Abstract
The potential of endophytic bacteria to act as biofertilizers and bioprotectants has been demonstrated, and considerable progress has been made in explaining their role in plant protection. In the present study, three endophytic bacterial strains (BHU 12, BHU 16 isolated from the leaves of Abelmoschus esculentus, and BHU M7 isolated from the leaves of Andrographis paniculata) were used which displayed high sequence similarity to Alcaligenes faecalis. The biofilm formation ability of these endophytic strains in the presence of okra root exudates confirms their chemotactic ability, an initial step for successful endophytic colonization. Further, reinoculation of spontaneous rifampicin-tagged mutants into okra seedlings revealed a CFU count above 105 cells g−1 of all three endophytic strains in root samples during the first 15 days of plant growth. The CFU count increased up to 1013 by 30 days of plant growth, followed by a gradual decline to approximately 1010 cells g−1 at 45 days of plant growth. Systemic endophytic colonization was further supported by 2, 3, 5-triphenyl tetrazolium chloride staining and fluorescence imaging of ds-RED expressing conjugants of the endophytic strains. The strains were further assessed for their plausible in vivo and in vitro plant growth-promoting and antagonistic abilities. Our results demonstrated that the endophytic strains BHU 12, BHU 16, and BHU M7 augmented plant biomass by greater than 40 %. Root and shoot lengths of okra plants when primed by BHU 12, BHU 16, and BHU M7 increased up to 34 and 14.5 %, respectively. The endophytic isolates also exhibited significant in vitro antagonistic potential against the collar rot pathogen Sclerotium rolfsii. In summary, our results demonstrate excellent potential of the three endophytic bacterial strains as biofertilizers and biocontrol agents, indicating the possibility for use in sustainable agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Okra (Abelmoschus esculentus L. Moench) is arguably an important vegetable crop throughout the world. Being endowed with an affluent source of various vitamins and minerals, it is presently considered as a secret weapon against the deadly disease diabetes (Sabitha and others 2011). This context makes okra as one of the most sought after vegetable crops throughout the world. India ranks first in Okra production with approximately seven states participating actively in its cultivation (NCPAH 2014, www.ncpahindia.com).
Okra’s need for high temperature and humidity makes it prone to a magnitude of insect pests and pathogens. Pesticidal dependence, a quick plausible respite, however worsens the scenario as the crop is harvested at short intervals and consumed fresh (Randhawa and others 2007). A plethora of reports confirm the role of microbes, such as plant growth-promoting rhizobacteria (PGPR), as appropriate alternatives. PGPR are characteristically defined as the indispensible component of the total soil microbial community having characteristic properties of aggressive colonization, plant growth stimulation, and biocontrol (Vessey 2003; Bhattacharyya and Jha 2012; Jain and others 2012; Singh and others 2013a, b). However, benefits conferred by endophytes acquire a competitive advantage over PGPR (Etesami and others 2014). For instance, the survivability and colonizability of PGPR largely depend upon their intrinsic physiological properties, biotic and abiotic factors of the soil, whereas such is not the same for endophytes. The endemic bacteria are in close contact with their host plants and thereby largely protected from the abiotic and biotic stress conditions in the soil (Hallmann and others 1997; Rajkumar and others 2009). Another conspicuous feature of marked prominence is that re-introduction of endophytic bacteria does not affect the indigenous bacterial populations within host plants, unlike the introduction of PGPR in soil which causes an exemplar shift in the soil microbial community (Conn and Franco 2004).
A range of benefits are provided by the endophytes to their hosts including plant growth promotion and enhanced plant mineral uptake (Malinowski and Belesky 2000; Barka and others 2002; Kang and others 2007), reduction of disease incidence through induction of systemic plant defenses (Bargabus and others 2002; Coombs and others 2004; Senthilkumar and others 2007; Bakker and others 2007; Mishra and others 2015), and synthesis of anti-herbivory products (Scott 2001; Melnick and others 2008). In this context, Sturz and Nowak (2000) suggested that the early establishment of endophytes within the host root system is essential for the generation of their beneficial attributes, which is otherwise potentially challenging owing to the high biodiversity of the indigenous soil microbiota. However, the compatibility of the endophytic microbes to the host plant plays a major role in their successful establishment within the plant tissues. Endophytes residing within the host tissues establish a so-called dual fitness trait; a synergistic partnership wherein the plant-secreted metabolites are taken up by the endophytic microbes owing to their intimate interaction with the host cells. In return, the microbes release plant growth-promoting compounds beneficial to the host (Hardoim and others 2012; Singh and others 2013a, b). Other constructive attributes of endophytic microbes include an enhancement in water retention, and increment in biomass attained by a delay in flowering and leaf senescence, which further fixes a greater amount of carbon within the host (Owen and Hundley 2004).
