Abstract
This review focuses on the efficiency of different water treatment processes for the removal of cyanotoxins from potable water. Although several investigators have studied full-scale drinking water processes to determine the efficiency of cyanotoxin inactivation, many of the studies were based on ancillary practice. In this context, “ancillary practice” refers to the removal or inactivation of cyanotoxins by standard daily operational procedures and without a contingency operational plan utilizing specific treatment barriers. In this review, “auxiliary practice” refers to the implementation of inactivation/removal treatment barriers or operational changes explicitly designed to minimize risk from toxin-forming algae and their toxins to make potable water. Furthermore, the best drinking water treatment practices are based on extension of the multibarrier approach to remove cyanotoxins from water. Cyanotoxins are considered natural contaminants that occur worldwide and specific classes of cyanotoxins have shown regional prevalence. For example, freshwaters in the Americas often show high concentrations of microcystin, anatoxin-a, and cylindrospermopsin, whereas Australian water sources often show high concentrations of microcystin, cylindrospermopsin, and saxitoxins. Other less frequently reported cyanotoxins include lyngbyatoxin A, debromoaplysiatoxin, and β-N-methylamino-l-alanine. This review focuses on the commonly used unit processes and treatment trains to reduce the toxicity of four classes of cyanotoxins: the microcystins, cylindrospermopsin, anatoxin-a, and saxitoxins. The goal of this review is to inform the reader of how each unit process participates in a treatment train and how an auxiliary multibarrier approach to water treatment can provide safer water for the consumer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To balance the removal economics against the risk of cyanotoxins in potable water, utility managers must understand the fate and transport of cyanotoxins in their system. Cyanotoxins are toxic, secondary metabolites produced by about 40 species of cyanobacteria. A confounding factor is that species of cyanobacteria capable of producing toxin may be present, but they may not be producing toxin. Visual identification by a trained taxonomist or molecular probes may be used to determine if the cyanobacteria have the potential for producing cyanotoxins [1]. In either case, quantitative cyanotoxin analysis is needed to determine if the cyanobacteria are actually producing the toxin [2]. A combination of microscopy, molecular probes, and quantitative cyanotoxin analyses is being used to investigate the environmental and physiological variables of cyanotoxin production.
Drinking water sources are unique and need to be investigated by each utility manager to determine the risk and the best management strategy for cyanotoxin risk reduction. To create a comprehensive cyanotoxin contingency plan, the manager must be familiar with the physical and chemical properties of the toxin, the nature of the cyanotoxin, i.e., intracellular or extracellular, growth and diurnal cyanobacteria bloom patterns, and effective treatment processes. Other treatment reviews include those of Yoo et al. [3], Chorus and Bartram [4], Oehrle and Westrick [5], Svrcek and Smith [6], Newcombe [7], Falconer [8], and Westrick [9].
Microcystins
Microcystins are the most prevalent class of cyanotoxins and the most frequently studied. The hepatotoxin microcystin originates from several genera of cyanobacteria: Microcystis, Anabaena, Planktothrix, Nostoc, and Anabaenopsis. The microcystins are very soluble in water and consist of over 80 reported variants; however, four microcystins (LR, RR, LA, and YR) are of special concern to the US Environmental Protection Agency (US EPA) and are on US EPA Contaminant Candidate List III, which was developed through a series of international panel discussions [10]. The World Health Organization (WHO) has set a provisional drinking water guideline of 1 ug/L for microcystin LR.
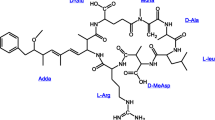
Microcystins are cyclic peptides made from seven amino acids with molecular masses around 1,000 Da. The conserved toxic moiety is 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (Adda) and the other mostly conserved unit is N-methyldehydroalanine (Mdha). The Adda functionality inhibits protein phosphatase. The main structural differences between microcystin variants such as LR, LA, RR, and YR arise from the substitution of single amino acids. From a treatment standpoint, the microcystins have three general areas subject to oxidation: the conjugated double bond in the Adda moiety; the single double bond in the Mdha moiety; and the side chain of the variant amino acids. Of the four priority microcystin variants, only the arginine and tyrosine amino acid side chains are potentially vulnerable to oxidation.
