Abstract
The luminescent europium complex [Eu(TFI)3(Phen)]·CHCl3 (TFI = 2-(2,2,2-trifluoroethyl)-1-indone, Phen = 1,10-phenanthroline) was incorporated into poly(vinylpyrrolidone) (PVP) matrixes and electrospun into nanofibers. The effect of [Eu(TFI)3(Phen)]·CHCl3 on the morphology and luminescence of composite nanofibers has been studied. FT-IR and TGA analyses of the composite nanofibers have been conducted and discussed. Further, the Judd–Ofelt theory is employed to study the effect of the dispersion of [Eu(TFI)3(Phen)]·CHCl3 and the interactions between the [Eu(TFI)3(Phen)]·CHCl3 molecules and neighboring chain segments of PVP. The results suggest that PVP led to the increased polarization degree of Eu3+ ions and significantly enhanced the electronic dipole-allowed transitions of Eu3+ ions, resulting in the enhancement of luminescent efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of importance for both fundamental studies and technological applications, one-dimensional (1D) nanostructures have attracted much attention in recent years [1]. A large number of fabrication and synthesis methods has been demonstrated for generating 1D nanostructures in the form of spirals, wires, rods, fibers, belts, tubes, and rings from various materials [2]. Electrospinning, among these methods, is rapidly attracting increased attention because of its simple electrostatic method for generating 1D nanostructures from inorganic–organic materials. In a customarily process, a solution is injected from a small nozzle under the influence of an electric field, travels for a certain distance in the air, and collected as a fiber mat on a grounded collector plate [3].
Some organo-lanthanide complexes, for example β-diketone europium complexes [4], are of distinct luminescent characteristics, such as sharp emission bands, long lifetimes, and high-luminescent quantum efficiencies. However, they have low-processing capability, e.g., weak mechanical strength and poor thermal stability, which impediment their applications in the fabrication of fluorescent probes and optical switches [5]. One potential solution is to incorporate the complexes into zeolites, polymers, and organically modified silicates [6]. It is known that the polymer-capped complexes have essentially improved the thermal stability, luminescent properties, and mechanical flexibility for the luminescent complexes [7]. Nevertheless, the syntheses of such copolymers between organic monomers and metal complexes are often difficult [8]. Previously, Zhang et al. have developed the composite nanofibers containing lanthanide complexes in a polymer matrix by electrospinning. The relative luminescent intensities enhanced proportionally with the increasing amount of the Eu-complex in the copolymers, and the thermal stability of the photoluminescence in the composite fibers was improved considerably over the pure complex [9]. As compared with the synthetic method of copolymerization, the technique of electrospinning is more straightforward for convenient fabrication of composites containing complexes. Thus, the similar distribution of luminescent complexes could be achieved by electrospinning and the technique would be a viable method for the preparation of innovative composites in the form of nanofibers with enhanced luminescent properties.
In this article, a luminescent lanthanide complex of [Eu(TFI)3(Phen)]·CHCl3 has been doped into PVP and the composite nanofibers with diameters of ~100 ± 25 nm containing [Eu(TFI)3(Phen)]·CHCl3/PVP have been successfully fabricated by electrospinning. Further, the effect of the dispersion of [Eu(TFI)3(Phen)]·CHCl3 and the PVP on the luminescent nanofibers have been expatiated.