The current work deals with isolation and identification of some endophytic bacteria from various agricultural crops and medicinal plants and evaluation of their biofertilizer and biocontrol efficacy in okra under greenhouse conditions. The screening was done on the basis of their plant growth-promoting traits and antagonistic potential against the collar rot pathogen Sclerotium rolfsii. S. rolfsii. Sacc. is a potentially harmful member of the sclerotial fungi having a wide host range of about 500 different plant species. Okra is one of the various hosts of this pathogen forming dark brown lesions on the stem, particularly at the collar region followed by progressive wilting of the stem and leaves (Koné and others 2012).
Materials and Methods
Isolation of Endophytic Bacterial Strains
Plant saplings (4 weeks old) were collected during the months of June–December (2012) from agricultural farms, and horticultural fields of Banaras Hindu University (B.H.U.), Varanasi, Uttar Pradesh, India (25° 28′ N, 82° 95′ E) and Karauta village, Bhadohi, Uttar Pradesh, India (25° 42′ N, 82° 57′ E). The plant tissues desired for isolation of endophytes included roots and the first nodal leaf. Surface sterilization of the desired tissue sections was carried out according to the method proposed by Jasim and others (2013). The tissues were washed thoroughly in tap water and were excised in 1 cm2 pieces. The sections were surface sterilized in 3 % sodium hypochlorite (NaClO) for 10 min followed by three thorough rinses in sterile distilled water. Maceration of the tissues was carried out in 0.85 % normal saline in the w/v ratio of 1:10 (tissue:saline) using a sterilized mortar-pestle. The macerate was further subjected to serial dilution and dilutions of 10−6, 10−7, and 10−8 were plated on nutrient agar (NA). Bacterial colonies formed on the NA plates after incubation at 28 °C for 24–48 h were further subcultured and purified. The isolates were stored on NA slants at 4 °C until further use.
Inoculum Preparation for Biopriming
Bacterial isolates (100 ml each) were grown in 250 ml conical flasks until the beginning of the stationary phase. The cultures were pelleted by centrifugation at 10,000 rpm for 10 min at 4 °C followed by washing of the culture pellets with 10 ml of 0.85 % sodium chloride solution to remove the residual culture broth. Finally, the pellets were suspended in 25 ml of 1 % carboxymethyl cellulose sodium salt (Merck) suspension and vortexed vigorously to obtain a homogenous cell density of about 4 × 108 (Jain and others 2013).
Gnotobiotic Plant Growth Promotion Assay
The growth-promoting ability of the isolates in okra seedlings was assessed in vitro by measuring the growth augmentation of bioprimed okra seedlings under gnotobiotic conditions. Healthy and uniform seedlings developed from okra seeds (A. esculentus cv. Sakata Prerna) and surface sterilized with 3 % NaClO for 10 min followed by three successive thorough rinses in sterilized water were inoculated with the suspensions of bacterial cultures for 2 h and placed in culture tubes containing a sterile sand-soil mixture as described by Botta and others (2013). The tubes were kept in the growth chamber for 45 days maintained at 28 ± 2 °C and relative humidity (RH) of 76 %. The control set consisted of water-soaked seedlings. The experiment was set up in triplicates.
Isolates expressing positive plant growth promotion under gnotobiotic conditions were selected for further experimentations.
Isolation of Pathogen S. rolfsii
Sclerotia of S. rolfsii Sacc. were collected from the collar region of okra (A. esculentus) plants exhibiting typical collar rot symptoms from the horticultural field of Banaras Hindu University (B.H.U.), Varanasi, India (25.28° N, 82.95° E). The sclerotia were surface sterilized using 1 % NaClO for 30 s followed by three thorough rinses in sterilized water. The surface-sterilized sclerotia were further cut using a sterilized scalpel and placed on potato dextrose agar (PDA) at 27 ± 2 °C for 4 days. The pathogen colony formed was further subcultured into fresh potato dextrose agar (PDA) medium.
Detection of In Vitro Antagonistic Activity of the Endophytic Strains Against S. rolfsii
Antagonistic activity against S. rolfsii was evaluated in vitro using the dual culture plate assay. The endophytic bacterial colonies were streaked individually on PDA plates, 2 cm from the edge of the plates. A 2 cm pathogen plug was placed on the opposite side of the bacterial inoculum at a distance of 2 cm from the other edge of the plate maintaining a 5 cm distance between the pathogen and the biocontrol endophytic strain. The plates were incubated at 27 ± 2 °C and the growth of the pathogen toward the bacterial colony was observed regularly. The experiment was set up in triplicate and inhibition of the pathogen was observed 4 days post incubation.
