Abstract
Glycation refers to the addition of a sugar moiety to a protein molecule and occurs during the Maillard reaction. Maillard reaction is initiated by the condensation of amino groups of proteins, peptides, and amino acids with carbonyl groups of reducing sugars. After subsequent elimination, degradation, and oxidation reactions in the later stages, so-called advanced glycation end products (AGEs) are formed. AGEs have been linked to many chronic and degenerative disorders and aging. The results of several animal and human studies confirm that dietary AGE levels have effects on AGE accumulation in body and further complications in degenerative diseases. In this chapter, following brief information about protein glycation, the consequences of glycation, and the contribution of dietary AGEs will be discussed. Formation routes of main glycation products in foods will be explained and their analysis methods will be summarized. Major mitigation strategies developed so far will be evaluated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
6.1 Introduction
Until after the 1940s, when there were some reports about the nutritional loss in milk powder due to the reaction between lactose and milk proteins, the consequences of Maillard reaction were not recognized [1]. Soon after it was understood that this reaction not only takes place during heating of foods but also in vivo, Maillard reaction has gained much more attention. With the identification of a nonenzymatic glycosylated variant of hemoglobin (HbA1c) in the blood of diabetic patients [2], “glycation” term was introduced to the literature. In the 1980s, Monnier and Cerami [3] supposed that the Maillard reaction of proteins could have a causative role in the aging of extracellular matrix proteins and related pathologies, and since then the interest in the field of the Maillard reaction in vivo has increased exponentially.
The chemistry behind the Maillard reaction/glycation is very complicated. Even in simple reaction systems, for example, in glucose and glycine solutions, many tens of reaction products are formed. Therefore, even in such simple systems, the Maillard reaction mechanisms have not been fully elucidated, and all the reaction products have still not been identified.
Maillard reaction/glycation affects many food quality parameters such as color, sensorial properties, textural properties, and protein functionality. However, the so-called advanced glycation end products (AGEs), which are formed at the later stages of the Maillard reaction during food processing, might have some undesired properties. The results of the animal and human studies confirm that dietary AGE levels have direct and indirect effects on AGE accumulation in the body and further complications in degenerative diseases. Therefore, inhibition of glycation reactions during food processing is an important issue since this may help to reduce the dietary intake of AGEs.
In this chapter, following brief information about protein glycation, the consequences of glycation and the contribution of dietary AGEs will be discussed. The formation routes of the main glycation products in foods will be explained, and their analysis methods will be summarized. The major mitigation strategies developed so far will be evaluated.
6.1.1 Protein Glycation
Glycation refers to the addition of a sugar moiety to a protein molecule and occurs during the Maillard reaction. In the Maillard reaction, the amine moiety from free amino acids, peptides, or proteins reacts with the carbonyl group of a reducing sugar, oxidized lipids, vitamin C, or quinones. Glycation takes place in three stages as commonly accepted; “early”, “intermediate”, and “advanced” stages. However, it should be noted that the reactions occur simultaneously depending on conditions [1].
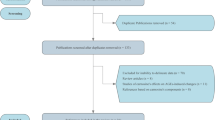
Glycation is initiated with the nucleophilic addition of amino groups of an amino acid-free or within a protein molecule to the carbonyl group of a reducing sugar such as glucose, fructose, lactose, or maltose. The covalent attachment results in the formation of a reversible and unstable Schiff base (Fig. 6.1). After the condensation reaction, the so-called Schiff base undergoes an arrangement to form an Amadori product (or Heyns product, if the reducing sugar is a ketose), which is the first stable product of the reaction. N-ε-fructosyllysine, N-ε-maltulosyllysine, or N-ε-lactulosyllysine are the major Amadori compounds generated in the early stage of protein glycation during food processing. In the intermediate stage of glycation, by the degradation of Amadori compounds via enolization and elimination reactions, reactive carbonyl species, known as dicarbonyl compounds or oxoaldehydes, are formed. Dicarbonyl compounds might also be formed by caramelization solely during food processing, and this might occur to a larger extent compared to their formation via the degradation of Amadori compounds. The formation of dicarbonyl compounds is discussed in Chap. 2. Some early glycation compounds and dicarbonyl compounds are shown in Fig. 6.2. The dicarbonyl compounds are very reactive, and hence they react immediately with the side chains of peptides and proteins to form advanced glycation end products (AGEs) in the advanced stage. The ε-amino group of lysine, guanidino group of arginine, sulfhydryl group of cysteine residues, and the N-terminal amino group of any amino acids are susceptible for the derivatization by 1,2-dicarbonyl compounds. When oxidation takes place with glycation, the products formed are also called glyco-oxidation products. The great variety of the carbonyl species produced through sugar autoxidation and lipid peroxidation results in a great variety of AGEs in food systems. So far, several glycation products such as N-ε-fructoselysine (FL), pyrraline, pentosidine, N-ε-carboxymethyllysine (CML), N-ε-carboxyethyllysine (CEL), S-carboxymethylcysteine, glyoxal lysine dimer (GOLD), methylglyoxal lysine dimer (MOLD), and 3-deoxyglucosone lysine dimer (DOLD) have been identified in processed foods [4,5,6,7]. The formation and occurrence of these compounds will be discussed in Sect. 6.2.
6.1.2 Factors Affecting Glycation
The extent of glycation and the formation of glycation products in food systems depend on several factors such as temperature, reaction time, reaction environment (water content, water activity), reactant species, pH of the reaction medium, the presence of oxygen, and protein conformation.
The extent of glycation is determined by the severity of the heat treatment, either by the increase in temperature or heating time. Glycation is accelerated by the increase in processing temperature. Mild heat treatment results mostly in the formation of Amadori products. However, when the processing temperature or time extends, subsequent degradation of Amadori compounds leads to the formation of dicarbonyl compounds and AGEs. The diversity of the amino acids within a protein molecule involved in the reaction increases by the increase in heating temperature.
Glycation proceeds at a higher rate in dry heating conditions than in aqueous conditions due to the dilution effect of the reactants in the aqueous environment. Since the condensation reaction between the carbonyl and amine group generates water [8], Amadori rearrangement product formation is restricted in the presence of water. The water in the reaction medium also affects the site-specificity of the reaction. In several studies, it was revealed that the lactosylation site of β-lactoglobulin differs when protein was heated in solution or in the dry state. 47Lys [9,10,11] and 100Lys [9, 12, 13] were found to be preferentially lactosylated during heating in solution, whereas 47Lys and 91Lys were lactosylated during the heating of β-lactoglobulin in the dry state [14, 15].
Due to the fact that water activity (aw) affects the molecular mobility of the reactants, protein conformation, surface area, dynamics, and accessibility of amino groups besides the dissolved oxygen concentration and pH of the medium, site-specific glycation is affected by aw [16]. In addition to these, the solvent and its contact with the protein matrix influence the electrostatic and biophysical properties of the protein [16]. Generally, browning is considered to occur at its maximum in aw values between 0.5 and 0.8 [17], and researchers showed increased glycation of proteins at intermediate aw values [18, 19]. The reaction rate is decreased at low values of aw due to the diffusional limitations of the reactants, and at high values of aw, the decrease is attributed to the dilution effect and inhibition by water. Relative humidity (water activity) affects the formation of dicarbonyl compounds, and the proportions of dicarbonyls formed differ for the samples heated at low, intermediate, and high relative humidities. For instance, it was shown that under high relative humidity values in a model system containing sodium caseinate and lactose, 3-deoxypentosulose and galactosyl 2-pentosulose were produced, whereas galactosyl hexosulose and 1,4-dideoxyhexosulose were produced in higher amounts under low relative humidities [20].
In terms of reactant species, the type of carbonyl source is very important. The reactivity of carbonyl compounds generally increases in the following order [21]:
-
Ketoses < aldoses
-
Polysaccharides < disaccharides < hexoses < pentoses < tetroses < trioses.
-
Oxoacids < saccharides < ketones < aldehydes < α-dicarbonyl compounds.
The carbonyl source has effects on the extent of glycation and site-specificity of the reaction. Aldose sugars are more reactive toward the lysine residues of proteins than ketose sugars, and these sugars prefer different glycation sites. Glycation of β-lactoglobulin was three to four times more efficient with glucose than that of with fructose [22]. 13Lys, 16Lys, 93/94Lys, 98Lys, 108Lys, 114Lys, and 122Lys residues of α-lactalbumin were glycated by allose and glucose, while 13Lys, 98/108Lys, and 114Lys were glycated with fructose and psicose [23]. Monosaccharides are generally more reactive than disaccharides; β-lactoglobulin was found to attach more galactose (up to 22 adducts) than lactose (up to 14 adducts) in a study where it was confirmed by LC/MS that the products were mainly the early glycation products [24]. Heating of β-casein with either glucose or glyoxal at 95 °C for 1 h in solution resulted in modification at 107Lys and 176Lys [25]. The Amadori product was formed preferentially on 176Lys rather than 107Lys, while the proportion of N-ε-carboxymethyllysine (CML) on both lysine residues was similar. 202Arg was found to be the main modification site of β-casein with glyoxal [25].
