Abstract
An incessant increase in global population along with a continuous augmentation in abiotic stress conditions, such as temperature, pH, salinity, etc., and limitation of natural resources has posed a serious threat to developing nations in terms of food security and enhanced nutritional value of the yield. Substantial crop losses in both qualitative and quantitative aspects due to the several prevalent phytopathogens are adding severity to the existing trouble. Confrontation with this ongoing problem initially led to the application of chemical fertilizers. However, hazardous aftereffects of the chemical fertilizers on the ecosystem have instigated a demand for a promising eco-friendly substitute that deals with both biotic and abiotic stresses. Rhizospheric microorganisms can be utilized as an effective alternative because they reside in soil and have the intrinsic property of upholding balanced ecosystem. These plant growth-promoting rhizobacteria (PGPRs) enhance plant growth even in poor and stressed environmental conditions by the formation of beneficial associations with the host through biological nitrogen fixation, phosphate solubilization, siderophore and hormone production, etc. They can also trigger host defense mechanism through induced systemic resistance (ISR). These PGPRs are also helpful for phytoremediation by various processes such as direct absorption, accumulation, etc. PGPRs are utilized in the fields of phytostimulation, biofertilization, and biocontrol activities. In the current chapter, we would aim to uphold the mechanisms opted by PGPR for effective plant growth promotion and defense under various abiotic as well as biotic stress conditions. In this context, we would also aim to delve in detail about the host-PGPR cross talk during the onset of stress conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
By observing the steep increase in population growth curve with respect to time, it is very easy to predict the upcoming demand of food, fiber, fodder, and biomass by continuously decreasing arable land due to various anthropogenic activities (Abhilash et al. 2013). With an enormously growing population and limited resources, a major problem in front of developing countries is to provide food security with ecosystem stability. Both biotic such as pathogenic microorganisms, pests, weeds, etc. and abiotic stresses including low and high temperature, drought, salinity, flooding, ultraviolet light, air pollution, heavy metals, etc. are adding pressure to the crop production. Approximately 7–15% of the crops are damaged by various soilborne fungi, oomycetes, bacteria, and nematodes through various mechanisms such as destroying and damaging of root tips and root hairs, the release of toxins, etc. (Oerke 2005; Singh et al. 2014; Mishra et al. 2015). Increasing salt level in both land and irrigating water is the main problem faced by arid and semiarid areas due to which plant shows stunted growth as the photosynthetic unit becomes unable to work properly. Similar physiological modulations can be observed in plants against other abiotic stresses which ultimately lead to crop loss. These stresses cause a noticeable decrease of 50–82% in agricultural productivity and raise hindrance for the cultivation of new crops. To cope up with the abovementioned problems of the food crisis, malnutrition, etc., producers become inclined toward the unbalanced use of agrochemicals as an economically reliable substitute for crop protection. The enormous application of these chemical agents has led to severe negative impacts which include the development of pathogen resistance against applied agents, accumulation in the ecosystem due to non-degradation of the compounds, and therefore entry into the food chain. There is an urgent need to sustainably enhance the quality of crop production to meet future requirements and also protect the remaining cultivable soil from further degradation and contamination. Further, owing to the increasing awareness among people about harmful effects of these residues as well as the unavailability of chemical solutions against some phyto ailments apart from the continuously and rampantly increasing cost of pesticides, the search for a safer and eco-friendly alternative started which gave rise to biological control measures.
Currently, biological measures are one of the most emerging and sustainable methods among both agronomist and environmentalists for integrated plant growth and nutrient management systems to ease the burden on the environment. Among the numerous practices employed, application of plant growth-promoting rhizobacteria (PGPRs) is a potential measure as it prevents the plant from various phytopathogens as well as enhances the plant growth-promoting attributes due to their strong colonization affinity.
5.2 Plant Growth-Promoting Rhizobacteria (PGPRs)
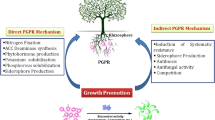
The rhizosphere upholds a variety of microorganisms which can be deleterious, neutral, or beneficial (Fig. 5.1). Among numerous microfauna present in the soil, about 2–5% of free-living and rhizosphere-competent microbes providing plant growth promotional attributes even in the presence of competing microbes and phytopathogens are known as the PGPRs (Kloepper and Schroth 1978). Along with nutrients and water uptake, the root system of the host plants also secretes a variety of compounds in the rhizosphere (Walker et al. 2003) The rhizosphere PGPRs enhance the sustainability of soil for production of crops through various biotic activities that increase the nutrient turn over which in turn improve the soil structure. The main property of the PGPR which makes them more efficient is turning over of nutrients through their mobilization which enhances the sustainability for cultivation (Ahemad et al. 2009; Chandler et al. 2008). Further, several reports justify the sequestration of heavy metals and degradation of xenobiotics such as herbicides, pesticides, etc. by PGPRs, thereby leading to effective bioremediation (Ahemad 2012; Ahemed and Malik 2011; Hayat et al. 2010; Glick 2012). In this context, it is significant to notify the pursual of research on a global scale to yield biocontrol agents with numerous beneficial traits such as management of phytopathogens, plant growth promotion, heavy metal detoxification, abiotic stress tolerance, pesticide tolerance, etc. for the enhancement of sustainable agriculture (Chaudhary et al. 2012; Vaishnav et al. 2014). With all the promising plant growth promotional and biocontrol attributes, PGPRs can be used as an effective and eco-friendly tool for enhancing the sustainability of production, restoration of contaminated land, nutritional and food security, carbon sequestration, phytoremediation of heavily contaminated soils, and biofuel and biomass production. Presently numerous symbiotic microbes such as Rhizobium spp. and Bradyrhizobium spp. as well as nonsymbiotic microbes including Pseudomonas, Bacillus, Azotobacter, Azospirillum, and Alcaligenes are known globally for their application as inoculants possessing plant growth and stress-tolerant attributes (Ma et al. 2011a, b; Wani and Khan 2010; Mayak et al. 2004; Ray et al. 2016a, b, 2018b).
5.3 Mechanisms Implicated by PGPR
5.3.1 Root Colonization
A significant drawback consistently associated with PGPRs is their poor field performance owing to the inconsistency of rhizosphere colonization, particularly under field conditions (Schroth and Hancock 1981; Thomashow 1996a, b). Efficient root colonization is the primary step for effective proliferation and survival in the presence of other rhizospheric microflora as well as for establishing competence that provides effective biocontrol, plant-microbe cross talk, and enhanced PGPR efficiency (Parke 1991; Wipps 1997; Lugtenberg and Dekkers 1999). As the rhizospheric soil behave as sink for nutrients, plants release root exudates with diverse chemical compounds such as specific sugars, organic acids, amino acids, etc. which act as chemoattractants for numerous active soil microbes and synchronize the microbial presence in close proximity of root surface (Rovira 1965; Welbaum et al. 2004; Dakora and Phillips 2002). Due to the presence of these exudates, the symbiotic association takes place with the nearby rhizospheric microbial communities that promote plant growth and in turn obtaining major nutrients, such as carbon, nitrogen, phosphorus, etc., through the chemical compounds released by roots and root hairs (Nardi et al. 2000). When the PGPRs reach the root through their motile structures in response to the exudates which is known as rhizospheric effect (Hiltner 1904), some of them colonize the surface of roots and root hairs without causing harmful effects, thereby inhibiting the invasion of phytopathogens by means of nutrient and niche competition, whereas many of them have the ability to enter endodermis after crossing the barrier and exist as endophytes in different organs of the host plant (Hallman et al. 1997; Duffy 2001; Turnbull et al. 2001; Compant et al. 2005; Gray and Smith 2005; Ray et al. 2018a).
