Abstract
Toxic cyanobacterial blooms (TCBs) are an environmental concern due to their ability to produce wide a range of hepatotoxins, neurotoxins, and dermatotoxins. Microcystins (MCs) are the most common toxin and are considered to be one of the most hazardous groups. The increasing occurrence and detection of MCs in recreation or drinking water sources pose a variety of challenges to water treatment. To ensure the safety of drinking water supplies, a variety of physical, chemical and biological processes, such as coagulation, flocculation, sedimentation, filtration, disinfection, adsorption, and biodegradation have been applied for removal of MCs. It is important to determine which type of MCs is present and whether the toxins reside within the cell or as extracellular to optimize treatment approaches. Conventional treatments using coagulation, flocculation, sedimentation, and filtration are effective for removing cyanobacteria intact cells. However, these methods are faced with the release of dissolved toxins as well as the requirement of regular backwashing. Dissolved MCs have been shown to be effectively removed by some techniques such as activated carbon adsorption or biological degradation. However, factors affecting the removal such as acclimation periods, biofilm composition, temperature, and water quality cannot be easily controlled. This chapter provides an overview of the current knowledge of MCs including occurrence, distribution, as well as current methods of their removal from drinking water.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Eutrophication of surface water, in particular through the excessive use of fertilizer and manure in agriculture as well as through sewage discharges are the major cause for the global occurrence of toxic cyanobacterial blooms (TCBs) (He et al. 2016; Preece et al. 2017). Recent evidence revealed that warmer conditions, rising CO2, and climate changes have enhanced TCBs in water systems (Visser et al. 2016). These blooms accumulated with high concentrations of cyanotoxins in drinking, recreational, and irrigation water bodies pose a serious hazard for wild and domestic animals as well as humans (Pham and Utsumi 2018). Among cyanotoxins, microcystins (MCs) are the most common and are considered to be one of the most hazardous groups in eutrophic freshwaters (Li et al. 2017). MCs are mainly produced by the three genera Microcystis, Dolichospermum, and Planktothrix, but they can be also produced by other cyanobacteria such as Aphanizomenon, Anabaenopsis, Aphanocapsa, Fischerella, Gomphosphaeria, Hapalosiphon, Nostoc, Phormidium, and Pseudanabaena in which Microcystis has been reported as the most common bloom-forming and the main producer of MCs in freshwater ecosystems worldwide (Preece et al. 2017).
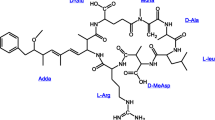
Microcystins are cyclic peptides with molecular weight (MW) ≈800–1100 Dalton (Da). They contain seven peptide-linked amino acids, with the two terminal amino acids of the linear peptide being condensed (joined) to form a cyclic compound (Fig. 2.1). Most congeners are with the general structure cyclo-(d-alanine-X-d-MeAsp-Y-Adda-d-glutamate-Mdha) in which R1 and R2 are variable l amino acids, d-MeAsp is d-erythro-b-methylas-partic acid, and Mdha is N-methyldehydroalanine (Pham and Utsumi 2018). The amino acid Adda, (2S,3S,8S,9S)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid, is the most unusual structure in this group of cyclic peptide toxins. There are over 100 MC congeners have so far been reported but the three most common are MC-LR, -RR and -YR, in which MC-LR is the most toxic one (Li et al. 2017). Chemical structures of the several most common MC variants are shown in Fig. 2.1. MCs were produced non-ribosomally by a multifunctional enzyme complex that includes peptide synthetase and polyketide synthase modules, both of which are encoded by the microcystin synthetase gene (mcy) cluster which contains 55 kb of DNA and has been revealed in several cyanobacterial genera (Pham et al. 2015).
Microcystins cause toxic effects to human, animals, and plants. Their adverse effects on different living organisms have been extensively studied and reviewed (Chen et al. 2016; Elisabete et al. 2016; McLellan and Manderville 2017). The inhibition and regulation of the expression of protein phosphatases (PPs) groups are well known as the principal mechanism of toxicity of MCs (Chen et al. 2016; Elisabete et al. 2016). PPs are well known for their regulated function to maintain homeostasis in the cell, inhibition of PPs may generate hyperphosphorylation, causing severe cell damage (Elisabete et al. 2016). This is an important post-transitional modification which can lead to excessive signaling and may resulted in cell proliferation, cell transformation, and tumor promotion (McLellan and Manderville 2017). In addition, MCs are known to induce oxidative stress, which caused by reactive oxygen species (ROS) such as superoxide anion (\( {{{\text{O}}_{2}}^{ \cdot - }} \)), hydrogen peroxide (H2O2), and hydroxyl radical (HO·), in both animal and plant (Elisabete et al. 2016). As a preventive step to protect the public health from adverse effects, the World Health Organization (WHO) recommends a provisional guideline value of 1 μg/L for MC-LR concentration in drinking water in 1998, and a chronic tolerable daily intake (TDI) of 0.04 μg/kg body mass per day for human consumption (Chorus and Bartram 1999).
2 The Occurrence of Microcystin in Freshwater Ecosystems
The occurrence of TCBs associated with cyanotoxins has been reported in eutrophic freshwaters worldwide, where cyclic hepatotoxic MCs are found in over 75% of cyanobacterial bloom cases (Harke et al. 2016). A summary of TCBs, toxin producers, their prevalence, and MC concentrations recorded worldwide are shown in Table 2.1. Through the literature reports, Microcystis is one of the most ubiquitous bloom-forming cyanobacterial genera in inland freshwaters (Lürling et al. 2017). Its blooms associated with toxins have been recorded in at least 108 countries and territories worldwide except Antarctica, and MCs have been detected in 79 of these locations (Harke et al. 2016). Due to the lower amount of publications from Eastern Europe, Africa, and South America reflecting the lack of monitoring campaigns in these regions, this may lead to the underestimation of the prevalence of toxic cyanobacterial blooms and the diversity of toxins worldwide (Merel et al. 2013). A recent study indicated an expansion of Microcystis, as previous documentation noted less than 30 countries with bloom recorded (Zurawell et al. 2005), suggesting that Microcystis has proliferated and dominated phytoplankton communities in a wide range of freshwater ecosystems in both temperate and tropical climates (Lürling et al. 2017). Likewise, MCs in freshwater blooms are found at higher concentrations than the other cyanotoxins (Lürling et al. 2017). MC levels in lakes can vary over orders of magnitude and can be strongly related to Microcystis abundance. However, MCs are produced by only toxic cyanobacterial strains, and cyanobacterial blooms are often comprised of toxic and nontoxic strains. Thus, total MC concentrations generally positively correlated with quantification of toxigenic cyanobacterial biomass (Singh et al. 2015; Dong et al. 2016).