Experimental section
The lanthanide complex of [Eu(TFI)3(Phen)]·CHCl3 was synthesized according to the literature [10]. The detailed synthesis and characterization are provided in Supporting Material. The electrospinning solution was prepared by first dissolving PVP (M w = 1.3 × 106 g mol−1) in a solvent mixture of N,N-dimethylformamide at the concentration of 14 wt%. Subsequently, varied amounts of [Eu(TFI)3(Phen)]·CHCl3 were added, making the contents of [Eu(TFI)3(Phen)]·CHCl3 to PVP equal to 1, 3, 5, 7, and 10 wt%. The mixture was stirred for 24 h to acquire uniform solutions. The solutions were then placed into 10 mL plastic syringes with a 90° blunt stainless-steel needle with inner diameter of 0.37 mm. The electrospinning setup by DC high-voltage generator is purchased from the BMEI CO Inc. During electrospinning, a positive high voltage of 15 kV was applied to the needle to maintain the flow rate at 0.1 mL h−1. The distance between the plate and the tip of the needle is 30 cm. The nanofibers were collected randomly as overlaid mats on an electrically grounded copper plate wrapped with aluminum foil. After electrospinning, the composite nanofiber mats were dried in a vacuum oven at room temperature for 12 h before characterizations.
A Hitachi S-4800 scanning electron microscope (SEM) was employed to examine the morphologies of the electrospinning nanofibers. Prior to SEM examination, the specimens were sputter-coated with gold to avoid charge accumulation. A JEM-2100 transmission electron microscope (TEM) was used to examine the dispersion of [Eu(TFI)3(Phen)]·CHCl3 in the composite nanofibers. Fluorescence microscope of Leica DM400 M with camera shot Leica DFC 425C was employed to study the luminescence of the composite nanofibers. FT-IR spectra were obtained on a Perkin-Elmer Spectrum One spectrophotometer by using KBr disks in the range of 4000–450 cm−1. Thermal analyses were conducted on a Perkin-Elmer STA 6000 with a heating rate of 10 ºC min−1 in a temperature range from 30 to 800 °C under atmosphere. Excitation and emission spectra were measured with an Edinburgh FLS 920 fluorescence spectrophotometer. Luminescence lifetimes were recorded on a single photon counting spectrometer from Edinburgh Instrument (FLS 920) with microsecond pulse lamp as the excitation. The data were analyzed by software supplied by Edinburgh Instruments. Both the slit widths for excitation and emission were set at 1.2 nm. The fluorescence dynamics of the samples was measured with an FLS 920 instrument (Edinburgh). During the measurements, an oscillograph was used to record the decay dynamics, and the 345 nm incident light generated from a microsecond flash-lamp, which was used as the excitation source.
Results and discussion
Morphology of composite nanofibers and dispersion of [Eu(TFI)3(Phen)]·CHCl3
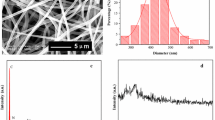
SEM photos reveal that the composite nanofibers consists of [Eu(TFI)3(Phen)]·CHCl3 and PVP with an average diameter of 100 ± 25 nm have been successfully prepared (Fig. 1b, c). However, the composite nanofibers are of slightly smaller diameters than that of the nanofibers for neat PVP (Fig. 1a) which is attributed to the presence of [Eu(TFI)3(Phen)]·CHCl3 leading to an increase of excess charge density in the electrospinning fibers. The surface of the composite nanofibers is smooth without identifiable particles, suggesting that the [Eu(TFI)3(Phen)]·CHCl3 might be uniformly dispersed in the nanofibers. The morphological structures and dispersion of [Eu(TFI)3(Phen)]·CHCl3 in the composite nanofibers were further characterized by TEM (Fig. 1d–f). The results indicated that almost no particles could be observed when the [Eu(TFI)3(Phen)]·CHCl3 content was lower than 3 wt% (Fig. 1d). However, when the content of [Eu(TFI)3(Phen)]·CHCl3 was increased up to 3 wt%, the nanoparticles with 30 nm could be occasionally identified at 3 and 5 wt%, indicating that the nanoparticles of [Eu(TFI)3(Phen)]·CHCl3 in the PVP matrices start to aggregate (Fig. 1e, f). This was mainly due to the following reasons: during electrospinning, the solutions contain uniformly dispersed [Eu(TFI)3(Phen)]·CHCl3 molecules and the rapid evaporation of the solvent concomitant fast solidification of the filaments (within tens of milliseconds) hindered the aggregation of [Eu(TFI)3(Phen)]·CHCl3, resulting in that the [Eu(TFI)3(Phen)]·CHCl3 predominantly existed as molecular clusters and nanoparticles with sizes of 30 nm or smaller in the composite nanofibers [11]. With increasing the [Eu(TFI)3(Phen)]·CHCl3 content, aggregates could be probably formed even in the electrospinning solutions, leading to the nanoparticles in the resultant composite nanofibers (Fig. 1f). The photos of fluorescence microscope (Fig. 1g–i) show the luminescent intensity increased with the content of [Eu(TFI)3(Phen)]·CHCl3 increase and reached its maximum at 3 wt% with extraordinary red light under ultraviolet irradiation.
