Abstract
Eu3+ doped polymer nanofibers were fabricated by electrospinning technique using various polymers such as poly(vinylidene fluoride) (PVDF), polyethylene oxide (PEO), poly(vinyl pyrrolidone) (PVP) to study the influence of polymer in their photoluminescence properties. As-fabricated nanofibers were characterized by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDX) and photoluminescence (PL). Spectral analysis of Polymer/Eu3+ nanofibers was based on their emission spectra. The photoluminescence property shows superior bright red emission spectra from the Eu3+ and relatively stronger hypersensitive behavior of the 5D0 → 7F2 transition. Eu3+ doped polymeric nanofibers are very much suitable for photoluminescent fabric designing in smart textiles. The enhanced properties of this photoluminescence indicated a more polarized chemical environment for the Eu3+ ions and greater hypersensitivity for the 5D0 → 7F2 transition, which showed the potential for application in various polymer optoelectronic devices.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Photoluminescent materials are the products which glow in the dark like photoluminescent paint, exit sign stickers which are used in offices, cinema halls which are helpful in power failure situation or used as safety purposes. Photoluminescent thread which embroidered on the clothes attracts more attention as compared to ordinary clothes. Apart from these, photoluminescence spectroscopy is of great significance in other fields of analysis, especially semiconductors in band gap determination, impurity levels and defect detection, recombination mechanisms, surface structure and excited states. Currently, functional and practical applications of this physical phenomenon in our daily life can be found in light-emitting diodes, lasers, lamps, solar cells, sensors, scintillates, electronic displays, etc. Photoluminescence spectroscopy is a widely used technique for characterization of the optical and electronic properties of semiconductors and molecules. Now a day these photoluminescent materials are specially used in designing fabric and in military purposes also. But all this is possible only because of rare earth ions, which are highly, valued for their unique properties, especially as optically active material in ionized state for lasers.
Among all the rare earth (RE) ions, the material Eu3+ is selected because europium metal ions exhibit a high yield of luminescence in the visible light region and extremely sharp emission bands due to their 4f electrons well shielded by closed 5s and 5p outer shells. The energy levels of the 4f shell have equal parity and hence electric dipole transitions are forbidden. RE ions can transform their absorption energy from the shorter wavelength region into luminescence energy in the longer wavelength region with high luminosity. The material Eu3+ is one of the most attractive rare earth ions due to its excellent luminescence properties that originate from the 4f–4f transition. Europium is used in the printing of euro banknotes. It glows red under UV light, and forgeries can be detected by the lack of this red glow. Europium is excellent at absorbing neutrons, making it valuable in control rods for nuclear reactors. Europium-doped plastic has been used as a laser material. It is also used in making thin super-conducting alloys. Because europium are strongly influenced by its half filed electron shell, it has second lowest melting point and it become superconductor when cooled below 1.8 K and compressed to above 80 GPa. A recent application of europium is in quantum memory chips which can reliably store information for days at a time; this could allow sensitive quantum data to be stored to a hard disk like device and shipped around. In the field of lighting, white light emitting diode (WLED), a new generation solid source has been highlighted due to its high luminous efficiency, low energy consumption and great potential in environmental protection. There is a tremendous growth in the development of WLED worldwide due to its various applications like lighting, motor vehicle and backlight for mobile panel and liquid crystal display.
To increase its optical property rare earth ions are doped with polymers. The polymer material is well recognized for its high transparency, flexible, excellent optical properties and simple synthesis process, which make the material to be perfect host for rare earth ions. The rare earth ions doped with polymers gives a new class of material which makes them applicable in wide range of new technologies. To design a fabric, new technology is used with the help of fibers made of materials at nanoscale [1,2,3]. These nanofibers can be easily fabricated by electrospinning method. Electrospinning is an effective and easy method for generating one dimensional nanofibers with diameter ranging from nanometer to micrometer. The electrospun nanofibers possess properties like high surface area to volume ratio, high aspect ratio (length to diameter), controlled pore size and superior mechanical performance. The superior mechanical properties associated with the electrospun nanofibers arise from the decrease in diameter that cannot be achieved through conventional spinning processes. It is a technique with which rich variety of materials such as polymers, organic, inorganic compounds can be used for fabrication of nanofibers. These photoluminescent electrospun nanofibers become the new trend in textile industry [4,5,6,7].
