Abstract
Esparto grass, known as alfa, is a renewable biomass widely distributed in southern and western Mediterranean basin. The present work focused on the isolation of pure cellulose from alfa stems, via different approaches, i.e., acidified sodium chlorite (NaClO2), totally chlorine free (TCF) or their combination, followed by the preparation of microcrystalline cellulose (MCC) using acid hydrolysis method. The obtained samples were characterized using infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetry (TGA) and differential scanning calorimeter (DSC). The FTIR spectroscopy exhibited the removal of lignin and hemicellulose after the delignification and alkaline treatments. The XRD data showed that all of the MCCs have higher crystallinity indexes (Alfa-MCC 73–82%) and belong to cellulose I type. From SEM images, it is clear that the different MCC particles presented rough surface and micro-sized particles. The DSC/TGA analyses revealed that MCC samples present better thermal stability than their respective cellulose ones, with higher temperature of decomposition (more than 350 °C). Moreover, the use of a combined process yields to MCC with higher crystallinity and better thermal stability. Consequently, based on these findings, the delignification with combined method can be considered as a promising approach to extract MCC from alfa fibers with outstanding features.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microcrystalline cellulose (MCC) is discovered for the first time in 1955 by Battista and smith from unsuccessful experiments [1, 2]. MCC is a fine crystalline powder, odorless, tasteless, which is normally derived from different lignocellulosic sources by the purified and partially depolymerized cellulose. MCC has particle size in the range between micrometers to hundreds micrometers. It has several interesting properties such as strength, high crystallinity, fibrous nature, lightness, stiffness, water insolubility, and renewability. These outstanding features make it more important to be used in several industrial applications such as pharmaceutical area including binders and adsorbents, cosmetic such as substitute and thickeners, food and beverage as anti-caking agents and stabilizers, biocomposites as reinforcement, among others [1,2,3,4,5,6]. Furthermore, more than ten (10) suppliers produce commercial microcrystalline cellulose worldwide [7]. Generally, commercial MCC is produced via hydrolysis of cotton and wood cellulose using dilute mineral acids. Wood is considered by far as the first principal source of cellulose, and consequently the most essential feedstock employed for the production MCC. Nonetheless, competition among several sectors like pulp, papermaking, furniture, building product and energy production, renders its exploitation a challenge to provide all area with required amount of wood at acceptable costs. Therefore, the exploration of non-wood cellulosic sources such as agricultural wastes, grass has attracted immense research interest of the scientific community [1, 8]. MCC can be isolated from different lignocellulosic sources. It can be produced from oil palm biomass [9], folder grass [10], waste paper [11], soybean hull [12], alfa grass [13], among others.
Alfa grass (Stipa tenacissima L), also called esparto grass or halfa in arab countries, is a perennial herb of the Gramineae family that is widely distributed in arid and semi-arid ecosystems of the southern and the western Mediterranean basin, mainly in the Maghreb [14,15,16]. This plant is abundant and considered as one of the most important natural sources in the Mediterranean basin, mainly in Algeria. In fact, Algeria is the major producer of alfa grass in the world with an estimated covered area of about 4.5 million hectares [13, 17]. Alfa fibers, as raw materials, are widely used in numerous fields such as animal feed, pulp and papermaking. Recently, it has been employed as reinforcement in the biodegradable composites fabrication. Owing to their availability and high content of cellulose (~ 44%) offer high prospective as an outstanding green feedstock for MCC isolation [18, 19].
MCC properties, which can be adapted to selective uses, are not only dependent on the nature of lignocellulosic sources or the procedure of hydrolysis, but also related to the different pretreatments used to extract the native cellulose from raw sources. Broadly, lignocellulosic sources require the removing of non-cellulosic components via the elimination of extractive components (fat, tannins, rosin, free sugars, flavonoids, terpenoids, terpene, fatty acid, and waxes), the delignification and bleaching processes. Such pre-treatments can be performed through different chemical, physical, biological, and combined methods [20]. These pretreatments permit the separation of pure and crystalline cellulose, ensure the break of the linkages existing between cellulose and non-cellulosic compounds (lignin and hemicellulose), decrease the degree of polymerization, promote the accessibility toward cellulose-rich fraction and increase the porosity, the inner surface and reactivity [21]. However, numerous pretreatments may negatively influence the process through the generation of toxic and hazardous wastes, imperfect separation, degradation, and loss of cellulose as well as of the high overall process expenses [22]. For these reasons, several studied are still in progress worldwide to understand the phenomena that can occur during the pretreatments, and optimize the efficiency and the easiness of the processes. MCC with different features can be obtained depending on the natural source, its origin and maturity, pretreatment and processing methodologies and reaction parameters [23].
The delignification process of fibers is commonly used to eliminate lignin, hemicellulose and other impurities from raw biomass [24,25,26]. The acidified sodium chloride delignification method is the conventional process commonly employed to remove the lignin [27]. Besides, recent approaches are based on the utilization of the green delignification with total chlorine free methods such as deep eutectic solvents, ionic liquids and hydrogen peroxide solutions [28]. Nevertheless, depending on the natural lignocellulosic source, hemicellulose, lignin and cellulose are organized in a complex hierarchical microstructure, establishing recalcitrant nature against chemicals and microbial treatments. Therefore, in order to prepare value-added cellulosic products, better separation approach of these components through the utilization of the aforementioned individual methods or the combined process of them is required [29, 30]. Moreover, the morphology, size, and other characteristics of the isolated cellulose depend closely on isolation approach and processing conditions as well as the possible pre- or post-treatments [23]. The opportunity of producing MCC with various features is considered fairly an exciting topic, which can promote the exploration of unexplored biomass. The benefits of the 3-D hierarchical structure of MCC and its physicochemical characteristics open new prospects in several applications [31,32,33].