5.3.2 Growth-Promoting Attributes
Post-effective establishment and colonization, PGPRs enhance the growth and increase the productivity of host plant through various direct and indirect methods such as nutrient acquisition, regulating plant hormone and synthesis of various beneficial metabolites (Glick 2012).
5.3.2.1 Biological Nitrogen Fixation
With 78% of the fraction in the atmosphere, nitrogen is the most essential macromolecule required for plant growth and development which is fixed in plant utilizable forms through biological nitrogen fixation (BNF). In this process, atmospheric nitrogen is converted to ammonia with the help of microorganism borne nitrogenase enzyme system (Kim and Rees 1994). Nitrogenase is a two-component complex metalloenzyme system comprising of dinitrogenase reductase as iron protein and dinitrogenase as a metal cofactor, and on their basis, three different nitrogen-fixing systems have been reported, namely, Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase (Dean and Jacobson 1992; Kim and Rees 1994). Majority of BNF is performed by Mo-nitrogenase present in most of the PGPRs carrying nitrogen fixation in nonleguminous plants through the establishment of nonobligate interaction (Glick et al. 1999; Bishop and Jorerger 1990). Microorganism involved in BNF can be broadly divided into (a) symbiotic association with leguminous and (b) nonleguminous plants and (c) free-living as well as associate nonsymbiotic endophytes such as Acetobacter, Azospirillum, Bacillus, Pseudomonas, etc. which fix a minor portion of atmospheric nitrogen. Majority of unavailable atmospheric nitrogen is fixed through symbiotic nitrogen fixers such as Rhizobia in leguminous and Frankia in the nonleguminous plant (Saxena and Tilak 1998; Bhattacharya and Jha 2012; Glick 2012). A number of studies revealed two third biological fixation of atmospheric nitrogen globally, and remaining requirements are fulfilled by the Haber-Bosch method (Rubio and Ludden 2008). Treatment of plants and soil with PGPRs having the nitrogen-fixing ability is an economical and ecologically sustainable substitute of chemical fertilizers (Ladha et al. 1997).
5.3.2.2 Phosphate Solubilization Activity
The soil is the most abundant reservoir of both organic and inorganic form of phosphorus, the most essential macronutrient for plant growth promotion after nitrogen (Khan et al. 2009). Regardless of such an enormous reservoir, plants, in general, face scarcity of phosphorus as the roots only absorb monobasic and dibasic forms of the ion, while a major portion of phosphorus present in insoluble forms such as inositol phosphate, phosphomonoester, and triesters remain unutilized (Bhattacharya and Jha 2012). To deal with unavailability, farmers apply numerous phosphatic fertilizers, but only a little amount is absorbed by the plant with the remaining portion being turned into insoluble complexes (Mckenzie and Roberts 1990). Among numerous rhizospheric microflora, phosphate-solubilizing microorganisms (PSM) including Bacillus, Enterobacter, Pseudomonas, Burkholderia, Flavobacterium, Rhizobium, Microbacterium, Serratia, etc. can be applied as a substitute for sustainable agriculture since they can convert unavailable form of phosphorus to available form through the activity of low molecular weight organic acids produced by PSM (Zaidi et al. 2009). These PSM also synthesize numerous phosphatases for mineralization of organic phosphorus through phosphoric ester hydrolysis (Glick 2012). Numerous beneficial effects such as mineralization, enhanced efficiency of BNF through nodule formation, increased uptake of trace elements, etc. have been observed in the host plants treated with single or amalgamated PGPRs having phosphate-solubilizing property (Ahemad and Khan 2012; Vikramal and Hamzehzarghani 2008; Zaidi et al. 2009; Ahmad et al. 2008).
5.3.2.3 Production of Phytohormones
Plant hormones are the organic compounds which act as chemical messengers generated through various metabolic processes in one portion and get distributed all over the system. They are concentration and target specifically for optimum growth and development of a plant in different environmental conditions and therefore also termed as a plant growth regulator. On the basis of previous studies, phytohormones have been classified into five major classes: auxins, cytokinins, gibberellin, abscisic acid, and ethylene. Among these, IAA is the supreme indigenous auxin which regulates cellular processes (such as division, expansion, and differentiation), regulation of genes, organ development, pigment formation, metabolite synthesis, stress resistance, and several tropic responses (Ryu and Patten 2008; Ashrafuzzaman et al. 2009). Previous studies have reported the production and release of IAA by approximately 80% of rhizospheric microorganism as their secondary metabolite which may alter the intrinsic production of phytohormone and also change the permeability of plant cell wall for enhanced release of root exudates (Glick 2012; Spaepen et al. 2007). Apart from growth and development processes, IAA is also involved in defense mechanism and plant-microbe interaction (Santner and Estelle 2009; Spaepen and Vanderleyden 2011). Numerous microflora such as Pseudomonas, Mycobacterium, Rhizobium, Bacillus, and Rhizobia uphold the ability to produce IAA and influence the numerous processes of host plant ranging from phytostimulation to pathogenesis (Mandal et al. 2007). PGPRs with IAA-producing abilities can be applied as biofertilizer and/or bioenhancers as they elevate root expansion through lateral and adventitious root formation, thereby increasing surface area for increased uptake of nutrient and water. Apart from regulating cellular processes, IAA also stimulates vascular bundle formation and nodule formation (Glick 2012). Enhancement in seed germination and physio-morphological changes have been reported in the orchids which were treated with IAA-producing PGPRs such as Azospirillum brasilense and Bradyrhizobium japonicum (Cassa’na et al. 2009).
