Abstract
The kinetic study of abies wood delignification by H2O2 in the medium acetic acid–water was first carried out in the presence of TiO2 catalyst under mild conditions: temperatures 70–100 °C, atmospheric pressure. The oxidative delignification process is described satisfactory by the first order equation in all temperature range. The rate constants varied between 0.80 and 12.3 × 10−3 min−1 and the activation energy was near 81 ± 0.21 kJ mol−1. The optimal parameters of the delignification process, providing the effective fractionation of abies wood on microcrystalline cellulose and soluble lignin, were established by experimental and numerical methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The manufacture of cellulose by sulfate and sulfite pulping is the most developed direction of chemical wood processing [1, 2]. These industrial pulping processes are based on the application of harmful and corrosive sulfur and chlorine-containing delignification reagents. Advanced processes of organosolv delignification, where organic and water–organic solvents are used, can become an alternative to the traditional pulping methods [3–5]. The most promising solvents, free from sulfur and chlorine, are organic acids (acetic and formic acids), alcohols (methanol, ethanol, butanol), acetone and other low-toxic organic compounds [6–9]. Organosolv methods of delignification causes less environmental damage and they are less energy-intensive, as compared to existing pulping processes.
The processes of organosolv delignification in the presence of environmentally safe oxidants, like oxygen and hydrogen peroxide [10–12], has drawn a lot of attention. The new organosolv processes do not always allow obtaining cellulose quality, required for its further chemical processing. However, they can be successfully used for the wood fractionation on cellulose and soluble lignin for the purpose of their further processing to chemicals and biofuels. It has been shown that some catalysts are able to intensify the processes of organosolv delignification [13, 14].
Some complexes of the transition metals, for example so-called polyoxometalates, can promote the processes of pulp and wood delignification by oxygen [15, 16]. However, the practical use of these catalysts is complicated by their high cost and the complexity of regeneration for reuse.
Sulfuric acid can act as a catalyst of wood oxidative delignification by H2O2 in acetic acid–water solvent [17]. But H2SO4 promotes the hydrolysis of polysaccharides decreasing the yield of cellulosic product. Besides, H2SO4 catalyst has such technological disadvantages as high toxicity and corrosion activity.
More technologically convenient solid TiO2 can catalyze an oxidative delignification of the wood by H2O2 at 120–130 °C and elevated pressure [18, 19]. Advantages of TiO2 application, as delignification catalyst, are stipulated by the absence of corrosion activity and toxicity, availability, low cost and lack of need for its regeneration. It is important to note that the catalytic properties of TiO2-based materials are sensitive to the method of their producing [20].
The different side reactions of low molecular mass products condensation with the formation of so-called “pseudo-lignin” [21] take place in the wood delignification processes at elevated temperatures. The contribution of condensation reactions can be reduced by acceleration the diffusion of lignin depolymerization products from the wood particles into solution.
In a previous paper [22], the authors have shown that the use of small particles of wood (sawdust), high liquid to wood ratio (LWR) and intensive mixing of reaction solution, allow to reduce externally diffusion limitations and to provide in the presence of 2% H2SO4 catalyst a high rate of aspen wood delignification by H2O2 under mild conditions (70–100 °C, atmospheric pressure).
The present work demonstrates a possibility of successful replacement the H2SO4 catalyst on non-toxic and non-corrosive solid TiO2 catalyst in the oxidative fractionation of abies wood on cellulose and soluble lignin under mild conditions. The kinetic study of abies wood sawdust delignification in the medium “H2O2–CH3COOH–H2O–TiO2 catalyst” at temperatures 70–100 °C, atmospheric pressure and under vigorous agitation was accomplished. By experimental and computational methods the optimal process conditions providing a high yield of quality cellulosic product, were determined.
Experimental
Air dry sawdust (fraction 2–5 mm) of abies wood (Abies sibirica L.), harvested in the forest area near Krasnoyarsk city, has been used as an initial raw material. The chemical composition of abies wood (wt% on abs. dry wood): cellulose—45.7, lignin—25.3, hemicelluloses—17.7, extractive substances—6.2, ash—0.5.