Cylindrospermopsin
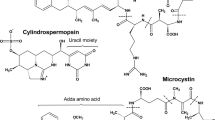
Cylindrospermopsin, a tricyclic alkaloid, is a common cyanotoxin consisting of a tricyclic guanidine moiety, hydroxymethyluracil, and sulfate with a molecular mass of 415 Da [8]. In the typical pH range of natural waters, cylindrospermopsin is a zwitterion, making it water soluble. Cylindrospermopsin originates from several genera of cyanobacteria: Cylindrospermopsis, Anabaena, Umezakia, and Aphanizomenon. The WHO [11], Falconer [8], and the USA [12] are evaluating toxicity data to determine if a drinking water guideline for cylindrospermopsin is warranted. Falconer [8] recommends a tentative guideline value of 1 ug/L for cylindrospermopsin. The uracil side chain of cylindrospermopsin inhibits protein translation and binds to DNA, causing strand breakage and promoting hepatotoxicity, cytoxicity, and genotoxicity. Of the three major functional groups, only the uracil may be chemically susceptible to oxidation.
Anatoxin-a
Anatoxin-a is a commonly occurring and potent neurotoxin. It is a bicylic secondary amine and the smallest of the cyanotoxins, with a molecular mass of 165 Da. Although the WHO has not determined a drinking water guideline for anatoxin-a, Fawell et al. [13] recommend a guideline concentration of 1 ug/L. Anatoxin-a is produced by three genera: Anabaena, Planktothrix, and Aphanizomenon. This cholinergic agonist mimics acetylcholine by having its amine and carbonyl functionality bind to the acetylcholine receptors. Anatoxin-a has two functional groups that are susceptible to oxidation, the amine and α,β-unsaturated ketone. The amine pK a is 9.4, which means that anatoxin-a is protonated and soluble in most natural waters and its oxidation may be pH-dependent.
Saxitoxins
These neurotoxins, commonly associated with “red tides,” are caused by blooms of marine dinoflagelletes and act as paralytic shellfish poisons. Saxitoxins have been found in several genera of freshwater cyanobacteria: Aphanizomenon, Anabaena, Lyngbya, and Cylindrospermopsis. Freshwater occurrences of saxitoxins are most noted for animal deaths; however, Australia does have a drinking water guideline of 3 ug/L of saxitoxin equivalence. Sixteen variants of saxitoxin have been reported. Saxitoxins are a group of carbamate alkaloid neurotoxins that are unsulfonated, monosulfonated, or disulfonated and are commonly referred to as saxitoxins, gonyautoxins, and C-toxins, respectively. This tricylic molecule has two guanidine groups with pK a values of 8.2 and 11.3. The cationic molecule dissolves readily in water. Only the two guanidine functional groups may be susceptible to oxidation.
Treatment considerations
Several factors influence the removal/inactivation of cyanotoxins from water. First and foremost among these factors is if the cyanotoxin is extracellular or intracellular. Cyanotoxins can be enclosed in the cell walls, exist intracellularly in the cytoplasm, or be released by excretion, cell lysis, or breakage, i.e., become extracellular cyanotoxins. Cyano metabolites and toxins increase chemical demand, disinfection by-product formation, and microbial growth in the distribution [14]. In addition, these metabolites often contribute off-tastes and odors, lowering the aesthetic quality of water. Treating intracellular toxin effectively will mean that particulate removal should remove intact cyanobacterial cells, whereas treating extracellular toxins follows strategies for removing natural organic matter or inactivating traditional chemical contaminants. The development of contingency treatment plans and the evaluation of each unit process will determine the success of producing a safe drinking water.