Thus, the aim of this work is to obtain an efficient approach to isolate pure cellulose from Algerian alfa grass via different processes, i.e., acidified sodium chlorite (NaClO2), totally chlorine free (TCF) or their combination, and then to prepare MCC using acid hydrolysis. The series of analyses encompassing FTIR, XRD, SEM, DSC, and TGA/DTG are performed to investigate the impact of the different delignification processes used on the physicochemical properties and the thermal stability of isolated MCCs. The characteristics of obtained products are also compared to those of the MCC reported in literature.
Materials and Methods
Materials
The esparto grass stems fibers exploited in this work was gathered from Oum El Bouaghi area in the east of Algeria at the end of the spring of 2019, near the maturity time of the product. For the removal of soil and other impurities, the raw material was rinsed with distilled water and then dried in the air. The obtained fibers were powdered, sieved into particle size of 250 μm and stored in a vacuum desiccator. Ethanol (96%), toluene (99.5%), hydrogen peroxide (H2O2, 30%), acetic acid glacial (CH3COOH, 96%), nitric acid (HNO3, 67%) and hydrochloric acid (HCl, 37%) were purchased from VWR Prolabo. Sodium hydroxide (NaOH) pellet and sodium chlorite (NaClO2) were supplied by Sigma Aldrich. All reagents are used without previous purification.
Methods
Preparation of Alfa Grass Cellulose
To extract alfa grass cellulose, many processes were applied to the raw fibers. The elimination of organic and hot water extractives, the delignification with different methods and the alkaline treatment to prepare three pure cellulose samples have been carried out. Firstly, the removal of toluene/ethanol and hot water extractives was performed through the soxhlet extraction, using 10 g of dried fibers and 220 ml of toluene/ethanol (1:2, v/v) mixture at fixed temperature over 6 h [34], followed by the treatment of obtained powder with hot water for 5 h. Then, the filtered residue is dried in the oven at 100 °C [35].
After that, the delignification stage was performed with three different methods (acidified sodium chlorite, totally chlorine free and there combination) to eliminate the lignin.
The first process is based on the acidified sodium chlorite NaClO2 delignification, which is employed following to the procedure of Ilyas et al. [24]. Briefly, 10 g of the treated alfa fibers are mixed with 325 mL of distilled water in a 500 ml round bottom flask. Then, 2 ml of acetic acid (to control the pH in the range of 3–4) and 4 g of sodium chlorite are added to the mixture for each an hour repeatedly for the duration of 7 h at 70 °C. After the filtration and rinsing with distilled water at several times, the recovered holocellulose named holocellulose-alfa-NaClO2 is dried in oven at 105 °C.
The second process deals with the use of the totally chlorine free (TCF) delignification, which was done by hydrogen peroxide under acidic and alkaline conditions. The hydrogen peroxide treatment under acidic condition was performed according the procedure reported by Kuznetsov et al. with some modification [36]. Succinctly, 10 g of the extractive-free alfa fibers are soaked in 150 mL of hydrogen peroxide (6%)/acetic acid (25%) mixture at 100 °C for 5 h. The residue obtained after filtration is rinsed by distilled water. In the second stage, this residue is placed in a 250 mL round bottom containing 150 mL of nitric acid (20%) /acetic acid (25%) mixture at 120 °C for 20 min. The derived product is then filtered and washed with distilled water [37]. In the final stage, the residue is treated with 200 mL of hydrogen peroxide (5%) /sodium hydroxide (4%) mixture at 50 °C for 90 min. The obtained holocellulose, named holocellulose-Alfa-TCF, is filtered, rinsed at the same time with distilled water then dried in oven at 105 °C overnight [38].
The last process concerns the use of the combined delignification, which was realized by the addition of acidified sodium chlorite before the treatment of fibers with the above TCF process. Concisely, 10 g of alfa fibers obtained after the hot water treatment and 6 g of sodium chlorite are placed in 350 mL of distilled water in 500 mL round bottom at 70 °C during 5 h. Simultaneously, 4 ml of acetic acid are added to the mixture to maintain the pH to 4.5. The white prepared holocellulose, named holocellulose-alfa-combined, is washed by distilled water during the filtration then dried at 100 °C overnight [39].
Furthermore, the three-holocellulose samples are treated with alkaline solution of sodium hydroxide to eliminate the non-cellulosic materials to produce three samples of pure cellulose. This treatment was accomplished via the method of Jiang et al. [25]. Briefly, the samples are placed in NaOH (5%) solution with a fraction of (1/20 w/v) for 24 h under magnetic stirring at room temperature. After that, it is heated up at 90 °C for 2 h. The formed cellulose samples are neutralized by an important amount of distilled water and diluted acetic acid, followed by a drying at 105 ° C in the oven overnight.