The delignification solution was composed of the “chemically pure” acetic acid (GOST 61-75), “medical grade” hydrogen peroxide (GOST 177-88) and distilled water (GOST 6709-72). All chemical reagents were purchased at the CJSC “Khimreactivsnab” (Russia).
Commercial TiO2 (GOST 9808-84) in rutil modification with an average particle size of about 10 microns and BET surface area 3 m2/g was used as the delignification catalyst.
Delignification of wood sawdust was carried out using 250 cm3 glass reactor equipped with mechanical stirrer, reflux condenser and thermometer. Abies wood sawdust (10 g) was placed into the glass reactor. Then, a mixture of glacial acetic acid (98 wt%), hydrogen peroxide (32 wt%), distilled water and TiO2 was added. The reaction mixture was vigorously stirred (700 rpm) under selected temperature (70–100 °C) for duration of 1–5 h. Composition of the reaction mixture was varied in the following range: hydrogen peroxide 3–7 wt%, acetic acid 20–35 wt%, liquid/wood ratio (LWR) −10 to 15. The concentration of TiO2 catalyst was kept at 1 wt% in all experiments. The solid product was separated under vacuum using a Buchner funnel, followed by washing with distilled water and drying at 105 °C until constant weight.
Such parameters as the residual lignin content and hemicelluloses content in cellulosic product were used to evaluate the delignification activity of TiO2 catalyst.
The cellulosic product yield was estimated by gravimetric method and calculated as follows:
Here Y is the yield of cellulosic product, wt%, m is the mass of abs. dry cellulosic product, g, m o is the mass of abs. dry wood, g.
The contents of cellulose, lignin and hemicelluloses were defined by chemical methods, generally accepted in wood chemistry [23].
X-ray diffraction analysis was carried out with the use of PANalytical X’Pert Pro diffractometer with Cu Kα source (λ = 0.154 nm) in the 2θ range 5–70° and scanning step width of 0.01°/scan. The cellulose samples were analyzed by the powder method in cuvette with 2.5 cm diameter. The crystallinity index (CI) was calculated from the ratio of the height between the intensity of the crystalline peak (I002–IAM) and the total intensity (I002) after the subtraction of the background signal [24]: CI = (I002–IAM)/(I002),where I002 is the height of the 002 peak (I002); IAM is the height of the minimum between the 002 and the 101 peaks.
FTIR spectra were recorded with Bruker Tensor—27 wavelengths in the range 4000–400 cm−1 in transmission mode. Samples of cellulose (4 mg for each) were prepared in tablet with matrix KBr. Spectral data were processed by OPUS/YR program (version 2.2).
Solid state 13C CP/MAS spectra were recorded with the use of Bruker Avance III spectrometer, operating at 150.9 MHz 13C resonance frequency. Samples were packed in 3.2 mm rotors and spun at 7.5 kHz. All spectra were aquired at room temperature (25 °C). Acquisition parameters were set as follows: acquisition time 0.33 ms, crosspolarization contact time 3 ms, 4096 accumulated scans with repetition interval 5 s. Chemical shifts were referenced relative to adamantane as external standard (methylene at δ13C = 29.5 ppm). Acquired free induction decays were multiplied with exponential window function with 25 Hz line broadening before Fourier transformation.
Result and discussion
To optimize the process of abies wood delignification, the influence of reaction conditions (temperature, concentrations of acetic acid and hydrogen peroxide, liquid/wood ratio, time) on the yield and composition of cellulosic product was studied.
The effect of TiO2 concentration on the process of peroxide delignification of wood at elevated temperatures (120–140 °C) was early studied by authors [18]. It was found that the optimal concentration of TiO2 catalyst providing the rather high yield of cellulosic product with low content of residual lignin corresponds to 1% TiO2 on the weight of absolutely dry wood. For this reason, the concentration of TiO2 1 wt% was used in the study of abies wood peroxide delignification under mild conditions (70–100 °C).
The impact of H2O2 and CH3COOH concentration on the yield and composition of cellulosic products obtained by abies wood delignification with 1% TiO2 catalyst was investigated at the fixed temperature, LWR and time.