There are numerous studies about the occurrence and distribution of MCs in lakes and reservoirs throughout the world. MC concentrations in surface waters have been reported from trace to several milligrams per liter. In temperate regions, high MC levels are often recorded during the summer period when heavy cyanobacteria blooms usually occurred (Turner et al. 2018). However, in tropical environments with sustained high temperatures, cyanobacterial blooms may occur at any time and persist for months (Singh et al. 2015; Pham et al. 2017). Recent evidence indicated that eutrophication and warmer conditions have enhanced cyanobacterial biomass and MCs concentration (Visser et al. 2016; Lürling et al. 2017). For instance, field and laboratory studies showed the level of MCs in Lake Taihu, China was nearly 20 times higher than previous records (Su et al. 2018). Several large and most important inland waters on Earth are increasingly experiencing severe TCBs associated with MCs, such as Lake Erie in USA (Rinta-Kanto et al. 2009), Lake Winnipeg in Canada (Binding et al. 2018), Lake Suwa in Japan (Chan et al. 2007), Lake Victoria in Kenya (Sitoki et al. 2012), Lakes Poyang (Zhang et al. 2015), Erhai (Yu et al. 2014), Chaohu (Yu et al. 2014), and Dianchi (Wu et al. 2014) in China, Dau Tieng and Tri An Reservoirs in Vietnam (Pham et al. 2017; Dao et al. 2016). Due to the temporal and spatial variation of MCs, it is difficult to accurately assess MC contamination within and among lake systems (Su et al. 2018). Each lake or reservoir has its own limnological and meteorological characteristics, which may lead to differences in the cyanobacterial composition, dominant species and congener in the water column (Su et al. 2018; Amé et al. 2010). In addition, because of differentiation in extraction and detection methodologies, comparing MC concentrations among water bodies is challenging. Nevertheless, extremely high levels of MCs from crude extract of bloom materials or from water column have been reported worldwide. For example, MCs concentration has been reported up to 7280 μg/g dry weight (dw) from central China (Chorus and Bartram 1999), or 7100 μg/g dw in Portugal waters (Vasconcelos et al. 1996). Very high levels of total MCs (including intracellular and extracellular) in water have also been documented up to 19,500 μg/L in Lake Suwa, Japan (Harke et al. 2016), 29,200 μg/L in Lake Oubeira, Algeria (Nasri et al. 2004), or 36,500 μg/L in Lake Horowhenua, New Zealand (Wood et al. 2006) (Table 2.1).
In general, MCs are first synthesized and retained more than 95% in toxic cells (intracellular or cell-bound), but they will be then released to the water after cell lysis or death, which results in accumulation of a high concentration of dissolved MCs (extracellular) in water column (Pham and Utsumi 2018). Thus, water column serves as an intermediate transmission compartment and is often the most contaminated with both intracellular and extracellular MCs. From here, MCs could contaminate to other aquatic compartments such as sediment, animals, aquatic, or terrestrial plants. MCs are chemically stable and can persist in water for several days or weeks after the bloom event (Preece et al. 2017). A ubiquitous distribution of MC in the aquatic environment has been summarized (Pham and Utsumi 2018). The main route of human exposure to MCs is the chronic and accidental ingestion of contaminated drinking water, although other routes such as consumption of contaminated food, dermal contact with toxins during recreational activities in recreational waters, or oral intake of cyanobacterial dietary supplements can be considered significant for some cultures and individuals (He et al. 2016).
3 The Occurrence of Cyanobacterial Toxins in Vietnamese Freshwaters
During the last two decades in Vietnam, most studies focused only on morphological characteristic and described the present or absent of cyanobacteria species. The most commonly cited potentially toxic cyanobacteria species is Microcystis aeruginosa, which has been morphologically described from Lake Thanh Cong (Hummert et al. 2001), the Huong River (Nguyen et al. 2007), and the Tri An Reservoir (Fig. 2.2a) (Dao et al. 2010). Other potentially toxic species such as Microcystis botrys and Microcystis wesenbergii have also been reported (Pham et al. 2015; Nguyen et al. 2007). Nguyen et al. (Nguyen et al. 2012) collected, morphologically characterized, and classified Microcystis strains in the middle and north of Vietnam, but provided no information on MC concentrations. The first report on cyanobacterial toxins in Vietnam was the study in Lake Thanh Cong, where extracts of M. aeruginosa contained MC-RR, MC-YR, MC-WR, and five minor compounds (Hummert et al. 2001), but no toxin concentration was given. However, during the past 10 years, the occurrence of cyanobacterial blooms and their toxin have been frequently reported from many water bodies in Vietnam (Fig. 2.2b, c). Nguyen et al. (Nguyen et al. 2007) reported a bloom of Microcystis spp. in a pond and several rivers in Thua Thien Hue, a central province in Vietnam, where total MC concentrations in the water were up to 76.2 µg/L. The authors also detected MC-LR and MC-RR in a culture biomass of Microcystis spp. with max concentration up to 4.12 mg MC/g dw (Nguyen et al. 2007). Dao et al. (Dao et al. 2016) reported MC concentrations up to 640 µg/g dw in bloom samples from the Tri An Reservoir. Duong et al. (2013, 2014) measured MCs concentration up to 1699 µg/g dw in bloom samples and 185 µg/L in water samples from the Nui Coc Reservoir and Hoan Kiem Lake, respectively. Pham et al. (2015) reported the present of three MCs includes MC-LR, MC-RR, and MC-YR with concentrations up to 2129 µg/g dw from either cyanobacterial strains or bloom biomass from the Dau Tieng Reservoir. Although MCs have been detected from several reservoirs used for drinking purpose, there are no facilities for removal of MCs from drinking water plant so far in Vietnam.
