Abstract
Different concentrations of bis-β-diketonate complex Eu2(BTP)3(Phen)2 (BTP = 1,3-bis(4,4,4-trifluoro-1,3-dioxobutyl)-phenyl and Phen = 1,10-phenanthroline) were doped into the poly(methylmethacrylate) (PMMA), forming a series of red Eu/PMMA luminescent nanofibers, via electrospinning technology. Various characterization techniques were employed to reveal the effect of Eu2(BTP)3(Phen)2 on the morphology, thermal stability, and luminescence of composite nanofibers. FT-IR spectra show the Eu2(BTP)3(Phen)2 complex was successfully doped into PMMA. The luminescent spectra of the composite nanofibers show strong characteristic emission of Eu3+ ions. Simultaneously, in comparison with the precursor complex Eu2(BTP)3(Phen)2, the Eu/PMMA nanofibers has a great improvement in thermal stability. Furthermore, the Judd-Ofelt theory and simulative constructions of the complex are employed to explain the effect of the dispersion of Eu2(BTP)3(Phen)2 and the interactions between the Eu2(BTP)3(Phen)2 complex and neighboring chain segments of PMMA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lanthanide ions have attracted considerable interest because of their technological applications in amplifiers for optical communications, optoelectronic, supramolecular, and luminescent probes for biological system and sensor uses [1]. However, the main disadvantage of lanthanide ions may be their low extinction coefficients owing to the parity-forbidden nature of the inner-shell f→f transition, necessitating the incorporation of chelating ligands that are strongly absorbing in a process appropriately named the antenna effect [2]. Ideal antenna ligands must be able to harvest light efficiently and have a suitable T1 energy for efficient energy transfer to the emissive state of the Ln3+ ions [3]. Many antenna ligands have been developed for lanthanide complexes [4]. Among the widely known ligands, the bis-β-diketonate ligand is one of the important “antennas” which can effectively transfer the intramolecular energy to the central ion [5]. However, another drawback of lanthanide ions may be the efficient nonradiative deactivation of their excited states by high energy oscillators such as O-H bonds from solvent molecules (H2O) [6]. In order to overcome this deficiency and improve the characteristics of light emission, the solvent molecules were replaced by the ancillary nitrogen ligands such as Phen, which has a high efficiency of light absorption [7]. Additionally, the lanthanide complexes also display weak mechanical properties and low thermal stability in their original state, which limited their applications in the fabrication of optical materials [8]. Hence, the complex will be incorporated into a matrix such as polymers to improve thermal stability and mechanical properties [9]. It is widely known that PMMA, one of the most ideal candidate, provides a series of advantages for the development of molecular materials, for instance, thermal and chemical stability, flexibility, versatility and biocompatibility, which can also influence the characteristic luminescence of Eu3+ ions [10, 11].
In addition, one-dimensional (1D) nanostructures have attracted tremendous interest in recent years due to their chemical, optical, and electrical properties [12]. By reducing the number of defects per unit length, a 1D nanostructure can render strong mechanical property that is essential for its nanoscale manipulation and further macro applications [13]. 1D nanostructures can be prepared by many methods such as template-directed methods [14], vapor-phase methods [15], self-assembly [16] and electrospinning [17], etc. Among the large number of fabrication methods demonstrated to generate 1D nanostructures, electrospinning has attracted rapidly increasing attention as a straightforward and simple method for forming inorganic superfine nanofibers from a polymer/inorganic composite precursor [18]. In the present work, the new complex/polymer composite material formed from the bis-β-diketonate Eu3+ complex were electrospun to form 1D nanofibers which will lead to significant flexibility, excellent optical properties, and thermal stability [19].
In this paper, a series of Eu2(BTP)3(Phen)2/PMMA nanofibers were successfully prepared through electrospinning. The effect of Eu2(BTP)3(Phen)2 on the morphology and luminescence of composite nanofibers has been studied. The luminescent properties of the nanofibers were investigated in comparison with that of the precursor complex. The effect of the dispersion of Eu2(BTP)3(Phen)2 and the interactions between the Eu2(BTP)3(Phen)2 molecules and neighboring chain segments of PMMA was also studied by the use of the Judd-Ofelt theory.