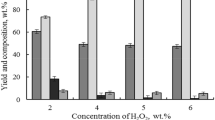
The increase of H2O2 concentration in the reaction mixture from 3 to 5 wt% significantly reduces the content of residual lignin and hemicelluloses in the cellulosic product (Table 1). But at the same time, the yield of cellulosic products is decreased from 69.7 to 52.6 wt% The same regularities were observed with the increase of acetic acid concentration (Table 2).
Acetic acid was used in the reaction mixture to facilitate the dissolution of the products of lignin oxidative depolymerization. When the concentration of CH3COOH in the reaction medium is less than 20 wt%, the obtained cellulosic product has a high content of residual lignin.
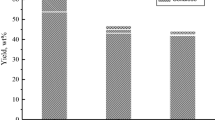
The rather high LWR (10–15) was used in the experiments in order to reduce the external diffusion limitations. The increase of LWR from 10 to 15 only slightly affects the yield of cellulosic product but reduces the content of residual lignin from 2.8 to 1.1 wt% and that of hemicelluloses-from 5.9 to 4.8 wt% (Fig. 1).
The kinetic study of abies wood delignification by H2O2 was accomplished in the temperature range of 70–100 °C. The variation of lignin concentration in the cellulosic product was used for calculating the rate constants of delignification process. A nonlinear fitting method was used for the processing of the kinetic data [25].
It was found that the process of oxidative delignification of abies wood by H2O2 in the presence of TiO2 catalyst is described satisfactory by the first order equation:
Here c is the concentration of lignin in the cellulosic product, wt%; c0 is the concentration of lignin in abies wood, wt%; k is the reaction rate constant, min−1; t is the time, min (Fig. 2).
The calculated rate constants of abies wood oxidative delignification with catalyst TiO2are presented in Table 3.
The value of activation energy of abies wood oxidative delignification process was determined using temperature dependence of the rate constants in Arrhenius coordinates (Fig. 3). The rather high value of activation energy (81 ± 0.21 kJ mol−1) points to the absence of significant of external diffusion limitations at the used conditions of abies wood oxidative delignification with TiO2 catalyst.
The numerical optimization of the abies wood oxidative delignification process with TiO2 catalyst was carried out with the Statigraphic application software, according to earlier described procedure [26]. The main purpose of the analysis was to find conditions for the most complete removal of lignin from wood, while maintaining a sufficiently high yield of cellulosic product.
As independent variables, the following factors have been selected: X1 is the the H2O2 concentration in reaction solution, wt%; X2 is the liquid to wood ratio. The other process parameters were fixed: temperature 100 °C, concentration of acetic acid 30 wt%; TiO2 1 wt%, time 4 h.
The following output parameters for optimization were selected: Y1 is the the cellulosic product yield, wt%; Y2 is the the cellulose content in the product, wt%; Y3 is the lignin content in the product, wt%
Optimization was performed with the use of generalized parameter of optimization (Wa), which was calculated using the following equation:
Here δ is the weight of the output parameter 0 ≤ δj ≤ 1; dj is the private utility function, which was calculated using the following equation:
Here φ 0(x) is the response of output parameter at the point X; y (+) j and y (−) j are the best and worst values of the output parameters, within the studied region.
Table 4 presents the data of conditions and results of experiments for the calculation of generalized parameter of optimization. When choosing weights δ, it was assumed that the residual lignin content is the most important parameter for assessing the quality of cellulosic product. For this reason, this parameter got the weight equal to 1. The weights of such parameters as yield of cellulosic product and cellulose content in the product were choosing equal 0.5.
The results of analysis of the influence of the main factors on the generalized parameter of optimization Wa are given in Table 5.
F-ratio variances (F ratio) and the level of significance (P value) characterize the contribution of each factor in the outcome of the process. The contribution of the factor is more significant when the F ratio is greater and the significance level (P value) is smaller.
The analysis of variances showed that the effect of both factors X1 and X2 on the generalized parameter of optimization is statistically significant (P value less than 0.05 and the confidence level is above 95%).
As a result of mathematical processing the following regression equation was obtained:
The response surface of the generalized parameter of optimization is presented in Fig. 4.
It was found that the generalized parameter of abies wood oxidative delignification process optimization is set to 0.777. This compiles with the following optimal parameters of delignification process: temperature 100 °C, concentrations in reaction solution of H2O2 5.9 wt% and of CH3COOH 30 wt%, LWR 15, duration 4 h.