Electrospun polymer nanofibers have been studied for use as medical or consumer products and in industrial or high-tech applications. Electrospun carbon nanofibers prepared from PAN have been used extensively to make bullet proof military jackets because of their properties of hardness as well as bending flexibility. Similarly, by using this introduced method for nanofibers manufacture, photoluminescent fibers can be prepared as a protective textile for soldiers, or as medical textiles or smart textiles using suitable polymers. With this background, we have reported in this paper by using polymers such as poly(vinylidene fluoride) (PVDF), polyethylene oxide (PEO), poly(vinyl pyrrolidone) (PVP) for doping Eu3+ ions into these polymers to fabricate Eu3+ doped polymer nanofibers by electrospinning technique and to study the influence of these polymers in their photoluminescence properties.
2 Experimental
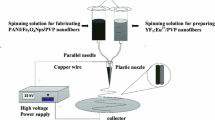
The starting material europium oxide (Eu2O3, 99.9%) was used as received without further purification for the preparation of europium ion doped polymer nanofibers. EuCl3 solution was prepared by dissolving the corresponding europium oxide (Eu2O3) in a dilute hydrochloric acid solution and stirring at 100 °C. During heating excess HCl was evaporated. EuCl3 powder was obtained after drying under vacuum [8]. For the preparation of PVDF/Eu3+ nanofibers by electrospinning, PVDF solution was prepared by using 1 g of PVDF (Mw = 40,000) powder in 10 mL N, N-dimethylformadide (DMF). Then solution was stirred for 5 h at room temperature. EuCl3 (0.5 g) was dissolved in the PVDF solution and rigorously stirred for 12 h at room temperature for homogenous mixing to obtain EuCl3/PVDF solution with 1:2 concentration. EuCl3/PVDF solution was then loaded into a syringe for electrospinning. The physical parameters of electrospinning were set by high voltage 17 kV between the needle of syringe and the grounded collector (aluminum foil) placed at a distance of 20 cm from needle. The solution flow rate was kept at 0.4 mL/h and maintained using computer control programming. PVDF/Eu3+ nanofibers were collected after 10 h and then dried in vacuum oven at 80 °C [9,10,11]. Then same procedure was repeated for the preparation of PEO/Eu3+ and PVP/Eu3+ nanofibers by electrospinning technique. Figure 10.1 shows the synthesis process for preparation of polymer/Eu3+ nanofibers. As-synthesized electrospun nanofibers were characterized by using Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray spectroscopy (EDX) performed on Carl Zeiss EVO-18 SEM-EDX and photoluminescence spectra were recorded on FP8200 spectrophotometer.
3 Results and Discussion
3.1 Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
SEM images and histogram of PVDF/Eu3+, PEO/Eu3+ and PVP/Eu3+ nanofibers are shown in Fig. 10.2a–c respectively. The diameter of the nanofibers was found to be 1200 nm, 672 nm and 584 nm for PVDF/Eu3+, PEO/Eu3+ and PVP/Eu3+ nanofibers respectively. All SEM images are having different magnification as these images have been provided by SEM user. However, with the help of histograms of as-synthesized electrospun nanofibers the average diameter can be obtained as mentioned. From SEM results, it is seen that PVP/Eu3+ electrospun nanofibers are very smooth, fine and smaller in diameter as compared to the nanofibers of PVDF/Eu3+ and PEO/Eu3+. This may be due to strong interaction between the polymer molecule chains of PVP and Eu3+ ions which avoids the formation of beads in nanofibers as demonstrated by Tang et al. [5]. These results are possible because PVP is good solvents i.e. it is soluble in various alcohols, such as methanol and ethanol. It has bearing active C=O groups, which made PVP to be able to use as carrier for preparation of many composite functional materials. If C=O groups of PVP coordinate with Eu3+ ions, it would influence the coordinative environment of the Eu3+ ions and change the energy transfer probabilities of the electric dipole transitions. PEO and PVDF are also water-soluble polymers heat resistance, resistance to chemical corrosion and low protein binding with high thermal stability with semi-crystalline nature because of that there may not be as good as interaction and hence having larger diameter [12].