Further screening of the potential endophytic strains was done on the basis of their positive in vitro antagonism against S. rolfsii.
Identification of Endophytic Bacterial Strains
Genomic DNA of the selected endophytic bacterial strains was isolated according to Wilson (1987) with slight modifications. The culture pellets formed by centrifugation of the bacterial culture were subjected to lysozyme (0.5 mg ml−1), SDS (2.3 %) and proteinase K (0.2 mg ml−1) treatment followed by heating at 55 °C for 2–3 h. The suspension was recentrifuged and genomic DNA from the supernatant was extracted using phenol–chloroform–isopropanol and precipitated using ethanol.
For taxonomic identification of the selected strains, the 16S rDNA was amplified using the universal bacterial primers (forward 5′ AGAGTTGATCYTCGCTC 3′) and (reverse 5′ GYTACCTTGTTACGACT 3′) obtained from Bangalore GeneI Pvt. Ltd., Bengaluru, Karnataka, India. The PCR conditions included initial denaturation at 94 °C for 4 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 15 min (Technie, Bengaluru) (Mishra and Nautiyal 2012). Further, a phylogenetic tree was constructed using MEGA 6 software to authenticate the classification.
Assay of In Vitro Plant Growth Promotion Ability of the Selected Endophytic Strains
The plant growth promotion ability of the endophytic isolates was estimated by measuring their probable in vitro phosphate solubilization ability as well as their indole-3-acetic acid (IAA) and siderophore production potential. For the IAA production assay, the isolates were inoculated in nutrient broth amended with tryptophan (5 mM) and incubated for 48 h at 28 °C and 120 rpm. The culture supernatants were used for colorimetric estimations as demonstrated by Bric and others (1991). Phosphate solubilization ability was evaluated following the method of Mehta and Nautiyal (2001). The isolates were inoculated in NBRIP medium (Nautiyal 1999) for 48 h at 28 °C and 120 rpm. The culture supernatants were subjected to stannous chloride reduction of the molybdo-phosphoric acid and the absorbance of the color developed was recorded at 660 nm against a reagent blank. Siderophore production ability was determined according to Bano and Musarrat (2003), whereas HCN production ability was ascertained by the method of Dastager and others (2009). Proteolytic activity was assayed using skim milk agar medium (Liu and others 2009), whereas ammonia production ability was assayed according to Ahmad and others (2008).
Assay of Colonization Efficiency of the Strains
In vitro colonization efficiency, in the presence of okra root exudates, was assessed through the biofilm formation assay. Root exudates of okra were extracted and collected according to Yao and Allen (2006). The ability of cellular adhesion of the endophytic isolates in the presence and absence of root exudates was assessed as demonstrated by Tan and others (2013). The strains were grown to the mid log phase in NB. Aliquots from the respective cultures were further inoculated in 1/10 diluted NB containing okra root exudates followed by inoculation (100 µl) into the wells of a PVC microtitre plate. The plate was sealed with parafilm and incubated at 28 °C for 48 h. After 48 h, the plate was inverted to remove the contents followed by staining with 1 % crystal violet (for 10 min) and destaining with 95 % ethanol. The amount of biofilm formed was assayed by recording the absorbance of bound crystal violet in ethanol at A590.
Generation of Spontaneous Rifampicin-Resistant Mutants
Spontaneous rifampicin-resistant mutants of the selected strains were generated according to Chauhan and Nautiyal (2010). Colonies growing at 100 µg ml−1 rifampicin were selected for further experimentations and were subcultured on fresh NA-rifampicin plates.
Establishment of the Endophytic Nature of the Isolates in Okra (A. esculentus)
Inoculum preparation of spontaneous rifampicin-resistant mutants of the selected strains which proceeded by biopriming of surface-sterilized, healthy, and uniform okra seedlings was carried out as described earlier. The bioprimed seedlings were placed in culture tubes containing a sterile sand-soil mixture. The tubes were kept in a growth chamber for 45 days at 28 ± 2 °C and 76 % relative humidity (RH). The control set consisted of water-soaked seedlings. The experiment was set up in triplicate.