5.3.2.4 ACC Deaminase
As a plant growth hormone, ethylene is a crucial metabolite generated endogenously by almost all plants and involved in conventional growth and development of host plant. Besides being involved in growth, ethylene is also confirmed as stress hormone as it affects plant growth through defoliation and other noticeable changes mainly in seedlings during biotic and/or abiotic stress conditions (Saleem et al. 2007; Bhattarcharya and Jha 2012). Numerous PGPRs including Acinetobacter, Achromobacter, Agrobacterium, Alcaligenes, Azospirillum, Bacillus, Burkholderia, Enterobacter, Pseudomonas, etc. enhance plant growth through ACC deaminase activity. ACC deaminase is a pyridoxal 5-phosphate (PLP)-dependent polymeric enzyme which was initially reported in soil bacterium Pseudomonas (Honma and Shimomura 1978). A remarkable amount of ACC is released by the plant as root exudates in the soil to maintain the endogenous and external balance which in turn is utilized by PGPRs having ACC deaminase activity, thereby enhancing their proliferation (Glick et al. 1998). The enzyme utilizes the immediate precursor of ethylene and 1-aminocyclopropane-1-carboxylate and hydrolyzes it to α-ketobutyrate and ammonia which is further consumed as carbon and nitrogen sources by PGPRs (Arshad et al. 2007; Glick et al. 1998; Honma and Shimomura 1978). Further, according to Glick (2005), ACC deaminase activity varies in different organisms, and those with high activity bind inclusively to plant surfaces. Due to ACC deaminase activity of PGPRs, the endogenous level of ethylene reduces which in turn provides resistance against several stresses such as drought, salinity, flooding, high temperature, heavy metals, aromatic hydrocarbons, high radiations, wounding, insect predation, phytopathogens, etc. (Glick 2012; Lugtenberg and Kamilova 2009). Root elongation, shoot growth promotion, enhanced uptake of NPK, and increased nodulation with mycorrhizal colonization are some of the observable changes seen in plants inoculated with PGPRs (Nadeem et al. 2007, 2009; Glick 2014; Kumari et al. 2016).
5.3.3 Synthesis of Allelochemicals
Along with the growth promotion, PGPRs provide biocontrol activity through the secretion of allelochemicals which includes antibiotics, siderophores, biocidal volatiles, lytic enzymes, etc. (Bais et al. 2004; Glick 1995; Sturz and Christie 2003; Vaishnav et al. 2015, 2017).
5.3.3.1 Siderophore Production
Iron is an essential nutrient for all living forms with certain exceptions (Neiland 1995). In the rhizospheric region under aerobic environment, the ferric form of iron gets converted into insoluble hydroxides and oxyhydroxides, thereby raising the problem of iron scarcity (Rajkumar et al. 2010). Under limiting and competitive environment, rhizospheric microorganisms synthesize intra- and extracellular water-soluble peptidic iron chelator of low molecular weight, i.e., siderophore with different side chains and functional groups behaving as ligands with a different affinity (Crosa and Walsh 2002). Different edaphic and environmental factors such as amount and type of iron, pH of the soil, availability of macronutrients, the concentration of trace elements, etc. can regulate the synthesis of siderophores (Duffy and Defago 2000). These molecules can be classified into three major groups, namely, catecholates, hydroxamates, and carboxylates, on the basis of ligands utilized in ferric ion chelation (Xie et al. 2006). The efficiency of siderophore depends on the association constant of their complex formation with ferric ions. Rhizospheric siderophores uphold the higher value of association constant, thereby generating a severe iron-deficient condition for the pathogenic microorganism. Siderophores function as solubilizing agents for iron under limiting condition by reducing ferric ions to a ferrous ion which are further transported to cell interior through the gated membrane system. After this phenomenon, siderophores either get recycled or destroyed (Indiragandhi et al. 2008; Rajkumaret al. 2010; Neilands 1995). Along with iron sequestration, siderophores uphold the ability to form stable complexes with hazardous heavy metals such as Pb, Cd, Zn, Cu, Al, and Ga and radionuclide such as U, Np, etc. which are of alarming concern to the environment (Neubauer et al. 2000; Kiss and Farkas 1998).
5.3.3.2 Lytic Enzymes
Production and secretion of numerous enzymes from rhizospheric microorganism are involved in disrupting pathogenic membranes through hyperparasitic activity (Chernin and Chet 2002). Previous studies revealed that different enzymes including hydrolase, chitinase, lipases, pectinase, etc. attack pathogenic microorganisms through different mechanisms. Chitinase inhibits further spread of pathogen through hindering elongation of germ tube and spore germination (Frankwoski et al. 2001; Ordentlich et al. 1988). Some of the specific enzymes such as laminarinase are released by PGPRs alone or in combination with other enzymes to restrict specific pathogenic microorganism (Lim et al. 1991). Certain forms of glucanase, i.e., β1–3, β1–4, and β1–6, along with certain proteases directly target the glucans present in the fungal cell wall and destroy its integrity (Valois et al. 1996; Simons et al. 1997; Frankowski et al. 2001; Kamensky et al. 2003).
5.3.3.3 Antibiotic Production
Among the various methods applied by rhizospheric microorganisms to check proliferation of phytopathogens, antibiosis including the production and secretion of antibiotics is most commonly applied (Glick et al. 2007a, b; Lugtenberg and Kamilova 2009; Whipps 2001). Antibiotics are low molecular weight heterogeneous organic compounds, or metabolites primarily governed by nutrient availability and other environmental factors (Thomashow 1996; Duffy 2001). Even at low concentrations, these metabolites possess antimicrobial, antiviral, insecticidal, cytotoxic, antioxidant, antitumor, antihelminthic, and plant growth-promoting properties (de Bruijn et al. 2007; Raaijmaker et al. 2010). Broadly, these antibiotics can be classified into volatile and nonvolatile compounds which are further grouped into various subclasses. Nonvolatile antibiotics include polyketides, heterocyclic nitrogenous compounds, phenylpyrrole, cyclic lipopeptides, lipopeptide, and amino polyols, whereas hydrogen cyanide, aldehydes, alcohols, ketones, and sulfides are grouped under volatile antibiotics (Defago 1993; de Souza et al. 2003; Nielsen and Sorensen 2003; Raaijmakerset al. 2002). Pseudomonas, Bacillus, Streptomyces, Burkholderia, Brevibacterium, and several other microorganisms have been reported to produce and secrete antibiotics of a broad spectrum range (Keel et al. 1997; Haas and Keel 2003; Bender et al. 1999; Sutherland et al. 1985; Anjaiah et al. 1998).
5.4 PGPR Resistance to Biotic and Abiotic Stresses
A thorough understanding of the various mechanisms undertaken by PGPRs, particularly to resist biotic or abiotic stresses, is of paramount importance, more so because of the congregative nature of stress imposition. This would include not only the molecular identification of the bacterial strains involved but also the physiological as well as molecular mechanisms employed during the host-PGPR interaction.
5.4.1 Biotic Stress
Rhizospheric microbiota, particularly PGPRs, enable the augmentation of the inherent ability of plants to defend themselves against phytopathogens, apart from being a suitable alternative against chemical fertilizers (Sarma et al. 2015; Jain et al. 2012; Spence et al. 2014). In this context, management of phytopathogens through a microbial consortium or the use of endophytes has shown much promise. While endophytes have the inherent ability to provide plant protection and immunity enhancement due to their tendency of remaining sheltered within the plant interior (Ray et al. 2018a, b), microbial consortia remain in the vicinity of environmental stress but a strong promise to combat phytopathogens (Whipps 2001; Gossen et al. 2001; Stockwell et al. 2011). Several reports justify the plant growth promotional and improved disease resisting potential of PGPRs, such as Pseudomonas spp., Trichoderma spp., Bacillus spp., etc., on a variety of host plants, such as chickpea, pea, pigeon pea, okra, radish, tomato, wheat, pepper, Arabidopsis, etc. (Duffy et al. 1996; Rudresh et al. 2005; Jetiyanon 2007; Kannan and Sureendar 2009; Jain et al. 2012; Singh et al, 2013; Chauhan and Bagyaraj 2015).