The cellulosic product obtained by oxidative delignification of abies wood at optimal parameters was studied by XRD, FTIR and 13C NMR methods.
The FTIR spectra of cellulosic product from abies wood and the commercial sample of MCC Vivapur are very similar (Fig. 5).
Both samples have an absorption bands attributed to microcrystalline cellulose [27, 28]. The absence of the cellulosic product from abies wood the peaks in the spectrum in the range 1509–1609 cm−1, which would correspond to C=C aromatic skeletal vibrations, indicates the removal of lignin from the cellulosic product. The absorption band, which corresponds to either the acetyl or uronic ester groups of hemicelluloses, normally appears in the region 1700–1740 cm−1 [29]. The presence of this band in the spectrum of cellulosic product from abies wood indicates that some part of hemicelluloses was not removed during abies wood delignification with H2O2 at 100 °C.
The absorption peak at 1430 cm−1corresponds to the CH2 bending vibration that is attributed to the “crystallinity band” in the cellulose [30]. The band at 893 cm−1 was attributed to the C–O–C stretching vibration of β-(1→4)-glycosidic linkages of cellulose, which was considered as an “amorphous band” in the cellulose [30]. The ratio of peak areas A1430/A893 was proposed as sensible to cellulose type I crystallinity [31]. Practically, the same values of this ratio for cellulose from abies wood (1.13) and for commercial MCC Vivapur(1.12) indicate the similar structures for both samples.
According to X-ray diffraction data, the cellulose from abies wood and the commercial MCC Vivapur have a crystal lattice typical for cellulose I [32] and the crystallinity indexes 0.70–0.75 (Fig. 6).
In order to compare the structure of cellulosic product from abies wood and MCC Vivapur 101, the 13C CP-MAS NMR solid state analysis was performed. The resonance lines in abies wood cellulosic product and MCC Vivapur 101 were almost similar (Fig. 7). Both spectra have resonance lines which indicate the presence of crystalline and amorphous forms of cellulose. Spectra were assigned after deconvolution procedure conducted on Bruker Topspin 3 software. Component line shapes were restricted to Gaussian form. Initial chemical shifts of peaks were set and assigned according to data from Wikberg and Maunu [33].
Peaks have been assigned based on the literature data [33, 34] and are displayed on the Table 6.
The similar kinetic parameters of peroxide delignification of aspen wood [22], birch wood [35] and abies wood in the presence of H2SO4 and TiO2 catalysts (the first order equations, the comparable values of activation energies 82–91 kJ mol−1) point to the identical mechanism of delignification of these wood species.
The deep oxidation of lignin with the disclosure of aromatic rings takes place in the process of wood peroxide delignification. The main final products of lignin degradation by hydrogen peroxide are dicarbonic acids (maleic, fumaric, oxalic acids), acetic and formic acids [36].
Hydrogen peroxide can interact with organic acids with the formation of peroxyacids: 
Peroxyacids are strong electrophilic oxidizers:
Both the peroxyacids and H2O2 can generate the hydroxyl (·OH) and peroxide (·OOH) radicals. Therefore, lignin oxidation in acetic acid medium can be carried out via radical and electrophilic mechanisms [36].
TiO2 can promote the destruction of lignin fragments via the indirect catalysis route, which includes the formation of hydroxyl and peroxide radicals from H2O2 on the TiO2 surface and their diffusion to wood particles [37]: 
To confirm the proposed mechanism, the dynamics of H2O2 decomposition in the medium “H2O2–CH3COOH–H2O” in the presence of TiO2 catalyst was studied (Fig. 8). It was found that the catalyst TiO2 promotes the decomposition of hydrogen peroxide in acetic acid–water medium.
Conclusion
The reduction of diffusion limitations by reducing the size of abies wood particles and the use of intensive agitation of the reaction mixture allowed to decrease the temperature of wood delignification by H2O2 with TiO2 catalyst and to provide an effective fractionation of wood on microcrystalline cellulose and soluble lignin at mild conditions.