Proteins react with carbonyl compounds primarily through the ε-amino group of lysine residues and, to a smaller extent, through the α-amino groups of N-terminal amino acids and other amino acid functional groups, such as the thiol group of cysteine and guanidine group of arginine. The availability of glycation sites within a protein molecule greatly influences the extent of reaction. Given the fact that the accessibility of glycation sites within a protein molecule depends on its conformation, any environmental factor affecting a protein’s conformation has an indirect effect on the glycation behavior. pH- or temperature-induced changes (including denaturation, aggregation, or hydrolysis) would have an effect on protein conformation and thus on the glycoforms produced. The composition of the reaction medium (the presence of lipids, minerals, other proteins, and reducing agents) and the molecular weight of the carbonyl attached to the protein influence the conformation of the protein [16]. Glycation of ovalbumin was favored when the tertiary structure was disrupted; after reducing the disulfide bonds, the number of glycated sites was increased from 7 to 12 in a dry state and from 1 to 2 in aqueous conditions [26].
Researchers suggested that the structural accessibility of lysine residues is the most important factor affecting the preferential glycation sites [27, 28]. Hydrogen bonding between the N-H of lysine residues with water and with the C=O with other amino acids in the polypeptide chain may protect lysine residues against glycation [29]. pKa values, phosphate and bicarbonate ions, and proximate amino acids have effects on the reactivity of lysine residues and play a role especially in the early stages of glycation [45,46,47,48]. The reactivity of a lysine residue within a protein sequence may be explained by their position adjacent to the neighboring basic amino acids in the primary or tertiary protein structure. The Maillard reaction is accelerated when an acidic amino acid is present near the lysine residue in the primary structure or in the 3D conformation. Also, amino acid residues of Ile, Leu, Phe, and Arg increase the lysine reactivity in lysine-containing dipeptides [30]. The presence of histidine or lysine residue near to lysine was shown to promote the glycation tendency of lysine [31,32,33].
The pH of the reaction medium is another factor affecting protein glycation. The rate of carbonyl-amine addition is related to the pKa value of the amino compound, which determines the concentration of reactive species at a certain pH. Lysine is the most reactive amino acid in a wide pH range, whereas aliphatic aromatic amino acids valine, leucine, and isoleucine are the least reactive ones. pH is a determining factor whether decomposition takes place by 1,2- or 2,3-enolization. 1,2-Enolization is favored that allows protonization of the Amadori product in acidic media, whereas 2,3-enolization dominates in alkaline solutions and in nonaqueous conditions [21]. Increase in pH led to increased glycation in several studies conducted with milk proteins. The molecular weight of fructosylated and glucosylated β-lactoglobulin was increased with the increase of pH from 5.0 to 8.0 [22]. Thirteen glucose molecules on average were attached to β-lactoglobulin at pH 5.0, while 14 glucose moieties were attached at pH 8.0 [22]. Isoelectric point of a protein is important in terms of glycation rate. Thomsen et al. [18] reported that increasing pH during the preparations of dry reaction media caused an increase in both the rate and the degree of lactosylation. The reaction solution prepared at pH 5.0 was less lactosylated than those prepared at pH 6.0 and 7.0. Since pH 5.0 is close to the pI of β-lactoglobulin, protein-protein interactions might have evolved, thus making it more difficult for free amino groups to react with lactose. The reactivity of amino groups was limited due to the low amount of reactive unprotonated amine groups at low pH. However, when the pH is increased, negative charges on the protein molecule increase, causing repulsion and a decrease in protein-protein interactions. Therefore, the amount of free reactive amino groups increases yielding an increased lactosylation [18].
The presence of oxygen in the medium also affects the glycation of proteins. More glucose attachment is favored in the presence of oxygen. Oxygen level has an important effect in the later stages of glycation; dicarbonyl compounds generated through glycoxidation also participate in the reaction, therefore increasing the glycation rate. It was reported in a study where lysozyme was heated at 50 °C for 14 days that due to the higher reactivity of dicarbonyl compounds (generated in the presence of oxygen) for the guanidine group of arginine residues, the involvement of arginine in glycation favored the glycation rate [34]. The reaction rate for the systems having the same conditions but containing fructose was lower than that of glucose, and this was explained by the fact that glucose was more susceptible to glycoxidation under dry heating conditions. It was stated that fructose mainly reacted with the ε-amino group of lysine residues, whereas glucose reacted with all primary amino groups and guanidine groups of arginine, as well. Due to the lower reactivity of fructose, a narrow distribution of glycoforms was obtained; however, for glucose, a higher glycation rate and a wider range of glycoforms were observed [34].
6.1.3 Consequences of Glycation and Contributions of Dietary AGEs
The glycation of protein is of particular importance for food chemistry since color development (such as the color of bread crust, roasted coffee, French fries, and fried onions) and aroma formation (roasted coffee and bakery products) are typical results of this reaction. On the contrary, undesired color formation as a quality defect (in the production of dried foods, milk powders, as well as fruits and vegetables), formation of off-flavors (such as cooked flavor in UHT milk), reduction of the nutritional value of foods (due to modification in amino acids), and formation of toxic compounds (such as HMF, acrylamide, and furan) are the drawbacks of glycation in food systems.
After the identification of the nonenzymatic glycosylated variant of hemoglobin, HbA1c, in the blood of diabetic patients [2], it was understood that glycation also takes place endogenously; since then, Maillard reaction has attracted attention in the field of biochemistry and medicine. In the human body, AGEs arise not only from glucose but also from the reactive products of glucose metabolism (such as glucose-6 phosphate, triose phosphates, and fructose-3-phosphate) and nonenzymatic degradation. Methylglyoxal, glyoxal, 3-deoxyhexuloses, transformation products of ascorbic acid, or some secondary decomposition products of lipid hydroperoxides react with proteins [21, 35]. AGEs have some undesirable consequences in terms of chronic and especially age-related disorders. They may take part in chronic and degenerative diseases, such as diabetes, renal failure [36], atherosclerosis [37, 38], and Alzheimer’s and Parkinson’s diseases [39, 40]. Glycation is increased in diabetes mellitus, where glyoxal, methylglyoxal, and 3-deoxyglucose, besides plasma glucose concentration, are increased and in uremia, where many α-oxoaldehydes are increased [41]. The body proteins of diabetic patients were found to be two to three times more glycated than those of healthy humans, due to the increased level of blood sugar [42]. Amadori products are the predominant form of circulating glycated protein in patients with diabetes [43, 44]. Uremic patients accumulate pentosidine or CML in the plasma and tissues [45]. The serum level of pentosidine was found to be 2.5 times greater in patients with diabetes and 23 times greater in patients with diabetes with end-stage renal disease [46]. It was stated that patients with advancing age, diabetes, and end-stage renal disease have a very high incidence of atherosclerotic vascular disease [38, 46]. An excess of blood or tissue AGEs is also associated with rheumatoid arthritis, amyloidosis, and Alzheimer’s and other neurodegenerative diseases [47].
AGEs are generally accumulated in long-lived proteins such as collagen and eye lens due to the low turnover of these proteins. Cataract is one of the most common consequences of diabetes. A high correlation was obtained between pentosidine cross-links and the degree of pigmentation in cataractous lenses, indicating that pentosidine formation in human lens leads to brunescent cataracts [48].
There are two major sources contributing to the total pool of AGEs in the body: AGEs that are consumed with foods and endogenous AGEs that are generated by the nonenzymatic glycation of proteins, lipids, and nucleic acids, especially under hyperglycemic conditions in diabetes [37, 49]. The interrelationship between dietary AGEs and AGEs in the body has been established with several animal and human studies. In a study [50] where laboratory rats were fed with glucose-lysine model food (containing AGEs) for 3 months, dietary dicarbonyl compounds from the diet or dietary CML itself were found to be responsible for CML accumulation in hearts and tendons. Moreover, regular consumption of dietary AGEs in healthy individuals promoted CML accumulation in some organs, such as cardiac tissue and tail tendon [50]. Feeding laboratory mice with high-AGE diet resulted in twofold higher plasma AGE levels than the levels of mice fed with low-AGE diet [51]. Proteinuria increased during feeding with high-AGE diets in remnant kidney models in rats [52, 53]. High-AGE diets were also shown to accelerate the progression of renal fibrosis [52]. In another study [54] where casein-linked lysinoalanine (LAL), N-ε-fructoselysine (FL), and N-ε-carboxymethyllysine (CML) were administered to rats at different doses for 10 days, it was concluded that kidneys were the predominant sites for accumulation and excretion of LAL, FL, and CML. It was also observed that the endogenous load of compound in either plasma or tissue was increased by its dietary intake [54]. In a mouse model of obesity, targeted reduction of the advanced glycation improved renal function and glycemic control in obesity [55].