Preparation of Alfa Grass Microcrystalline Cellulose
The alfa cellulose samples are treated with acid hydrolysis according to the procedure of Trache et al. with some modifications [13]. The acid hydrolysis of cellulose samples was realized using 2.5 m of hydrochloric HCl acid with the ratio of (1/20 w/v) at temperature of 100 °C for 30 min.
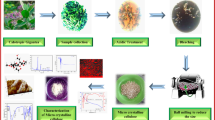
Afterwards, the resulted samples of microcrystalline cellulose were rinsed with distilled water and NaOH (0.5 m) solution to achieve a neutral pH of 7. The obtained product dried at 65 °C for 48 h was snowy-white in appearance. The overall steps of the extraction process of MCC and digital images of each product are displayed in Fig. 1.
Characterization
Chemical Composition
The chemical composition of alfa grass fibers was determined according to the Technical Association of the Pulp and Paper Industry (TAPPI) standard methods and previous investigations [40, 41]. The ash content is calculated using the standard T 211 om-07, the toluene/ethanol solvent extractives content is measured using T 204 cm-07, the hot water extractives are quantified with the standard T-257, the lignin content is quantified using the standard T-222 om-06, then the α-cellulose content is evaluated with the standard T 203 cm-99. The holocellulose content was determined according the procedure reported by Wise et al. [42], whereas, using the difference between holocellulose and α-cellulose is considered as the hemicellulose content. The quantification values were found by repeating each procedure three times.
FTIR analysis
FTIR spectroscopy was used to study the changes of functional groups and chemical structure transformation of the different samples. The dried samples (0.1–-2 mg) were pelletized with KBr (100 mg) and were analyzed using Parkin Elmer FTIR spectrometer. The spectra were recorded in transmittance band mode, in the wave number range of 400–4000 cm− 1 by averaging of 64 scans at resolution of 4 cm− 1. Prior to each evaluation, background spectra were determined at ambient temperature after that deducted automatically from the sample spectrum.
X-Ray Diffraction Examination
X-Ray Diffraction analysis was carried out to investigate the structure and to obtain all the diffractograms of the different samples of cellulose and MCC. The results were collected using a PANalytical X’Pert PRO Multipurpose diffractometer by Cu Kα radiation at a generator voltage of 45 kV and current of 40 mA conditions. An X’celerator detector was performed to register the data over a 2θ angular range of 5–50° with a step size of 0.017°/ 2θ and a count time of 50.1650 s at each step. The crystalline indexes of raw fibers and both cellulose and microcrystalline cellulose samples were determined from different diffractograms, according to Segal’s equation.
where CrI is the crystallinity index, I200 is the maximum intensity of the 200 peak at 2θ = 22° and Iam is the intensity at 2θ = 18°.
From the different diffractograms obtained using 200 lattice plane, the size of crystallite was calculated via Scherrer equation.
where D (h k l) is the crystallite size (nm), k is the Scherrer constant (0.9), λ is the X-ray wavelength (1.5418 cm− 1), βh k l is the full width at half maximum intensity (FWHM) of the diffraction peak and θ is the half of the Bragg angle [27].
Scanning Electron Microscopy (SEM)
Surface morphologies of both cellulose and microcrystalline cellulose samples were obtained with FEI Quanta 250 scanning electron microscope tool at a 10 mm working distance and 10 kV accelerating voltage. Dry powders of different cellulosic samples are placed on double-sided conductive adhesive tape. Then, they were analyzed using the secondary electrons (SE) for morphology. The particle size of the samples was calculated with Image J software [24].
Thermal Analysis
The thermal features of both cellulose and MCC samples were determined with thermogravimetric analysis (TGA) and a differential scanning calorimetry (DSC). The TGA experiments were carried out by means of a TGA Q500 V20.13 Build 39, while the DSC ones have been assessed with a calibrated Perkin Elmer DSC 8000 analyzer. All of the samples were weighted to 5 mg for used in the different analyses, which are investigated in the temperature range of 200 to 450 ° C with a heating rate of 10 ° C min− 1. The different analyses were carried out under the inert nitrogen gas atmosphere conditions.
Results and Discussion
Chemical Composition
The composition of alfa fibers is summarized in Table 1. They are composed of 44.36% cellulose followed by hemicellulose with 22.89% and lignin with 18.87%. Furthermore, the amounts of the toluene-ethanol extractives, the hot water extractives and the ash are, respectively, 4.8%, 2.76% and 3.45%. The chemical composition of alfa grass employed in present study had similar cellulose amount compared to alfa grass exploited in other studies [13, 19, 43,44,45,46]. In addition, it can be seen that our alfa fibers has a similar chemical composition than posidonia ocianica brown algae [2] and sugar palm [24].