The colonizing efficiency of the strains was evaluated on the basis of CFU count on rifampicin-amended NA medium. Roots, shoots, and leaves of 15-day-old plants were excised using a sterilized blade and surface sterilized with 3 % NaClO solution. Further, the tissues were macerated using 0.85 % normal saline under sterile conditions. The macerate was serially diluted followed by plating of appropriate dilutions (10−4, 10−6, and 10−8) on NA medium amended with 50 µg ml−1 rifampicin to establish the authenticity of endophytic nature of the isolates. Inoculations of bacterial strains were made in triplicate. The above procedure was repeated up to 45 days at an interval of 15 days.
2, 3, 5-Triphenyl Tetrazolium Chloride (TTC) Staining of the Endophytic Strains
One-month-old okra seedlings bioprimed with the selected endophytic strains, growing under gnotobiotic conditions were surface sterilized in 3 % NaClO for 10 min followed by three subsequent washings in sterile deionized water. Root, shoot, and leaf tissues surface sterilized with 3 % NaClO for 10 min followed by three thorough rinses in sterile distilled water were soaked in TTC-malate buffer (0.625 gl−1 malate was dissolved in 0.05 M phosphate buffer and to the sterilized solution 1.5 gl−1 TTC was added). After 3 days, tissue sections (~50 µm) were prepared using a sterilized scalpel and observed for pinkish red spots of bacterial colonies at 10× and 100× magnifications under a light microscope (Nikon DS-fi1, Japan) (Thomas and Sekhar 2014).
Generation of Fluorescent Conjugant of the Endophytic Strains
A transconjugant of the strain BHU 12 with Escherichia coli DB3.1 expressing the ds-RED plasmid was prepared according to Nautiyal and others (1989). Minimal basal salt medium M9 amended with 5 µg ml−1 each of kanamycin and neomycin, 50 µg ml−1 each of ampicillin and streptomycin, 0.4 % glucose, 1 mM magnesium sulfate, and 0.25 mM calcium chloride was used for the selective growth of conjugants (BHU 12a) and to avoid the growth of auxotrophs. The conjugants were further subcultured on NA medium to determine the stability of the strains. The conjugant was inoculated onto surface-sterilized and aseptically germinated okra seedlings as described earlier. The inoculated seedlings were further transferred to culture tubes containing a sterile sand-soil mixture. The entire setup was incubated in a growth chamber for 15 days at 28 ± 2 °C and RH of 76 %, respectively. The control set consisted of water-soaked seedlings. The experiment was done in triplicates.
For microscopic observation, thin sections (~50 µm) were prepared aseptically from 10-day-old seedlings. A Nikon Eclipse E200 epifluorescence microscope fitted with a TRITC/rhodamine/PI, DM565 dichroic mirror, and BA605/55 barrier filter was used for red excitation. The sections were observed using a NIKON DS-F12 camera under 40X and 100X magnification.
Greenhouse Experiment
Experimental Setup
Inoculum preparation of the three endophytic strains, that is, BHU 12, BHU 16, and BHU M7 was carried out as described earlier. The greenhouse experiments were conducted in the Department of Mycology and Plant Pathology, Institute of Agricultural Sciences, B.H.U., Varanasi (25° 26′ N, 82° 99′ E). The plant tests were set up in pots (15 cm × 10 cm) having unsterilized soil and consisting of three replicates with five seedlings per replicate. The treatments included uninoculated okra as control, BHU 12-treated okra, BHU 16-treated okra, and BHU M7-treated okra seeds. The treatments were drenched every alternate day with their respective strains containing 8.0 log10 CFU ml−1, whereas the control set was drenched with NB. Irrigation was done to maintain the moisture content up to approximately 20 % as and when required.
Plant samples were analyzed at the day 30 and 45 for basic parameters of growth promotion, that is, root length, shoot length, leaf area, fresh weight (30 and 45 days), and plant biomass (30 and 45 days). Fresh weight was assessed by weighing the plants immediately post harvesting, whereas plant biomass was attained by oven-drying the plants at 80 °C for 2–3 days until a constant weight was achieved (Verwijst and Telenius 1999; Pandey and others 2012) The total chlorophyll content was determined by homogenizing the leaf tissues in 80 % acetone and recording the absorbance at 645 and 663 nm (Arnon 1949). The compatibility of the strains in live soil was assessed by serial dilution of the homogenate and plating on NA medium augmented with rifampicin (100 µg ml−1) as described by Nautiyal and others (2010).
Statistical Analysis
The data were expressed as means of three replications ± standard deviations (S.D.). One-way ANOVA was performed to compare the means with p ≤ 0.05 using SPSS ver. 16 software (SPSS, Inc., Chicago, IL, USA).