Ten percent of consumed dietary AGEs were reported to be absorbed by humans, and this was correlated with the circulating and tissue levels of AGEs [56]. Dietary AGE level of healthy people showed correlation with the circulating AGE levels, such as CML and methylglyoxal, as well as with oxidative stress markers [57]. Furthermore, reduction of AGEs in the diet in diabetes patients [58] and kidney disease patients [59, 60] or healthy individuals [61] also reduced the markers of oxidative stress and inflammation. In a human study where people consumed a standard diet (high amounts of AGE-containing diet) or steamed diet (low in AGEs) for a month, the urinary CML excretion was found to be 40% higher and fasting plasma CML was 7% higher in the standard diet group. This suggested that dietary CML was absorbed in the intestines and rapidly excreted, confirming the results obtained in animals [62].
The results of the animal and human studies confirm that dietary AGE levels have direct and indirect effects on AGE accumulation in the body and further complications in degenerative diseases. Therefore, inhibition of glycation reactions during food processing is an important issue since this may help to reduce the dietary intake of AGEs. The methods useful for the mitigation of glycation will be discussed in Sect. 6.4.
6.2 Occurrence of AGEs in Foods
As stated above, Amadori products are the early products of protein glycation. The condensation reaction between a carbonyl moiety and an amine residue of a protein results in the formation of an unstable Schiff base. After the condensation reaction, the so-called Schiff base undergoes an arrangement to form an Amadori product (N-substituted 1-amino-1-deoxy-2-ketoses), which is the first stable product of the reaction (Figs. 6.3 and 6.4). In the case of food proteins, the ε-amino group of lysine is the most susceptible target for the attack of carbonyls; so the product formed is mostly lysine derivatives such as N-ε-fructosyllysine, N-ε-lactulosyllysine, and N-ε-maltulosyllysine; however, the N-terminal α-amino acids also react to the Amadori compounds. Free amino acids are also significantly modified [63], but it will not be discussed in this chapter.
Amadori products are quantitatively the most prevalent glycation products in many food systems. Depending upon the temperature and time of processing or storage of a food product, up to 70% of lysine might react to the Amadori product [4]. The formation of N-ε-fructosyllysine causes the loss of nutritional quality of proteins, since lysine bioavailability is decreased due to lysine modification. Therefore, furosine formation is investigated in many foods for the evaluation of the nutritional quality of heat-treated foods. Furosine content is measured as the quality indicator of milk products, honey, cereals, pasta, and several other food products [63,64,65,66,67]. It is also used for regulatory purposes; in mozzarella cheese, the furosine content indicates the addition of heat-treated cow’s milk to the original product made from low temperature-treated buffalo’s milk [68].
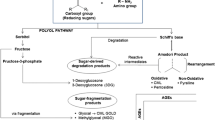
Furosine concentration in foods does not always correlate well with the severity of heat treatment; it does not increase linearly with heat damage. Amadori compounds may degrade via enolization and elimination reactions in the intermediate stages of glycation, forming dicarbonyl compounds. These dicarbonyl compounds are so reactive that they immediately react with the amine residues of proteins to form the advanced glycation end products. The Amadori compound is also oxidized to form the advanced glycation compounds. CML is the first and the most common amino acid derivative of the advanced glycation that was quantified in foods and a major AGE structure formed in vivo. It can be formed through various pathways as shown in Fig. 6.5 [69]. In the autoxidative pathway, glyoxal is derived from glucose and then reacts with lysine residues to form CML [70]. In the Namiki pathway, CML is formed by the reaction with lysine residues and glyoxal derived from Schiff base [71]. In another pathway, Amadori product is oxidized to form CML [72].
Pathways of CML formation. (Adapted from Han et al. [69])
CML is present in a range of heat-treated foods such as dairy products [73,74,75,76], cereals and bakery products [7, 77], meat [74, 78, 79], and nuts [80, 81]. Lipid oxidation occurs simultaneously during heating in some food products, and lipid oxidation products (highly reactive aldehydes and ketones, such as glyoxal) may be involved in the formation of AGEs. In a study [82], vegetable and fish oils were treated under accelerated storage conditions and cooking conditions, and it was found that fish oils with polyunsaturated fatty acids produced more glyoxal than vegetable oils. Glyoxal derived from lipid oxidation participated in food-derived CML formation [82]. Fu et al. [83] also showed that CML was formed in vitro during copper-catalyzed oxidation of PUFA in the presence of protein. Therefore, during thermal processing, CML may be formed through one or more of the mentioned pathways, depending on the food composition (precursors) and process conditions.
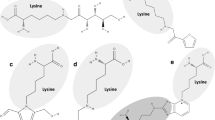
N-ε-carboxyethyllysine (CEL) (Fig. 6.6), which is formed by the reaction between methylglyoxal and lysine, is a homolog of CML and is found in several food products. He et al. [7] reported CEL levels ranging between 225 and 820 mg/kg protein in bread crust, between 159 and 452 mg/kg protein in biscuits, and between 146 and 373 mg/kg protein in fried dough sticks. Commercial sterilization of chicken, beef, and pork meat was found to increase protein-bound CML and CEL levels significantly [79]. The amounts of protein-bound CML and CEL in fish muscle increased as the heating (100 °C) time increased [84]. In a study where the effect of irradiation on CML and CEL formation and its relationship with lipid oxidation in meat products during storage was investigated [85], a linear correlation was found between the loss of polyunsaturated fatty acids content and the increase in CML and CEL contents in the irradiated beef samples during 6 weeks of storage. It was indicated that irradiation-induced lipid oxidation promotes CML and CEL formation through oxidation pathway [85].
Another important AGE, pyrraline (Fig. 6.6), which is the product of lysine and 3-deoxyglucosone, is found in high heat treatment-applied foods, such as bread crust (up to 3.7 g/kg protein), cookies (120 mg/kg protein), dried carrot products (up to 378 mg/kg protein), or roasted peanuts (up to 382 mg/kg protein) [80, 86, 87]. Considerable amounts of pyrraline were also reported in beer [88] and peptide-enriched drinks [89].
Pronyllysine results from lysine side chains and acetylformoin (Fig. 6.6) and was quantified up to 62 mg/kg in the crust and 6 mg/kg in the crumb of bread [90,91,92], whereas 0.43 mg/kg in Pilsner-type pale beer and 0.92 mg/kg in dark beer [90].
Argpyrimidine [N-δ-(5-hydroxy-4,6-dimethylpyrimidine-2-yl)-L-ornithine] is an AGE derived from the reaction of methylglyoxal with arginine residues (Fig. 6.6). It was detected as a free amino acid derivative form in beer [88, 93].
Pentosidine is a cross-linker formed by the reaction of pentose with the lysine and arginine residues of proteins (Fig. 6.6). In milk, up to 5 mg pentosidine/kg protein was detected in some samples of sterilized and UHT milk, whereas higher amounts up to 23 mg/kg protein were obtained in alkali-treated bakery products, such as pretzels. The highest amount of pentosidine was found in roasted coffee, ranging from 11 to 40 mg/kg protein [94]. 10–158 μg/100mL of pentosidine was detected in soy sauce, sour-sweet sauce, barbecue sauce, or tomato sauce, and meat treated with sauces also contained high amounts of pentosidine after baking and frying [95].
DOLD, GOLD, and MOLD (Fig. 6.6), the lysine dimers resulting from the reaction between two lysine side chains and two molecules of 3-deoxyglucosone, glyoxal and methylglyoxal, respectively, were found in the enzymatic hydrolysates of bakery products and boiled egg white in the mg/kg level, together with the cross-links between lysine and arginine (DODIC, GODIC, MODIC) [96]. The concentrations of MODIC and GODIC were found to be almost five times higher than those of their corresponding imidazolium compounds, MOLD and GOLD. 151 mg MODIC/kg protein was found maximum in butter biscuit samples [96]. Soy sauce-based seasonings were also found to contain up to 0.19 mg/L GOLD and up to 0.30 mg/L MOLD in the free form [97].
Imidazolinones are formed by the reactions of the guanidine group of arginine residues with dicarbonyl compounds, such as methylglyoxal and 3-deoxyglucosone (Fig. 6.6). The acid-labile imidazolinone resulting from the reaction between peptide-bound arginine and methylglyoxal was quantified in alkali-treated bakery products [98]. The amounts of imidazolone after complete enzymic digestion ranged between 9 and 13 mg/g protein, indicating that between 20 and 30% of the arginyl residues might react with methylglyoxal during the bakery process [98]. Traces of methylglyoxal-dihydroxyimidazoline were detected at 124Arg of β-lactoglobulin in sterilized and evaporated milk and small amounts of methylglyoxal-imidazolinone were shown to be present at 40Arg and 124Arg in more severely heated products [99]. In different beer types, 35.5–136.6 mg/kg protein MG-H1 was detected [88], whereas free forms of MG-H1 were also determined up to 2.47 mg/L in beer and beer-type liquors [88, 97] and up to 7.75 mg/L in soy sauce-based seasonings [97].