SEM images (a–c) and TEM images (d–f) show the representative morphological structures of neat PVP nanofibers (a) and composite nanofibers with [Eu(TFI)3(Phen)]·CHCl3 contents of 1 wt% (d), 3 wt% (b, e), and 5 wt% (c, f), respectively. Fluorescence microscope images (g–i) show the representative morphological structures of composite nanofibers with [Eu(TFI)3(Phen)]·CHCl3 contents of 1 wt% (g), 3 wt% (h), and 5 wt% (i)
FT-IR
FT-IR spectra of PVP, composite fibers, and complex [Eu(TFI)3(Phen)]·CHCl3 (Fig. 2) suggest that the peak at 1660 cm−1 is assigned to the C=O stretching band of PVP, while, this band is red shifted to 1652 cm−1 in the Eu/PVP fibers, which suggests that oxygen atoms of the carbonyl group of PVP are interacted with Eu3+ ion in [Eu(TFI)3(Phen)]·CHCl3.
Thermal properties
TGA analysis (Fig. 3) reveal that a weight loss of 8.3 wt% occurred below 100 °C for Eu/PVP nanofibers is assigned to loss of residual N,N-dimethylformamide, and the weight loss of 8.2 wt% below 160 °C for [Eu(TFI)3(Phen)]·CHCl3 is assigned to loss of the crystallined CHCl3. The decomposition temperatures (T d) for complex [Eu(TFI)3(Phen)]·CHCl3 are 321 and 434 °C. There are two decomposition points for Eu/PVP nanofibers in the TGA that the T d values are about 387 °C (25 wt%) and 451 °C (41 wt%), which suggest that the thermal stabilities of Eu/PVP nanofibers are better than that of the [Eu(TFI)3(Phen)]·CHCl3. The improved thermal stability for Eu/PVP owe to the chemical bondings between the oxygen atoms of the carbonyl groups in PVP and the Eu3+ ion in [Eu(TFI)3(Phen)]·CHCl3.
Luminescent properties
The luminescent properties of the [Eu(TFI)3(Phen)]·CHCl3/PVP composite nanofibers were then investigated, and the results were compared with those of the neat [Eu(TFI)3(Phen)]·CHCl3. The excitation spectra for various samples are shown in Fig. 4. The excitation top point is 384 nm in the neat complex of [Eu(TFI)3(Phen)]·CHCl3. Interestingly, such an excitation for the [Eu(TFI)3(Phen)]·CHCl3/PVP composite nanofibers is blue shifted obviously and split into two bands centered at 350 and 368 nm, respectively. This mean that the site symmetry of the Eu3+ ions in complex [Eu(TFI)3(Phen)]·CHCl3 became lower than that of the Eu3+ ions in the composite nanofibers because of the influences of the neighboring chain segments of PVP [12]. In addition, both 7F1 → 5D1 and 7F0 → 5D2 excitations for the composite nanofibers disappeared, suggesting that the f → f inner-shell transitions for the composite nanofibers were quenched via nonradiative energy transfers [13].