Optimizing the treatment process to remove fragile particulates is very important since approximately 95% of anatoxin-a, saxitoxin variants, and the microcystin variants are found intracellularly during the growth stage of the bloom [4]. When cell growth slows and the population begins to senesce, a larger proportion of intracellular toxin is released into the water. Bloom collapse occurs when environmental conditions become unfavorable for cyanobacteria, such as the lack of nutrients, extreme temperatures, predation, viral attack, flow/mixing changes, or when humans add chemicals such as algaecides. Cylindrospermopsin is naturally released from the cyanobacterial cells; the reported ratio is about 50% intracellular and 50% extracellular [15]. Chemical and physical properties of the cyanotoxins such as the hydrophobicity, hydrophilicity, molecular size, and functional groups affect the efficiency of treatment of extracellular cyanotoxins. The priority cyanotoxins listed from most hydrophilic to most hydrophobic are saxitoxin, cylindrospermopsin, anatoxin-a, and microcystin variants RR, YR, LR, and LA. The discussions that follow review and organize the published peer-reviewed literature in a manner so that drinking water managers may develop a multibarrier treatment train to remove/inactivate cyanotoxins while meeting their other goals under the disinfection by-product rule, microbial regulations, or the lead and copper rule.
Intake
There are three management strategies drinking water utilities may undertake to minimize consumer exposure to cyanotoxins: (1) use an alternate supply; (2) adjust intake depth; and (3) intake water treatment. For drinking water utilities that have access to more than one source water the simplest strategy is to change sources. Most utilities do not have multiple sources that are of sufficient size to supply 100% of the water demand during peak bloom times. An alternative strategy relies on knowledge of bloom ecology and water column dynamics [11]. Some cyanobacteria blooms occur at specific depths in the water column and cyanobacteria can regulate their buoyancy in a diurnal pattern. It is possible for utilities to draw water from different depths or at specific times to avoid drawing contaminated water and cells into the treatment plant. Often drinking water monitoring protocols call for samples to be taken at the intake; however, very little research has been done in the area of diurnal vertical buoyancy patterns and their temporal impact on the source water quality and plant operations throughout the day. A report by Werblow [16] reports that some drinking water utilities are monitoring source water at specific depths several times a day and implementing this information into their treatment process; however, the authors could not find peer-reviewed papers on diurnal patterns relevant to water treatment. Determining the diurnal depth patterns of different cyanobacteria in different types of source waters is especially important in determining the risk associated with a poorly mixed intake.
Oxidants are often added at the intake to (1) reduce taste and odor compounds, (2) discourage biological growth (zebra mussels, biofilm, and algae) on the intake pipe, (3) reduce the production of disinfection by-products (4) stimulate coagulation, and (5) remove manganese. The addition of an oxidant at the intake poses several questions with respect to cyanotoxin removal. The first concern is to prevent lysing the cells. The general consensus is that cyanotoxin removal is maximized by removing intact cells [3, 8, 11]. The question remains of whether an oxidant can be added at the intake that will not lyse the cells and still help oxidize the extracellular dissolved toxins. Chlorination at the intake is no longer commonly practiced because of the formation of chlorinated disinfection by-products and the risk of lysing algal cells [17, 18]. Four recent papers by Miao and Tao [19], Huang et al. [20], Cheng et al. [21], and Ho et al. [22] investigated the treatment of cyanobacterial cells with ozone and potassium permanganate. The ozonation of Microcystis aeruginosa and Oscillatoria tenuisa resulted in cell wall damage which released cytoplasm, microcystins, and volatile taste and odor compounds[19, 20]. In contrast Ho et al. [22] demonstrated that KMnO4 can be used at the intake without releasing intracellular saxitoxins or taste and odor compounds. Cheng et al. [21] also reported that KMnO4 inactivated Cylindrospermopsis cells with no release of toxin. Both of these results were in agreement with an early article by Schmidt et al. [23] and Chen and Yeh [24]. These publications suggest that KMnO4 is an effective oxidant that helps coagulate cells and achieves other treatment objectives, whereas ozone reacts with the cyanobacterial cell membrane, causing cell lysis and the release of cyanotoxins.