FTIR Spectroscopy
FTIR analysis was mainly performed to determine the functional groups of all samples at each step of microcrystalline cellulose production. Moreover, FTIR spectroscopy was carried out to prove the elimination of lignin and hemicellulose. The FTIR spectra of the prepared samples, i.e., Alfa, Alfa-after Soxhlet extraction, Alfa-after hot water extraction, holocellulose Alfa-NaClO2, holocellulose Alfa-TCF, holocellulose Alfa-combined, Alfa-C-NaClO2 (f), Alfa-C-TCF, Alfa-C-combined, Alfa -MCC-NaClO2, Alfa -MCC-TCF, Alfa -MCC-combined, are displayed in Fig. 2. From the FTIR spectra, it can be clearly illustrated that relatively all samples showed the identical functional groups, where the delignification and alkaline treatments do not destroy the cellulose structure. The absorbance band about 3405 cm− 1 is attributed to stretching vibration of –OH groups [10, 47, 48]. The absorbance peak at 2922 cm− 1 is referred to aliphatic saturated –CH2 stretching vibration, indicating the absence of α-cellulose degradation in the raw alfa plant [49]. The band situated at 2851 cm− 1 is assigned to the asymmetric and symmetric stretching vibration of the CH2 group of hemicellulose in alfa grass. The spectra of pure cellulose and MCC samples proved the reduced of this band after the delignification with sodium chlorite and the alkaline treatment, while the FTIR spectra revealed the total absence of this band after using the delignification with either totally chlorine free or the combined process. The pretreatment with sodium chlorite seems to be less efficient because of the biomass recalcitrance. Although, the pretreatments via the free chlorine or combined process are revealed to be more effective. More explicitly, the acidified sodium chlorite treatment dissolves and eliminates favorably lignin without significantly impacting the elimination of hemicelluloses, where the lignin dissolution increases via the primary oxidizing of phenolic rings to produce quinonoid and muconic acid derivatives or methyl/methylene groups in the allyric position, generating carbonyl or carboxylic groups [50, 51]. Nevertheless, the free chlorine delignification with hydrogen peroxide under alkaline conditions yields to a high degree of lignin removal, where the hydroperoxide anions (HOO−) are responsible for the lignin chromophore groups’ elimination by a selective attack of ethylenic and carbonyl groups. However, other radical species such as (OH−) are responsible for the hemicellulose solubilization to produce extremely purified cellulose [52]. The peak about 1739 cm− 1 corresponds to either acetyl or uronic ester groups of hemicellulose or ester linkage of the carboxylic groups of the ferulic and p-coumeric acids of lignin and/or hemicellulose in alfa grass. This peak is disappeared, whatever the delignification process used. The employed treatments can cause cellulose swelling, which in turn breaks the intermolecular bonds of hydrogen between cellulose and hemicellulose [53]. The peaks at 1512 cm− 1 and 1250 cm− 1 are assigned to C = C of aromatic vibration and C=O of lignin, respectively. The total removal of lignin is confirmed by the disappearance of these two latter peaks after the different delignification processes, which are used in this study. The observed peak at 1643 cm− 1 is linked to the absorbed water owing to interactions between moisture and cellulose. The absorption at around 898 cm− 1 is known as C-H rock vibration in cellulose β-glucosides. The absorption peak at 1430 cm− 1 is associated to crystalline band, which is referred to CH2 symmetric stretching [37, 54], the increased of this peak in alfa cellulose and alfa-MCC indicates the removal of the amorphous regions. Following to literature and the obtained results, it can be deduced that the different cellulosic samples are effectively isolated, whatever the delignification process used [13, 23, 55]. In addition, the produced MCCs from alfa grass using hydrochloric acid hydrolysis have identical chemical composition as that obtained in the literature [13].
Crystalline Structure Analysis
Cellulose includes the crystalline and amorphous regions that are bonded with intra/intermolecular linkages (van der Waals forces) [54, 56, 3]. The crystalline structure and the crystallinity index of different cellulose and MCC samples isolated from alfa fibers were obtained with X-ray diffraction analysis. The XRD diffractogrammes of Alfa, Alfa-C-NaClO2, Alfa -C-TCF, Alfa -C-combined, Alfa-MCC-NaClO2, Alfa -MCC-TCF and Alfa-MCC-combined are displayed in Fig. 3. Moreover, from the different diffractograms of cellulose and MCCs samples, it can be checked out the presence of cellulose type I, with the presence of peaks at 14.9° (1ī0), 16.3° (110), 22.6° (200) and 34.5° (004). Without the existence of cellulose type II, owing to the absence of peak (200) with doublet intensity. These peaks become more defined upon chemical treatments, as expected. Furthermore, the diffraction peak at about 22.6° is appeared narrower in MCCs patterns, proving the increase of the MCC samples crystallinity in comparison with the crystallinity of pure cellulose samples. It is evident that the three delignification processes applied had no influence on the structure of different cellulose samples and MCCs. This finding corroborates well the FTIR results as well. The crystallinity indexes of all samples are calculated and summarized in Table 2. Observably, the cellulose samples presented higher crystallinity index compared to that of the raw fibers of alfa grass (61.38%) due to the removal of amorphous non-cellulosic components [57]. Hence, it is necessary to note that raw alfa (61.38%) and alfa cellulose (65.20%) exhibited slightly higher crystallinity indexes compared to that alfa cellulose (59%) prepared by Trache et al. [13]. This finding can be attributed to the alfa origin, maturity as well as the cellulose isolation process used. In addition, from the different obtained diffractograms depicted in Fig. 3 and the calculated data reported in Table 2, it is obvious that the crystallinity indexes of microcrystalline cellulose samples have higher values compared to their respective native cellulose samples. This finding is referred to the degradation of the amorphous parts during the acid hydrolysis [9, 54, 55, 58]. Moreover, the cellulose and MCC samples prepared with the combined delignification method showed the highest crystallinity indexes in comparison with those prepared via the totally free chlorine process and acidified chlorite method, respectively. However, the acidified chlorite process presents the lower crystallinity indexes. The acid hydrolysis with HCl releases a great quantity of protons, which provoke the deterioration of disordered regions of cellulose in a cellulosic microstructure. Afterwards, they destroy the β1,4-glycosidic bonds to achieve the levelling-off polymerization degree, conducting to higher crystalline MCC. Nevertheless, the acid hydrolysis of celluloses pretreated with the two other processes, where the hemicelluloses are not completely removed, causes a selective solubilization of the amorphous hemicellulose than the amorphous parts of cellulose [59, 60]. Thus, the used acid hydrolysis is not just employed to hydrolyze the amorphous parts of cellulose molecules but also eliminates the residual amount of amorphous hemicelluloses. Consequently, the crystallinity of MCC produced with combined/ HCl method is higher than that of the two other MCCs (acidified NaClO2 and totally chlorine free process). Besides that, the higher crystallinity value found with the combined delignification/ HCl will confer better features for MCC for several potential applications. It is worthy to note that the obtained crystallinity values of MCC in the present work are higher than that reported by Abu-Thabit for MCC derived from acid hydrolysis from date seeds of the date palm tree, which is 70% [61]. Furthermore, the prepared MCC samples with CrI% of falls within the crystallinity range of the commercial MCC, which is in the range of 55–80% [23].