In the emission spectra (Fig. 5), the bands at 580, 590, 612, 656, and 704 nm in [Eu(TFI)3(Phen)]·CHCl3 were assigned to the 5D0 → 7FJ (J = 0, 1, 2, 3, and 4) transitions, respectively, and the 5D0 → 7F2 hypersensitive transition at 612 nm was the most intense, indicating there was a highly polarized chemical environment around the Eu3+ ions [14]. It is worth mentioning that the integrated intensity ratios of 5D0 → 7F2 to 5D0 → 7F1 (I02/I01), which indicated the asymmetry of the coordination sphere of Eu3+ ions, were much higher for the composite nanofibers than that for the neat [Eu(TFI)3(Phen)]·CHCl3 [15]. The ratios of the neat [Eu(TFI)3(Phen)]·CHCl3 and composite nanofibers with [Eu(TFI)3(Phen)]·CHCl3 contents of 1, 3, and 5 wt% were calculated to be 12.26, 17.54, 15.92, and 14.84, respectively. The results suggested that the degree of polarization for [Eu(TFI)3(Phen)]·CHCl3 was enhanced when the complex was impregnated into the PVP matrix, and the local environment around the Eu3+ ions was more disordered, which further led to higher probability for the electronic dipole-allowed transitions [16].
Figure 6 shows the integrated emission intensity of I02 for the 5D0 → 7F2 transition as a function of the [Eu(TFI)3(Phen)]·CHCl3 content in the composite nanofibers. The emission intensity increased with increasing the [Eu(TFI)3(Phen)]·CHCl3 and reached its maximal value at ~3 wt%, this was followed by typical emission concentration quenching with further increasing of the [Eu(TFI)3(Phen)]·CHCl3. Such a quenching phenomenon was due to the deactivation of the 5D0 and 5D1 states through the electrostatic multipolar interactions and by the exciton migration through the Förster dipole–dipole mechanism. The mechanism has been illustrated in the Supporting Material. Compared with the composite polymers prepared via copolymerization, the electrospun composite nanofibers achieved higher [Eu(TFI)3(Phen)]·CHCl3 contents without inducing the concentration quenching. This was because the desired dispersion of [Eu(TFI)3(Phen)]·CHCl3 in the matrix of the composite nanofibers might be even more preferred than the distribution of [Eu(TFI)3(Phen)]·CHCl3 units along macromolecular chains [17]. In the composite nanofibers, most of [Eu(TFI)3(Phen)]·CHCl3 dispersed in the nanofiber matrix as molecular states and nanoparticles with sizes smaller than 30 nm when the content of [Eu(TFI)3(Phen)]·CHCl3 was low and the exciton migration through the diffusion-induced collision among Eu3+ ions was negligible. When the content of [Eu(TFI)3(Phen)]·CHCl3 was high, on the other hand, some aggregates with sizes of tens of nanometers formed, which led to high Eu3+ ion concentrations locally and were responsible for the emission concentration quenching [18].