Some AGEs and their concentrations in different food products are given in Table 6.1.
6.3 Analysis Methods
Monitoring of glycation is challenging given the complexity of the reaction. Until today, many techniques have been used to determine the AGEs in food products and the body, including methods that use only simple absorbance measurements or more sophisticated instruments.
Due to the formation of brown-colored products in the Maillard reaction, the absorbance at 420 nm increases by the degree of glycation; hence, absorbance measurements at 420 nm might give an idea about the extent of glycation [104, 105]. Fluorescence measurements at 340–350 nm excitation and 400–440 nm emission have also been carried out to monitor protein glycation [106,107,108,109]; however, only AGEs with fluorescent properties such as pentosidine and crossline can be detected by this method, whereas nonfluorescent AGEs such as CML and CEL cannot be detected. Fluorescamine (4-phenylspiro [furan-2 (3H, 1-phtalan]-3–3′-dion) assay, which is based on the reaction between this reagent and the primary amino groups of protein and amino acids [110], also gives an idea about the extent of glycation. The resulting fluorescence decreases in case of glycation due to the decrease in the free amino groups [110].
Immunological detection and quantification of protein glycation based on ELISA [56, 111,112,113,114] have been widely used in biomedical and food science investigations. Although the ELISA method is easy and rapid, it is not regarded as reliable at present since the precision and accuracy are not high. The results are expressed in arbitrary units rather than actual concentrations. The method requires the use of specific antibodies for each compound, and furthermore, the food matrix affects the specificity of the assay.
Determination and quantification of glycation products might be performed more precisely by HPLC with UV-DAD detectors, LC-MS/MS, and GC-MS. Some AGEs such as furosine and CML are regarded as indicators of glycation, and they have been used as markers for the extent of glycation. Furosine has been used as a reliable indicator of thermal damage in foods since its detection in 1966 [68]. It is one of the first identified early glycation products in foods and is the most common chemical indicator of the Amadori product [68]. Furosine is formed during the acid hydrolysis of the Amadori products, N-ε-lactulosyllysine, N-ε-fructosyllysine, and N-ε-maltulosyllysine and tagatosyllysine [115]. Generally, food products are hydrolyzed by using concentrated acids, such as 6 N or 8 N hydrochloric acid (Fig. 6.7). The yield of furosine from the Amadori compounds during acid hydrolysis is variational between different Amadori compounds but is considered to be constant under controlled conditions. Different yields ranged from 20% to 30% after hydrolysis in 6 N hydrochloric acid, from 29% to 46% after hydrolysis in 7.8 N hydrochloric acid, and from 46% to 51% after hydrolysis with 8 N hydrochloric acid [115, 116]. If the corresponding conversion factors are known, then monitoring of the Amadori product formation in foods may be evaluated. Similarly, other Amadori compounds may be converted into N-(2-furoylmethyl) amino acids (FMAAs) by acid hydrolysis and then may be measured by RP-HPLC [67, 86].
CML is frequently used as a marker for AGE formation in food. Chemical analyses of CML concentrations in food products include extraction of the compound from the food and determination of its level by immunochemical assays or instrumental methods [117]. High-performance liquid chromatography (HPLC), gas chromatography coupled with mass spectrometry (GC-MS), and liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) might be used for the identification and determination of CML. For the determination of protein-bound CML, acid hydrolysis is applied to release CML from the protein. Since CML might be formed from fructosyllysine, sample preparation should be performed with extreme care to avoid any potential undesirable reactions, which might give rise to artifactual CML formation and thus an overestimation of the real content. Therefore, it has been proposed to initially reduce fructosyllysine residues into hexitollysine by sodium borohydride to prevent this process [73, 76]. Delatour et al. [75] proposed that enzymatic digestion might be performed to prevent the artifactual formation of CML mediated by fructosyllysine. However, they concluded that a slight overestimation of CML with enzymatic digestion might be observed. Determination of CML in food products may also be performed by GC analysis [77, 118].
N-ε-carboxyethyllysine (CEL), which is formed by the reaction between methylglyoxal and lysine, is a homolog of CML. Its content may be determined after acid hydrolysis or enzymatic hydrolysis by HPLC and LC-MS/MS methods [7, 81, 84, 101, 119, 120].
Pyrraline, which is the product of lysine and 3-deoxyglucosone, was first identified by amino acid analysis in heated skim-milk powder [121]. Pyrraline amount may be quantified using HPLC techniques either in free form or in protein-bound form after enzymatic hydrolysis, since the pyrrole compound is labile during acid and alkaline hydrolysis [1, 89, 122].
Mass spectrometry is widely used for the analysis of glycation products. Pronyllysine can be determined with HRGC/MS [90,91,92]; argpyrimidine with LC-MS/MS [81, 88] or high-resolution GC-MS [93]; pentosidine with LC-MS/MS [81] or HPLC with a fluorescence detector [95]; DOLD, GOLD, MOLD, DODIC, GODIC, MODIC, and methylhydroimidazolones with LC-MS [96]; and MG-H1 with HPLC-ESI-MS/MS [88, 99].
The extent of glycation of a protein molecule could be determined by mass spectrometric techniques. ESI-MS and MALDI-MS have been used to evaluate the glycation extent and glycoforms of proteins in different processing conditions [15, 123,124,125]. In most cases, it was shown that only one or two sugar units were attached to proteins after heating in solution state, whereas multiple glycoforms were obtained in the dry state [15, 123,124,125]. Mass spectrometry also enables the determination of the glycation sites of the protein molecule. Formation of lactulosyllysine at 47Lys, 138Lys, and 141Lys and also methionine sulfoxide at 7Met, 24Met, and 145Met in β-lactoglobulin was detected by using MALDI-TOF-MS coupled to electrophoretic protein separation and in gel digestion with the endoproteinase AspN [126]. CML formation was shown at different lysine residues of β-lactoglobulin such as 47Lys, 60Lys, 91Lys, 135Lys, and CEL formation at 69/70Lys and 91Lys by using ultrahigh-performance liquid chromatography tandem mass spectrometry [99]. Traces of methylglyoxal-dihydroxyimidazoline were detected at 124Arg in sterilized and evaporated milk, and small amounts of methylglyoxal-imidazolinone were shown to be present at 40Arg and 124Arg in severely heated products [99].
The use of mass spectrometry also allows the enlightenment of reaction mechanisms for the inhibition of glycation. The ability of phenolic compounds to trap carbonyl compounds and the ability of oxidized forms of catechins to react with the amino groups of proteins were revealed by using different mass spectrometric techniques such as high-resolution ESI-TOF/MS and ESI-ion trap MS [127,128,129,130]. The mechanisms of inhibition of glycation will be discussed in the next section.
6.4 Mitigation Strategies
The human organism has a certain protective mechanism to fight against AGE formation. There are chemical and biochemical processes including enzymatic and immune responses. Enzymes such as glyoxalases, aldehyde reductases, aldehyde dehydrogenases, amadoriases, and fructosamine 3-phosphokinases are responsible for the suppression of glycation reactions in the body and the repair of glycated proteins [21]. Nonetheless, in such cases, mainly in the increased level of carbonyl and oxidative stress, these protective mechanisms might be insufficient to struggle with the consequences of glycation. Therefore, AGE inhibitors are used for the treatment of the consequences of glycation.
The medical concept of glycation inhibition includes any mechanism delaying or preventing glycation reactions in vivo. The principle of the inhibition is based on the following strategies [131]:
-
Anti-glycation strategies involving scavenging hydroxyl radicals and superoxide radicals to attenuate oxidative stress and reducing the generation of reactive carbonyl compounds.
-
Blocking the carbonyl or dicarbonyl attachment to proteins.
-
Metal ion chelation since AGE formation is related to the presence of transition metal ions.
-
Breaking the cross-linked structures in AGEs.
Pharmaceuticals used as AGE inhibitors (such as aminoguanidine or pimagedine) might cause adverse effects such as gastrointestinal disturbance, anemia, and flu-like symptoms [132, 133]. Therefore, several natural compounds have been investigated for their inhibitory effects on glycation. Food-derived compounds such as spermin and spermidine [134,135,136], chlorogenic acid [137, 138], and isoflavonoid glycoside puerarin [139] have been shown to exert in vivo anti-glycation effects in human and animal studies.
In a model system composed of bovine serum albumin and glucose/fructose, incubated at 37 °C for 7 days, wild berries were shown to have anti-glycation activity in a concentration-dependent manner, and reduction in the AGE formation was positively correlated with the total phenolic content and related to radical scavenging capacity [140]. In another study [141], vegetable seed extracts were found to exhibit anti-AGE activity in protein-glucose assay (37 °C, 21 days), ranging from 20 to 92% inhibition, while peach and pomegranate extracts exhibited the highest anti-AGE activity in protein-methylglyoxal assay (37 °C, 14 days), ranging from 0 to 79% inhibition [141]. Presence of white grape skin extracts yielded a reduction in the formation of fluorescent AGEs in bovine serum albumin-fructose model system incubated at 37 °C for 3 days [142].