Results
Isolation and Characterization of Bacterial Endophytes
A total of 150 endophytic strains were collected from all healthy plants selected for isolation over a period of 6 months. For isolation of endophytic bacterial strains, plants were harvested and processed as described earlier. The isolated endophytes were further screened on the basis of their gnotobiotic plant growth promotion and antagonism against the collar rot pathogen, S. rolfsii. The selected strains were further assessed on the basis of their in vitro plant growth promotion attributes. Among the collected isolates, endophytic strains BHU 12, BHU 16 (isolated from A. esculentus leaf), and BHU M7 (isolated from Andrographis paniculata leaf) expressing significant plant growth promotion and positive antagonism against S. rolfsii were selected with the aim of harnessing their biofertilizer potential. All three endophytic strains produced IAA, HCN, siderophore, and solubilized phosphate apart from their inherent antagonistic potential against S. rolfsii (Table 1). Figures 1 and 2 elaborate the relative in vitro IAA production, phosphate solubilization, and their plant growth promotion under gnotobiotic conditions, whereas Fig. 3 displays the prominent antagonistic effect against S. rolfsii by the three strains in comparison to control. Strain BHU 16 was found to be the highest IAA producer (30.93 µg ml−1) and phosphate solubilizer (30.58 µg ml−1) followed by strains BHU M7 (26.13 µg ml−1; 25.88 µg ml−1) and BHU 12 (25.46 µg ml−1; 23.53 µg ml−1), respectively.
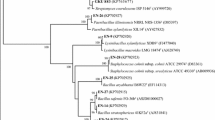
These characteristics clearly support that the endophytic strains have potential biofertilizer and biocontrol capacity in vitro. To find out the genetic relatedness and also to identify the endophytic strains (BHU 12, BHU 16, and BHU M7) at the molecular level, 16S ribosomal DNA was amplified and the product size (~1.5 kb) was sequenced. The endophytic strains (BHU 12, BHU 16, and BHU M7) were identified as having greater than 95 % sequence similarity to Alcaligenes faecalis (Gen Bank accession: KT013264, KT013265, and KT013266, respectively) (Fig. 4, Table 1).
Colonization Efficiency of the Endophytic Strains
To determine the colonization efficiency of the endophytic strains, a biofilm formation assay on solid surfaces was performed on microtitre plates and after 48 h of incubation, biofilm formation was quantified by the crystal violet staining method. Figure 5 indicates the biofilm formation of the wild type of endophytic isolates in the presence and absence of okra root exudates. Endophytic strain BHU 16 colonized the rhizoplane with the highest degree of colonizability in presence of okra root exudates followed by endophytic strains BHU M7 and BHU 12.
Effects of root exudates (R.E.) on biofilm formation by the three endophytic strains, BHU 12, BHU 16, and BHU M7, respectively, compared to non-root exudate-amended NB. NB was amended with 10 % R.E. Different letters on the bars indicate significant difference between the isolates according to LSD at p < 0.05
Establishment of the Endophytic Identity of the Strains
TTC staining of the host roots revealed colonization of the endophytic isolates in the cortical region with traces in the vascular area as well (Fig. 6a–h). The fluorescence microscopic images of the root and shoot cross sections clearly revealed the presence of endophytic isolates in the cortical as well as in vascular regions (Fig. 6i–l). However, the CFU count of the three endophytic strains on NA-Rif plates in comparison to control suggested that the strain BHU M7 was the most efficient endophytic colonizer in the root region followed by strain BHU 16 and strain BHU 12 (Fig. 7). Endophytic strain BHU 12, however, displayed slow systemic colonization in the host, particularly in the aerial parts of the plant. This suggests that strain BHU 12 displays an initial slow chemotactic potential to okra root exudates but once it colonizes, it perfectly adapts within the host tissues as evident by the growth promotion parameters.
TTC-malate buffer-treated root tissue displaying pink stained bacterial strains BHU12 (b), BHU16 (c), and BHUM7 (d) under 10× magnification. 100× magnification of the cortical cells displaying endophytic colonization of BHU12 (f), BHU16 (g), and BHUM7 (h). Control (a, e) showing absence of any form of colonization. The seedlings were grown under gnotobiotic conditions for 30 days before treatment with TTC-malate buffer solution. j, k, l Fluorescent microscopic images of root and shoot cross sections, respectively, of BHU 12a (conjugant expressing the ds-RED protein) inoculated okra plants. i Uninoculated control displaying bright-field image of root cross section of unprimed okra plants
Growth-Promoting Attributes of the Endophytic Strains
In the presence of endophytic strains BHU 12, BHU 16, and BHU M7, the host okra plant displayed 8.25, 27, and 34.37 % and 14.5, 11.56, and 4.34 % increase in root and shoot length, respectively, during 30 days of the growth period. Treatment with BHU 12, BHU 16, and BHU M7 enhanced the fresh weight by 26, 33, and 20 %, respectively. The total biomass was augmented by 48.84, 60, and 40 %, respectively, as compared to control. Significant enhancement in the leaf area and chlorophyll content was also observed, suggesting an augmentation in photosynthetic activities (Figs. 8 and 9).