Judd–Ofelt analysis and luminescent quantum efficiency
The Judd–Ofelt theory is a useful tool for analyzing f–f inner shell electronic transitions [19]. The Judd–Ofelt parameters Ω λ (λ = 2, 4, and 6) are interaction parameters of ligand fields, in which Ω 2 is sensitive to the sequence and symmetry of ligand fields [20]. The values of Ω 2 and Ω 4 were determined by experiments, from the 5D0 → 7F2 and 5D0 → 7F4 transitions (Fig. 6) using the magnetic dipole transition of 5D0 → 7F1 as the reference (Fig. 7). The specific calculations and detailed principles were provided in the Supporting Material, and the calculated values of Ω 2 and Ω 4 for the composite nanofibers and the neat complex [Eu(TFI)3(Phen)]·CHCl3 are listed in Table 1. It was evident that the composite nanofibers are of higher Ω 2 values than that for the neat [Eu(TFI)3(Phen)]·CHCl3, suggesting an enhancement of the 5D0 → 7F2 hypersensitive transition and an increase of the covalence degree in the first coordination shell of Eu3+ ions [21]. This was also due to the change of the chemical environment surrounding Eu3+ ions, which was induced by the intermolecular interactions between neighboring chain segments of PVP and [Eu(TFI)3(Phen)]·CHCl3 [18] (Fig. 8). The higher values of Ω 4 for the composite nanofibers as compared with that of the neat [Eu(TFI)3(Phen)]·CHCl3 indicated a perturbation on the coordination effect of the bidentate TFI by the steric factors from the surrounding PVP. Furthermore, the values of Ω 2 and Ω 4 decreased with increasing the [Eu(TFI)3(Phen)]·CHCl3 content, suggesting that the effect of neighboring PVP chain segments on the ligand fields of Eu3+ was gradually weakened. Such a phenomenon was attributed to the uniform dispersion of [Eu(TFI)3(Phen)]·CHCl3 in the nanofiber matrix too. When the content of [Eu(TFI)3(Phen)]·CHCl3 was lower than 3 wt%, most of the [Eu(TFI)3(Phen)]·CHCl3 existed as molecular complex and the chemical environment around the Eu3+ ions was significantly affected by the surrounding PVP, whereas such an influence reduced gradually with the increase of the complex content resulting in the formation of aggregates [22].
Term scheme for Eu3+ ion and the relevant luminescent transitions between the 5D0 state and the 7FJ manifold. The solid lines indicate the induced electric dipole (ED) transitions, the dotted line is the magnetic dipole (MD) transition, and the dashed lines are the transitions that are forbidden in both ED and MD schemes
The luminescent quantum efficiency (Φ) for the 5D0 → 7F0–4 transitions of Eu3+ ions fundamentally determines the luminescent properties of the complex, and Φ is defined as A RAD/(A RAD + A NR), where A RAD is the total radiative transition rate of the 5D0 → 7F0–4 transitions and A NR is the nonradiative transition rate [23]. The A RAD, A NR, and Φ values for the neat complex and the composite nanofibers were calculated based upon the Judd–Ofelt theory, and the detailed calculations are provided in the Supporting Material. The typical luminescence decay curves for the excited Eu3+ ions at the 5D0 state under 345 nm excitation are illustrated in Fig. 9, and they were described in terms of a single-exponential function within the experimental errors [24]. The obtained lifetime values (τ obs) and A RAD, A NR, and Φ values of the neat [Eu(TFI)3(Phen)]·CHCl3 and the composite nanofibers are listed in Table 1. The A RAD values of Eu3+ ion for the composite nanofibers increased approximately 149–323 cm−1 as compared to that for the neat [Eu(TFI)3(Phen)]·CHCl3 due to the enhanced electronic dipole transition, making the Φ values of Eu3+ ion significantly improved [25]. Intriguingly, both the A RAD and the Φ values of Eu3+ ion in the composite nanofibers decreased with increasing the [Eu(TFI)3(Phen)]·CHCl3 content. This was mainly due to the following two reasons. (1) The status of the uniform dispersion of [Eu(TFI)3(Phen)]·CHCl3 in the nanofiber matrix. When the complex content was low, most of the [Eu(TFI)3(Phen)]·CHCl3 complex dispersed uniformly in the nanofiber matrix as molecular complexes, which maximized the luminescence of individual [Eu(TFI)3(Phen)]·CHCl3 molecule and considerably enhanced the luminescent efficiency [26]. With the increase of the [Eu(TFI)3(Phen)]·CHCl3 content, some aggregates formed in the fiber matrix. The exciton migration between the [Eu(TFI)3(Phen)]·CHCl3 molecules resulted in the luminescence quenching of the complex, which further led to the decrease of A RAD and Φ values, (Table 1). (2) The interactions between the [Eu(TFI)3(Phen)]·CHCl3 molecules and neighboring chain segments of PVP led to the increased polarization degree of Eu3+ ions, which significantly enhanced the electronic dipole-allowed transitions of Eu3+ ions. Accordingly, the composite nanofibers exhibited higher values of A RAD and Φ as compared with the neat [Eu(TFI)3(Phen)]·CHCl3 [27]. As the [Eu(TFI)3(Phen)]·CHCl3 content increased, the [Eu(TFI)3(Phen)]·CHCl3 aggregates formed in the fiber matrix. The interaction between the particles and neighboring chain segments of PVP became weaker, which led to the decrease of the A RAD and Φ values of Eu3+ ions [28]. The above results demonstrated that the composite nanofibers with a low content of [Eu(TFI)3(Phen)]·CHCl3 uniformly distributed in the PVP matrix significantly outperformed the neat [Eu(TFI)3(Phen)]·CHCl3 in achieving a high-luminescent efficiency, and the composite nanofibers could be an innovative type of nanomaterial for applications in the fields of optical switches and fluorescent probes.