Maillard reaction and glycation have particular importance for the food industry. These reactions affect the organoleptic properties, color development, protein functionality, and nutritional properties of the product. Since glycation reactions are also responsible for the desired flavor and color development, mitigation of glycation in food products is a challenging issue.
The factors affecting glycation was discussed thoroughly in Sect. 6.1.2. Any reaction conditions or environmental factors affecting the rate of glycation such as reactant species, water activity, pH, and oxygen status would affect the progression of glycation; thus, by altering these parameters, glycation could be mitigated. However, addition of functional ingredients able to inhibit glycation is the most frequently used strategy in different food products and food model systems. Table 6.2 summarizes the strategies used for mitigation of glycation in food products and model systems.
6.4.1 Use of Polyphenols
Polyphenols are the most widely studied natural ingredients used as anti-glycation agents in food systems. Anti-glycation effect was mostly attributed to their antioxidant activities and their dicarbonyl trapping functions. Antioxidants act as AGE inhibitors, presumably through metal-ion chelation and sequestration of free-radical species, yielding attenuation of oxidative stress [162, 163] and also by trapping carbonyl compounds formed in the intermediate stages of glycation.
Addition of 600 mg and 1000 mg of grape seed extract, which is rich in catechins and proanthocyanidins, to bread (500 g) led to over 30% and 50% reduction, respectively, in the CML content of bread crust [150]. The effect was attributed to strong antioxidant activities of these compounds. Addition of ferulic acid to sponge cake baked at 190 °C for 30 min was found to lower the level of CML and CEL significantly, and the anti-glycation activity was attributed to the free-radical scavenging activity in the intermediate stage of glycation [151]. In the study of Zhang et al. [153], addition of phloretin, naringenin, epicatechin, chlorogenic acid, and rosmarinic acid to the glucose-casein model system showed inhibition on the formation of fluorescent AGEs and CML during heating at 120 °C for 2 hours. Chlorogenic acid, being the most potent inhibitor among the phenolics studied, was found to lower glyoxal and methyl glyoxal formation due to its antioxidant activity. The same phenolics in cookie models had positive correlation between glyoxal formation and antioxidant activity; however, methylglyoxal concentration was found to be unaffected [154]. In a recent study [164], negative correlation was observed between total phenolic compounds and the glyoxal, methylglyoxal, and diacetyl concentrations after baking, indicating the ability of phenolic compounds to trap α-dicarbonyl compounds during baking of cookies made of different cereal species. It was concluded that colored corn flour could be the source of natural dietary anti-glycation agents due to the good abilities of their phenolic compounds to trap C2, C3, and C4 α-dicarbonyl compounds [164].
Genistein was shown to inhibit the cross-links of the glycated β-lactoglobulin and suppress the formation AGEs in a dose-dependent manner by trapping reactive dicarbonyl compounds. By using LC-MS, both mono- and di-methylglyoxal adducts of genistein were detected in the β-lactoglobulin–methylglyoxal assay [155]. Quercetin was also shown to have the ability to trap dicarbonyl compounds in bovine serum albumin-methylglyoxal (or glyoxal) model systems [156]. Catechins were shown as potent dicarbonyl trapping agents in many studies. Maillard reaction model system studies have revealed that catechins sequester reactive dicarbonyl compounds through electrophilic aromatic substitution reactions, primarily on A-ring of flavan-3-ols [157,158,159,160]. Catechins have also been reported as trapping agents for the reactive imine intermediates linked to the Maillard reaction [161].
Besides their antioxidant actions and carbonyl trapping functions, polyphenols also may inhibit the glycation through blocking the amine residues of proteins in certain conditions. At alkaline conditions, polyphenols are oxidized to their corresponding quinone forms. Quinone, being a reactive electrophilic intermediate, can readily undergo attack by nucleophiles such lysine, methionine, cysteine, and tryptophan residues in a protein chain [165, 166].
In a study [143], soy glycinin or bovine serum albumin was incubated at 60 °C for 60 min at pH 12 with ferulic acid, and then fructose was added into the model systems and incubated for further 60 min. Ferulic acid was found to reduce fluorescent AGEs and CML formation by nearly 90% and 85%, respectively [143]. Similar results were reported for the use of soy isoflavone-rich extract (containing daidzein, glycitein, and genistein) at oxidizing conditions (60 °C for 1 or 16 hours at pH 12) in the soy glycinin-fructose model system [145]. It was suggested that the formation of early MR products might be inhibited by the conjugation of isoflavones to the active site of glycation, while AGE formation might be modulated by the trapping of dicarbonyl intermediates and oxygen radical species [145]. Pretreatment of ovalbumin with green tea infusion under oxidized conditions (pH 9.0, 50 °C, 1 h) was shown to be effective in reducing furosine and CML formation in the ovalbumin-glucose model system due to the reduction in the free lysine concentration of ovalbumin [144]. It was explained that the quinone forms of green tea polyphenols might react with the free amino groups of ovalbumin under alkaline conditions (Fig. 6.8). Thereby, the concentration of glycation products occurring during heating of ovalbumin and glucose decreased due to the modified lysine moieties in ovalbumin [144]. A similar explanation was also given for the antiglycoxidative mechanism of chlorogenic acid in a model system composed of bovine serum albumin and methylglyoxal [146]. Evidence of binding between BSA and multiple chlorogenic acids and/or its derivative molecules (isomers and oxidation products) was found. It was also concluded that methylglyoxal and chlorogenic acid competed for free amine groups, which prevents methylglyoxal from binding to BSA, resulting in an effective decrease in AGE formation [146].
By using high-resolution ESI-TOF mass spectrometry and isotope labeling technique, various glycine adducts of catechins were shown for the reaction between glycine and (+)-catechin at 120 °C for 70 min under oxidative conditions [127]. Detailed MS/MS analysis confirmed that amino acids were added to oxidized B-ring of (+)-catechin through the formation of Schiff bases [127]. Similarly, Yin et al. [128] stated that the inhibitory effect of tea polyphenols on MR might also be correlated with their ability to react with amino acids. It was explained that due to the strong electrophilic nature of quinones, the epicatechin quinone could react with lysine by a Michael-type addition, where lysine is added at the C-5 or C-2 position of the B-ring of epicatechin. It was concluded that tea catechins, epicatechin and epigallocatechin gallate, inhibited the formation of intermediary radicals by the Maillard reaction, by competing with glucose for lysine [128].
6.4.2 Modifications on Physical Structure
The physical structure of proteins affects its glycation tendency. The availability of glycation sites might be changed by the modifications of the protein molecule. A possible anti-glycation mechanism could be due to the physical protection of proteins against glycation by polyphenols. Hydrogen bonding between the phenolic hydroxyl groups and the amine and carboxyl groups of protein is involved in the protein-phenolic interactions. Hydrophobic interaction between the nonpolar regions of the phenolic molecules and the nonpolar domains of the protein may be responsible for weak interactions between the phenolic compounds and proteins [167, 168]. The anti-glycation mechanism involves noncovalent interactions with phenolics and proteins, making the glycation targets on protein molecule inaccessible to react in glycation [169, 170]. Vlassopoulos et al. [169] showed a reduction in fructosamine production in the phenolic preincubated albumin; on the contrary, addition of phenolic acids in the reaction solution throughout the incubation period had no significant effect on fructosamine production compared to glucose alone. It was suggested that physical protection from glycation through protein-phenolic acid interaction is the most likely anti-glycative mechanism especially in oxidative environments. Akıllıoğlu & Gökmen [147] showed that glycation of casein could be reduced by the complexation of casein with epicatechin prior to heating, causing a reduction in the available glycation sites by steric hindrance. Moreover, it was stated that when casein molecule was disintegrated by high-shear treatment before introducing epicatechin, better interaction between epicatechin and casein due to the exposed hydrophobic regions led to a further decrease in the advanced stages of glycation [147]. In the casein-calcium complexes prepared prior to heating in an aqueous solution state, calcium ions acted as cross-linking agents forming bridges between the casein micelles that make it difficult for carbonyl compounds to bind to the glycation sites on protein [147]. In a study where casein glycation was investigated in terms of micellar integrity [149], significantly higher amounts of CML were observed in nonmicellar casein than in the casein micelles after heating for 4 h. The lower amount of CML formation in casein micelle was attributed to the higher amounts of calcium when compared to sodium caseinate suspensions [149].
To evaluate the inhibition of glycation and to determine the mechanism of anti-glycation agents, Akıllıoğlu and Gökmen [148] proposed a kinetic approach, which was similarly derived from the enzyme inhibition kinetic analysis. The kinetic analysis allowed the estimation of the activity of anti-glycation agents comparatively through the calculation of related kinetic constants, in addition to the interpretation of the possible inhibition mechanisms. The effects of tannic acid and calcium ions on the formation of furosine in the ovalbumin-glucose model system in the dry state or aqueous conditions were determined to be noncompetitive [148], which is consistent with the published data about their noncovalent interactions with proteins.