In general, several unit processes in a drinking water plant act synergistically to remove abiotic particulates such as silts and clays as well as biotic particulates such as bacteria and parasites. The standard treatment train is coagulation, flocculation, and sedimentation, followed by filtration. The performance of these unit processes is usually tracked and optimized with particle counters or surrogate monitoring parameters such as turbidity, streaming current, and zeta potential. Coagulation, flocculation, and dissolved air flotation (DAF) is more effective than sedimentation for cyanobacteria-rich waters and has been reviewed by Hrudey et al. [11], Falconer [8], and Knappe et al. [14]. Recent publications by Teixeira and Rosa [25, 26] focused on optimizing both coagulation/flocculation/sedimentation and coagulation/floculation/DAF and the impact of natural organic matter. They also reported DAF as a more effective treatment process for Microcystis removal. During a cyanobacterial bloom, these unit treatment processes can be managed as an auxiliary barrier to effectively remove intracellular cyanotoxins. Each of these unit treatment processes must be optimized to remove intact cyanobacterial cells.
To the best knowledge of the authors, no studies investigating lime precipitation as a separate drinking water process to remove cyanobacteria are available. However, two treatment studies by Hrudey et al. [11] and Lam et al. [28] evaluated drinking water treatment trains with lime. These studies did show removal of cyanobacteria without lysing the cells. The authors of this paper would classify lime soda precipitation treatment as an ancillary treatment process; further investigation is needed to determine dosage and robustness to consider this treatment process an auxiliary treatment.
A logical place to add a treatment barrier is after sedimentation of either floc or precipitate. Lime-softened water has a high pH and low carbonate concentration, leading to large pH variations, which could make some of the normal cyanotoxin barriers inefficient. No ancillary or auxiliary investigations were identified on lime-softened water.
Recent advances over the last two decades in membrane technology have allowed membrane filtration to become a viable drinking water treatment process. Two types of membrane filtration, microfiltration and ultrafiltration, are commonly used to remove particulate contaminants from drinking water. Microfiltration and ultrafiltration pore size varies from 0.075 to 3.0 µm and 0.1 to 0.001 µm, respectively. Studies have shown that pretreatment increases the removal efficiency of algal cells and lessens membrane fouling [29–32]. A review of pretreatment for low-pressure membrane filtration by Huang et al. [33] addresses how pretreatment for removal of cyanobacteria may impact the membrane. Studies suggest that both microfiltration and ultrafiltration as either stand-alone treatments or as a replacement for conventional sand filtration are highly effective at removing intact cyanobacterial cells [34–36]. Chow et al. [34] and Gijsbertsen-Abrahamse et al. [35] evaluated the removal of Microcytsis, unicellular, colonial cyanobacteria, and Planktothrix, filamentous cyanobacteria, respectively. More research is needed to determine the shear sensitivities and toxin release for other cyanobacteria. A summary of intact algal cell removal performance is given in Table 1.
Extracellular cyanotoxins
Extracellular cyanotoxin removal by common drinking water treatment processes is divided into three sections: physical removal; chemical inactivation; and biological inactivation. Physical removal of extracellular cyanotoxins includes adsorption by activated carbon and exclusion by membrane filtration. Chemical and biological inactivation includes biological activity and ultraviolet light unit processes and oxidants.
Activated carbon
Common precursors for large-scale production of activated carbon are coal, coconut, and wood. The different precursors and activation processes result in adsorptive capabilities that can be varied and the activated carbons can be made somewhat selective for specific contaminants. Carbons may be characterized by the ratio of micro to meso to macro pore structure. Two types of activated carbon are used in the drinking water industry; powdered activated carbon is generally used as a temporary treatment for transient contaminants and granular activated carbon (GAC) is used in fixed beds to reduce natural organic matter, taste and odor compounds, and synthetic organic compounds from industrialized source waters.