Conclusion
[Eu(TFI)3(Phen)]·CHCl3/PVP composite nanofibers with an average diameter of 100 ± 25 nm have been successfully fabricated by electrospinning. TEM analyses indicated that the [Eu(TFI)3(Phen)]·CHCl3 complex predominantly existed as molecular complexes in the composite nanofibers when the content of [Eu(TFI)3(Phen)]·CHCl3 was lower than 3 wt%. Luminescence studies revealed that such a level of dispersion of [Eu(TFI)3(Phen)]·CHCl3 in the composite nanofibers resulted in the distinguishable enhancement of the 5D0 → 7F2 hypersensitive transition, leading to a considerably higher luminescent efficiency of the Eu3+ ions. Spectroscopic parameters (Ω 2, Ω 4, A RAD, and A NR) analysis suggests that the interactions between the [Eu(TFI)3(Phen)]·CHCl3 molecules and neighboring chain segments of PVP led to the increased polarization degree of Eu3+ ions and significantly enhanced the electronic dipole-allowed transitions of Eu3+ ions, resulting in the enhancement of luminescent efficiency. The thermal stability of the composite fibers is much better than that of the precursor complex [Eu(TFI)3(Phen)]·CHCl3 because of the modification of the polymer matrixes. This study provided a new way in the design and fabrication of innovative composite nanomaterial containing luminescent lanthanide complexes, and developed 1D nanomaterial with potential important applications, particularly in fluorescent probes and optical switches.
References
Xia Y, Yang P, Sun Y, Wu Y, Mayers B, Gates B, Yin Y, Kim F, Yan H (2003) Adv Mater 15(5):353. doi:10.1002/adma.200390087
Greiner A, Wendorff JH (2007) Angew Chem Int Ed 46(30):5670. doi:10.1002/anie.200604646
Thompson CJ, Chase GG, Yarin AL, Reneker DH (2007) Polymer 48(23):6913. doi:10.1016/j.polymer.2007.09.017
Wang Y, Jones H, Evans PV (1998) J Mater Sci 33(21):5205. doi:10.1023/A:1004483904519
Adadurov AF, Zhmurin PN, Lebedev VN, Kovalenko VN (2011) Appl Radiat Isot 69(10):1475. doi:10.1016/j.apradiso.2011.05.022
Zhang X, Wen S, Hu S, Zhang L, Liu L (2010) J Rare Earths 28(3):333. doi:10.1016/S1002-0721(09)60108-3
Verlan VI, Iovu MS, Culeac I, Nistor Y, Turta CI, Zubareva VE (2011) J Non Cryst Solids 357(3):1004. doi:10.1016/j.jnoncrysol.2010.10.022
Zhu H, Zuo D (2009) J Phys Chem C 113(24):10402. doi:10.1021/jp900242j
Zhang H, Song H, Dong B, Han L, Pan G, Bai X, Fan L, Lu S, Zhao H, Wang F (2008) J Phys Chem C 112(25):9155. doi:10.1021/jp7115005
Li J, Li H, Yan P, Chen P, Hou G, Li G (2012) Inorg Chem 51(9):5050. doi:10.1021/ic202473b
Wang S-J, Hu J-B, Wang Y–Y, Luo F (2013) J Mater Sci 48(2):805. doi:10.1007/s10853-012-6799-y