The chief mechanism behind stimulation of the innate defense response of host plants by PGPRs is through induction of induced systemic resistance, operating in response to a microbial elicitor (Shoresh et al. 2010). In this context, Jain et al. (2012) reported enhancement of defense enzymes, particularly peroxidase, polyphenol oxidase, superoxide dismutase, glucanase, chitinase, etc., as well as phenol accumulation and lignin deposition in response to priming with a consortial mixture of PGPRs. In another study by Jain et al. (2015), the microbial consortia have been reported to recuperate the oxidative burst pathway inhibited by oxalic acid, the chief pathogenic factor of Sclerotium rolfsii/Sclerotinia sclerotiorum. Thus, the above studies clearly justify that PGPRs not only induce an augmented form of defense response within the host but also enable the quenching of factors responsible for induction of oxidative stress response within the host (Hammerschmidt 2005; Singh et al. 2013).
5.4.2 Abiotic Stress
Stress in nature is not a single phenomenon but a cumulative effect of various minor and major factors acting in togetherness (Mahajan and Tuteja 2005). While several natural stresses, such as drought, salt, flooding, and high/low temperature, have resulted in lowering of plant growth, certain anthropogenic activities have led to an additional confrontation with heavy metal stress, thereby declining crop yield and productivity by a significant level (Ramegowda and Senthil-Kumar 2015). Further, heavy metals sediment in soils and lead to groundwater contamination, thereby causing human health hazards. In other words, abiotic stresses may be considered as a root cause of loss of yield of several major crops (Bray 2004).
5.4.2.1 Drought Stress
Incessant reduction of rainfall year after year has led to a significant lowering of soil moisture content. Currently, even temperate regions are devising novel strategies to enhance the use of soil moisture content (Bray 2004; Farooq et al. 2009; Azcon et al. 2013; Panwar et al. 2014). Plant photosynthesis and nutrient uptake depend on a large scale on water availability in soil. Drastic reduction of soil moisture content or appearance of drought conditions severely hampers the basic requirements of the plant. For instance, water scarcity simultaneously increases the solute concentration within the plant cells, or a reduction in water potential, which in turn affect shoot and root elongation of plants. Further, water deficiency lowers carbon dioxide access by plants, thereby resulting in reactive oxygen species formation, such as superoxide, peroxide, and hydroxyl radical within plant cells, which in turn leads to apoptotic cell death of the plant (Sgherri et al. 2000).
In the above context, PGPR, such as Pseudomonas mendocina and Glomus intraradices or G. mosseae, was reported to release catalase enzyme and quench ROS produced within lettuce plants grown under severe drought conditions (Kohler et al. 2008). Thus PGPR may be considered as augmentation of defense enzymes in plants, such as peroxidase, polyphenol oxidase, etc. which further lead to protection of plant cell membrane and genomic DNA from oxidative damage (Bowler et al. 1992). Apart from individual PGPR, microbial consortia play a greater role in redemption from drought stress and in the improvement of plant growth. For instance, according to Figueiredo et al. (2008), a consortial mixture of beneficial PGPRs improved the overall health and nodulation of Phaseolus vulgaris under drought conditions as compared to inoculation with Rhizobium only. While report suggested PGPR treatment recuperated leaf water potential, biomass content, as well as sugar, proline, and amino acid content and loss of electrolyte leakage from plants (Sandhya et al. 2010; Vaishnav et al. 2018), treatment with consortial mixture of PGPR (Bacillus lentus, Pseudomonadales sp., and Azospirillum brasilense) augmented antioxidant activity as well as photosynthetic capacity along with the aforementioned properties in Ocimum basilicum (Heidari and Golpayengani 2012). Moreover, according to Stefan et al. (2013), consortial inoculation of PGPR improved superoxide dismutase and peroxidase activity in runner bean.
5.4.2.2 Salinity Stress
Presence of excessive amount of cations, such Na+, K+, Ca2+, Mg2+, etc., as well as anions, such as Cl−, CO32−, NO3−, SO42−, and HCO3−, in agricultural soils may be defined as saline stress (Yadav et al. 2011). As per the US Department of Agriculture (USDA) standards, soil having an electrical conductivity (EC) 4 dS m−1 or higher may be considered as saline soil (Seidahmed et al. 2013). Numerous reports imply saline stress as the chief cause of (a) development of drought-like situation on owing to shortage of water; (b) development of the payment of high ionic content in plants, thereby perturbing the normal physiological pathway; and (c) unavailability of other soil nutrients due to high salt concentration (Vaishnav et al. 2016). Munns (2002) reported stunted growth in plants exposed to salt stress due to lowering of water content with a simultaneous elevation in salt content. Further, accumulation of Na+ ion content within host tissues led to additional necrosis (Parida and Das 2005) apart from interfering with the root cell plasma membrane, thereby causing stunted root growth and nutrient uptake (Yadav et al. 2011).
In the above context, priming of plants with PGPRs offers a plausible respite against salt stress (Kumari et al. 2015). Han and Lee (2005) reported that priming of lettuce plants with Serratia sp. and Rhizobium sp. did not adversely affect the growth and physiological parameters of the plant under salt stress conditions. Similarly, an enhanced nodule formation was observed in common bean and soybean at 25 mM salt concentrations upon priming with a consortial mixture of R. tropici (CIAT899) or R. etli(ISP42) and Ensifer fredii (Sinorhizobium) SMH12 and HH103 with Chryseobacterium balustinum Aur9 (Estevezi et al. 2009). In another report by Bano and Fatima (2009), priming of maize varieties with Pseudomonas sp. and Rhizobium sp. augmented plant growth promotional parameters even under salt stress. Similarly, a significant increase in growth promotional parameters of wheat plants under salinity stress was observed upon priming with a consortium of Pseudomonas fluorescens, Enterobacter cloacae, Serratia ficaria, and P. putida (Nadeem et al. 2013a, b).
5.4.2.3 Heavy Metal Stress
The industrial revolution, as well as some of the anthropogenic activities, has resulted in a significant increase in heavy metals and radionuclides in the soil. Few among these such as molybdenum (Mo), iron (Fe), and manganese (Mn) are reported to be essential for the photosystem, yet others, such as cadmium (Cd), mercury (Hg), chromium (Cr) etc., are particularly considered as nonessential elements. Extreme accumulation of particularly the nonessential elements not only affects the soil microflora (Oliveira and Pampulha 2006; Wani and Khan 2010; Cheng 2003) but also get translocated to different photo organelles, thereby causing disruption of membranes and simultaneous disintegration of cell organelles as well as a complete collapse of the essential physiological functions, such as photosynthesis, protein synthesis, etc. (Bray 2004; Morsy et al. 2013). Various studies have particularly focused on PGPR as effective bioremediation as well as enhancers of plant growth (He and Yang 2007; Madhaiyan et al. 2007). Dary et al. (2010) suggested augmented yield, biomass, as well as nitrogen content in plants treated with consortia of Bradyrhizobium sp., Ochrobactrum cytisi, and metal-tolerant Pseudomonas sp. In yet another report by Singh et al. (2010), mung bean treated with metal-tolerant PGPR exhibited augmentation in growth and biomass when grown in cadmium-infected soil. Similarly, Marques et al. (2013) reported lower metal accumulation within tissues of Helianthus annuus treated with Ralstonia eutropha and Chryseobacterium hispalense when grown in Cd- and Zn-infected soil.