Figure 10.3 shows EDX spectra of PVDF/Eu3+, PEO/Eu3+ and PVP/Eu3+ nanofibers, which indicates the peak for element Eu confirming its existence in the prepared PVDF/Eu3+, PEO/Eu3+, PVP/Eu3+nanofibers. PVDF/Eu3+ contain maximum concentrations of the elements like fluorine and carbon indicates their presence due to PVDF polymer. In PEO/Eu3+ nanofibers, peaks of carbon, oxygen, europium and chlorine are observed along with the peak for element Eu confirming its existence in PEO/Eu3+ nanofibers. From the EDX spectrum PVP/Eu3+, the europium peak can be detected confirming its existence in PVP/Eu3+. Hence when Eu3+ ions are doped in the polymer PVP the peaks of Europium and chlorine are clearly visible, along with the contents carbon and nitrogen of PVP.
3.2 Photoluminescence
The excitation of all synthesized Eu3+ doped polymer nanofibers was registered at room temperature by monitoring the luminescence intensity of the 5D0 → 7F2 transition at 615 nm. Figure 10.4a shows the excitation and emission spectra of PVDF/Eu3+ nanofibers. It is observed that the nanofibers exhibit an intense broad excitation band at 411 nm. In the emission spectra of PVDF/Eu3+ nanofibers, sharp peak at around 615 nm is assigned due to 5D0 → 7F2 electric dipole transition [8, 13, 14]. The 5D0 → 7F2 transition is responsible for the typical red luminescence observed in europium (III).
Figure 10.4b shows the excitation and emission spectra of PEO/Eu3+ nanofibers. It is observed that the nanofibers exhibit an intense broad excitation band at 411 nm. In emission spectra, peaks at around 592 and 613 nm are assigned to 5D0 → 7FJ transition (J = 1, 2) respectively. The 5D0 → 7F2 transition is a called as “hypersensitive transition” (electric dipole transitions). Transition 5D0 → 7F2 shows a very high intense peak at 613 nm. Hyper-sensitive transitions obey the selection rules |S| = 0, |L| ≤ 2 and |J| ≤ 2 which are same as the selection rules for a quadrupole transition. The intensity of the hypersensitive transition 5D0 → 7F2 is often used as a measure for the asymmetry of the Eu3+ site i.e. intensity is much more affected by the environment of Eu3+ ions. Another peak is because of 5D0 → 7F1 transition called as magnetic dipole transitions. Laporte selection rule is only applicable for this type of transition. The intensity of magnetic dipole transition is very weak as compared to electric dipole transitions. The weak emission intensity of 5D0 → 7F1 transition at 592 nm is because of independent of the coordination environment of the Eu3+ [15]. Figure 10.4c shows the excitation and emission spectra of PVP/Eu3+ nanofibers. Transition 5D0 → 7F2 shows a very high intense peak at 615 nm. Intensity is much more affected by the environment of Eu3+ ions. It is observed that the intensity of PVP/Eu3+ nanofibers is greater than the other polymer nanofibers. It may be due to the smaller the range of nanofibers higher will be the surface area of PVP/Eu3+ nanofibers, so that more emission centers Eu3+ could be excited. And hence the intensity of PVP/Eu3+ nanofibers had a significant increase [16, 17]. It is observed that when europium ion is doped with polymers the intensity of emitting light increased because of the well homogeneous distribution of the Eu3+ ions with the polymers as well as high surface area of the nanofibers due to which more emission centres could be excited. So, the emission intensity of the nanofibers had a significant increase [18].
Photoluminescence (PL) spectra of all synthesized Eu3+ doped polymer nanofiber s were further qualitative analysed by Judd-Ofelt theory for the study of f–f inner shell electronic transitions and its intensity parameters [19]. Judd-Ofelt intensity parameters for transitions are denoted by Ωλ (λ = 2, 4 and 6) which are obtained from emission data of electronic transitions 5D0 → 7F2, 5D0 → 7F4 and 5D0 → 7F6 respectively. Judd-Ofelt intensity parameters (Ωλ) are important for the investigation of local structure and bonding in the vicinity of rare earth ions. ARAD is the total radiative transition rate of the 5D0 → 7F0-4 transitions. Judd-Ofelt theory is a useful tool for analyzing f–f inner shell electronic transitions. The intensity parameter (Ωλ) gives the emission intensity of the prepared material and it can be calculated by using radiative transition rate (ARAD) which are well explained by Zhang et al. [20].