Experimental section
Materials and solution properties
Poly (methyl methacrylate) (PMMA M w = 120 000) was obtained from Tianjin Chemical Reagent Factory Damao (China). N,N-dimethylformamide (DMF) purchased from Tianjin Chemical Reagent Factory (China) was used as a solvent to prepare the electrospinning solution. Europium oxide (Eu2O3, 99.99 %) and sodium hydride (60 %, A. R.) were purchased from Helire (Shanghai, China) and Tianjin Chemical Reagent Factory (China), respectively.
EuCl3·6H2O and Eu2(BTP)3(Phen)2 complex were prepared according to procedure previously described [20, 21]. EuCl3·6H2O was synthesized by dissolving lanthanide oxide in a slight excess of hydrochloric acid. The spinning solutions were prepared by first dissolving PMMA in DMF solution at the concentration of 18 wt% at ambient temperature. Subsequently, the Eu2(BTP)3(Phen)2 complexes were added (contents of Eu2(BTP)3(Phen)2 to PMMA equal to 5, 7, 9, 11, 13, and 15 wt%) into the above mixture solution and stirred for 6 h at 50 °C.
Preparation of luminescent nanofibers
The solutions were then placed into 1-mL plastic syringes attached to a stainless-steel needle with inner diameter of 0.37 mm. The electrospinning setup with a DC high-voltage generator was purchased from the BMEI Co., Inc. In the preparation of Eu2(BTP)3(Phen)2/PMMA nanofibers, the voltage applied was 13 kV, and the distance between the collector and the tip of the needle was 20 cm. The nanofibers were collected as randomly overlaid mats on an electrically grounded aluminum foil. After electrospinning, the composite nanofiber mats were dried in a vacuum oven at room temperature for 12 h before characterizations.
Characterization
SEM image of the electrospinning nanofibers was obtained through the scanning electron microscopy (SEM) (Hitachi S-4800) working at 20 kV of acceleration voltage. Prior to SEM examination, the specimens were sputter-coated with gold to avoid charge accumulation. FT-IR spectral data were recorded on a PerkinElmer Spectrum One spectrophotometer in the range of 4000–400 cm−1 using KBr disks. Fluorescence microscope of Leica DM400 M with camera shot Leica DFC 425C was employed to study the luminescence of the composite nanofibers. Thermal analyses were conducted on a PerkinElmer STA 6000 with a heat in grate of 10 °C min−1 in a temperature range from 30 to 800 °C under atmosphere. Excitation and emission spectra were measured with an Edinburgh FLS 920 fluorescence spectrophotometer. Luminescence lifetimes were recorded on a single photon counting spectrometer from Edinburgh Instrument (FLS 920) with microsecond pulse lamp as the excitation source. The data were analyzed by software supplied by Edinburgh Instruments. Both the slit widths for excitation and emission were set at 2.0 nm. The fluorescence dynamics of the samples were measured with an FLS 920 instrument (Edinburgh). During the measurements, an oscillograph was used to record the decay dynamics, and the 335 nm incident light generated from a microsecond flash lamp, which was used as the excitation source.
Results and discussion
Morphology of composite nanofibers and dispersion of Eu2(BTP)3(Phen)2
SEM images of a typical electrospun sheet demonstrated the random distribution of fibers, which reveal that the composite nanofibers consists of Eu2(BTP)3(Phen)2 and PMMA with an average diameter of 565 ± 33 nm have been successfully prepared (Fig. 1a–c). The surface of the composite nanofibers is smooth without identifiable particles, suggesting that the Eu2(BTP)3(Phen)2 might be uniformly dispersed into the nanofibers.
SEM images (a–c) show the representative morphological structures of neat PMMA nanofibers and composite nanofibers with Eu2(BTP)3(Phen)2 contents of 11 wt% (b),13 wt% (c), respectively. Fluorescence microscope images (d–f) show the representative morphological structures of composite nanofibers with Eu2(BTP)3(Phen)2 contents of 11 wt% (d), 13 wt% (e), and 15 wt% (f)
The images of fluorescence microscope (Fig. 1d–f) show the luminescent intensity reached its maximum at 11 wt% and decreased with the content of Eu2(BTP)3(Phen)2 increasing from 11 to 15 wt% with extraordinary red light under ultraviolet irradiation. Additionally, the bright spot in Fig. 1e, f mainly owe to the aggregation of Eu2(BTP)3(Phen)2 formed in the electrospinning solutions, leading to the nanoparticles in the resultant composite nanofibers.