The kinetic study of abies wood delignification by H2O2 in the medium acetic acid–water was first studied in the presence of TiO2 catalyst under mild conditions: temperatures 70–100 °C, atmospheric pressure. The oxidative delignification process is described satisfactory by the first order equation in all temperature range.
The rate constants are varied between 0.80 and 2.3 × 10−3 min−1 and the activation energy is near 81 kJ mol−1.
By experimental and numerical optimization the parameters of delignification process providing a high yield (53.3 wt%) of quality cellulosic product were established: temperature 100 °C, concentration of H2O2 5.9 wt%, of CH3COOH 30 wt%, LWR 15, duration 4 h.
According to XRD, FTIR and 13C CP-MAS NMR data, the cellulose obtained by abies wood oxidative delignification with 1 wt% TiO2 catalyst has the structure of microcrystalline cellulose.
References
Johan G, Fogelbolm CJ (2000) Chemical pulping, papermaking science and technology book 6A. Tappi Press, Finland
Sixta H (2006) Hand book of pulp. Wiley, Weinheim
JiménezL Pérez A, Rodríguez A, de la Torre MJ (2006) New raw materials and pulping processes for production of pulp and paper. Afinidad 63(525):362–369
Rodríguez A, Jiménez L (2008) Pulping with organic solvents other than alcohols. Afinidad 65(535):188–196
Sixta H, Harms H, Dapia S, Parajo JC, Puls J, Saake B, Fink HP, Röder T (2004) Evaluation of new organosolv dissolving pulps. Part I: preparation, analytical characterization and viscose processability. Cellulose 11:73–83
Zhao X, Heide E, Zhang T, Liu D (2010) Delignification of sugarcane bagasse with alkali and peracetic acid and characterization of the pulp. Bioresources 5:1565–1580
López F, Alfaro A, Jiménez L, Rodríguez A (2006) Alcohols as organic solvents for obtainment of cellulose pulp. Afinidad 523:174–182
Villaverde JJ, Ligero P, Vega A (2015) Fractionation of Miscanthus x Giganteus via modification of the Formacell process. Ind Crops Prod 77:275–281
Ligero P, Vega A, Villaverde JJ (2010) Delignification of Miscanthus x Giganteus by the Milox process. Bioresour Technol 101:3188–3193
Suchy M, Argyropoulos D (2001) Catalysis and activation of oxygen and peroxi dedelignification of chemical pulps: a review. ACS Symp Ser 785:2–43
SongYo Wi SG, Kim HM, Bae HJ (2016) Cellulosic bioethanol production from Jerusalem artichoke (Helianthus tuberosus L.) using hydrogen peroxide-acetic acid (HPAC) pretreatment. Bioresour Technol 214:30–36
Dussan K, Girisuta B, Haverty D, Leahy JJ, Hayes MH (2014) The effect of hydrogen peroxide concentration and solid loading on the fractionation of biomass in formic acid. Carbohydr Polym 111:374–384
Das S, Lachenal D, Marlin N (2013) Production of pure cellulose from Kraft pulp by a totally chlorine-free process using catalyzed hydrogen peroxide. Ind Crops Prod 49:844–850
Ramadoss G, Muthukumar K (2015) Influence of dual salt on the pretreatment of sugarcane bagasse with hydrogen peroxide for bioethanol production. Chem Eng J 260:178–187
Gaspar AR, Gamelas JAF, EvtuguinD NetoCP (2007) Alternatives for lignocellulosic pulp delignification using polyoxometalates and oxygen: a review. Green Chem 9:717–730
Popova NR, Tortseva TV, Bogolitsyn KG (2013) Catalytic delignification of cellulose fibers by hydrogen peroxide in presence of polyoxometalates. Russ J Appl Chem 86:1275–1279
Kuznetsov BN, Kuznetsova SA, Danilov VG, Yatsenkova OV, Petrov AV (2011) A green one-step process of obtaining microcrystalline cellulose by catalytic oxidation of wood. Reac Kinet Mech Cat 104:337–343
Kuznetsov BN, Kuznetsova SA, Danilov VG, Yatsenkova OV (2008) Catalytic properties of TiO2 in wood delignification by acetic acid–hydrogen peroxide mixture. React Kinet Catal Lett 94:311–317