6.5 Concluding Remarks
Since glycation reactions are also responsible for the desired flavor and color development, mitigating glycation in food products is a challenging issue. Thus, particular attention must be paid to the beneficial aspects of the Maillard reaction. Generally, addition of a functional food ingredient is preferred rather than changing the process conditions, to retain the sensorial and textural characteristics of the product. However, the concentration of the inhibitor agent is very important in terms of avoiding any deleterious side effects. It is necessary to know the concentration of the inhibitor agent to be added to food and the kinetics of the reactions taking place in the presence of the inhibitor agent. Unfortunately, most of the studies undertaken until now do not give concentration-dependent inhibition information. Further studies are needed in this regard.
The alternative techniques or agents used for processing yield different products during glycation. For instance, complexation of protein and oxidized phenolic compounds might result in the reduction of the bioavailability of the protein. Due to the fact that lysine is an essential amino acid, there are health concerns about the bioavailability of modified lysine residues in the protein. Therefore, researches with advanced analytical tools should be performed for the identification of neo-formed compounds, and the effects of new techniques should be evaluated in terms of protein digestibility and amino acid bioavailability.
References
Henle T et al (2008) Maillard reaction of proteins and advanced glycation end products (AGEs) in food. Wiley, New Jersey, pp 215–242
Rahbar S et al (1969) Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 36(5):838–843
Monnier VM, Cerami A (1981) Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science 211(4481):491–493
Henle T (2005) Protein-bound advanced glycation end products (AGEs) as bioactive amino acid derivatives in foods. Amino Acids 29(4):313–322
Poulsen MW et al (2013) Advanced glycation end products in food and their effects on health. Food Chem Toxicol 60:10–37
Zeng J, Davies MJ (2005) Evidence for the formation of adducts and S-(carboxymethyl)cysteine on reaction of alpha-dicarbonyl compounds with thiol groups on amino acids, peptides, and proteins. Chem Res Toxicol 18(8):1232–1241
He J et al (2013) Simultaneous determination of N ε-(carboxymethyl) lysine and N ε-(carboxyethyl) lysine in cereal foods by LC–MS/MS. Eur Food Res Technol 238(3):367–374
van Boekel MA (2001) Kinetic aspects of the Maillard reaction: a critical review. Nahrung/Food 45:150–159
Chevalier F et al (2001) Maillard glycation of beta-lactoglobulin with several sugars: comparative study of the properties of the obtained polymers and of the substituted sites. Dairy Sci Technol 81(C7):651–666
Leonil J, Molle D, Fauquant J, Maubois JL, Pearce RJ, Bouhallab S (1997) Characterization by ionization mass spectrometry of lactosyl β-lactoglobulin conjugates formed during heat treatment of milk and whey and identification of one lactose-binding site. J Dairy Sci 80:2270–2281
Meltretter J et al (2007) Site-specific formation of Maillard, oxidation, and condensation products from whey proteins during reaction with lactose. J Agric Food Chem 55(15):6096–6103
Fogliano V, Monti SM, Visconti A, Randazzo G, Facchiano AM, Colonna G, Ritieni A (1998) Identification of a β-lactoglobulin lactosylation site. Biochim Biophys Acta 1388:295–304
Siciliano R et al (2000) Modern mass spectrometric methodologies in monitoring milk quality. Anal Chem 72(2):408–415
Morgan F et al (1998) Lactolation of beta-lactoglobulin monitored by electrospray ionisation mass spectrometry. Int Dairy J 8(2):95–98
Fenaille F et al (2004) Solid-state glycation of beta-lactoglobulin by lactose and galactose: localization of the modified amino acids using mass spectrometric techniques. J Mass Spectrom 39(1):16–28
Oliver CM (2011) Insight into the glycation of milk proteins: an ESI- and MALDI-MS perspective (review). Crit Rev Food Sci Nutr 51(5):410–431
Labuza TP, Baisier WM (1992) The kinetics of nonenzymatic browning. In: Schwartzberg HG, Hartel RW (eds) Physical chemistry of foods. Dekker, New York, pp 595–649
Thomsen MK et al (2012) Effect of water activity, temperature and pH on solid state lactosylation of β-lactoglobulin. Int Dairy J 23(1):1–8
Martinez-Alvarenga MS et al (2014) Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate – Maltodextrin conjugates. Food Hydrocoll 38(16609336):110–118
Pan GG et al (2006) α-dicarbonyl compounds formed by nonenzymatic browning during the dry heating of caseinate and lactose. J Agric Food Chem 54(18):6852–6857
Velisek J (2014) Saccharides. In: Velısek J (ed) The chemistry of food. Wiley, West Sussex
Broersen K et al (2004) Glycoforms of ?-lactoglobulin with improved thermostability and preserved structural packing. Biotechnol Bioeng 86(1):78–87
Sun Y et al (2005) Evaluation of the site specific protein glycation and antioxidant capacity of rare sugar-protein/peptide conjugates. J Agric Food Chem 53(26):10205–10212
Fenaille F et al (2003) Solid-state glycation of beta-lactoglobulin monitored by electrospray ionisation mass spectrometry and gel electrophoresis techniques. Rapid Commun Mass Spectrom 17(13):1483–1492
Lima M et al (2009) Ultra performance liquid chromatography-mass spectrometric determination of the site specificity of modification of beta-casein by glucose and methylglyoxal. Amino Acids 36(3):475–481
Huang X et al (2013) Increase of ovalbumin glycation by the Maillard reaction after disruption of the disulfide bridge evaluated by liquid chromatography and high resolution mass spectrometry. J Agric Food Chem 61(9):2253–2262
Brown EM et al (1988) Accessibility and mobility of lysine residues in.beta.-lactoglobulin. Biochemistry 27(15):5601–5610
Sawyer L, Kontopidis G, Wu S-Y (1999) β-Lactoglobulin – a three-dimensional perspective. Int J Food Sci Technol 34:409–418
JW B et al (1989) In: Baynes JW, Monnier VM, Liss AR (eds) The Amadori product on protein: structure and reactions, New York, pp 43–67
Mennella C et al (2006) Glycation of lysine-containing dipeptides. J Pept Sci 12(4):291–296
Shilton BH, Walton DJ (1991) Sites of glycation of human and horse liver alcohol dehydrogenase in vivo. J Biol Chem 266(9):5587
Shilton BH, Campbell RL, Walton DJ (1993) Site specificity of glycation of horse liver alcohol dehydrogenase in vitro. Eur J Biochem 215(3):567–572
Fogliano V et al (1998) Identification of a beta-lactoglobulin lactosylation site. Biochim Biophys Acta 1388:295–304
Yeboah FK et al (2000) Monitoring glycation of lysozyme by electrospray ionization mass spectrometry. J Agric Food Chem 48(7):2766
Baynes JW (2001) The role of AGEs in aging: causation or correlation. Exp Gerontol 36(9):1527–1537
Henle T (2007) Dietary advanced glycation end products--a risk to human health? A call for an interdisciplinary debate. Mol Nutr Food Res 51(9):1075–1078
Wang Z et al (2012) Advanced glycation end-product Nepsilon-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis 221(2):387–396
Sakata N et al (1999) Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis 142(1):67
Münch G et al (1998) Alzheimer’s disease--synergistic effects of glucose deficit, oxidative stress and advanced glycation end products. J Neural Transm 105(4–5):439–461
Li J et al (2012) Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci 317(1–2):1–5
Thornalley PJ et al (2003) Quantitative screening of advanced glycation end products in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 375(3):581–592
Ahmed N et al (2005) Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 48(8):1590–1603
HF B et al (1978) The glycosylation of hemoglobin: relevance to diabetes mellitus. Science 200(4337):21–27
Wells-Knecht KJ et al (1994) 3-Deoxyfructose concentrations are increased in human plasma and urine in diabetes. Diabetes 43(9):1152–1156
Schwenger V et al (2015) Advanced glycation end products (AGEs) as uremic toxins. Mol Nutr Food Res 45(3):172–176
Raj DS et al (2000) Advanced glycation end products: a Nephrologist’s perspective. Am J Kidney Dis 35(3):365–380
Sasaki N et al (1998) Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol 153(4):1149–1155
Nagaraj RH et al (1991) High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A 88(22):10257–10261
Uribarri J et al (2005) Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 1043:461–466
Roncero-Ramos I et al (2014) An advanced glycation end product (AGE)-rich diet promotes Nepsilon-carboxymethyl-lysine accumulation in the cardiac tissue and tendons of rats. J Agric Food Chem 62(25):6001–6006
Lin R-Y et al (2003) Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 168(2):213–220
Feng JX et al (2007) Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int 71(9):901–911
Sebekova K et al (2003) Effects of a diet rich in advanced glycation end products in the rat remnant kidney model. Am J Kidney Dis 41(3 Suppl 1):S48–S51