4 Current Approaches to Microcystins Removal
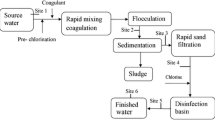
As TCBs and their toxins in freshwater increase in frequency, the protection of water supplies becomes more challenging (He et al. 2016). Therefore, there is a need for alternate water treatment technologies to remove MCs to reduce the risk from toxic cyanobacterial blooms in drinking water. MCs removal by common drinking water treatment (DWT) processes usually include a sequence of fundamental and optional processes. The most basic treatment steps for a high-quality surface water resource would typically consist of coarse filtration followed by clarification to remove natural organic matter (NOM) and disinfection to inactivate pathogens (Fig. 2.3). These techniques could be divided into two categories: those based on the retention of contaminants (coagulation, flocculation, sand filtration, adsorption, etc.), and those based on the degradation of contaminants (biodegradation, advanced oxidation, etc.) (Merel et al. 2013). Although multiple technologies have been developed for removal of MC, this chapter will discuss those currently in use for general water treatment.

Source: Adapted and modified from Merel et al. (2013)
Basic steps for microcystins (MCs) removal in drinking water treatment. (*)The barrier which is mainly responsible for MCs removal.
4.1 Coagulation, Flocculation, and Sedimentation
Conventional methods for water treatment such as coagulation, flocculation, sedimentation, and filtration are frequently used in DWT. The traditional coagulation process involves the addition and rapid mixing of a metal salt compound (e.g., aluminum sulfate, ferric chloride) with raw water (He et al. 2016). These reactions produce a variety of precipitates that facilitate the agglomeration of suspended particles, which enhances removal during sedimentation.
Cyanobacteria are microscopic organisms with negative charges on the cell membrane that can be roughly considered as colloids and removed by conventional methods. For example, up to 90% removal can be achieved on cultured of Microcystis spp. (Merel et al. 2013). Due to the positive buoyancy, low specific density, motility, variable morphologies, the removal of some cyanobacteria genera may be more challenging (Merel et al. 2013). For those positive buoyancy, the application of dissolved air flotation (DAF), which uses air injected at the bottom of the reactor to carry the cells to the surface where they can be removed by scrapping, could also efficiently remove cyanobacteria instead of sedimentation (Teixeira et al. 2010). Previous studies have shown that coagulation and flocculation are effectively removed cyanobacteria cells or intracellular MC but do not remove extracellular one (Teixeira and Rosa 2007; Sun et al. 2012). In addition, physical perturbations involved in coagulation processes may result in cyanobacterial cell lysis and a direct increase in dissolved MC concentration. Coagulation and sedimentation are typically followed by the process of rapid or slow sand filtration by using sand, gravel, and/or anthracite. The traditional purpose of a rapid sand filtration is to remove any remaining particles in the water following sedimentation (He et al. 2016).
Direct and rapid filtration was not effective in removing cyanobacterial cells and extracellular MC, but slow sand filters were shown to remove both cyanobacteria and their toxins during water treatment. For example, slow sand filters can remove up to 99% of the cells of Planktothrix agardhii (Grützmacher et al. 2002). In addition, slow sand filtration possibly develops a biofilm on the top of the filter, due to its lower loading rate, resulting in biodegradation of extracellular MCs (Grützmacher et al. 2002; Bourne et al. 2006). Grützmacher et al. (2002) found that more than 90% of extracellular MC were removed during slow sand filtration, primarily due to the biodegradation on or inside the filter bed. Thus, rapid filtration is not enough effective method for removal of cyanobacterial cells and extracellular MC, but slow sand filtration could improve the treatment (Grützmacher et al. 2002; Ho et al. 2006). However, this water treatment requires regular backwashing of the filters and if this process is performed inadequately, plugging of the filter and toxin release from the lysed cyanobacterial cells entrained in filter beds are significant problems. Coagulation and filtration alone do not lead to a substantial reduction of toxicity but are potentially very useful if combined with other treatment techniques (Pantelíc et al. 2013).
4.2 Membrane Filtration
Membrane filtrations involve pressure-driven filtration through small pores to remove contaminants not typically removed through physical coarse filtration (Roegner et al. 2014). The term membrane filtration includes four categories characterized by the pore size of the associated membrane: microfiltration (MF) (0.1–10 μm), ultrafiltration (UF) (1–100 nm), nanofiltration (NF) (around 1 nm), and reverse osmosis (RO) (0.1 nm) (Merel et al. 2013). These retention techniques have received great attention for their application to remove micropollutants in DWT. MF and UF techniques are effective for removing cyanobacteria intact cells as well as intracellular toxins. For instance, Merel et al. (2013) showed that both kinds of membranes can remove up to 98% of the cells of the toxic cyanobacteria M. aeruginosa. Previous studies revealed that MF and UF with a molecular weight cutoff (MWCO) of 100 kDa rejected cyanobacterial cells but not the MCs (Gijsbertsen-Abrahamse et al. 2006; Lee and Walker 2008). However, MF and UF techniques are not expected to remove extracellular MC and require costly pumping of water as well as face fouling and cell lysis problems.
To date, MF and UF processes appear adequately to remove cyanobacterial biomass if backwashing, cleaning, and disinfection occur between runs (Roegner et al. 2014). Both kinds of MF and UF processes can be applied to remove extracellular toxins previously adsorbed on powdered activated carbon (PAC) (Dixon et al. 2011). On the contrary, RO and NF membranes are effective for removing MCs via size exclusion since the pore size of these membranes MWCO ~100 Da for RO, and 150−200 Da used for NF is smaller than the molecular weight of MCs (~1000 Da). For example, Teixeira and Rosa (2006) found that NF reduced more than 95% of MC-LR. Neumann and Weckesser (1998) observed that 95% and 99% of MC-LR and -RR were removed by using NF and RO membranes, respectively. Although membrane filtration seems to be a promising method to remove both cyanobacteria and MC during DWT, they require a high cost and high technique level of maintenance to prevent membrane fouling by NOM and cyanobacterial cells (Lee and Walker 2008).