Morphology
The morphological investigation of all the alfa cellulose and MCC samples was performed using SEM. The micrographs of (a) Alfa -C-NaClO2, (b) Alfa -MCC-NaClO2, (c) Alfa -C-TCF, (d) Alfa-MCC-TCF, (e) Alfa A-C-combined and (f) Alfa-MCC-combined are depicted in Fig. 4. The cellulose images illustrate the clean smoother surfaces, long and uniform individualized fibers. This finding referred to the removal of non-cellulosic components like lignin, hemicellulose and other impurities (a, c and e samples) after the different treatments used during the isolation of cellulose [62]. These obtained results corroborate the FTIR analysis that confirmed the removal of lignin and hemicellulose. The diameter sizes of all the extracted cellulose samples and MCCs were calculated by ImageJ processing software (IJ 1.46) from the SEM images, where more than 50 fibers have been exploited to certify reproducible data. From the data of Table 2, it is obvious that the pure cellulose extracted using NaClO2 delignification showed the higher average diameter in comparison with that obtained with TCF process. This finding is assigned to the impact of acids mixture (HNO3/ CH3COOH) at high temperature and to the effect of hydrogen peroxide oxidation under alkaline conditions, which can deteriorate the cellulose molecules [38, 63, 64]. However, the combined process presented lower diameter owing to its efficient removal of non-cellulosic material, particularly lignin and hemicellulose. In addition, the MCC micrographs present irregular rod-like shape with rough surface due to the HCl hydrolysis influence that causes the degradation of cellulose fibers [23]. In the other hand, the MCCs micrographs show that MCC fibers have lower average length compared to cellulose particles, which can be assigned to the fragmentation of pyranose bonds that lead to the deterioration of cellulose amorphous regions [13]. Moreover, the MCC particles prepared using the combined process have a lower diameter size in comparison with the two other individual processes (Table 2). These results confirmed once again that the different delignification treatments have an effect on the hydrolysis influence. The same remarks was obtained with XRD results. Furthermore, it is interesting to point out that the prepared MCC samples have relatively similar average diameters as those obtained in the literature for alfa fibers, reeds, algae and commercial MCC [2, 65].
Thermal Stability
The thermal stability of cellulose and microcrystalline cellulose materials are crucial to estimate their performance and their use in several industries. The thermal properties were investigated using DSC and TGA/ DTG analyses [23, 55, 66, 67]. The thermal parameters of all cellulose samples are reported in Table 2.
The DSC curves of cellulose and MCC samples are displayed in Figs. 5 and 6, respectively. All samples present endothermic phenomena. The peak at about 250 and 380 °C is referred to the highest decomposition of different cellulose samples due to the cleavage of the 1,4-glycosidic bond of cellulose biopolymer followed by the generation of levoglucosan and charring. The appearance of this peak at high temperature proves the total elimination of non-cellulosic components via the different chemical treatments used in this study. On the other hand, it is worthy to indicate that the native cellulose prepared with the combined process shows a higher temperature of degradation compared to the two other individuals delignification treatments. This result corroborate well the FTIR findings, which present the disappearance of peak at 2860 cm− 1. In addition, MCC samples show high temperatures of decomposition with respect to their respective native celluloses. This finding is due to the higher degree of crystallinity of MCC particles as obtained with XRD data. Therefore, several authors previously mentioned that the higher molecular ordering yields to higher thermal decomposition properties and consequently better thermal stability [2, 4, 65]. Hence, it is essential to note that the utilization of combined delignification method leads to efficient acid hydrolysis and provides to produce the MCC with good thermal stability in comparison with the use of either totally chlorine free or sodium chlorite delignification methods.