Somoza V et al (2006) Dose-dependent utilisation of casein-linked lysinoalanine, N(epsilon)-fructoselysine and N(epsilon)-carboxymethyllysine in rats. Mol Nutr Food Res 50(9):833–841
Harcourt BE et al (2011) Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int 80(2):190–198
Koschinsky T et al (1997) Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A 94(12):6474–6479
Uribarri J et al (2007) Circulating glycotoxins and dietary advanced glycation end products: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 62(4):427–433
Vlassara H et al (2002) Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A 99(24):15596–15601
Uribarri J (2003) Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol 14(3):728–731
Uribarri J et al (2003) Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis 42(3):532–538
Vlassara H et al (2009) Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab 94(11):4483–4491
Birlouez-Aragon I et al (2010) A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr 91(5):1220–1226
Sanz ML et al (2003) 2-Furoylmethyl amino acids and hydroxymethylfurfural as indicators of honey quality. J Agric Food Chem 51(15):4278–4283
Erbersdobler HF et al (1987) Determination of furosine in heated milk as a measure of heat intensity during processing. J Dairy Res 54(1):147–151
Rufián-Henares JA et al (2007) Assessing nutritional quality of milk-based sport supplements as determined by furosine. Food Chem 101(2):573–578
Delgado-Andrade C et al (2007) Lysine availability is diminished in commercial fibre-enriched breakfast cereals. Food Chem 100(2):725–731
Castillo MDD et al (1999) Early stages of Maillard reaction in dehydrated orange juice. J Agric Food Chem 47(10):4388
Erbersdobler HF, Somoza V (2007) Forty years of furosine – forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol Nutr Food Res 51(4):423–430
Han L et al (2013) Hydroxyl radical induced by lipid in Maillard reaction model system promotes diet-derived N(epsilon)-carboxymethyllysine formation. Food Chem Toxicol 60:536–541
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J 245(1):243–250
Glomb MA, Monnier VM (1995) Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem 270(17):10017–10026
Ahmed MU, Thorpe SR, Baynes JW (1986) Identification of N-epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem 261:4889–4894
Drusch S et al (1999) Determination of N ϵ -carboxymethyllysine in milk products by a modified reversed-phase HPLC method. Food Chem 65(4):547–553
Assar SH et al (2009) Determination of Nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 36(2):317–326
Delatour T et al (2009) Analysis of advanced glycation end products in dairy products by isotope dilution liquid chromatography-electrospray tandem mass spectrometry. The particular case of carboxymethyllysine. J Chromatogr A 1216(12):2371–2381
Fenaille F et al (2006) Modifications of milk constituents during processing: a preliminary benchmarking study. Int Dairy J 16(7):728–739
Charissou A et al (2007) Evaluation of a gas chromatography/mass spectrometry method for the quantification of carboxymethyllysine in food samples. J Chromatogr A 1140(1–2):189–194
Hull GLJ et al (2012) Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem 131(1):170–174
Sun X et al (2015) Formation of advanced glycation end products in ground beef under pasteurisation conditions. Food Chem 172:802–807
Wellner A et al (2011) Glycation compounds in peanuts. Eur Food Res Technol 234(3):423–429
Zhang G et al (2011) Determination of advanced glycation end products by LC-MS/MS in raw and roasted almonds (Prunus dulcis). J Agric Food Chem 59(22):12037–12046
Fujioka K, Shibamoto T (2004) Formation of genotoxic dicarbonyl compounds in dietary oils upon oxidation. Lipids 39(5):481
Fu MX et al (1996) The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 271(17):9982–9986
Niu L et al (2017) Free and protein-bound Nε -carboxymethyllysine and Nε -carboxyethyllysine in fish muscle: biological variation and effects of heat treatment. J Food Compost Anal 57:56–63
Yu L et al (2016) Effect of irradiation on Nε-carboxymethyl-lysine and Nε-carboxyethyl-lysine formation in cooked meat products during storage. Radiat Phys Chem 120:73–80
Wellner A et al (2011) Formation of Maillard reaction products during heat treatment of carrots. J Agric Food Chem 59(14):7992–7998
Hellwig M, Henle T (2012) Quantification of the Maillard reaction product 6-(2-formyl-1-pyrrolyl)-l-norleucine (formyline) in food. Eur Food Res Technol 235(1):99–106
Hellwig M et al (2016) Free and protein-bound Maillard reaction products in beer: method development and a survey of different beer types. J Agric Food Chem 64(38):7234–7243
Liang Z et al (2016) Determination of free-form and peptide bound pyrraline in the commercial drinks enriched with different protein hydrolysates. Int J Mol Sci 17(7)
Somoza V et al (2005) Influence of feeding malt, bread crust, and a pronylated protein on the activity of chemopreventive enzymes and antioxidative defense parameters in vivo. J Agric Food Chem 53(21):8176
Lindenmeier M et al (2002) Structural and functional characterization of pronyl-lysine, a novel protein modification in bread crust melanoidins showing in vitro antioxidative and Phase I/II enzyme modulating activity. J Agric Food Chem 50(24):6997–7006
Lindenmeier M, Hofmann T (2004) Influence of baking conditions and precursor supplementation on the amounts of the antioxidant pronyl-L-lysine in bakery products. J Agric Food Chem 52(2):350–354
Glomb MA, Rösch D, Nagaraj RH (2001) Nδ-(5-hydroxy-4,6-dimethylpyrimidine-2-yl)-l-ornithine, a novel methylglyoxal−arginine modification in beer. J Agric Food Chem 49:366–372
Henle T et al (1997) Detection and quantification of pentosidine in foods. Zeitschrift für Lebensmitteluntersuchung und – Forschung A 204(2):95–98
P-c C et al (2009) Analysis of glycative products in sauces and sauce-treated foods. Food Chem 113(1):262–266
Biemel KM et al (2001) Identification and quantitative evaluation of the lysine-arginine crosslinks GODIC, MODIC, DODIC, and glucosepan in foods. Mol Nutr Food Res 45(3):210
Nomi Y et al (2016) Simultaneous quantitation of advanced glycation end products in soy sauce and beer by liquid chromatography-tandem mass spectrometry without ion-pair reagents and derivatization. J Agric Food Chem 64(44):8397–8405
Henle T et al (1994) Detection and identification of a protein-bound imidazolone resulting from the reaction of arginine residues and methylglyoxal. Z Lebensm Unters Forsch 199(1):55–58
Meltretter J et al (2014) Modified peptides as indicators for thermal and nonthermal reactions in processed milk. J Agric Food Chem 62(45):10903–10915
Henle T, Zehetner G, Klostermeyer H (1995) Fast and sensitive determination of furosine. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung 200:235–237
Troise AD et al (2015) Quantification of Nepsilon-(2-Furoylmethyl)-L-lysine (furosine), Nepsilon-(Carboxymethyl)-L-lysine (CML), Nepsilon-(Carboxyethyl)-L-lysine (CEL) and total lysine through stable isotope dilution assay and tandem mass spectrometry. Food Chem 188:357–364
Schwietzke U, Schwarzenbolz U, Henle T (2009) Influence of cheese type and maturation time on the early Maillard reaction in cheese. Czech J Food Sci 27:S140–S1S2
Schwietzke U et al (2011) Quantification of Amadori products in cheese. Eur Food Res Technol 233(2):243–251
Labuza TP, Saltmarch M (2010) Kinetics of browning and protein quality loss in whey powders during steady state and nonsteady state storage conditions. J Food Sci 47(1):92–96
CGA D et al (1998) Indication of the Maillard reaction during storage of protein isolates. J Agric Food Chem 46(2):2485–2489
Morales FJ et al (1996) Fluorescence associated with Maillard reaction in milk and milk-resembling systems. Food Chem 57(3):423–428
Suárez G et al (1995) Fructated protein is more resistant to ATP-dependent proteolysis than glucated protein possibly as a result of higher content of Maillard fluorophores. Arch Biochem Biophys 321(1):209–213
FJ M, MAJSvan B (1998) A study on advanced Maillard reaction in heated casein/sugar solutions: fluorescence accumulation. Int Dairy J 7(11):675–683
Yanagisawa K et al (1998) Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metab Clin Exp 47(11):1348–1353
VA Y et al (1992) A fluorescamine-based assay for the degree of glycation in bovine serum albumin. Food Res Int 25(4):269–275
Uribarri J et al (2010) Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 110(6):911–916. e12
Dittrich R et al (2006) Concentrations of Nε-carboxymethyllysine in human breast milk, infant formulas, and urine of infants. J Agric Food Chem 54(18):6924
Šebeková K et al (2001) Plasma levels of advanced glycation end products in healthy, long-term vegetarians and subjects on a western mixed diet. Eur J Nutr 40(6):275–281
Miyazawa N et al (1998) Immunological detection of fructated proteins in vitro and in vivo. Biochem J 336. ( Pt 1(2):101
Mehta BM, Deeth HC (2016) Blocked lysine in dairy products: formation, occurrence, analysis, and nutritional implications. Compr Rev Food Sci Food Saf 15(1):206–218