4.3 Activated Carbon
Manufactured from wood, coal, peat, and coconut shell, activated carbon (AC) has a high porosity and a large surface area, typically ranging from 600 to 1200 m2/g, which enables to adsorb contaminants from water (Donati et al. 1994; Huang et al. 2007). AC in both powdered activated carbon (PAC) and granular activated carbon (GAC) forms has been extensively used for decades to remove pollutants in drinking water and wastewaters (Donati et al. 1994; Huang et al. 2007; Drogui et al. 2012). Adsorption by AC, either GAC or PAC is considered as the best available technology and commonly used for trace organic contaminants removal from surface water. In particular, MCs are effectively adsorbed into AC (Donati et al. 1994; Mohamed et al. 1999). By far, as a single technology, AC most effectively removes dissolved MCs from a water source, with reported levels of up to 99% removal (Roegner et al. 2014). AC is effectively adsorbed extracellular MC but neither cyanobacteria nor intracellular MC. The removal efficiency of cyanotoxins may depend on the kind of adsorbent employed (Donati et al. 1994; Huang et al. 2007; Newcombe and Nicholson 2004). PAC with mesopores range from 2 to 50 nm in diameter is sizeable surface areas for adsorptions, while micropores (<2 nm) hold fewer adsorption sites and limit water flow, making them less effective (Roegner et al. 2014). Indeed, Donati et al. (1994) investigated the adsorption of MC-LR on eight activated carbons, and adsorbents with the largest volume of mesopores (pore diameter in the range of 2–50 nm) were shown to be the most efficient.
Water quality has a strong influence on the removal capability of cyanotoxins by AC since NOM can compete with contaminants and limit their adsorption. Indeed, Lambert et al. (1996) observed a clear difference when compare PAC adsorption isotherms of MC-LR ultrapure water versus surface water. The isotherms obtained with surface water or previously used AC exhibit an alteration of the slope indicating much lower adsorption capacity for MC (Lambert et al. 1996). Other factors influence the removal capability of AC is the dose of adsorbent and contact time. For effective MC removal, very high doses of PAC are required (10 μg/L MC requite >200 mg PAC/L) at a contact time from 1 to 12 h (Pantelíc et al. 2013; Ho et al. 2011).
Although AC can efficiently retain cyanotoxins, AC filtration displays a limited lifetime for all contaminants including MCs (Pantelíc et al. 2013). It needs to be changed frequently vary between 2 months to 1 year depending on the type of toxin and the water quality, and the removing efficiency will decrease with time (Ho et al. 2011). Therefore, complete adsorption would require a high amount of different adsorbent types, what significantly increases treatment costs. AC filtration is safe because no by-products were produced during treatment process. Consequently, AC should not be considered as an individual remediation measure but as a part of a multi-barrier approach (Roegner et al. 2014).
4.4 Advanced Oxidation Processes
Advanced oxidation processes such as ozonation and UV photolysis are also a very efficient process for the rapid and complete destruction of MCs from water (Westrick et al. 2010; He et al. 2015). Ozone is one of the most commonly used oxidants in water treatment due to its effective and fast reaction agent. Ozone has been widely utilized for disinfection and oxidation purposes. It has been recently applied at a two-stage treatment including pre- and post-ozonation in water treatment plants (Pantelíc et al. 2013). Ozone is one of the most effective and fastest agents for the destruction of intracellular and extracellular cyanotoxin fraction. Indeed, Westrick et al. (2010) reported that nearly 100% of MC in natural water was oxidized by ozonation (0.22 mg/L ozone) within a short treatment time (15 s). The ability to oxidate MCs depends on pH values. At pH values below 7.0, ozone can be very effective for MC-LR oxidation; however, about pH 7.0, oxidation will be minimal and may not achieve desired removal (Pantelíc et al. 2013). By studying the intermediates and by-products pathways from ozonation degradation of MC-LR in aqueous solution, Chang et al. (2014) demonstrated that ozone reacted with two sites of MC-LR: the diene carbon bonds in the Adda side chain and the Mdha amino acid in the cyclic structure. The fragment from the Adda side chain oxidative cleavage could be further oxidized to an aldehyde.
UV irradiation is a potential process for drinking water disinfection. Although sunlight alone cannot cause the degradation of the cyanotoxins, UV light in the range 240–280 nm results in inactivating microorganisms by inducing DNA alteration (Westrick et al. 2010). UV photolysis is effective for the destruction of MCs, but high UV radiation dosage (1530−20,000 mJ/cm2) is required for the successful UV photolysis of MCs, which is impractical for full-scale water treatment (Westrick et al. 2010). MC-LR and -RR degraded much more rapidly when the toxins were exposed to UV and UVC (100–280 nm) light at wavelengths around their absorption maxima (238–254 nm) (He et al. 2016). Recent studies suggested that the UV/O3 process was a more effective method for the removal and mineralization of MC-LR in water, compared with UV- and O3-alone processes (Chang et al. 2015). The degradation pathways of the MC-LR during UV/O3 process involved isomerization, hydroxylation, and oxidative cleavage of the Adda side chain, oxidation of Mdha and decarboxylation of MeAsp and Glu, in which the oxidation of Adda moiety was the dominant reaction (Chang et al. 2015). While UV radiation mainly resulted in the isomerization of Adda moiety and the decarboxylation of MeAsp and Glu, O3 oxidation resulted in the oxidation and cleavage of Adda and Mdha (Chang et al. 2015).
Although ozonation and UV photolysis contribute significantly to MCs removal, the potential problem of ozonation is the generation of toxic by-products due to incomplete oxidation (He et al. 2016). Due to high doses required, low to medium pressure lamp, UV treatment is not recommended as a viable treatment barrier for MCs. The disadvantages of these treatment techniques also include the high cost of ozonation equipment, highly corrosive and toxicity of ozone, as well as higher level of maintenance and operator skill (Westrick et al. 2010).
4.5 Biodegradation
Although less commonly employed compared to physical and chemical treatment processes, biodegradation is increasing attention since it has been proven as an environmentally benign and cost-efficient method for MCs removal. Current research advances for MC biodegradation were discussed in detail by Li et al. (2017). Biological treatment is most often employed in combination with the filtration process in DWT (He et al. 2016). Microorganisms capable of degrading MCs have been described in the literature for almost two decades. Nowadays, various organisms originated from diverse ecosystems including prokaryotes (e.g., bacteria) and eukaryotes with the ability to degrade MCs in water have been identified, with the majority identified as Sphingomonas and Sphingopyxis genera belonging to α-Proteobacteria class (Li et al. 2017). The degradation pathways and enzymatic processes are fully characterized for strains within α, β, and γ-proteobacteria, including Sphingomonas, Stentophomonas, Sphingopoxyis, and Methylobacillus (He et al. 2016).