From the emission spectra of Eu3+ doped polymer nanofibers, the experimental intensity parameters Ω2 was determined by using the 5D0 → 7F2 transition by expressing the emission intensity I0J in terms of the surface ‘S’ under the emission curve. The radiative transition rate (ARAD) emitting level 5D0 was determined from intensity I0J and used it for the determination of intensity parameters Ω2 [20]. Intensity parameters Ω2 for Eu3+ doped polymer nanofibers are reported in Table 10.1. It was found that the emission wavelength for all Eu3+ doped polymer nanofibers is nearly same as 615 nm which is the characteristics of 5D0 → 7F2 transition called hypersensitive transition. The International Commission on Illumination (CIE) chromaticity coordinates of PVDF/Eu3+, PEO/Eu3+ and PVP/Eu3+ nanofibers are calculated by using PL spectra and represented in Fig. 10.5. Purpose of colorimetry is to express color as numerical values and colorimetry is nothing but a technique of color measurement. The coordinates of CIE indicates that what is the actual color of the prepared materials. The location of coordinates indicates the intensity of the color. The prepared material emits red color and their coordinates indicates the less and high intensity of the material. If the coordinate is more towards x and less towards y then the intensity of red light emission is more. CIE values for PVDF/Eu3+, PEO/Eu3+ and PVP/Eu3+nanofibers are calculated to an average (0.65, 0.35), which is closed to the standard red chromaticity of Nation Television Standard Committee (NTSC) (x = 0.68, y = 0.32). The CIE chromaticity coordinates for these PVDF/Eu3+, PEO/Eu3+ and PVP/Eu3+nanofibers are located in the red region. It can be clearly seen from the diagram that all the nanofibers give an intense red emission due to the presence of relatively intense 615 nm lines (5D0 → 7F2) under 411 nm excitation.
4 Conclusion
Eu3+ doped polymer nanofibers were fabricated by electrospinning and comparatively studied their photoluminescence properties for fabric designing. Among all nanofibers, PVP shows good photoluminescence because Eu3+ ions dispersed in the PVP molecule chain and there could be some more interaction between the C=O group of PVP and Eu3+ ions. Due to high surface area to volume ratio and outstanding fluorescent property, Eu3+ doped polymer nanofibers showed potential applications in preparation of various polymer optoelectronic devices and nanofabric in textiles industries which show photoluminescent properties for making new kind of light emitting clothes.
References
H. Timothy, G. Froerer, Photoluminescence in analysis of surfaces and interfaces, in Encyclopedia of Analytical Chemistry (2006), pp. 9209–9239
V. Prajzler, V. Lyutakov, I. Hutte, J. Oswald, V. Jerabek, Optical and spectroscopic properties of polymer layers doped with rare earth ions source, in Advances in Lasers and Electro Optics, ed. by N. Costa, A. Cartaxo (2010). ISBN 978-953-307-088-9, p. 838
S. Itankar, M. Dandekar, S. Kondawar, B. Bahirwar, Eu3+ doped polystyrene and polyvinylidene fluoride nanofibers fabricated by electrospinning for photoluminescent fabric design. J. Luminescence 32 (2017)
S. Tang, C. Shao, M.R. Yichun, Electrospun nanofibers of poly(acrylonitrile)Eu3+ and their photoluminescent properties. J. Phys. Chem. Solids 71, 273–278 (2010)
S. Tang, C. Shao, Y.C. Li, Electrospun nanofibers of poly (vinyl pyrrolidone)/Eu3+ and its photoluminescence properties. Chin. Chem. Lett. 18, 465–468 (2007)