Krause R et al (2003) Studies on the formation of furosine and pyridosine during acid hydrolysis of different Amadori products of lysine. Eur Food Res Technol 216(4):277–283
Nguyen HT et al (2014) N ϵ-(carboxymethyl)lysine: a review on analytical methods, formation, and occurrence in processed food, and health impact. Food Rev Int 30(1):36–52
Charissou A et al (2007) Kinetics of formation of three indicators of the Maillard reaction in model cookies: influence of baking temperature and type of sugar. J Agric Food Chem 55(11):4532–4539
Tareke E et al (2013) Isotope dilution ESI-LC-MS/MS for quantification of free and total Nε-(1-Carboxymethyl)-l-Lysine and free Nε-(1-Carboxyethyl)-l-Lysine: Comparison of total Nε-(1-Carboxymethyl)-l-Lysine levels measured with new method to ELISA assay in gruel samples. Food Chem 141:4253–4259
Jiao Y et al (2017) N(epsilon)-(carboxymethyl)lysine and N(epsilon)-(carboxyethyl)lysine in tea and the factors affecting their formation. Food Chem 232:683–688
Henle T, Klostermeyer H (1993) Determination of protein-bound 2-amino-6-(2-formyl-1-pyrrolyl). Z Lebensm Unters Forsch 196(1):1–4
Schwarzenbolz U et al (2016) Free Maillard reaction products in milk reflect nutritional intake of glycated proteins and can be used to distinguish “organic” and “conventionally” produced milk. J Agric Food Chem 64(24):5071
Hau J, Bovetto L (2001) Characterisation of modified whey protein in milk ingredients by liquid chromatography coupled to electrospray ionisation mass spectrometry. J Chromatogr A 926(1):105–112
French SJ et al (2002) Maillard reaction induced lactose attachment to bovine beta-lactoglobulin: electrospray ionization and matrix-assisted laser desorption/ionization examination. J Agric Food Chem 50(4):820–823
Akıllıoğlu HG et al (2017) Monitoring protein glycation by electrospray ionization (ESI) quadrupole time-of-flight (Q-TOF) mass spectrometer. Food Chem 217:65–73
Meltretter J et al (2008) Identification and site-specific relative quantification of beta-lactoglobulin modifications in heated milk and dairy products. J Agric Food Chem 56(13):5165–5171
Guerra PV, Yaylayan VA (2014) Interaction of flavanols with amino acids: postoxidative reactivity of the B-ring of catechin with glycine. J Agric Food Chem 62(17):3831–3836
Yin J et al (2014) Epicatechin and epigallocatechin gallate inhibit formation of intermediary radicals during heating of lysine and glucose. Food Chem 146(1):C48–C55
Sang S, Shao X, Bai N, Lo CY, Yang CS, Ho CT (2007) Tea Polyphenol (−)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem Res Toxicol 20:1862–1870
Schilling S et al (2010) Characterization of covalent addition products of chlorogenic acid quinone with amino acid derivatives in model systems and apple juice by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 22(4):441–448
Wu CH et al (2011) Inhibition of advanced glycation end product formation by foodstuffs. Food Funct 2(5):224–234
Freedman BI et al (1999) Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control Clin Trials 20(5):493–510
Williams ME (2004) Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr Diab Rep 4(6):441–446
Méndez JD, Leal LI (2004) Inhibition of in vitro pyrraline formation by L-arginine and polyamines. Biomed Pharmacother 58(10):598–604
Mendez JD, Balderas FL (2006) Inhibition by L-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed Pharmacother 60(1):26–31
Jafarnejad A et al (2008) Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life Sci 82(5):301–307
Kim J et al (2011) Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch Pharm Res 34(3):495–500
Kim YS et al (2011) Preventive effect of chlorogenic acid on lens opacity and cytotoxicity in human lens epithelial cells. Biol Pharm Bull 34(6):925–928
Gasser P et al (2011) Glycation induction and antiglycation activity of skin care ingredients on living human skin explants. Int J Cosmet Sci 33(4):366–370
Harris CS et al (2014) Investigating wild berries as a dietary approach to reducing the formation of advanced glycation end products: chemical correlates of in vitro antiglycation activity. Plant Foods Hum Nutr 69(1):71–77
Mesías M et al (2012) Antiglycative effect of fruit and vegetable seed extracts: inhibition of AGE formation and carbonyl-trapping abilities. J Sci Food Agric 93(8):2037–2044
Sri Harsha PSC et al (2014) Protective ability of phenolics from white grape vinification by-products against structural damage of bovine serum albumin induced by glycation. Food Chem 156:220–226
Silván JM et al (2011) Control of the Maillard reaction by ferulic acid. Food Chem 128(1):208–213
Cömert ED et al (2017) Mitigation of ovalbumin glycation in vitro by its treatment with green tea polyphenols. Eur Food Res Technol 243(1):11–19
Silvan JM et al (2014) Glycation is regulated by isoflavones. Food Funct 5(9):2036–2042
Fernandez-Gomez B et al (2015) New knowledge on the antiglycoxidative mechanism of chlorogenic acid. Food Funct 6(6):2081–2090
Akıllıoğlu HG, Gökmen V (2014) Effects of hydrophobic and ionic interactions on glycation of casein during Maillard reaction. J Agric Food Chem 62(46):11289–11295
Akıllıoğlu HG, Gökmen V (2016) Kinetic evaluation of the inhibition of protein glycation during heating. Food Chem 196:1117–1124
Moeckel U et al (2016) Glycation reactions of casein micelles. J Agric Food Chem 64(14):2953–2961
Peng X et al (2010) The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem 119(1):49–53
Srey C et al (2010) Effect of inhibitor compounds on Nε-(Carboxymethyl)lysine (CML) and Nε-(Carboxyethyl)lysine (CEL) formation in model foods. J Agric Food Chem 58(22):12036–12041
Wang J et al (2009) Protein glycation inhibitory activity of wheat bran feruloyl oligosaccharides. Food Chem 112(2):350–353
Zhang X et al (2014) Treatment of proteins with dietary polyphenols lowers the formation of AGEs and AGE-induced toxicity. Food Funct 5(10):2656–2661
Zhang X et al (2014) Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J Agric Food Chem 62(7):1643–1648
Kong Y et al (2015) Glycation of β-lactoglobulin and antiglycation by genistein in different reactive carbonyl model systems. Food Chem 183:36–42
Li X et al (2014) Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J Agric Food Chem 62(50):12152–12158
Totlani VM, Peterson DG (2005) Reactivity of epicatechin in aqueous glycine and glucose maillard reaction models: quenching of C2, C3, and C4 sugar fragments. J Agric Food Chem 53(10):4130–4135
Totlani VM, Peterson DG (2007) influence of epicatechin reactions on the mechanisms of Maillard product formation in low moisture model systems. J Agric Food Chem 55(2):414–420
Totlani VM, Peterson DG (2006) Epicatechin carbonyl-trapping reactions in aqueous maillard systems: identification and structural elucidation. J Agric Food Chem 54(19):7311–7318
Noda Y, Peterson DG (2007) Structure−reactivity relationships of flavan-3-ols on product generation in aqueous glucose/glycine model systems. J Agric Food Chem 55(9):3686–3691
Bin Q et al (2012) Influence of phenolic compounds on the mechanisms of pyrazinium radical generation in the Maillard reaction. J Agric Food Chem 60(21):5482–5490
Peng X et al (2011) Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct 2(6):289–301
Reddy VP, Beyaz A (2006) Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov Today 11(13):646–654
Kocadağlı T et al (2016) Formation of α-dicarbonyl compounds in cookies made from wheat, hull-less barley and colored corn and its relation with phenolic compounds, free amino acids and sugars. Eur Food Res Technol 242(1):51–60
Hurrell RF, Finot P-A (1984) Nutritional consequences of the reactions between proteins and oxidized polyphenolic acids. In: Friedman M (ed) Nutritional and toxicological aspects of food safety. Springer, Boston, pp 423–435
Rawel HM et al (2001) Reactions of phenolic substances with lysozyme — physicochemical characterisation and proteolytic digestion of the derivatives. Food Chem 72(1):59–71
Ali H(2002) Protein-phenolic interactions in food
Oh HI et al (1980) Hydrophobic interaction in tannin-protein complexes. J Agric Food Chem 28(2):394–398
Vlassopoulos A et al (2014) Protein–phenolic interactions and inhibition of glycation–combining a systematic review and experimental models for enhanced physiological relevance. Food Funct 5(10):2646–2655
Xiao J, Kai G (2012) A review of dietary polyphenol-plasma protein interactions: characterization, influence on the bioactivity, and structure-affinity relationship. Crit Rev Food Sci Nutr 52(1):85–101
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Akıllıoğlu, H.G., Gökmen, V. (2019). Advanced Glycation End Products (AGEs). In: Wang, S. (eds) Chemical Hazards in Thermally-Processed Foods. Springer, Singapore. https://doi.org/10.1007/978-981-13-8118-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-13-8118-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8117-1
Online ISBN: 978-981-13-8118-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)