Previous studies have demonstrated that aerobic biodegradation is the main natural attenuation mechanism for MCs (Bourne et al. 2006; Ho et al. 2006). However, recent researches revealed that MCs can be rapidly degraded under anaerobic condition with natural sediments as inoculum (Chen et al. 2010; Zhao et al. 2017). This suggested that both aerobic and anaerobic biodegradation are important in the natural degradation of MCs. The biodegradation pathway for MC-LR has been elucidated. Bourne et al. (Bourne et al. 1996) explored that Sphingomonas sp. ACM-3962 degraded MC-LR by using three enzymes. The first enzyme, microcystinase, cleaves the Arg–Adda peptide bond in the toxin and converts the cyclic MC-LR to a linear form. The second enzyme hydrolyzes the Ala–Leu bond, converting the linearized MC-LR into a tetrapeptide. And the last one breaks the tetrapeptide into smaller peptides and amino acids, which are used for constructing new proteins or enzymes (Bourne et al. 1996). The fact that the by-products from MC-LR degradation are nontoxic compared with parent MC-LR. A few years later, Bourne et al. (Bourne et al. 2001) identified a gene cluster, mlrA, mlrB, mlrC , and mlrD, involved in the degradation of MC-LR from the first isolated MC-degrading bacterium, Sphingomonas sp. ACM-3962. The authors determined that the mlrA gene encoded an enzyme responsible for the hydrolytic cleaving of the cyclic structure of MC-LR (Bourne et al. 2001). Hydrolysis of linearized MC-LR to the tetrapeptide intermediate is catalyzed by mlrB, a putative serine peptidase. Tetrapeptide cleavage is accomplished via mlrC, also a putative metallopeptidase (Fig. 2.4). The final gene, mlrD, encoded for a putative transporter protein that may support for active transport of MC and/or its degradation products into or out of the cell (Bourne et al. 2001).

Source: Adapted and modified from Li et al. (2017)
Biodegradation pathway of MC-LR by Sphingomonas sp.
Since more and more MC-degrading bacteria are being identified indicates that MC-degrading bacteria may be prevalent in the natural environment. Unfortunately, estimating rates of toxin removal in complex natural environments from laboratory experiments containing isolates or consortia is not straightforward (He et al. 2016), microbial consortia grown on biofilm seemed to be more effective at MC removal than isolated strains such as Sphingomonas sp. (Bourne et al. 2006). In addition, biodegradation is effective for only extracellular MC and degradation rates in natural environments containing a mixture of cyanotoxins could be more different than rates measured for isolates grown on individual toxins under laboratory conditions (He et al. 2016). Although biodegradation of MCs from water provides a reliable, cost-effective purification system, this treatment requires long reaction time of hours to days to complete degradation. Therefore, biological degradation should be used in conjunction with other treatment methods such as filtration, PAC, or GAC to meet the WHO recommended guidelines.
5 Conclusion
Toxic cyanobacterial blooms continue to plague eutrophic waters worldwide. The occurrence of TCBs of Microcystis associated with the hepatotoxic MCs appears to be expanding, with hundred countries or territories around the world. MCs are ubiquitous in the eutrophic environment. As a result, humans are increasing exposed to cyanobacterial toxins through drinking water consumption. Thus, control and abatement TCBs are critical issues faced by the scientist community. There is also a need for further efforts to curb eutrophication of freshwater resources. Numerous strategies have been emerged to prevent or eliminate blooms of cyanobacteria and MCs. To meet the WHO drinking water guideline, it is important that DWT has to remove both intracellular and extracellular MCs. While the conventional method efficiently removes cyanobacteria cells or intracellular MCs and in some case increasing extracellular MCs, combining AC absorption, biodegradation as well as advanced oxidation processes should ensure the removal of the most common extracellular MCs. However, MCs contain for hundred structures and variants, no single treatment has been proven to simultaneously remove all the MCs in a mixture. Although individual MCs can be efficiently removed or transformed by at least one treatment step during the production of drinking water, the efficient management of MCs in DWT should be based on a multi-barrier approach. Water treatment appears to be successful in the term of overcome consequence, a sustainable approach strategy should be a prevention of TCBs in surface waters.
References
He X, Liu Y-L, Conklin A, Westrick J, Weavers LK, Dionysiou DD, Lenhart JJ, Mouser PJ, Szlag D, Walker HW (2016) Toxic cyanobacteria and drinking water: impacts, detection, and treatment. Harmful Algae 54:174–193. https://doi.org/10.1016/j.hal.2016.01.001
Preece EP, Hardy FJ, Moore BC, Bryan M (2017) A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 61(Supplement C):31–45. https://doi.org/10.1016/j.hal.2016.11.006
Visser PM, Verspagen JMH, Sandrini G, Stal LJ, Matthijs HCP, Davis TW, Paerl HW, Huisman J (2016) How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 54:145–159. https://doi.org/10.1016/j.hal.2015.12.006
Pham T-L, Utsumi M (2018) An overview of the accumulation of microcystins in aquatic ecosystems. J Environ Manag 213:520–529. https://doi.org/10.1016/j.jenvman.2018.01.077
Li J, Li R, Li J (2017) Current research scenario for microcystins biodegradation—a review on fundamental knowledge, application prospects and challenges. Sci Total Environ 595(Supplement C):615–632. https://doi.org/10.1016/j.scitotenv.2017.03.285
