-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)