The TGA and DTG thermograms of the different dried cellulose samples and MCCs are displayed in Figs. 7 and 8. From the different curves, it is evident that the thermograms of the different analyzed samples show one thermal decomposition at high temperature between 250 and 380 °C, which is caused by several immediate processes such as depolymerization, dehydration, decarboxylation, and decomposition of glycosyl units followed by the formation of a charred residue [54, 23]. In addition, the TGA/DTG curves of different samples showed the same trend in comparison with obtained DSC results. From Table 2 data, it is clear that MCC samples exhibited better thermal stability compared to their respective pure celluloses. Furthermore, it is worthy to mention that the samples prepared using the delignification with NaClO2 and TCF treatment displayed lower thermal stability compared to those obtained by the combined process. This lower thermal stability presented by NaClO2 delignification method can be referred to the residual amount of hemicellulose and lignin that start to decompose at lower temperature. In addition, it is reported in another work that the thermal decomposition of MCC derived from alfa through sodium hypochlorite delignification and HCl hydrolysis occurred at 351.39 °C [13], which is lower than that obtained in our present work using the combined delignification process, which is more thermostable.
Besides, the presence of hemicellulose particles, which are naturally prone to the degradation, leads to the low efficiency of the elimination of cellulose amorphous regions during the acid hydrolysis. The prepared MCC samples display comparable thermal properties of commercial MCC and MCC isolated from other parts of date palm such as date seed (353 °C) and fruit bunch stalk (364.2 °C) and fall within the reported decomposition range of cellulose (315–400 °C) [23, 61]. Overall, the prepared alfa grass MCC using a combined delignification process/ HCl acid hydrolysis presents higher thermal stability, which makes it a promising candidate to prepare high-value products.
Conclusion
Alfa plant, which is rich in cellulose, is an available grass in Mediterranean basin mainly in the Maghreb and can be a considered as prominent source for MCC preparation. In this study, cellulose and MCC samples were successfully prepared from alfa grass with three different delignification media/HCl acid hydrolysis. The FTIR results demonstrate that the different delignification methods used influence the purity of cellulose without any impact in cellulose chemical structure. The combined delignification that consists of sodium chloride followed by chlorine-free treatment generates cellulose with a higher purity compared to that obtained with the individual processes. The SEM micrographs of the different MCC samples showed compact and rough microstructures with rod-like shape of microfibrils. The XRD analysis revealed that the isolated MCC samples presented typical cellulose I allomorph, and the crystallinity index ranged from 73 to 80%. Moreover, this study demonstrated that alfa grass MCC prepared with the combined process presented relatively better features with higher crystallinity, smaller micro-sized structure and higher thermal stability, making it promising candidate for many applications in reinforced polymer composites, pharmaceutics, medical, automotive industries, packaging, smart and energetic materials.
References
Trache D (2017) Microcrystalline cellulose and related polymer composites: synthesis, characterization and properties. In: Thakur VK, Thakur MK, Kessle MR (eds) Handbook of composites from renewable materials, structure and chemistry. Wiley, New York, pp 61–92
Tarchoun AF, Trache D, Klapötke TM (2019) Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int J Biol Macromol 138:837–845
Khalil HA, Lai TK, Tye YY, Paridah M, Fazita MN, Azniwati A et al (2018) Preparation and Characterization of Microcrystalline Cellulose from Sacred Bali Bamboo as Reinforcing Filler in Seaweed-based Composite Film. Fibers Polym 19(2):423–434
Owolabi AF, Haafiz MM, Hossain MS, Hussin MH, Fazita MN (2017) Influence of alkaline hydrogen peroxide pre-hydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int J Biol Macromol 95:1228–1234
Yuliasmi S, Pardede T, Syahputra H, editors. Comparison of microcrystalline characterization results from oil palm midrib alpha cellulose using different delignization method. IOP Conference Series: Materials Science and Engineering; 2017: IOP Publishing
Lamaming J, Chew SC, Hashim R, Sulaiman O, Sugimoto T (2017) Extraction of microcrystalline cellulose from oil palm trunk. J Jpn Inst Energy 96(11):513–518
Thoorens G, Krier F, Leclercq B, Carlin B, Evrard BJIJoP, (2014) Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int J Pharm 473(1–2):64–72
Trache D, Hussin MH, Haafiz MM, Thakur VK (2017) Recent progress in cellulose nanocrystals: sources and production. Nanoscale 9(5):1763–1786
Haafiz MM, Eichhorn S, Hassan A, Jawaid M (2013) Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr Polym 93(2):628–634