5.5 Application and Future Prospects
Application of PGPR such as Pseudomonas spp., Bacillus spp. Rhizobium spp., Mesorhizobium, Bradyrhizobium, Azospirillum, Azotobacter, etc. has been reported to increase seed weight, yield, plant height, leaf area, shoot dry weight, and root growth significantly in several crops, such as maize, mung bean, soybean, wheat, groundnut, chickpea, cotton, and Brassica spp. (Ahemad and Khan 2010; Ahemad and Kibret 2014; Gholami et al. 2009; Zahir et al. 2010). Mechanisms, such as nitrogen fixation, phosphate solubilization, potassium solubilization, siderophore biosynthesis, IAA production, ACC deaminase synthesis, cytokinin, and gibberellin production, are responsible for plant growth promotion and enhanced crop yield (Bashan and Holguin 1997). Plant disease management mediated by PGPR will curtail the pesticide load and reduce disease in an eco-friendly manner, particularly by posing competition for nutrients, induced systemic resistance, metabolites production, etc. (Lugtenberg and Kamilova 2009). Accumulation of hazardous substances possesses a major threat to the environment. Phytoremediation involves the use of plants or plant product to degrade hazardous substances accumulated in the environment (Cunningham et al. 1995). The compromised growth of plants at contaminated sites can be overcome by application of PGPR (Burd et al. 2000). PGPRs, such as Agrobacterium radiobacter, Azospirillum spp., Pseudomonas spp., Enterobacter spp., have been reported to speed up detoxification of contaminants, including cadmium, lead, nickel, chromium, and zinc by increased uptake as well as promotion of growth and biomass accumulation in barley, maize, rye, canola, and tomato grown on contaminated site (Belimov et al. 1998; Belimov and Dietz 2000; Hoflich and Metz 1997; Burd et al. 1998; Lucy et al. 2004). Further, PGPR can survive and promote plant growth in a colder climate with the help of antifreeze proteins and aid in survival under salinity and drought stress by ACC deaminase mediated lowering of ethylene level (De Freitas and Germida 1990; Hamaoui et al. 2001; Vaishnav et al. 2016). PGPR has the ability to promote plant growth under abiotic stresses such as drought, flood, extreme temperature, high light, the presence of toxic metals and organic contaminants, and radiation and biotic stresses: insect predation, the nematodes, fungi, bacteria, and viruses (Glick 2012). Thus the above property of PGPR equips it as potential biofertilizer, biocontrol agent, psychostimulant, and phytoremediator.
Continuously increasing demand for food grain production, the simultaneous buildup of chemical residue in the food chain has led to environmental pollution. The shift toward environmental friendly methods of disease management has thus become the need of the hour. In this context, according to Tewari and Arora (2013), future research needs to be directed toward bioengineering of rhizospheric biology to achieve the desired level of crop yield by manipulating microbes as well as their microclimate. Development of ready-to-use formulation of microbial consortia could be quiet effective over its single products in plant stress reduction. Researches need to be focused on optimizing shelf life, conditions for growth, enhanced crop yield, tolerance to unfavorable environmental conditions, and development of cost-effective PGPR products affordable to farmers. The molecular and biotechnological approaches need to be exploited to explore the rhizospheric biology and attain the desired level of microbial disease control. Bioinoculants of higher efficacy need to be developed for high-value crops such as flowers, fruits, and vegetables. Further, according to Nadeem et al. (2013), the low-temperature stress may be recuperated by exploiting ice-nucleating plant growth-promoting rhizobacteria (Nadeem et al. 2013). In addition, researches need to be focused on potassium-solubilizing plant growth-promoting rhizobacteria for an augmented utilization of potassium, the third most essential macronutrient after nitrogen and phosphorus. A better understanding of plant growth-promoting rhizobacteria needs to be developed regarding the mechanism of action, plant growth promotion, ecology, and growth-stimulating effect on the plant. These will help us in the identification, screening, and development of potential commercial formulations to combat phytopathogens and maintain a sustainable agroecosystem (Nelson 2004; Gupta et al. 2015).
5.6 Conclusion
After having a glance of applications and future prospects, we can conclude that PGPRs have a multidimensional approach in favor of living organisms and the environment. Their efficiency can further be enhanced through their optimization and acclimatization in the provided space. Different inoculation system can be applied on PGPR to maintain their establishment and improve their efficiency. After the competency test, strains with the different feature can be used in combination to survive diverse and extreme environmental condition. Further detailed studies will come up with a more potent rhizobacterial strain to survive diverse ecological situations. Studies at the genetic level can provide us with a next-generation solution through forward or reverse genetics. On a precise note, PGPRs either in combination or alone could be a better and safer alternative to the chemical means.