Pham T-L, Dao T-S, Shimizu K, Lan-Chi D-H, Utsumi M (2015) Isolation and characterization of microcystin-producing cyanobacteria from Dau Tieng Reservoir, Vietnam. Nova Hedwigia 101(1–2):3–20. https://doi.org/10.1127/nova_hedwigia/2014/0243
Chen L, Chen J, Zhang X, Xie P (2016) A review of reproductive toxicity of microcystins. J Hazard Mater 301:381–399. https://doi.org/10.1016/j.jhazmat.2015.08.041
Elisabete V, Vitor V, Alexandre C (2016) New insights on the mode of action of microcystins in animal cells—a review. Mini Rev Med Chem 16(13):1032–1041. https://doi.org/10.2174/1389557516666160219130553
McLellan NL, Manderville RA (2017) Toxic mechanisms of microcystins in mammals. Toxicol Res 6(4):391–405. https://doi.org/10.1039/C7TX00043J
Chorus I, Bartram J (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management, Published on behalf of WHO, Spon Press, London
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20. https://doi.org/10.1016/j.hal.2015.12.007
Lürling M, van Oosterhout F, Faassen E (2017) Eutrophication and warming boost cyanobacterial biomass and microcystins. Toxins 9(2):64. https://doi.org/10.3390/toxins9020064
Merel S, Villarín MC, Chung K, Snyder S (2013a) Spatial and thematic distribution of research on cyanotoxins. Toxicon 76:118–131. https://doi.org/10.1016/j.toxicon.2013.09.008
Zurawell RW, Chen H, Burke JM, Prepas EE (2005) Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. J Toxicol Environ Health B Crit Rev 8(1):1–37. https://doi.org/10.1080/10937400590889412
Singh S, Rai PK, Chau R, Ravi AK, Neilan BA, Asthana RK (2015) Temporal variations in microcystin-producing cells and microcystin concentrations in two fresh water ponds. Water Res 69:131–142. https://doi.org/10.1016/j.watres.2014.11.015
Dong X, Zeng S, Bai F, Li D, He M (2016) Extracellular microcystin prediction based on toxigenic Microcystis detection in a eutrophic lake. Sci Rep 6:20886. https://doi.org/10.1038/srep20886
Turner AD, Dhanji-Rapkova M, O’Neill A, Coates L, Lewis A, Lewis K (2018) Analysis of microcystins in cyanobacterial blooms from freshwater bodies in England. Toxins 10(1):39. https://doi.org/10.3390/toxins10010039
Pham T-L, Dao T-S, Tran N-D, Nimptsch J, Wiegand C, Motoo U (2017) Influence of environmental factors on cyanobacterial biomass and microcystin concentration in the Dau Tieng Reservoir, a tropical eutrophic water body in Vietnam. Ann Limnol—Int J Lim 53:89–100. https://doi.org/10.1051/limn/2016038
Su X, Steinman AD, Xue Q, Zhao Y, Xie L (2018) Evaluating the contamination of microcystins in Lake Taihu, China: the application of equivalent total MC-LR concentration. Ecol Indic 89:445–454. https://doi.org/10.1016/j.ecolind.2017.11.042
Rinta-Kanto JM, Konopko EA, DeBruyn JM, Bourbonniere RA, Boyer GL, Wilhelm SW (2009) Lake Erie Microcystis: relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 8(5):665–673. https://doi.org/10.1016/j.hal.2008.12.004
Binding CE, Greenberg TA, McCullough G, Watson SB, Page E (2018) An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J Great Lakes Res 44(3):436–446. https://doi.org/10.1016/j.jglr.2018.04.001
Chan WS, Recknagel F, Cao H, Park H-D (2007) Elucidation and short-term forecasting of microcystin concentrations in Lake Suwa (Japan) by means of artificial neural networks and evolutionary algorithms. Water Res 41(10):2247–2255. https://doi.org/10.1016/j.watres.2007.02.001
Sitoki L, Kurmayer R, Rott E (2012) Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (Lake Victoria, Kenya). Hydrobiologia 691(1):109–122. https://doi.org/10.1007/s10750-012-1062-8
Zhang D, Liao Q, Zhang L, Wang D, Luo L, Chen Y, Zhong J, Liu J (2015) Occurrence and spatial distributions of microcystins in Poyang Lake, the largest freshwater lake in China. Ecotoxicology 24(1):19–28. https://doi.org/10.1007/s10646-014-1349-9
Yu G, Jiang Y, Song G, Tan W, Zhu M, Li R (2014a) Variation of Microcystis and microcystins coupling nitrogen and phosphorus nutrients in Lake Erhai, a drinking-water source in Southwest Plateau, China. Environ Sci Pollut Res 21(16):9887–9898. https://doi.org/10.1007/s11356-014-2937-1
Yu L, Kong F, Zhang M, Yang Z, Shi X, Du M (2014b) The dynamics of Microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins (Basel) 6(12):3238–3257. https://doi.org/10.3390/toxins6123238
Wu Y, Li L, Gan N, Zheng L, Ma H, Shan K, Liu J, Xiao B, Song L (2014) Seasonal dynamics of water bloom-forming Microcystis morphospecies and the associated extracellular microcystin concentrations in large, shallow, eutrophic Dianchi Lake. J Environ Sci 26(9):1921–1929. https://doi.org/10.1016/j.jes.2014.06.031
Dao T-S, Nimptsch J, Wiegand C (2016) Dynamics of cyanobacteria and cyanobacterial toxins and their correlation with environmental parameters in Tri An Reservoir, Vietnam. J Water Health 14:669–712. https://doi.org/10.2166/wh.2016.257
Amé MV, Galanti LN, Menone ML, Gerpe MS, Moreno VJ, Wunderlin DA (2010) Microcystin–LR, –RR, –YR and –LA in water samples and fishes from a shallow lake in Argentina. Harmful Algae 9(1):66–73. https://doi.org/10.1016/j.hal.2009.08.001
Vasconcelos VM, Sivonen K, Evans WR, Carmichael WW, Namikoshi M (1996) Hepatotoxic microcystin diversity in cyanobacterial blooms collected in Portuguese freshwaters. Water Res 30(10):2377–2384. https://doi.org/10.1016/0043-1354(96)00152-2
Nasri AB, Bouaicha N, Fastner J (2004) First report of a microcystin-containing bloom of the cyanobacteria Microcystis spp. in Lake Oubeira, eastern Algeria. Arch Environ Contam Toxicol 46(2):197–202. https://doi.org/10.1007/s00244-003-2283-7