J. Liang, F. Xie, X. Ren, Y. Chen, B. Chen, F. Guo, Temperature dependent luminescence of a europium complex incorporated in poly(methyl methacrylate). Mol. Biomol. Spectrosc. 116, 317–320 (2013)
M. Dandekar, S. Kondawar, S. Itankar, D. Nandanwar, Luminescence properties of electrospun nanofibers of europium complex Eu(TTA)3phen/polymers. Procedia Mater. Sci. 10, 580–587 (2015)
X. Li, G. Shen, X. Jin, M. Liu, L. Shi, J. Lu, Novel polyimide containing 1,10-phenanthroline and its europium (III) complex: synthesis, characterization and luminescence properties. J. Mater. Sci. 51, 2072–2078 (2016)
S. Tang, S. Shao, S. Li, Electrospun nanofibers of poly (vinyl pyrrolidone)/Eu3+ and its photoluminescence properties. Chin. Chem. Lett. 18, 465–468 (2007)
S. Tang, C. Shao, Y. Liu, R. Mu, Electrospun nanofibers of poly(acrylonitrile)/Eu3+ and their photoluminescence properties. J. Phys. Chem. Solids 71, 273–278 (2010)
Z. Zhang, Y. Long, H. Yin, B. Sun, J. Zheng, H. Zhang, X. Ji, C. Gu, Electrospun fluorescein/polymer composite nanofibers and their photoluminescent properties. Chin. Phys. B. 21, 097805-1-6 (2012)
S. Itankar, M. Dandekar, S. Kondawar, B. Bahirwar, Comparative photoluminescent study of PVDF/Eu3+ and PEO/Eu3+ electrospun nanofibers in photonic fabric. AIP Conf. Proc. 2104, 1 (2019)
S. Itankar, M. Dandekar, S. Kondawar, B. Bahirwar, Synthesis and characterization of electrospun Eu3+ doped poly(methylmethacrylate) nanofibers. Int. J. Res. Biosci. Agric. Technol. 1, 419–425 (2014)
M. Mondragon, G. Trujillo, I. Moggio, E. Arias, Luminescent polylactic acid and polysulfone electrospun fibers containing europium (III) complexes. Eur. Polymer J. 80, 126–133 (2016)
J. Zhao, W. Zang, E. Xie, Z. Ma, A. Zhao, Z. Liu, Structure and photoluminescence of Ga2O3:Eu3+ nanofibers prepared by electrospinning. Appl. Surf. Sci. 275, 4968–4972 (2011)
R. Argauer, C. White, Fluorescent compounds for calibration of excitation and emission unit of spectroflurometer. Anal. Chem. 36, 368–371 (1964)
K. Sivaiah, S. Buddhudu, Light emission in Tb3+ and Eu3+ polymer films. Indian J. Pure Appl. Phys. 49, 377–381 (2011)
L. Saravanan, S. Diwakar, R. Mohankumar, A. Pandurangan, R. Jayavel, Synthesis, structural and optical properties of PVP encapsulated CdS nanoparticles. Nanomater. Nanotechnol. 1, 42–48 (2011)
Y. Tao, P. Yan, C. Wang, G. Li, Luminescent electrospun composite nanofibers of [Eu(TFI)3(Phen)].CHCl3/polyvinylpyrrolidone. J. Mater. Sci. 48, 6682–6688 (2013)
X. Zhang, S. Wen, L. Zhang, L. Liu, Electrospinning preparation and luminescence properties of Eu(TTA)3phen/polystyrene composite nanofibers. J. Rare Earths 28, 333–338 (2010)
Acknowledgements
This work was supported by the Department of Science and Technology (DST, New Delhi, India) Support under DST-FIST Program, Grant No. SR/FST/PSI-178/2012(C).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Itankar, S.G., Dandekar, M.P., Koinkar, P.M., Kondawar, S.B. (2020). Influence of Polymer in Photoluminescence Properties of Electrospun Eu3+ Doped Polymer Nanofibers. In: Murakami, RI., Koinkar, P., Fujii, T., Kim, TG., Abdullah, H. (eds) NAC 2019. Springer Proceedings in Physics, vol 242. Springer, Singapore. https://doi.org/10.1007/978-981-15-2294-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-2294-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2293-2
Online ISBN: 978-981-15-2294-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)