Kalita RD, Nath Y, Ochubiojo ME, Buragohain AK (2013) Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L.) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids and Surfaces B 108:85–89
Okwonna OO (2013) The effect of pulping concentration treatment on the properties of microcrystalline cellulose powder obtained from waste paper. Carbohydr Polym 98(1):721–725
Merci A, Urbano A, Grossmann MVE, Tischer CA, Mali SJ (2015) Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res Int 73:38–43
Trache D, Donnot A, Khimeche K, Benelmir R, Brosse N (2014) Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr Polym 104:223–230
García-Fayos P, Gasque MJ (2006) Seed vs. microsite limitation for seedling emergence in the perennial grass Stipa tenacissima L. (Poaceae). Food Res Int 30(2):276–82
Cerdà AJJoAE (1997) The effect of patchy distribution of Stipa tenacissima L.on runoff erosion. J Arid Environ 36(1):37–51
El-Abbassi FE, Assarar M, Ayad R, Bourmaud A, Baley C, Manufacturing (2020) A review on alfa fibre (Stipa tenacissima L.): from the plant architecture to the reinforcement of polymer composites. J Compos Part A 128:105677
Bessadok A, Marais S, Gouanvé F, Colasse L, Zimmerlin I, Roudesli S et al (2007) Effect of chemical treatments of Alfa (Stipa tenacissima) fibres on water-sorption properties. J Compos Sci Technol 67(3–4):685–697
Triki A, Dittmer J, Hassen MB, Arous M, Bulou A, Gargouri M (2016) Spectroscopy analyses of hybrid unsaturated polyester composite reinforced by Alfa, wool, and thermo-binder fibres. J Polym Sci Ser A 58(2):255–264
Bouiri B, Amrani M, Chemistry E (2010) Elemental chlorine-free bleaching halfa pulp. J Ind Eng Chem 16(4):587–592
Trache D, Tarchoun AF, Derradji M, Hamidon TS, Masruchin N, Brosse N et al (2020) Nanocellulose: from fundamentals to advanced applications. Front Chem 8:192
Kargarzadeh H, Ahmad I, Thomas S, Dufresne A (2017) Handbook of nanocellulose and cellulose nanocomposites. Wiley, New York
Phanthong P, Reubroycharoen P, Hao X, Xu G, Abudula A, Guan G (2018) Nanocellulose: extraction and application. Carbon Resour Convers 1(1):32–43
Trache D, Hussin MH, Chuin CTH, Sabar S, Fazita MN, Taiwo OF et al (2016) Microcrystalline cellulose: Isolation, characterization and bio-composites application—a review. Int J Biol Macromol 93:789–804
Ilyas R, Sapuan S, Ishak M (2018) Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr Polym 181:1038–1051
Jiang F, Hsieh Y-L (2015) Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydrate Polym 122:60–8
Liao JJ, Abd Latif NH, Trache D, Brosse N, Hussin MH (2020) Current advancement on the isolation, characterization and application of lignin. Int J Biol Macromol 162:285–1024
Robles E, Fernandez-Rodriguez J, Barbosa AM, Gordobil O, Carreno NL, Labidi J (2018) Production of cellulose nanoparticles from blue agave waste treated with environmentally friendly processes. Carbohydr Polym 183:294–302
Cheng F, Zhao X, Hu Y (2018) Lignocellulosic biomass delignification using aqueous alcohol solutions with the catalysis of acidic ionic liquids: a comparison study of solvents. Bioresour Technol 249:969–975
Ren H, Shen J, Pei J, Wang Z, Peng Z, Fu S et al (2019) Characteristic microcrystalline cellulose extracted by combined acid and enzyme hydrolysis of sweet sorghum. Cellulose 26(15):8367–8381
Yiin CL, Ho S, Yusup S, Quitain AT, Chan YH, Loy ACM et al (2019) Recovery of cellulose fibers from oil palm empty fruit bunch for pulp and paper using green delignification approach. Bioresour Technol 290:121797
Garba ZN, Lawan I, Zhou W, Zhang M, Wang L, Yuan Z (2020) Microcrystalline cellulose (MCC) based materials as emerging adsorbents for the removal of dyes and heavy metals–a review. Sci Total Environ 717:135070
Kian LK, Saba N, Jawaid M, Fouad H (2020) Characterization of microcrystalline cellulose extracted from olive fiber. Int J Biol Macromol
Wardhono EY, Kanani N, Alfirano A (2020) A simple process of isolation microcrystalline cellulose using ultrasonic irradiation. J Dispersion Sci Technol 41(8):1217–1226
Standard T (1997) Solvent extractives of wood and pulp. TAPPI T.204
Kilic A, Niemz P (2012) Extractives in some tropical woods. Eur J Wood Wood Product 70(1–3):79–83
Kuznetsov B, Sudakova I, Garyntseva N, Djakovitch L, Pinel C (2017) Kinetic studies and optimization of abies wood fractionation by hydrogen peroxide under mild conditions with TiO2 catalyst. Reac Kinet Mech Catal 120(1):81–94
Rosa SM, Rehman N, de Miranda MIG, Nachtigall SM, Bica CI (2012) Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohyd Polym 87(2):1131–1138
Sun J, Sun X, Zhao H, Sun R (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab 84(2):331–339
Khandanlou R, Ngoh GC, Chong WT (2016) Feasibility study and structural analysis of cellulose isolated from rice husk: Microwave irradiation, optimization, and treatment process scheme. BioResources. 11(3):5751–66
Reddy KO, Uma Maheswari C, Muzenda E, Shukla M, Rajulu AV (2016) Extraction and characterization of cellulose from pretreated ficus (peepal tree) leaf fibers. J Nat Fibers 13(1):54–64
Hu Y, Tang L, Lu Q, Wang S, Chen X, Huang B (2014) Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 21(3):1611–8
Wise LE (1946) Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Paper Trade 122:35–43