References
Abhilash PC, Dubey RK, Tripathi V, Srivastava P, Verma JP, Singh HB (2013) Remediation and management of POPs-contaminated soils in a warming climate: challenges and perspectives. Environ Sci Pol 20(8):5879–5885
Ahemad M (2012) Implications of bacterial resistance against heavy metals in bioremediation: a review. IIOABJ 3:39–46
Ahemad M, Khan MS (2010) Comparative toxicity of selected insecticides to pea plants and growth promotion in response to insecticide-tolerant and plant growth promoting Rhizobium leguminosarum. Crop Prot 29:325–329
Ahemad M, Khan MS (2012) Alleviation of fungicide-induced phytotoxicity in green gram Vigna radiata (L.) Wilczek using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi J Biol Sci 19:451–459
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: a current perspective. J King Saud Univ Sci 26(1):1–20
Ahemad M, Malik A (2011) Bioaccumulation of heavy metals by zinc resistant bacteria isolated from agricultural soils irrigated with wastewater. J Bacteriol 2:12–21
Ahemad M, Khan MS, Zaidi A, Wani, PA (2009) Remediation of herbicides contaminated soil using microbes. Microbes in Sustainable Agriculture, pp 261–284
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163(2):173–181
Anjaiah V, Koedam N, Nowak-Thompson B, Loper JE, Höfte M, Tambong JT, Cornelis P (1998) Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 and Tn 5 derivatives toward Fusarium spp. and Pythium spp. Mol Plant Microbe Int 11(9):847–854
Arshad M, Saleem M, Hussain S (2007) Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol 25(8):356–362
Ashrafuzzaman M, Hossen FA, Ismail MR, Hoque A, Islam MZ, Shahidullah SM, Meon S (2009) Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth. Afr J Biotechnol 8:7
Azcon R, Medina A, Aroca R, Ruiz-Lozano JM (2013) Abiotic stress remediation by the arbuscular mycorrhizal symbiosis and rhizosphere bacteria/yeast interactions. In: de Bruijn FJ (ed) Molecular microbial ecology of the rhizosphere. Wiley, Hoboken, pp 991–1002
Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9(1):26–32
Bano A, Fatima M (2009) Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol Fertil Soil 45:405–413
Bashan Y, Holguin G (1997) Azospirillum-plant relationships: environmental and physiological advances (1990–1996). Can J Microbiol 43:103–121
Belimov AA, Dietz K (2000) Effect of associative bacteria on element composition of barley seedlings grown in solution culture at toxic cadmium concentrations. Microbiol Res 155:113–121
Belimov AA, Kunakova AM, Gruzdeva EV (1998) Influence of soil pH on the interaction of associative bacteria with barley. Microbiology 67:463–469
Bender CL, Alarcón-Chaidez F, Gross DC (1999) Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63(2):266–292
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350
Bishop PE, Jorerger RD (1990) Genetics and molecular biology of an alternative nitrogen fixation system. Plant Mol Biol 41:109–125
Bowler C, van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Mol Biol 43:83–116
Bray EA (2004) Genes commonly regulated by water deficit stress in Arabidopsis thaliana. J Exp Bot 55:2331–2341
Burd GI, Dixon DG, Glick BR (1998) A plant-growth promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46:237–245
Cassana F, Perriga D, Sgroya V, Masciarellia O, Pennab C, Lunaa V (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E 109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45:28–35
Chandler D, Davidson G, Grant WP, Greaves J, Tatchell GM (2008) Microbial biopesticides for integrated crop management: an assessment of environmental and regulatory sustainability. Trends Food Sci Technol 19:275–283
Chaudhary V, Prasanna R, Nain L, Dubey SC, Gupta V, SinghR JS, Bhatnagar AK (2012) Bioefficacy of novel cyanobacteria-amended formulations in suppressing damping off disease in tomato seedlings. World J Microbiol Biotechnol 28:3301–3310
Chauhan H, Bagyaraj DJ (2015) Inoculation with selected microbial consortia not only enhances growth and yield of French bean but also reduces fertilizer application under field condition. Sci Horticult 197:441–446
Cheng S (2003) Effects of heavy metals on plants and resistance mechanisms. Environ Sci Pollut Res 10:256–264
Chernin L, Chet I (2002) Microbial enzymes in biocontrol of plant pathogens and pests. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology, and applications. Marcel Dekker, New York, pp 171–225
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66(2):223–249
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. In: Food security in nutrient-stressed environments: exploiting plants’ genetic capabilities. Springer, Dordrecht, pp 201–213
Dary M, Chamber-Parez MA, Palomares AJ, Pajeuelo E (2010) In situ phytostabilization of heavy metal polluted soils using Lupinus luteus inoculating with metal resistant plant growth promoting rhizobacteria. J Hazard Mater 177:323–330
De Bruijn I, de Kock MJ, Yang M, de Waard P, van Beek TA, Raaijmakers JM (2007) Genome-based discovery, structure prediction and functional analysis of cyclic lipopeptide antibiotics in Pseudomonas species. Mol Microbiol 63(2):417–428
De Freitas JR, Germida JJ (1990) Plant growth promoting rhizobacteria for winter wheat. Can J Microbiol 36:265–272
de Souza JT, de Boer M, de Waard P, van Beek TA, Raaijmakers JM (2003) Biochemical, genetic, and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl Environ Microbiol 69:7161–7172
Dean DR, Jacobson MR (1992) Biochemical genetics of nitrogenase. In: Stacey G, Burris H, Evans HJ (eds) Biological nitrogen fixation. Chapman and Hall, New York, pp 763–834
Défago G (1993) 2, 4-Diacetylphloroglucinol, a promising compound in biocontrol. Plant Pathol 42(3):311–312
Duffy BK (2001) Competition. In: Maloy OC, Murray TD (eds) Encyclopedia of plant pathology. Wiley, New York, pp 243–244
Duffy BK, Défago G (2000) Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol 66(8):3142–3150
Duffy BK, Simon A, Weller DM (1996) Combination of Trichoderma koningii with fluorescent pseudomonads for control of take-all on wheat. Phytopathology 86:188–194
Estevezi J, Dardanellii MS, Megiase M, Rodriguez-Navarro DN (2009) Symbiotic performance of common bean and soybean co-inoculated with rhizobia and Chryseobacterium balustinum Aur9 under moderate saline conditions. Symbiosis 49:29–36
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Figueiredo MVB, Burity HA, Martinez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by coinoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
Frankowski J, Lorito M, Scala F, Schmidt R, Berg G, Bahl H (2001) Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch Microbiol 176:421–426
Gholami A, Shahsavani S, Nezarat S (2009) The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Int J Biol Life Sci 1:35–40
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:2109–2117
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251(1):1–7
Glick BR (2012) Plant growth-promoting Bacteria: mechanisms and applications. Hindawi Publishing Corporation, Scientifica
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169(1):30–39
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190(1):63–68
Glick BR, Patten CL, Holguin G, Penrose GM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London
Glick BR, Cheng Z, Czarny J, Duan J (2007a) Promotion of plant growth by ACC deaminase-producing soil bacteria. In: New perspectives and approaches in plant growth-promoting Rhizobacteria research. Springer, Dordrecht, pp 329–339
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007b) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26(5–6):227–242
Gossen BD, Rimmer SR, Holley JD (2001) First report of resistance to benomyl fungicide in Sclerotinia sclerotiorum. Plant Dis 85:1206
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37(3):395–412
Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7(2):096–102
Haas D, Keel C (2003) Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu Rev Phytopathol 41:1117–1153