Kuznetsov BN, Kuznetsova SA, Danilov VG, Yatsenkova OV (2009) Influence of UV pretreatment on the abies wood catalytic delignification in the medium “acetic acid–hydrogen peroxide–TiO2”. React Kinet Catal Lett 97:295–300
Fernández-Rodrígueza C, Dona-Rodrígueza JM, González-Díaza O, Secka I, Zerbani D, Portillob D, Perez-Pena J (2012) Synthesis of highly photoactive TiO2 and Pt/TiO2 nanocatalysts for substrate-specific photocatalytic applications. Appl Catal B 125:383–389
Hu F, Jung S, Radauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12
Kuznetsov BN, Sudakova IG, Garyntseva NV, Djakovitch L, Pinel C (2013) Kinetic study of aspen-wood sawdust delignification by H2O2 with sulfuric acid catalyst under mild conditions. Reac Kinet Mech Cat 110:271–280
Sjoöstroöm E, Aleŕn R (1999) Analytical methods in wood chemistry pulping and papermaking, Springer, Berlin
Park S, Baker JO, Himmel ME, Parilla PA, Jonson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulose performance. Biotechnol Biofuels 3:10
Lente G (2015) Deterministic kinetics in chemistry and systems biology. Springer, New York. ISBN 978-3-319-15481-7
Sudakova IG, Garyntseva NV, Yatsenkova OV, Kuznetsov BN (2013) Optimization of aspen wood delignification by H2O2 with sulfuric acid catalyst. J Sib Fed Univ Chem 6:76–84
Adel AM, El-Wahab ZH, Ibrahim AA, Al-Shemy MT (2011) Characterization of microcrystalline cellulose prepared from lignocellulosic materials part II: physicochemical properties. Carbohydr Polym 83:676–687
Fan M, Dai D, Huang B (2012) Fourier transform infrared spectroscopy for natural fibres. In: SalihSalih (ed) Fourier transform—materials analysis. In Tech, Rijeka. doi:10.5772/35482
Xiang LY, Mohammed MAP, Baharuddin AS (2016) Characterisation of microcrystalline cellulose from oil palm fibers for food applications. Carbohydr Polym 148:11–20
Shankar S, Rhim JW (2016) Preparation of nanocellulose from microcrystalline cellulose: the effect on the performance and properties of agar based composite films. Carbohydr Polym 135:18–26
Yuvraj P, Chauhan RS, Sapkal VS, Zamre GS (2009) Microcrystalline cellulose from cotton rags (Waste from garment and hosiery industries. Int J Chem Sci 7:681–688
Moran JI, Alvarez VA, Cyras VP, Vazquez A (2008) Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 15:149–159
Wikberg H, Maunu SL (2004) Characterization of thermally modified hard- and softwoods by 13C CPMAS NMR. Carbohydr Polym 58:461–466
Zuckerstätter G, Schild G, Wollboldt P, Röder T, Weber HK, Sixta H (2009) The elucidation of cellulose supramolecular structure by 13C CP-MAS NMR. Lenzing Ber 87:38–46
Garyntseva NV, Sudakova IG, Kuznetsov BN (2015) Study of birch wood catalytic delignification by hydrogen peroxide at atmospheric pressure. J Sib Fed Univ. Chem 8:422–429
Ma R, XuYa Zhang X (2015) Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 8:24–51
Kuznetsov BN, Tarabanko VE, Kuznetsova SA (2008) New catalytic methods for obtaining cellulose and other chemical products from vegetable biomass. Kinet Catal 49:517–526
Acknowledgements
The reported study was supported by the Russian Science Foundation (Grant No. 16-13-10326).NMR experiments were conducted on BrukerAvance III spectrometer at Krasnoyarsk Regional Centre of Research Equipment SB RAS. The authors wish to thank Alexander Kondrasenko for the help with NMR experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuznetsov, B.N., Sudakova, I.G., Garyntseva, N.V. et al. Kinetic studies and optimization of abies wood fractionation by hydrogen peroxide under mild conditions with TiO2 catalyst. Reac Kinet Mech Cat 120, 81–94 (2017). https://doi.org/10.1007/s11144-016-1100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1100-z