Both microcystin and cylindrospermopsin can be absorbed by activated carbon with high mesopore capacity [17, 37]. The microcystin study also suggested that the microcystin variants may have different adsorption efficiencies; the order for the four variants from least to most adsorbent was reported to be RR, YR, LR, and LA. Both studies successfully used a homogenous surface diffusion model to predict the rate of cyanotoxin adsorption by powdered activated carbon. This type of model uses both isotherm and kinetic data to predict large-scale performance and has been widely used for synthetic organic compounds in the past.
Saxitoxins are lower molecular mass cyanotoxins. Studies by Newcombe [7] and Ho et al. [22] suggest activated carbons with a large fraction of pores that are smaller than 1 nm (micropore) will have the greatest capacity to adsorb saxitoxins. Good-quality steam-activated wood, coconut, or coal-based carbon are usually the best. In the past, a comparison of activated carbon was not feasible because of the cost of saxitoxin analysis; a new commercially available saxitoxin ELISA kit makes it economically feasible for a site-specific activated carbon comparison. Otherwise, a general rule to follow is that carbons that are good at removing taste and odor compounds such as 2-methylisoborneol and geosmin are also effective for removing saxitoxins [17].
GAC can be used either as a filter medium or as an adsorber. GAC filters are designed to remove particulates, adsorb chemicals, and biodegrade organic contaminants. The GAC filter medium is replaced after several years of service. In contrast, GAC adsorbers are used to remove organic contaminates by adsorption and the GAC adsorber medium is replaced or regenerated when total organic carbon breakthrough is high. Within 2 months of the start of service, Newcombe [17] reported microcystin in the GAC column effluent at 80% total organic carbon breakthrough. This suggests that GAC filters, which are expected to have a service life of 2 years or more, are an ineffective barrier but GAC adsorbers with proper regeneration or replacement can be used as an auxiliary barrier for microcystin.
Newcombe [17] and Orr et al. [38] investigated the effectiveness of GAC at removing variants of saxitoxin. Both investigations reported GAC was highly effective at removing STX and less effective at removing C1 and C2. GTX2/3 removal was variable between the two waters that were studied. Both Newcombe and Orr et al. considered GAC an effective barrier for saxitoxins.
Since microcystins and cylindrospermopsin appear to adsorb to the mesopores of activated carbon, the authors hypothesize that GAC filters will not be a good a barrier for cylindrospermopsin; however, GAC adsorbers with regeneration that maintains the spatial, physical, and chemical characteristics of the pore or replacement can be an auxiliary treatment process barrier. Very little work has been performed on the adsorption of anatoxin-a to activated carbon. More systematic studies are needed to determine which activated carbon type, dosage, and contact time is appropriate.
Membrane filtration
Reverse osmosis, nanofiltration, and ultrafiltration processes separate contaminants by size and charge depending on the physical/chemical characteristics of the membrane. Nanofiltration and reverse osmosis filtration studies report from 82% to complete microcystin removal [39, 40]. A reverse osmosis membrane study using between 10 and 130 ug/L of microcystin LR and RR removed more than 95% of the cyanotoxins from the waters [41, 42]. More recently, Gijsbertsen-Abrahamse et al. [35] examined the removal of four microcystins (LR, RR, YR, and LA) and anatoxin-a by a Trisep membrane with a molecular mass cutoff of 200 Da. The membrane showed at least 96% removal of all the cyanotoxins. Only anatoxin-a and microcystin RR were detected in the nanomembrane permeate. On the basis of the experimental result presented in that paper, these authors predict a rejection of 90% at full scale for the cyanotoxins tested. Teixeira and Rosa [25] investigated the removal of microcystin and anatoxin-a with a negatively charged nanofiltration membrane with a molecular mass cutoff of 150 g/mol. The main mechanisms involved in anatoxin-a rejection were electrostatic interactions and steric hindrance, whereas for microcystin rejection the mechanism was believed to be by steric hindrance alone. Very little work has looked at the efficiency of nanofiltration and reverse osmosis filtration to remove cylindrospermopsin and saxitoxins. On the basis solely of cutoff values, nanofiltration and reverse osmosis may effectively remove cylindrospermopsin and saxitoxins.