FT-IR measurement analysis
FT-IR spectra of PMMA, Eu/PMMA composite fibers, Eu2(BTP)3(Phen)2 complex, and BTP (Fig. 2) reveal that the peak at 1735 cm−1 is assigned to the C=O stretching band of PMMA, while this band is red shifted to 1732 cm−1 in the Eu/PMMA fibers, which suggests that oxygen atoms of the carbonyl group of PMMA are interacted with Eu3+ ions in the complex Eu2(BTP)3(Phen)2. The absence of the band in the region of 3000–3500 cm−1 implies that H2O molecules have been substituted by Phen in the complex Eu2(BTP)3(Phen)2 and the Eu/PMMA fibers. A weak absorption peak at 1627 cm−1 in the Eu/PMMA fibers which was assigned to the overtone of the C=O stretching mode in BTP shows the complex Eu2(BTP)3(Phen)2 was successfully doped into PMMA.
Thermal properties of the nanofibers
To determine the thermal stability of the composite fiber samples, the thermogravimetric analysis (TGA) experiments were performed; the results are shown in Fig. 3. The thermal decomposition of the Eu2(BTP)3(Phen)2 composite fibers begins at around 346 °C which shows an enhancement of 15 °C for the decomposition temperature (T d) in comparison with the Eu2(BTP)3(Phen)2 complexes. It is also shown that the undoped PMMA polymer fiber decomposes in a one-step event and its degradation starts at 286 °C. Similarly, the PMMA polymer fiber doped with the Eu2(BTP)3(Phen)2 complex also presented a curve of decomposition with one single decomposition event. The weight loss of Eu2(BTP)3(Phen)2/PMMA composite nanofiber occurs over a wide temperature interval (346–505 °C) which exhibits an increase about 60 °C compared with the undoped PMMA polymer fiber. The improved thermal stability for the Eu/PMMA fibers can be attributed to the chemical bonding between the oxygen atoms of the carbonyl groups in PMMA and the Eu3+ ions in Eu2(BTP)3(Phen)2. The result also proves that PMMA as polymer matrix can provide an excellent and stable chemical environment for Eu3+ complexes, because the rigid chain segments of polymer limit the vibration of organic ligands, enhancing relative independence of the doped molecules.
Effects of complex concentration on the luminescence of nanofibers
The luminescent properties of the solid neat complex of Eu2(BTP)3(Phen)2 and the Eu2(BTP)3(Phen)2/PMMA composite nanofibers were recorded at room temperature. The excitation spectra for various samples are shown in Fig. 4. The excitation top point is 365 nm in the neat complex of Eu2(BTP)3(Phen)2 (Fig. 4) which is blue shifted and split into two bands centered at 335 and 346 nm in the Eu2(BTP)3(Phen)2/PMMA nanofibers. This indicated that the site symmetry of the Eu3+ ions in the complex composite nanofibers was lower than that of the Eu3+ ions in the neat complex Eu2(BTP)3(Phen)2 for the influences of the neighboring chain segments of PMMA [22]. Additionally, both 7F0→5D2 and 7F1→5D1 excitations for the composite nanofibers disappeared, suggesting that the f→f inner-shell transitions for the composite nanofibers were quenched via non-radiative energy transfers [23]. However, the luminescent intensity of the Eu2(BTP)3(Phen)2/PMMA nanofibers decreased when the content of Eu2(BTP)3(Phen)2 was over 11 wt%, indicating that the nanoparticles of Eu2(BTP)3(Phen)2 in the PMMA matrices start to aggregate slightly. This was mainly because the Eu2(BTP)3(Phen)2 predominantly existed as molecular clusters and/or nanoparticles when the content of the complex was high enough. During electrospinning, the solutions contain uniformly dispersed Eu2(BTP)3(Phen)2 molecules and the rapid evaporation of the solvent concomitant fast solidification of the filaments (within tens of milliseconds) hindered the aggregation of Eu2(BTP)3(Phen)2 [24].