Brahim SB, Cheikh RB, Technology (2007) Influence of fibre orientation and volume fraction on the tensile properties of unidirectional Alfa-polyester composite. Compos Sci Technol 67(1):140–147
Hattalli S, Benaboura A, Ham-Pichavant F, Nourmamode A, Castellan A, Stability (2002) Adding value to Alfa grass (Stipa tenacissima L.) soda lignin as phenolic resins 1. Lignin characterization. Polym Degrad Stab 76(2):259–264
Zaafouri K, Ziadi M, Farah RB, Farid M, Hamdi M, Regaya I (2016) Potential of Tunisian Alfa (Stipa tenassicima) fibers for energy recovery to 2G bioethanol: Study of pretreatment, enzymatic saccharification and fermentation. Biomass Bioenergy 94:66–77
Paiva M, Ammar I, Campos A, Cheikh RB, Cunha A (2007) Alfa fibres: mechanical, morphological and interfacial characterization. Compos Sci Technol 67(6):1132–1138
Hussin MH, Husin NA, Bello I, Othman N, Bakar MA, Haafiz MM (2018) Isolation of microcrystalline cellulose (MCC) from oil palm frond as potential natural filler for PVA-LiClO4 polymer electrolyte. Int J Electrochem Sci 13:3356–3371
Zhao T, Chen Z, Lin X, Ren Z, Li B, Zhang Y (2018) Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr Polym 184:164–170
Hermawan D, Lai TK, Jafarzadeh S, Gopakumar DA, Hasan M, Owolabi FT et al (2019) Development of seaweed-based bamboo microcrystalline cellulose films intended for sustainable food packaging applications. BioResources 14(2):3389–3410
Liu Y, Nie Y, Lu X, Zhang X, He H, Pan F et al (2019) Cascade utilization of lignocellulosic biomass to high-value products. Green Chem 21(13):3499–3535
Reddy KO, Maheswari CU, Dhlamini M, Mothudi B, Kommula V, Zhang J et al (2018) Extraction and characterization of cellulose single fibers from native african napier grass. Carbohydr Polym 188:85–91
Ditzel FI, Prestes E, Carvalho BM, Demiate IM, Pinheiro LA (2017) Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr Polym 157:1577–1585
Bian J, Peng F, Peng X-P, Peng P, Xu F, Sun R-C (2012) Acetic acid enhanced purification of crude cellulose from sugarcane bagasse: structural and morphological characterization. BioResources 7(4):4626–4639
Bian J, Peng F, Peng X-P, Peng P, Xu F, Sun R-C (2012) Acetic acid enhanced purification of crude cellulose from sugarcane bagasse: structural and morphological characterization. BioResources 7(4):4626–4639
Trache D, Khimeche K, Donnot A, Benelmir R, editors. FTIR spectroscopy and X-ray powder diffraction characterization of microcrystalline cellulose obtained from alfa fibers. 39th Edition of the Joint European Days on Equilibrium between Phases; 2013
-Das K, Ray D, Bandyopadhyay N, Sengupta S (2010) Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, nanoindentation, TGA and SEM. J Polym Environ 18(3):355–363
Kumar R, Hu F, Hubbell CA, Ragauskas AJ, Wyman CE (2013) Comparison of laboratory delignification methods, their selectivity, and impacts on physiochemical characteristics of cellulosic biomass. Bioresour Technol 130:372–381
Ramli R, Junadi N, Beg MD, Yunus RM (2015) Microcrystalline cellulose (MCC) from oil palm empty fruit bunch (EFB) fiber via simultaneous ultrasonic and alkali treatment. Chem Mol Nucl Mater Metall Eng 9(1):8–11
Wyman CE, Decker SR, Himmel ME, Brady JW, Skopec CE, Viikari L (2004) Hydrolysis of cellulose and hemicellulose. In: Dumitriu S (ed) Polysaccharides: Structural diversity and functional versatility. Boca Raton, CRC Press, pp 1023–62
Kishani S, Vilaplana F, Xu W, Xu C, Wågberg L (2018) Solubility of softwood hemicelluloses. Biomacromol 19(4):1245–1255
Abu-Thabit NY, Judeh AA, Hakeem AS, Ul-Hamid A, Umar Y, Ahmad A (2020) Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int J Biol Macromol 155:730–739
Xiang LY, Mohammed MAP, Baharuddin AS (2016) Characterisation of microcrystalline cellulose from oil palm fibres for food applications. Carbohydr Polym 148:11–20
Francis R, Rodriguez S, Bose S, Granzow S, Evans T, editors. The critical role of transition metals in high-temperature peroxide (PO) bleaching. Annual Meeting-Technical Sction Canadian Pulp and Paper Association; 1998: Canadian Pulp & Paper ASSN-Technical Section
Ramos E, Calatrava S, Jiménez LJA (2008) Bleaching with hydrogen peroxide. A review. Afinidad 65(537)
Tarchoun AF, Trache D, Klapötke TM, Derradji M, Bessa W (2019) Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 26(13–14):7635–7651
Azubuike CP, Okhamafe AO (2012) Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int J Recycling Org Waste Agric 1(1):9
Bessa W, Trache D, Derradji M, Tarchoun AF (2020) Non-isothermal curing kinetics of alkali-treated alfa fibers/polybenzoxazine composites using differential scanning calorimetry. Chem Sel 5(18):5374–5386
Acknowledgements
The authors gratefully acknowledge the Ecole Militaire polytechnique for the financial support and the necessary facilities for the accomplishment of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beroual, M., Boumaza, L., Mehelli, O. et al. Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Isolated from Esparto Grass Using Different Delignification Approaches. J Polym Environ 29, 130–142 (2021). https://doi.org/10.1007/s10924-020-01858-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01858-w