Hallman J, Quadt-Hallman A, Mahafee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hamaoui B, Abbadi JM, Burdman S, Rashid A, Sarig S, Okon Y (2001) Effects of inoculation with Azospirillum brasilense on chickpeas Cicer arietinum and faba beans Viciafaba under different growth conditions. Agronomie 21:553–560
Hammerschmidt R (2005) Phenols and plant-pathogen interactions: the saga continues. Physiol Mol Plant Pathol 66:77–78
Han HS, Lee KD (2005) Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res J Agric Biol Sci 1:210–215
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
He ZL, Yang XE (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8(3):192–207
Heidari M, Golpayengani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci 11:57–61
Hiltner LT (1904) On every experiences and problems in the field of soil bacteriology and under special supervision of the foundation and Broche. Job Deut Landw Ges Berlin 98:59–78
Hoflich G, Metz R (1997) Interactions of plant-microorganism associations in heavy metal containing soils from sewage farms. Bodenkultur 48:239–247
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42(10):1825–1831
Indira Gandhi P, Anandham R, Madhaiyan M, Sa TM (2008) Characterization of plant growth–promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Curr Microbiol 56(4):327–333
Jain A, Singh S, Sarma BK, Singh HB (2012) Microbial consortium mediated reprogramming of defense network in pea to enhance tolerance against Sclerotinia sclerotiorum. J Appl Microbiol 112:537–550
Jain A, Singh A, Singh S, Singh HB (2015) Biocontrol agents-mediated suppression of oxalic acid-induced cell death during Sclerotinia sclerotiorum-pea interaction. J Basic Microbiol 55(5):601–606
Jetiyanon K (2007) Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biol Control 42:178–185
Kamensky M, Ovadis M, Chet I, Chernin L (2003) Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biol Biochem 35(2):323–331
Kannan V, Sureendar R (2009) Synergistic effect of beneficial rhizosphere microflora in biocontrol and plant growth promotion. J Basic Microbiol 49:158–164
Keel C, Défago G, Gange AC, Brown VK (1997) Interactions between beneficial soil bacteria and root pathogens: mechanisms and ecological impact. In: Multitrophic interactions in terrestrial system. Blackwell Science, Oxford, pp 27–47
Khan MS, Zaidi A, Wani PA, Oves M (2009) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 7:11–19
Kim J, Rees DC (1994) Nitrogenase and biological nitrogen fixation. Biochemistry 33(2):389–397
Kiss T, Farkas E (1998) Metal-binding ability of desferrioxamine B. J Incl Phenom Macro 32(2–3):385–403
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. In Station de pathologie vegetable etphyto-bacteriology (ed) Proceedings of the 4th international conference on plant pathogenic bacteria, vol. II. Gilbert-Clarey, Tours, France, pp 879–882
Kohler J, Hernandez JA, Caravaca F, Roldan A (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Func Plant Biol 35:141–151
Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK (2015) Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J Plant Growth Regul 34(3):558–573
Kumari A, Goyal RK, Choudhary M, Sindhu SS (2016) Effects of some plant growth promoting rhizobacteria (PGPR) strains on growth and flowering of chrysanthemum. J Crop Weed 12(1):7–15
Ladha JK, De Bruijn FJ, Malik KA (1997) Introduction: assessing opportunities for nitrogen fixation in rice-a frontier project. Plant Soil 194(1–2):1–10
Lim HS, Kim YS, Kim SD (1991) Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol 57(2):510–516
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 86(1):1–25
Lugtenberg BJ, Dekkers LC (1999) What makes Pseudomonas bacteria rhizosphere competent? Environ Microbiol 1(1):9–13
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Ma Y, Rajkumar M, Luo Y, Freitas H (2011a) Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J Hazard Mater 195:230–237
Ma Y, Rajkumar M, Vicente JA, Freitas H (2011b) Inoculation of Ni-resistant plant growth promoting bacterium Psychrobacter sp. strain SRS8 for the improvement of nickel phytoextraction by energy crops. Int J Phytoremediation 13:126–139
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444(2):139–158
Mandal SM, Mondal KC, Dey S, Pati BR (2007) Optimization of cultural and nutritional conditions for indole-3-acetic acid (IAA) production by a Rhizobium sp. isolated from root nodules of Vignamungo (L.) Hepper. Res J Microbiol 2:239–246
Marques APGC, Moreira H, Franco AR, Rangel AOSS, Castro PML (2013) Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria – effects on phytoremediation strategies. Chemosphere 92:74–83
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166(2):525–530
McKenzie RH, Roberts TL (1990) Soil and fertilizers phosphorus update. In: Proceedings of Alberta soil science workshop proceedings, Feb. 20–22, Edmonton, Alberta, pp 84–104
Mishra S, Singh A, Keswani C, Saxena A, Sarma BK, Singh HB (2015) Harnessing plant-microbe interactions for enhanced protection against phytopathogens. In: Plant microbes symbiosis: applied facets. Springer, New Delhi, pp 111–125
Morsy AA, Salama KHA, Kamel HA, Mansour MMF (2013) Effect of heavy metals on plasma membrane lipids and antioxidant enzymes of Zygophyllum species. Eurasia J Biosci 6:1–10
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2007) Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol 53(10):1141–1149
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2009) Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can J Microbiol 55(11):1302–1309
Nadeem SM, Naveed M, Zahir ZA, Asghar HN (2013a) Plant-microbe interactions for sustainable agriculture: fundamentals and recent advances. In: Arora NK (ed) Plant-microbe symbiosis: fundamentals and advances. Springer, India, pp 51–103
Nadeem SM, Zahir ZA, Naveed M, Nawaz S (2013b) Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann Microbiol 63:225–232
Nardi S, Concheri G, Pizzeghello D, Sturaro A, Rella R, Parvoli G (2000) Soil organic matter mobilization by root exudates. Chemosphere 41(5):653–658
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
Nelson LM (2004) Plant growth promoting rhizobacteria (PGPR): prospects for new inoculants. Crop Manag 3(1):0–0
Neubauer U, Furrer G, Kayser A, Schulin R (2000) Siderophores, NTA, and citrate: potential soil amendments to enhance heavy metal mobility in phytoremediation. Int J Phytoremediation 2(4):353–368
Nielsen TH, Sørensen J (2003) Production of cyclic lipopeptides by Pseudomonas fluorescens strains in bulk soil and in the sugar beet rhizosphere. Appl Environ Microbiol 69(2):861–868
Oerke EC (2005) Crop losses to pests. J Agric Sci 144:31–43
Oliveira A, Pampulha ME (2006) Effects of long-term heavy metal contamination on microbial characteristics. J Biosci Bioeng 102:157–161
Ordentlich A, Elad Y, Chet I (1988) The role of chitinase of Serratia marcescens in biocontrol of Sclerotium rolfsii. Phytopathology 78(1):84–88
Panwar M, Tewari R, Nayyar H (2014) Microbial consortium of plant growth-promoting rhizobacteria improves the performance of plants growing in stressed soils: an overview. In: Khan MS et al (eds) Phosphate solubilizing microorganisms. Springer International Publishing, Switzerland, pp 257–285
Parida AK, Das AB (2005) Salt stress and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–249
Parke JL (1991) Root colonization by indigenous and introduced microorganisms. In: Keister DL, Gregan PB (eds) The rhizosphere and plant growth. Kluwer Academic Publishers, Dordrecht, pp 33–42
Raaijmakers JM, Vlami M, De Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 81(1–4):537
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34(6):1037–1062
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149
Ramegowda V, Senthil-Kumar M (2015) The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol 176:47–54