In the emission spectra (Fig. 5), the peak intensities at 580, 590, 612, 652, and 701 nm were assigned to the J = 0, 1, 2, 3, and 4 transitions, respectively. Additionally, the 5D0→7F2 hypersensitive transition at 612 nm originated from the Eu3+ ions was the most intense emission, suggesting there was a highly polarized chemical environment around the Eu3+ ions [25]. The intensity ratios (I02/I01) between the electric dipole transition (5D0→7F2) and the magnetic dipole transition (5D0→7F1), a measure of the asymmetry of the local environment of Eu3+ ions [26], are shown in Table 1. It is worth mentioning that the ratio of the neat Eu2(BTP)3(Phen)2 is almost only half of that of the composite nanofibers. This suggests that the symmetry of the coordination sphere for the Eu3+ ions was more disordered, and the degree of polarization was higher in the composite fibers as compared to the pure Eu2(BTP)3(Phen)2, which further led to higher probability for the electronic dipole-allowed transitions [27].
Figure 6 shows the integrated emission intensity of the electric dipole transition (5D0→7F2) as a function of the Eu2(BTP)3(Phen)2 content in the composite nanofibers. The emission intensity enhanced with increasing content of the Eu2(BTP)3(Phen)2 and reached its maximum value at 11 wt%. The further intensity decreased with the increasing content of the complex because of typical emission concentration quenching which was due to the deactivation of the 5D0 and 5D1 states through the electrostatic multipolar interactions and the exciton migration through the Foürster dipole-dipole mechanism. The mechanism has been illustrated in the Supporting Material. The electrospun composite nanofibers, compared with the composite polymers prepared by copolymerization, achieved higher contents without inducing the concentration quenching owing to the desired dispersion of Eu2(BTP)3(Phen)2 in the matrix of the composite nanofibers might be even more preferred than the distribution of Eu2(BTP)3(Phen)2 units along macromolecular chains [28]. On one hand, when the content of Eu2(BTP)3(Phen)2 was low in the composite nanofibers, most of Eu2(BTP)3(Phen)2 dispersed as molecular states and/or nanoparticles and the exciton migration through the diffusion-induced collision among Eu3+ ions was negligible. On the other hand, when the content of Eu2(BTP)3(Phen)2 was high, some aggregates with sizes of tens of nanometers formed, which led to high Eu3+ ion concentrations locally and were responsible for the emission concentration quenching [29].
Judd-Ofelt analysis and luminescent quantum efficiency
The Judd-Ofelt theory is a widely accepted tool for exploring f→f inner shell electronic transitions in Ln3+ complexes [30]. The Judd-Ofelt parameters Ωλ (λ = 2, 4, and 6) are interaction parameters of ligand fields, in which Ω2 is sensitive to the chemical microenvironment around the Eu3+ ions [31]. The values of Ω2 and Ω4 can be estimated from the oscillator strengths of the 5D0→7F2 and 5D0→7F4 transitions in the emission spectrum (Fig. 5), using the magnetic dipole transition of 5D0→7F1 as the reference [32] (Fig. 7). Table 1 presents the Judd-Ofelt intensity parameters (Ω2, Ω4), radiative transition rate (ARAD), non-radiative transition rate (ANR), fluorescence lifetime (τobs), and luminescence quantum efficiency (Ф) for the 5D0 state of the Eu3+ ions in the neat complex Eu2(BTP)3(Phen)2 and the composite nanofibers. The high-resolution emission spectrum of the composite nanofiber with Eu2(BTP)3(Phen)2 contents of 11 wt% (Fig. 8) is given to determine the integrated area of 5D0→7FJ (J = 1, 2, 3, and 4) transition. The large values of Ω2 might be interpreted as a consequence of the hypersensitive behavior of the 5D0→7F2 transition [33] which suggests that the chemical environment of the Eu3+ ions is highly polarizable. The Ω4 parameter values, less sensitive to the coordination environment than Ω2, reflect a rigid chemical environment surrounding the Eu3+ ions [34]. The specific calculations and detailed principles were provided in the Supporting Material. The higher Ω2 values of the composite nanofibers than that of the neat Eu2(BTP)3(Phen)2 suggested an enhancement of the 5D0→7F2 hypersensitive transition [35]. This was also attributed to the change of the chemical environment surrounding Eu3+ ions, which was induced by the intermolecular interactions between neighboring chain segments of PMMA and Eu2(BTP)3(Phen)2 [36]. The higher values of Ω4 for the composite nanofibers as compared with that of the neat Eu2(BTP)3(Phen)2 indicated a perturbation on the coordination effect of the bis-bidentate BTP by the steric factors from the surrounding PMMA.