Recently, Lee and Walker [30] evaluated two types of ultrafiltration membranes: cellulose acetate and polyethersulfone membranes. The cellulose membrane, with 20-kDa molecular mass cutoff, did not reject or adsorb microcystin LR. The polyethersulfone with 20-kDa molecular mass cutoff, adsorbed microcystin LR and after 60 min no more microcystin LR was removed from the system. Lee and Walker [30] predicted the adsorption was based on hydrophobic interaction. Finally, a polyethersulfone membrane with 5-kDa molecular mass cutoff reduced microcystin LR by about 8%, which was comparable to reduction of the 1-kDa test compound, poly(ethene glylcol). This suggests that size exclusion was the primary rejection mechanism and that the repulsive charge interactions between the microcystin and the membrane were secondary. These experiments clearly show that ultrafiltration is not a reliable treatment barrier for cyanotoxins. Both molecular mass cutoff and intermolecular interactions, van der Waals, dipole-dipole, hydrogen bonding, and electrostatic interaction, play important roles in determining the rejection criteria of the membrane. More research is needed to determine the mechanism of cyanotoxin membrane rejection because if the rejection is based on intermolecular interactions, then membrane rejection may also be pH- or variant-dependent.
Biologically active filtration
Although biological activity has been recognized for years as a somewhat unintentional ancillary treatment, in the last 20 years researchers have explicitly investigated using biological activity as an auxiliary treatment barrier. Although biological activity occurs throughout the drinking water processes, most investigations have focused on different types of filtration. Biologically active river bank filtration and both slow and rapid filtration have been reported to remove/inactivate microcystins [3, 43–45] and cylindrospermopsin [46]. Acclimation prior to degradation is dependent on the conditioning and induction of a microbial population capable of metabolizing microcystins [47–50]. Other environmental variables include temperature, pH, and predation [17]. Grutzmacher et al. [45] conducted two full-scale experiments to study the removal of microcystins by biologically active slow sand filtration. During the summer months, with intact Schmutzdecke (biofilm) the removal of dissolved microcystin was more than 95% , whereas during the autumn months the removal declined to less than 65%. This decline was most likely attributable to a decrease in temperature. Bourne et al. [43] performed a pilot scale inoculation study using a bacterium (MJ-PV) which had been previously demonstrated to degrade microcystin LR. They demonstrated that inoculation may provide the initial rapid degradation of microcystin through shortening the acclimation phase. Recently, Kayal et al. [51] assessed the fate of five saxitoxin variants through biologically active filter media from two treatment facilities. Although a decrease in the concentration of the less toxic variants was reported, increased concentrations of the more toxic variants were also reported. These results suggest biotransformation from a less toxic to a more toxic saxitoxin may occur in biologically active filters. Little work has been performed on anatoxin-a because of an earlier publication by Rapala et al. [50] that stated anatoxin-a is readily biodegraded in natural waters. It is the opinion of the authors that since physiological conditions must be maintained, the potential of biologically active filtration as an auxiliary barrier is limited; we view biological activity as always being an ancillary barrier.
UV disinfection
The absorption of UV energy can break molecular bonds without chemical addition and is used to inactivate many pathogens in drinking water. Normally, the UV treatment process uses a low to medium pressure lamp with UV doses between 10 and 40 mJ/cm2 for disinfection. Work by Tsuji et al. [52], Chorus and Bartram [4], and Senogles et al. [53] suggests that microcystin, anatoxin-a, and cylindrospermopsin can undergo photolytic destruction at doses that range from 1,530 and 20,000 mJ/cm2, which are orders of magnitude higher than that needed for disinfection. Low-pressure narrow-band mercury vapor lamps at 254 nm degraded cylindrospermopsin above 643 mJ/cm2 [21]. Because of the high doses required, low to medium pressure lamp UV treatment is not recommended as a viable treatment barrier for cyanotoxins.