Ray S, Singh S, Sarma BK, Singh HB (2016a) Endophytic Alcaligenes isolated from horticultural and medicinal crops promotes growth in okra (Abelmoschus esculentus). J Plant Growth Regul 35(2):401–412
Ray S, Singh V, Singh S, Sarma BK, Singh HB (2016b) Biochemical and histochemical analyses revealing endophytic Alcaligene sfaecalis mediated suppression of oxidative stress in Abelmoschus esculentus challenged with Sclerotium rolfsii. Plant Physiol Biochem 109:430–441
Ray S, Mishra S, Bisen K, Singh S, Sarma BK, Singh HB (2018a) Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathway. Microbiol Res 207:100–107
Ray S, Singh J, Rajput RS, Singh HB, Singh S (2018b) Endophytic Bacteria: an essential requirement of phyto nutrition. Nutr Food Sci Int J 5(2):1–5
Rovira AD (1965) Interactions between plant roots and soil microorganisms. Annu Rev Microbiol 19(1):241–266
Rubio LM, Ludden PW (2008) Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu Rev Microbiol 62:93–111
Rudresh DL, Shivaprakash MK, Prasad RD (2005) Effect of combined application of Rhizobium, phosphate solubilizing bacterium and Trichoderma spp. on growth, nutrient uptake and yield of chickpea (Cicer aritenium L.). Appl Soil Ecol 28:139–146
Ryu R, Patten CL (2008) Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by 4 TyrR in Enterobacter cloacae UW5. Am Soc Microbiol 190(Suppl 21):1–35
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:10635–10648
Sandhya V, Ali SZ, Grover M, Reddy G, Venkatesvarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. J Plant Growth Regul 62:21–30
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signaling. Nature 459(7250):1071
Sarma BK, Yadav SK, Singh S, Singh HB (2015) Microbial consortium-mediated plant defense against phytopathogens: readdressing for enhancing efficacy. Soil Biol Biochem 87:25–33
Saxena AK, Tilak KVBR (1998) In: Verma AK (ed) Free-living nitrogen fixers: its role in crop production. Microbes for health, wealth and sustainable environment. Malhotra Publ Co, New Delhi, pp 25–64
Schroth MN, Hancock JG (1981) Selected topics in biological control. Annu Rev Microbiol 35(1):453–476
Seidahmed HA, Ballal ME, Mahgoub A (2013) Sodicity tolerance of Moringa olifera, Acacia senegal and Acacia tortilis subspp. raddiana seedlings. J Nat Resour Environ Stu 1:4–6
Sgherri CLM, Maffei M, Navari-Izzo F (2000) Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol 157:273–279
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrolagents. Annu Rev Phytopathol 48:21–43
Simons M, Permentier HP, de Weger LA, Wijffelman CA, Lugtenberg BJJ (1997) Amino acid synthesis is necessary for tomato root colonization by Pseudomonas fluorescens strain WCS365. Mol Plant-Microbe Interact 10:102–106
Singh NK, Rai UN, Tewari A, Singh M (2010) Metal accumulation and growth response in Vigna radiata L. inoculated with chromate tolerant rhizobacteria and grown on tannery sludge-amended soil. Bull Environ Contam Toxicol 84:118–124
Singh A, Jain A, Sarma BK, Upadhyay RS, Singh HB (2013) Rhizosphere microbes facilitate redox homeostasis in Cicer arietinum against biotic stress. Ann Appl Biol 163(1):33–46
Singh A, Jain A, Sarma BK, Upadhyay RS, Singh HB (2014) Beneficial compatible microbes enhance antioxidants in chickpea edible parts through synergistic interactions. LWT-Food Sci Technol 56(2):390–397
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:4
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:4425–4448
Spence C, Alff E, Johnson C (2014) Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol 14:130
Stefan M, Munteau N, Stoleru V, Mihasan M (2013) Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status, and yield of runner bean. Rom Biotechnol Lett 18:8132–8143
Stockwell VO, Johnson KB, Sugar D, Loper JE (2011) Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 101:113–123
Sturz AV, Christie BR (2003) Beneficial microbial allelopathies in the root zone: the management of soil quality and plant disease with rhizobacteria. Soil Tillage Res 72(2):107–123
Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR (1985) Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother 27(4):495–498
Tewari S, Arora NK (2013) Transactions among microorganisms and plant in the composite rhizosphere. In: Arora NK (ed) Plant-microbe symbiosis: fundamentals and advances. Springer, New Delhi, pp 1–50
Thomashow LS (1996a) Biological control of plant root pathogens. Curr Opin Biotechnol 7(3):343–347
Thomashow M (1996b) Ecological identity: becoming a reflective environmentalist. MIT Press, Cambridge, MA
Turnbull GAJ, Morgan AW, Whipps JM, Saunders JR (2001) The role of bacterial motility in the survival and spread of Pseudomonas fluorescens in soil and in the attachment and colonization of wheat roots. FEMS Microbiol Ecol 36:21–31
Vaishnav A, Jain S, Kasotia A, Kumari S, Gaur RK, Choudhary DK (2014) Molecular mechanism of benign microbe-elicited alleviation of biotic and abiotic stresses for plants. In: Gaur RK, Sharma P (eds) Approaches to plant stress and their management. Springer, New Delhi, pp 281–295
Vaishnav A, Kumari S, Jain S, Varma A, Choudhary DK (2015) Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J Appl Microbiol 119:539–551
Vaishnav A, Kumari S, Jain S, Varma A, Tuteja N, Choudhary DK (2016) PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J Basic Microbiol 56(11):1274–1288
Vaishnav A, Varma A, Tuteja N, Choudhary DK (2017) Characterization of bacterial volatiles and their impact on plant health under abiotic stress. In: Choudhary DK, Sharma AK, Agarwal P, Varma A, Tuteja N (eds) Volatiles and food security. Springer, Singapore, pp 15–24
Vaishnav A, Kasotia A, Choudhary DK (2018) Role of functional bacterial phylum proteobacteria in Glycine max growth promotion under abiotic stress: a Glimpse on case study. In: Choudhary DK, Kumar M, Prasad R, Kumar V (eds) In silico approach for sustainable agriculture. Springer, Singapore, pp 17–49
Valois D, Fayad K, Barasubiye T, Garon M, Dery C, Brzezinski R, Beaulieu C (1996) Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot. Appl Environ Microbiol 62(5):1630–1635
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48:3262–3267
Welbaum GE, Sturz AV, Dong Z, Nowak J (2004) Managing soil microorganisms to improve the productivity of agro-ecosystems. Crit Rev Plant Sci 23:2175–2193
Whipps JM (1997) Developments in the biological control of soil-borne plant pathogens. Adv Bot Res Acad Press 26:1–134
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Xie X, Wang J, Yuan H (2006) High-resolution analysis of catechol-type siderophores using polyamide thin layer chromatography. J Microbiol Met 67(2):390–393
Yadav S, Irfan M, Ahmed A, Hayat S (2011) Causes of salinity and plant manifestations of salt stress: a review. J Environ Biol 32:667–685
Zahir ZA, Shah MK, Naveed M, Akhter MJ (2010) Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiate L. under salt stress conditions. J Microbiol Biotechnol 20:1288–1294
Zaidi A, Khan M, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung 56(3):263–284
Acknowledgment
JS is grateful to CSIR for providing financial support in form CSIR-JRF fellowship. PS and RSR are thankful to UGC for providing financial assistance in the form of UGC-RET fellowship. SR and HBS are thankful to Department of Science and Technology (DST) for awarding project grant (NRDMS/SC/ST/40/016).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, J., Singh, P., Ray, S., Rajput, R.S., Singh, H.B. (2019). Plant Growth-Promoting Rhizobacteria: Benign and Useful Substitute for Mitigation of Biotic and Abiotic Stresses. In: Sayyed, R., Arora, N., Reddy, M. (eds) Plant Growth Promoting Rhizobacteria for Sustainable Stress Management . Microorganisms for Sustainability, vol 12. Springer, Singapore. https://doi.org/10.1007/978-981-13-6536-2_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-6536-2_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6535-5
Online ISBN: 978-981-13-6536-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)