Furthermore, with increasing content of the Eu2(BTP)3(Phen)2, the values of Ω2 and Ω4 decreased, suggesting that the effect of neighboring PMMA chain segments on the ligand fields of Eu3+ were gradually weakened. Such a phenomenon was attributed to the uniform dispersion of Eu2(BTP)3(Phen)2 in the nanofiber matrix. When the content of Eu2(BTP)3(Phen)2 was lower than 13 wt%, most of the Eu2(BTP)3(Phen)2 existed as molecular complex and the chemical environment around the Eu3+ ions was significantly affected by the surrounding PMMA, whereas such an influence reduced gradually with the increase of the complex content resulting in the formation of aggregates [37].
Simulative microchemical environment of Eu3+ ions in fiber
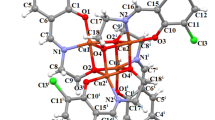
Two bis-β-diketone ligands, 1,3-bis(3-phenyl-3-oxopropanoyl)benzene (BPB) [38] and BTP, which bear two conjugated diketonate binding sites linked by a 1,3-phenylene spacer (Fig. 9a), were published. The molecular structures of the bis-β-diketone Eu3+ complexes represent with molecular models as neutral triple-stranded dinuclear lanthanide helices. The construction of the Eu2(BTP)3(Phen)2 complex model was based on the Eu2(BTP)3bpy2 complex crystal structure [21], and no solvent molecules were considered. In the structure of the model 1 (Fig. 9b), each central Eu3+ ion is coordinated with six oxygen atoms from three BTP ligands and two nitrogen atoms from Phen. Because of different molecular structure of the Eu2(BTP)3(Phen)2 complex in PMMA, the quantum efficiency of Eu/PMMA is lower than that of the pure complex (Table 1). The diverse steric configurations of the hybrid complex (Fig. 9c) are because the steric hindrance caused by the tremendous polymeric structure in such a short unit of the carbon chains, which could increase the level of non-radiative transition rate and restrict the efficiency of the intramolecular transfer mechanism. Specifically, in Fig. 9c, two groups of ester carbonyls were attracted by two Eu3+ ions and insert the space of Eu2(BTP)3(Phen)2. One of them (O3 and O4) has less influence on the structure of Eu2(BTP)3(Phen)2 than another (O1 and O2), because there is no methyl or other group of PMMA in the space of the complex. On the other hand, not only two ester carbonyls, but also two methyls (C1 and C2) are located on the same monomer, respectively. In such a microscopic structure, the distance of CH should be considered. Both C1 and C2 can influence the steric configuration of the complex by steric hinder.
Conclusion
In summary, we had successfully fabricated Eu2(BTP)3(Phen)2/PMMA nanofibers with great thermal stability by electrospinning. The microstructure of the fibers, obtained by SEM, showed the preparation of continuous fibers with a homogeneous morphology and an average diameter of 565 ± 33 nm. Fluorescence spectra and Judd-Ofelt parameters indicated that the Eu2(BTP)3(Phen)2/PMMA nanofibers had a maximum luminescence intensity at the content of 11 wt%. Meanwhile, spectroscopic parameters (Ω2, Ω4, ARAD, and ANR) analysis suggested that the increase of polarization degree and the enhancement of the electronic dipole-allowed transitions of Eu3+ ions were caused by the interactions between the Eu2(BTP)3(Phen)2 molecules and neighboring chain segments of PMMA. In addition, the thermal stability of the composite nanofibers was much better than that of the pure complex Eu2(BTP)3(Phen)2 because of the addition of the polymer matrixes. This study provide a new and useful way in the fabrication of innovative composite nanomaterial containing luminescent bis-β-diketone Eu3+ complexes, and the development of 1D nanomaterials could find important applications, especially in optical communications and luminescent probes.