Discussion
Endophytic bacteria still remain a largely unexploited reservoir, particularly in the context of novel antifungal compounds and other plant growth-promoting attributes (Rosenblueth and Martínez-Romero 2006; Strobel and Daisy 2003; Lodewyckx and others 2002; Sturz and Nowak 2000). Intergeneric exchange between the host and its intrinsic endophyte leads to the acquisition of several host properties (Kusari and Spiteller 2011). This suggests endophytes as plausible resources for various human-benefitting drugs, particularly medicinal plant endophytes. Though extensive reports are available on the endophytic nature of several bacterial genera, such as Pseudomonas, Bacillus, Enterobacter, and Agrobacterium (Hallmann and others 1997), yet to our knowledge, no comprehensive analysis has been performed on endophytic Alcaligenes spp. inhabiting the roots of okra.
Several features, singly or cumulatively, yield efficient plant growth promotion, such as, enhancement of the plant nutritional level, production of siderophores, phytohormones, and volatile compounds (Maksimov and others 2011; Ryan and others 2008; Ryu and others 2004). The three endophytic strains exhibited several prominent plant growth-promoting characteristics such as synthesis of IAA and solubilization of tricalcium phosphate. Recently, Sayyed and Chincholkar (2009) have reported a prominent siderophore-producing strain of A. faecalis which shows plant growth promotion and antagonistic capabilities against the collar rot pathogen S. rolfsii. The endophytic strains are shown to produce hydrogen cyanide which may function as a major antipathogenic metabolite. Apart from in vitro plant growth-promoting attributes, the endophytic isolates positively influence growth of the aboveground and the belowground regions of okra seedlings, in the initial period of its development (30- and 45-day-old seedlings). The bioprimed plants in contrast to their unprimed control counterparts exhibited an increment in the foliar area in addition to the stem height. The increase in foliar area is consistent with the higher photosynthetic rate as reported by Leite and others (2013).These effects are directly co-related with increased root surface area which leads to an augmentation in the absorption of nutrients and water from soil (Falcäo and others 2014).
The rhizosphere abounds in nutritionally rich exudates (Compant and others 2010). The abundant supply of organic carbon in the form of root exudates within the rhizospheric region serves as the refuge for growth and multiplication of bacteria in the bulk soil (Babalola 2010). Following initial colonization, the bacteria move to the primary sites of entry of the roots, that is, the sites of lateral root emergence, root tips, and/or wounds inflicted by pathogens or predators (Hardoim and others 2008). The three endophytic strains exhibited significantly high rates of chemotaxis toward okra root exudates. Interestingly, BHU M7, isolated from A. paniculata leaves also exhibited significant chemotactic ability toward okra root exudates, suggesting its ability to colonize the rhizospheric region of the okra plant.
After the initial colonization, the strains were tested for their ability to establish themselves endophytically within the host interior. Endophytic strain BHU 12 exhibited a slower systemic colonization as compared to endophytic strains BHU 16 and BHU M7. To assess the spatial distribution of the respective endophytic strains, the presence of the endophytic strains was analyzed in parts of roots, stems, and leaves. The inoculated Alcaligenes was isolated from every part of the plant analyzed and the strains exhibited significant colonization in the stem and leaf portions. Considering the antagonistic effect of the isolates (Fig. 3), the presence of these endophytic strains in okra plants may be helpful in protecting it from pathogens such as the extremely destructive S. rolfsii. Furthermore, the systemic localization of the Alcaligenes strain might help prevent the establishment of other pathogens.
The investigation conducted so far shows that A. faecalis isolates BHU 12, BHU 16, and BHU M7 have the potential to be employed not only as biofertilizers for okra production but also as potent biocontrol agents. Because innate endophytes are believed to inherit several of the host properties as proposed by the xenohormesis hypothesis, hence bioprospecting of endophytes offers marvelous scope to discover natural products with therapeutic values apart from their biofertilizer attributes (Kusari and Spiteller 2011). Furthermore, it appears as an interesting opportunity for further investigation on plant–endophyte–pathogen interactions, as well as a potentially valuable source for novel antifungal compounds.