Moudam O, Rowan BC, Alamiry M, Richardson P, Richards BS, Jones AC, Robertson N (2009) Chem Commun (43):6649
Ravi Kumar V, Veeraiah N, Appa Rao B, Bhuddudu S (1998) J Mater Sci 33(10):2659. doi:10.1023/A:1004321706627
Francis B, Ambili Raj DB, Reddy MLP (2010) Dalton Trans 39(34):8084
Li H-F, Yan P-F, Chen P, Wang Y, Xu H, Li G-M (2012) Dalton Trans 41(3):900
Kai J, Parra DF, Brito HF (2008) J Mater Chem 18(38):4549
Gerasimova VI, Antoshkov AA, Zavorotny YS, Rybaltovskii AO, Lemenovskii DA (2013) J Lumin 134:339. doi:10.1016/j.jlumin.2012.08.024
Ma D-Y, Zeng H-P, Li Y-W, Li J (2009) Solid State Sci 11(6):1065. doi:10.1016/j.solidstatesciences.2009.02.009
Chau P, Ryu K, Yo C (1998) J Mater Sci 33(5):1299. doi:10.1023/A:1004350314959
Wada A, Watanabe M, Yamanoi Y, Nishihara H (2008) Chem Commun 0(14):1671
Liu X, Hu Y, Wang B, Su Z (2009) Synth Met 159(15–16):1557. doi:10.1016/j.synthmet.2009.04.013
Rocha J, Carlos LD, Paz FAA, Ananias D (2011) Chem Soc Rev 40(2):926. doi:10.1016/j.jssc.2012.06.004
Zhong HX, Hong JM, Cao XF, Chen XT, Xue ZL (2009) Mater Res Bull 44:623
Xiao S–S, Zheng X-J, Yan S-H, Deng X-B, Jin L-P (2010) CrystEngComm 12(10):3145
Xu C-J, Li B-G, Wan J-T, Bu Z-Y (2011) Spectrochim Acta Part A Mol Biomol Spectrosc 82(1):159. doi:10.1016/j.saa.2011.07.027
Yang C, Xu J, Ma J, Zhu D, Zhang Y, Liang L, Lu M (2012) Polym Chem 3(9):2640
Fang Z-L, Zheng S-R, Fan J, Zhang W-G (2012) Inorg Chem Commun 20:122. doi:10.1016/j.inoche.2012.02.032
Li YK (2011) Spectrochim Acta Part A Mol Biomol Spectrosc 79(2):356. doi:10.1016/j.saa.2011.03.012
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 21272061, 21072049, and 21072050), Heilongjiang Province (No. 2010td03), and Heilongjiang University (2010hdt d-08 and 2010hdtd-11).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2013_7468_MOESM1_ESM.docx
FT-IR, UV–Vis absorption spectra and molecular structure of [Eu(TFI)3(Phen)]·CHCl3, Judd–Ofelt analysis of neat [Eu(TFI)3(Phen)]·CHCl3 complex and [Eu(TFI)3(Phen)]·CHCl3/PVP, fluorescence dynamics and discussion of the surrounding media on the radiative lifetime of Eu3+ ion, and the sensitization luminescence and quenching mechanism of the [Eu(TFI)3(Phen)]·CHCl3/PVP composite fibers are supplied in the Supporting Material . (DOCX 17300 kb)
Rights and permissions
About this article
Cite this article
Tao, Y., Yan, P., Wang, C. et al. Luminescent electrospun composite nanofibers of [Eu(TFI)3(Phen)]·CHCl3/polyvinylpyrrolidone. J Mater Sci 48, 6682–6688 (2013). https://doi.org/10.1007/s10853-013-7468-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7468-5