References
Bünzli J-CG (2010) Lanthanide luminescence for biomedical analyses and imaging. Chem Rev 110:2729–2755
Woodward AW, Frazer A, Morales AR, Yu J, Moore AF, Campiglia AD, Jucov EV, Timofeeva T, Belfield KD (2014) Two-photon sensitized visible and near-IR luminescence of lanthanide complexes using a fluorene-based donor-π-acceptor diketonate. Dalton Trans 43:16626
Park H-J, Ko S-B, Wyman IW, Wang S (2014) Selective sensitization of Eu(III) and Tb(III) emission with triarylboron-functionalized dipicolinic acids. Inorg Chem 53:9751
D’Aléo A, Pointillart F, Ouahab L, Andraud C, Maury O (2012) Charge transfer excited states sensitization of lanthanide emitting from the visible to the near-infra-red. Coord Chem Rev 256:1604
Kai J, Felinto MC, Nunes LA, Malta OL, Brito HF (2011) Intermolecular energy transfer and photostability of luminescence-tuneable multicolour PMMA films doped with lanthanide-β-diketonate complexes. J Mater Chem 21:3796–3802
Li D, Tian X, Hu G, Zhang Q, Wang P, Sun P, Tian Y (2011) Synthesis, crystal structures, photophysical properties, and bioimaging of living cells of Bis-β-diketonate phenothiazine ligands and its cyclic dinuclear complexes. Inorg Chem 50:7997
Freund C, Porzio W, Giovanella U, Vignali F, Pasini M, Destri S, Mech A, Di Pietro S, Di Bari L, Mineo P (2011) Thiophene based europium β-diketonate complexes: effect of the ligand structure on the emission quantum yield. Inorg Chem 50:5417
Feng J, Zhang H (2013) Hybrid materials based on lanthanide organic complexes: a review. ChemSoc Rev 42:387
Hudson ZM, Sun C, Helander MG, Chang Y-L, Lu Z-H, Wang S (2012) Highly efficient blue phosphorescence from triarylboron-functionalized platinum(II) complexes of N-heterocyclic carbenes. J Am Chem Soc 134:13930
Chen X-Y, Yang X, Holliday BJ (2008) Photoluminescent europium-containing inner sphere conducting metallopolymer. J Am Chem Soc 130:1546
Peng H, Stich MIJ, Yu J, L-n S, Fischer LH, Wolfbeis OS (2010) Luminescent europium(III) nanoparticles for sensing and imaging of temperature in the physiological range. Adv Mater 22:716
Leong WL, Vittal JJ (2010) One-dimensional coordination polymers: complexity and diversity in structures, properties, and applications. Chem Rev 111:688
Xie JL, Guo CX, Li CM (2014) Construction of one-dimensional nanostructures on graphene for efficient energy conversion and storage. Energy Environ Sci 7:2559
Martin CR (1994) Nanomaterials: a membrane-based synthetic approach. Science 266:1961
Law M, Goldberger J, Yang P (2004) Semiconductor nanowires and nanotubes. Annu Rev Mater Res 34:83
Hartgerink JD, Beniash E, Stupp SI (2001) Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294:1684
Park J, Karim M, Kim I, Cheong I, Kim J, Bae D, Cho J, Yeum J (2010) Electrospinning fabrication and characterization of poly(vinyl alcohol)/montmorillonite/silver hybrid nanofibers for antibacterial applications. Colloid Polym Sci 288:115
Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X (2014) Electrospinning of polymeric nanofibers for drug delivery applications. J Contr Release Off J Contr Release Soc 185:12
Kuriki K, Koike Y, Okamoto Y (2002) Plastic optical fiber lasers and amplifiers containing lanthanide complexes. Chem Rev 102:2347
Bassett AP, Magennis SW, Glover PB, Lewis DJ, Spencer N, Parsons S, Williams RM, De Cola L, Pikramenou Z (2004) Highly luminescent, triple- and quadruple-stranded, dinuclear Eu, Nd, and Sm(III) lanthanide complexes based on Bis-diketonate ligands. J Am Chem Soc 126:9413
Shi J, Hou Y, Chu W, Shi X, Gu H, Wang B, Sun Z (2013) Crystal structure and highly luminescent properties studies of bis-β-diketonate lanthanide complexes. Inorg Chem 52:5013
Moudam O, Rowan BC, Alamiry M, Richardson P, Richards BS, Jones AC, Robertson N (2009) Europium complexes with high total photoluminescence quantum yields in solution and in PMMA. Chemical Communications 6649