References
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163(2):173–181
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24(1):1
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32(11):1559–1570
Bakker PA, Pieterse CM, Van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 97(2):239–243
Bano N, Musarrat J (2003) Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr Microbiol 46(5):0324–0328
Bargabus RL, Zidack NK, Sherwood JE, Jacobsen BJ (2002) Characterization of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides, biological control agent. Physiol Mol Plant Path 61(5):289–298
Barka EA, Gognies S, Nowak J, Audran JC, Belarbi A (2002) Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol Control 24(2):135–142
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350
Botta AL, Santacecilia A, Ercole C, Cacchio P, Del Gallo M (2013) In vitro and in vivo inoculation of four endophytic bacteria on Lycopersicon esculentum. New Biotechnol 30(6):666–674
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57(2):535–538
Chauhan PS, Nautiyal CS (2010) The purB gene controls rhizosphere colonization by Pantoea agglomerans. Lett Appl Microbiol 50(2):205–210
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42(5):669–678
Conn VM, Franco CM (2004) Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl Environ Microbiol 70(11):6407–6413
Coombs JT, Michelsen PP, Franco CM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29(3):359–366
Dastager SG, Deepa CK, Puneet SC, Nautiyal CS, Pandey A (2009) Isolation and characterization of plant growth-promoting strain Pantoea NII-186 from Western Ghat forest soil, India. Lett Appl Microbiol 49(1):20–25
Etesami H, Hosseini HM, Alikhani HA, Mohammadi L (2014) Bacterial biosynthesis of 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase and Indole-3-Acetic Acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul 33(3):654–670
Falcäo LL, Silva-Werneck JO, Vilarinho BR, da Silva JP, Pomella AWV, Marcellino LH (2014) Antimicrobial and plant growth-promoting properties of the cacao endophyte Bacillus subtilis ALB629. J Appl Microbiol 116(6):1584–1592
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43(10):895–914
Hardoim P, Nissinen R, van Elsas JD (2012) Ecology of bacterial endophytes in sustainable agriculture. In: Maheshwari DK (ed) Bacteria in agrobiology: plant probiotics. Springer, Heidelberg, pp 97–126
Hardoim PP, van Overbeek LS, van Elas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16(10):463–471
Jain A, Singh S, Sarma BK, Singh HB (2012) Microbial consortium–mediated reprogramming of defense network in pea to enhance tolerance against Sclerotinia sclerotiorum. J Appl Microbiol 112(3):537–550
Jain A, Singh A, Singh S, Singh HB (2013) Microbial consortium-induced changes in oxidative stress markers in pea plants challenged with Sclerotinia sclerotiorum. J Plant Growth Regul 32(2):388–398
Jasim B, Jimtha CJ, Jyothis M, Radhakrishnan EK (2013) Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul 71(1):1–11
Kang SH, Cho H, Cheong H, Ryu C, Kim JF, Park S (2007) Two bacterial entophytes eliciting both plant growth promotion and plant defense on pepper (Capsicum annum L.). J Microbiol Biotechnol 17(1):96
Koné D, Mohamed D, Soro S, Bi BB, Kouadio YJ, Ji P (2012) First Report of Southern Blight of Okra (Abelmoschus esculentus) Caused by Sclerotium rolfsii in Côte d’Ivoire. Acta Phytopathol Entomol Hung 47:191–202
Kusari S, Spiteller M (2011) Are we ready for industrial production of bioactive plant secondary metabolites utilizing endophytes? Nat Prod Rep 28(7):1203–1207
Leite HAC, Silva AB, Gomes FP, Gramacho KP, Faria JC, de Souza JT, Loguercio LL (2013) Bacillus subtilis and Enterobacter cloacae endophytes from healthy Theobroma cacao L. trees can systemically colonize seedlings and promote growth. Appl Microbiol Biotechnol 97(6):2639–2651
Liu CH, Chiu CS, Ho PL, Wang SW (2009) Improvement in the growth performance of white shrimp, Litopenaeus vannamei, by a protease-producing probiotic, Bacillus subtilis E20, from natto. J Appl Microbiol 107(3):1031–1041
Lodewyckx C, Vangronsveld J, Porteous F, Moore ER, Taghavi S, Mezgeay M, der Lelie DV (2002) Endophytic bacteria and their potential applications. Crit Rev Plant Sci 21(6):583–606
Maksimov IV, Abizgil’dina RR, Pusenkova LI (2011) Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Appl Biochem Microbiol 47(4):333–345
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40(4):923–940