Wood SA, Holland PT, Stirling DJ, Briggs LR, Sprosen J, Ruck JG, Wear RG (2006) Survey of cyanotoxins in New Zealand water bodies between 2001 and 2004. N Z J Mar Freshwater Res 40(4):585–597. https://doi.org/10.1080/00288330.2006.9517447
Nasri H, Bouaïcha N, Harche MK (2007) A new morphospecies of Microcystis sp. forming bloom in the Cheffia dam (Algeria): seasonal variation of microcystin concentrations in raw water and their removal in a full-scale treatment plant. Environ Toxicol 22:347–356. https://doi.org/10.1002/tox.20275
Giannuzzi L, Sedan D, Echenique R, Andrinolo D (2011) An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar Drugs 9:2164–2175. https://doi.org/10.3390/md9112164
Kemp A, John J (2006) Microcystins associated with Microcystis dominated blooms in the Southwest wetlands, Western Australia. Environ Toxicol 21(2):125–130. https://doi.org/10.1002/tox.20164
Bittencourt-Oliveira MC, Piccin-Santos V, Moura AN, Aragão-Tavares NKC, Cordeiro-Araújo MK (2014) Cyanobacteria, microcystins and cylindrospermopsin in public drinking supply reservoirs of Brazil. An Acad Bras Cienc 86:297–309. https://doi.org/10.1590/0001-3765201302512
Lins RPM, Barbosa LG, Minillo A, Ceballos BSO (2016) Cyanobacteria in a eutrophicated reservoir in a semi-arid region in Brazil: dominance and microcystin events of blooms. Rev Bras Bot 39:583–591. https://doi.org/10.1007/s40415-016-0267-x
Almanza V, Parra O, Carlos E, Bicudo DM, Carolina B, Beltran J, Figueroa R, Urrutia R (2016) Occurrence of toxic blooms of Microcystis aeruginosa in a central Chilean (36° Lat. S) urban lake. Rev Chil Hist Nat 89:1–12. https://doi.org/10.1186/s40693-016-0057-7
Sabart M, Pobel D, Briand E, Combourieu B, Salencon MJ, Humbert JF, Latour D (2010) Spatiotemporal variations in microcystin concentrations and in the proportions of microcystin-producing cells in several Microcystis aeruginosa populations. Appl Environ Microbiol 76(14):4750–4759. https://doi.org/10.1128/AEM.02531-09
Tomioka N, Imai A, Komatsu K (2011) Effect of light availability on Microcystis aeruginosa blooms in shallow hypereutrophic Lake Kasumigaura. J Plankton Res 33(8):1263–1273. https://doi.org/10.1093/plankt/fbr020
Oberholster PJ, Myburgh JG, Govender D, Bengis R, Botha A-M (2009) Identification of toxigenic Microcystis strains after incidents of wild animal mortalities in the Kruger National Park, South Africa. Ecotoxicol Environ Saf 72(4):1177–1182. https://doi.org/10.1016/j.ecoenv.2008.12.014
Conradie KR, Barnard S (2012) The dynamics of toxic Microcystis strains and microcystin production in two hypertrofic South African reservoirs. Harmful Algae 20:1–10. https://doi.org/10.1016/j.hal.2012.03.006
Miller MA, Kudela RM, Mekebri A, Crane D, Oates SC, Tinker MT, Staedler M, Miller WA, Toy-Choutka S, Dominik C, Hardin D, Langlois G, Murray M, Ward K, Jessup DA (2010) Evidence for a novel marine harmful algal bloom: cyanotoxin (microcystin) transfer from Land to Sea Otters. PLoS ONE 5:e12576. https://doi.org/10.1371/journal.pone.0012576
Duong T, Jähnichen S, Le T, Ho C, Hoang T, Nguyen T, Vu T, Dang D (2014) The occurrence of cyanobacteria and microcystins in the Hoan Kiem Lake and the Nui Coc reservoir (North Vietnam). Environ Earth Sci 71(5):2419–2427. https://doi.org/10.1007/s12665-013-2642-2
Hummert C, Dahlmann J, Reinhardt K, Dang H, Dang D, Luckas B (2001) Liquid chromatography-mass spectrometry identification of microcystins in Microcystis aeruginosa strain from lake Thanh Cong, Hanoi, Vietnam. Chromatographia 54:569–575. https://doi.org/10.1007/BF02492180
Nguyen TTL, Cronberg G, Annadotter H, Larsen J (2007) Planktic cyanobacteria from freshwater localities in Thuathien-Hue Province, Vietnam II. Algal biomass and microcystin production. Nova Hedwigia 85:35–49. https://doi.org/10.1127/0029-5035/2007/0085-0035
Dao TS, Cronberg G, Nimptsch J, Do-Hong L-C, Wiegand C (2010) Toxic cyanobacteria from Tri An Reservoir, Vietnam. Nova Hedwigia 90(3–4):433–448. https://doi.org/10.1127/0029-5035/2010/0090-0433
Nguyen LAV, Tanabe Y, Matsuura H, Kaya K, Watanabe MM (2012) Morphological, biochemical and phylogenetic assessments of water-bloom-forming tropical morphospecies of Microcystis (Chroococcales, Cyanobacteria). Phycol Res 60(3):208–222. https://doi.org/10.1111/j.1440-1835.2012.00650.x
Duong T, Le T, Dao T-S, Pflugmacher S, Rochelle-Newall E, Hoang T, Vu T, Ho C, Dang D (2013) Seasonal variation of cyanobacteria and microcystins in the Nui Coc Reservoir, Northern Vietnam. J Appl Phycol 25(4):1065–1075. https://doi.org/10.1007/s10811-012-9919-9
Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O (2013b) State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int 59:303–327. https://doi.org/10.1016/j.envint.2013.06.013
Teixeira MR, Sousa V, Rosa MJ (2010) Investigating dissolved air flotation performance with cyanobacterial cells and filaments. Water Res 44:3337–3344. https://doi.org/10.1016/j.watres.2010.03.012
Teixeira MR, Rosa MJ (2007) Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa Part II. The effect of water background organics. Sep Purif Technol 53:126–134. https://doi.org/10.1016/j.seppur.2006.03.017
Sun F, Pei HY, Hu WR, Ma CX (2012) The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem Eng J 193:196–202. https://doi.org/10.1016/j.cej.2012.04.043
Grützmacher G, Böttcher G, Chorus I, Bartel H (2002) Removal of microcystins by slow sand filtration. Environ Toxicol 17:386–394. https://doi.org/10.1002/tox.10062
Bourne DG, Blakeley RL, Riddles P, Jones GJ (2006) Biodegradation of the cyanobacterial toxin microcystin LR in natural water and biologically active slow sand filters. Water Res 40(6):1294–1302. https://doi.org/10.1016/j.watres.2006.01.022