Ravi Kumar V, Veeraiah N, Appa Rao B, Bhuddudu S (1998) Optical absorption and photoluminescence properties of Eu3+-doped ZnF2–PbO–TeO2 glasses. J Mater Sci 33:2659
Wang S-J, Hu J-B, Wang Y-Y, Luo F (2013) Coating graphene oxide sheets with luminescent rare-earth complexes. J Mater Sci 48:805
Francis B, Ambili Raj DB, Reddy MLP (2010) Highly efficient luminescent hybrid materials covalently linking with europium(iii) complexes via a novel fluorinated β-diketonate ligand: synthesis, characterization and photophysical properties. Dalton Trans 39:8084
Qi S, Huang Y, Li Y, Cai P, Kim SI, Seo HJ (2014) Probe spectrum measurements of Eu3+ ions as a relevant tool for monitoring in vitro hydroxyapatite formation in a new borate biomaterial. J Mater Chem B 2:6387
Kai J, Parra DF, Brito HF (2008) Polymer matrix sensitizing effect on photoluminescence properties of Eu3+-β-diketonate complex doped into poly-β-hydroxybutyrate (PHB) in film form. J Mater Chem 18:4549
Lima AC, Sher P, Mano JF (2012) Production methodologies of polymeric and hydrogel particles for drug delivery applications. Expert Opin Drug Deliv 9:231
Smirnov VA, Sukhadolski GA, Philippova OE, Khokhlov AR (1999) Use of luminescence of europium ions for the study of the interaction of polyelectrolyte hydrogels with multivalent cations. J Phys Chem B 103:7621
Chau PTM, Ryu KH, Yo CH (1998) Influence of the technological conditions on the luminescence of Eu3+ ions in Sr2SnO4. J Mater Sci 33:1299
Wada A, Watanabe M, Yamanoi Y, Nishihara H (2008) Modification of the luminescence spectra of chloro (tetrapyridylcyclotetramine) europium complexes by fine tuning of the Eu-Cl distance with outer-sphere counterions in the solid state, in a polymer matrix and in solution. Chemical Communications 1671
Carlos LD, Messaddeq Y, Brito HF, Sá Ferreira RA, de Zea BV, Ribeiro SJL (2000) Full-color phosphors from europium(III)-based organosilicates. Adv Mater 12:594
Görller-Walrand C, Fluyt L, Ceulemans A, Carnall WT (1991) Magnetic dipole transitions as standards for Judd-Ofelt parametrization in lanthanide spectra. J Chem Phys 95:3099
Divya V, Freire RO, Reddy MLP (2011) Tuning of the excitation wavelength from UV to visible region in Eu3+-β-diketonate complexes: comparison of theoretical and experimental photophysical properties. Dalton Trans 40:3257
Liu X, Hu Y, Wang B, Su Z (2009) Synthesis and fluorescent properties of europium–polymer complexes containing 1,10-phenanthroline. Synth Met 159:1557
Zhang X, Wen S, Hu S, Chen Q, Fong H, Zhang L, Liu L (2010) Luminescence properties of Eu(III) complex/polyvinylpyrrolidone electrospun composite nanofibers. J Phys Chem C 114:3898
Tian Y, Chen B, Li X, Zhang J, Tian B, Sun J, Cheng L, Zhong H, Zhong H, Hua R (2012) Solvothermal synthesis and tunable luminescence of Tb3+, Eu3+ codoped YF3 nano- and micro-crystals with uniform morphologies. J Solid State Chem 196:187
Bassett AP, Magennis SW, Glover PB, Lewis DJ, Spencer N, Parsons S, Williams RM, De Cola L, Pikramenou Z (2004) Highly luminescent, triple-and quadruple-stranded, dinuclear Eu, Nd, and Sm (III) lanthanide complexes based on bis-diketonate ligands. J Am Chem Soc 126:9413
Acknowledgments
We are grateful for the financial support by the National Natural Science Foundation of China (No. 51303045) and the Education Department of Heilongjiang Province of China (No. 12521413).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 63 kb)
Rights and permissions
About this article
Cite this article
Gu, H., Hou, Y., Xu, F. et al. Electrospinning preparation, thermal, and luminescence properties of Eu2(BTP)3(Phen)2 complex doped in PMMA. Colloid Polym Sci 293, 2201–2208 (2015). https://doi.org/10.1007/s00396-015-3614-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3614-8