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43(1):51–56
Melnick RL, Zidack NK, Bailey BA, Maximova SN, Guiltinan M, Backman PA (2008) Bacterial endophytes: Bacillus spp. from annual crops as potential biological control agents of black pod rot of cacao. Biol Control 46(1):46–56
Mishra S, Nautiyal CS (2012) Reducing the allelopathic effect of Parthenium hysterophorus L. on wheat (Triticum aestivum L.) by Pseudomonas putida. Plant Growth Regul 66(2):155–165
Mishra S, Singh A, Keswani C, Saxena A, Sarma BK, Singh HB (2015) Harnessing plant-microbe interactions for enhanced protection against phytopathogens. In: Arora NK (ed) Plant microbes symbiosis: applied facets. Springer, New Delhi, pp 111–125
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270
Nautiyal CS, van Berkum P, Sadowsky MJ, Keister DL (1989) Cytochrome mutants of Bradyrhizobium induced by transposon Tn5. Plant Physiol 90(2):553–559
Nautiyal CS, Chauhan PS, Bhatia CR (2010) Changes in soil physico-chemical properties and microbial functional diversity due to 14 years of conversion of grassland to organic agriculture in semi-arid agroecosystem. Soil Till Res 109(2):55–60
Owen NL, Hundley N (2004) Endophytes–the chemical synthesizers inside plants. Sci Prog 87(2):79–99
Pandey PK, Yadav SK, Singh A, Sarma BK, Mishra A, Singh HB (2012) Cross-species alleviation of biotic and abiotic stresses by the endophyte Pseudomonas aeruginosa PW09. J Phytopathol 160(10):532–539
Rajkumar M, Ae N, Freitas H (2009) Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 77(2):153–160
Randhawa MA, Anjum FM, Ahmed A, Randhawa MS (2007) Field incurred chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol residues in fresh and processed vegetables. Food Chem 103(3):1016–1023
Rosenblueth M, Martiner-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe In 19(8):827–837
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278(1):1–9
Ryu CM, Murphy JF, Mysore KS, Kloepper JW (2004) Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J 39(3):381–392
Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K (2011) Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J Pharm Bioallied Sci 3(3):397
Sayyed RZ, Chincholkar SB (2009) Siderophore-producing Alcaligenes feacalis exhibited more biocontrol potential Vis-à-Vis chemical fungicide. Curr Microbiol 58(1):47–51
Scott B (2001) Epichloë endophytes: fungal symbionts of grasses. Curr Opin Microbiol 4(4):393–398
Senthilkumar M, Govindasamy V, Annapurna K (2007) Role of antibiosis in suppression of charcoal rot disease by soybean endophyte Paenibacillus sp. HKA-15. Curr Microbiol 55(1):25–29
Singh A, Sarma BK, Upadhyay RS, Singh HB (2013a) Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol Res 168(1):33–40
Singh RK, Malik N, Singh S (2013b) Improved nutrient use efficiency increases plant growth of rice with the use of IAA-overproducing strains of endophytic Burkholderia cepacia strain RRE25. Microb Ecol 66(2):375–384
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol R 67(4):491–502
Sturz AV, Nowak J (2000) Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl Soil Ecol 15(2):183–190
Tan S, Yang C, Mei X, Shen S, Raza W, Shen Q, Xu Y (2013) The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl Soil Ecol 64:15–22
Thomas P, Sekhar AC (2014) Live cell imaging reveals extensive intracellular cytoplasmic colonization of banana by normally non-cultivable endophytic bacteria. AoB Plants 6:plu002
Verwijst T, Telenius B (1999) Biomass estimation procedures in short rotation forestry. Forest Ecol Manag 121(1):137–146
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255(2):571–586
Wilson K (1987) Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol 2(4):1–5
Yao J, Allen C (2006) Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J Bacteriol 188(10):3697–3708
Acknowledgments
Shatrupa Ray is thankful to Banaras Hindu University, Varanasi, for award of RET-UGC fellowship. HBS and BKS are grateful to Indian Council of Agricultural Research for providing financial assistance under ICAR-Seed Project scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Rights and permissions
About this article
Cite this article
Ray, S., Singh, S., Sarma, B.K. et al. Endophytic Alcaligenes Isolated from Horticultural and Medicinal Crops Promotes Growth in Okra (Abelmoschus esculentus). J Plant Growth Regul 35, 401–412 (2016). https://doi.org/10.1007/s00344-015-9548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9548-z