Ho L, Meyn T, Keegan A, Hoefel D, Brookes J, Saint CP, Newcombe G (2006) Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res 40:768–774. https://doi.org/10.1016/j.watres.2005.12.009
Pantelíc D, Svirčev Z, Simeunović J, Vidović M, Trajković I (2013) Cyanotoxins: characteristics, production and degradation routes in drinking water treatment with reference to the situation in Serbia. Chemosphere 91(4):421–441. https://doi.org/10.1016/j.chemosphere.2013.01.003
Roegner AF, Brena B, González-Sapienza G, Puschner B (2014) Microcystins in potable surface waters: toxic effects and removal strategies. J Appl Toxicol 34:441–457. https://doi.org/10.1002/jat.2920
Gijsbertsen-Abrahamse AJ, Schmidt W, Chorus I, Heijman SGJ (2006) Removal of cyanotoxins by ultrafiltration and nanofiltration. J Membr Sci 276(1):252–259. https://doi.org/10.1016/j.memsci.2005.09.053
Lee J, Walker HW (2008) Mechanisms and factors influencing the removal of microcystin-LR by ultrafiltration membranes. J Membr Sci 320(1):240–247. https://doi.org/10.1016/j.memsci.2008.04.007
Dixon MB, Richard Y, Ho L, Chow CWK, O’Neill BK, Newcombe GA (2011) Coagulation-powdered activated carbon-ultrafiltration—multiple barrier approach for removing toxins from two Australian cyanobacterial blooms. J Hazard Mater 186:1553–1559. https://doi.org/10.1016/j.jhazmat.2010.12.049
Teixeira MR, Rosa MJ (2006) Neurotoxic and hepatotoxic cyanotoxins removal by nanofiltration. Water Res 40(15):2837–2846. https://doi.org/10.1016/j.watres.2006.05.035
Neumann U, Weckesser J (1998) Elimination of microcystin peptide toxins from water by reverse osmosis. Environ Toxicol Water Qual 13(2):143–148. https://doi.org/10.1002/(sici)1098-2256(1998)13:2%3c143::aid-tox5%3e3.0.co;2-7
Donati C, Drikas M, Hayes R, Newcombe G (1994) Microcystin-LR adsorption by powdered activated carbon. Water Res 28(8):1735–1742. https://doi.org/10.1016/0043-1354(94)90245-3
Huang WJ, Cheng BL, Cheng YL (2007) Adsorption of microcystin-LR by three types of activated carbon. J Hazard Mater 141:115–122. https://doi.org/10.1016/j.jhazmat.2006.06.122
Drogui P, Daghrir R, Simard MC, Sauvageau C, Blais JF (2012) Removal of microcystin-LR from spiked water using either activated carbon or anthracite as filter material. Environ Technol 33:381–391. https://doi.org/10.1080/09593330.2011.575186
Mohamed ZA, Carmichael WW, An J, El-Sharouny HM (1999) Activated carbon removal efficiency of microcystins in an aqueous cell extract of Microcystis aeruginosa and Oscillatoria tenuis strains isolated from Egyptian freshwaters. Environ Toxicol 14:197–201. https://doi.org/10.1002/(SICI)1522-7278(199902)14:1%3C197:AID-TOX25%3E3.0.CO;%202-6
Newcombe G, Nicholson B (2004) Water treatment options for dissolved cyanotoxins. J Water Supply Res T 53(4):227–239. https://doi.org/10.2166/aqua.2004.0019
Lambert TW, Holmes CFB, Hrudey SE (1996) Adsorption of microcystin-LR by activated carbon and removal in full scale water treatment. Water Res 30:1411–1422. https://doi.org/10.1016/0043-1354(96)00026-7
Ho L, Lambling P, Bustamante H, Duker P, Newcombe G (2011) Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Water Res 45(9):2954–2964. https://doi.org/10.1016/j.watres.2011.03.014
Westrick JA, Szlag DC, Southwell BJ, Sinclair J (2010) A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal Bioanal Chem 397(5):1705–1714. https://doi.org/10.1007/s00216-010-3709-5
He X, De La Cruz AA, Hiskia A, Kaloudis T, O’Shea K, Dionysiou DD (2015) Destruction of microcystins (cyanotoxins) by UV-254 nm-based direct photolysis and advanced oxidation processes (AOPs): influence of variable amino acids on the degradation kinetics and reaction mechanisms. Water Res 74:227–238. https://doi.org/10.1016/j.watres.2015.02.011
Chang J, Chen Z-L, Wang Z, Shen J-M, Chen Q, Kang J, Yang L, Liu X-W, Nie C-X (2014) Ozonation degradation of microcystin-LR in aqueous solution: intermediates, byproducts and pathways. Water Res 63:52–61. https://doi.org/10.1016/j.watres.2014.06.007
Chang J, Chen Z-L, Wang Z, Kang J, Chen Q, Yuan L, Shen J-M (2015) Oxidation of microcystin-LR in water by ozone combined with UV radiation: the removal and degradation pathway. Chem Eng J 276:97–105. https://doi.org/10.1016/j.cej.2015.04.070
Chen X, Yang X, Yang L, Xiao B, Wu X, Wang J, Wan H (2010) An effective pathway for the removal of microcystin LR via anoxic biodegradation in lake sediments. Water Res 44(6):1884–1892. https://doi.org/10.1016/j.watres.2009.11.025
Zhao D, Cao X, Huang R, Zeng J, Wu QL (2017) Variation of bacterial communities in water and sediments during the decomposition of Microcystis biomass. PLoS ONE 12(4):e0176397. https://doi.org/10.1371/journal.pone.0176397
Bourne DG, Jones GJ, Blakeley RL, Jones A, Negri AP, Riddles P (1996) Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Appl Environ Microbiol 62(11):4086–4094 (0099-2240/96/$04.0010).
Bourne DG, Riddles P, Jones GJ, Smith W, Blakeley RL (2001) Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environ Toxicol 16:523–534. https://doi.org/10.1002/tox.10013
Acknowledgements
This work was founded by the International Foundation for Science (IFS) under grant number “I-2-A-6054-1”.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pham, TL., Dang, T.N. (2019). Microcystins in Freshwater Ecosystems: Occurrence, Distribution, and Current Treatment Approaches. In: Bui, XT., Chiemchaisri, C., Fujioka, T., Varjani, S. (eds) Water and Wastewater Treatment Technologies. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3259-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-3259-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3258-6
Online ISBN: 978-981-13-3259-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)