Oxidants
In light of Lawton and Robertson’s [54] overview of potential reaction schemes for the oxidation of microcystin LR, this section of the review discusses the more recent literature which determines the reaction rates and mechanisms of the commonly used oxidants and cyanotoxins. Primary oxidants in drinking water are chlorine, ozone, hydroxyl radical, chloramines, potassium permanganate, and chlorine dioxide. These oxidants are commonly used before chemical addition, before filtering, or after filtering as disinfectants. With more countries making more stringent regulations for disinfection by-products, which are measured most frequently by the amount of halogenated compound formed from chlorination, chlorite and chlorate from chlorine dioxide and bromate from ozone, drinking water utilities are changing and combining oxidants to meet these regulations. Oxidation demand is dependent on the individual characteristics of the source water. Total organic carbon, temperature, pH, and potentially even treatment chemicals can change the oxidant demand, which may lower their effectiveness to inactivate cyantoxins. For example, ozone may be applied before and after coagulation to reduce the amount of halogenated compounds formed upon chlorination or the pH of the treatment water may be increased to 9, so lower amounts of halogenated compounds will be formed. Insufficient oxidation of cyanotoxins in the drinking water supply of a hemodialysis clinic in Brazil led to the death and chronic illness of several patients [55]. The Palm Island Mystery disease of 1979, a severe outbreak of hepatoenteritis in an Australian Aboriginal community, has been attributed to the release of the cell-bound cylindrospermopsin in a water supply treated with copper sulfate and minimal inactivation from treatment [15]. This section reviews the effectiveness and application of these oxidants for the inactivation of the cyanotoxins (Table 2).
Chlorine has played an important role in drinking water disinfection over the last 100 years. More recently, chlorine has been investigated to determine if it can be used as auxiliary treatment for organic contaminants such as cyanotoxins. Inactivation of organic contaminants by chlorine is usually pH-dependent because the pK a of hypochlorous acid is 7.6. Two publications proposed chlorine inactivation mechanisms for several variants of microcystin [56, 57]. Acero et al. [56] reported that microcystins LR, RR, and YR react with chlorine at the same rate and have the same reduction in toxicity, suggesting that the hydroxylation of the Adda moiety is the primary site for oxidation. In contrast, Ho et al. [57] reported that chlorine reaction rates for microcystins YR, RR, LR, and LA were different, with the order YR > RR > LR > LA suggesting the oxidation of different amino acids played an important role in the deactivation of the microcystins [57]. Regardless of the mechanism, both reports suggested that chlorination with pH <8.0 is an effective mechanism for inactivation.
Cylindrospermopsin can also be inactivated by chlorination between pH 6 and 9 [53, 58]. Two chlorinated by-products have been identified as a chlorouracil, 5-chloro-cylinderspermopsin [53], and a carboxylic acid derivative [64]. These chlorinated by-products produced fatty vacuolations on the liver in mice [70]. Unlike microcystin, cylindrospermopsin was reported by Senogles et al. [53] to be more susceptible to inactivation at higher pHs when the amine on the uracil is not protonated. However, in a more recent study, Rodriguez et al [60] reported that the maximum rate of inactivation of cylindrospermopsin is at pH 7, with an apparent second-order rate constant of 1,265 M−1 s−1.
The inactivation of anatoxin-a by chlorine has been reported as a very slow process by both Hrudey et al. [11] and Rodriguez et al. [67]. The more recent study of Rodriguez et al. [67], determined the apparent second-order rate constant at pH 7 and 20 °C to be 0.71 M−1 s−1. On the basis of the results from both of these investigations, chlorine is not a suitable oxidant for the inactivation of anatoxin-a.
Work by Nicholson et al. [61] suggests that the degradation of saxitoxins by chlorine is also pH-dependent; however, in contrast with the microcystins, higher pH values increase the inactivation rate of saxitoxin. The saxitoxin order of reactivity with chlorine was STX5 = dcSTX > STX > GTX3 = C2 > GTX2, with the most toxic saxitoxin being the most susceptible. Although in this study the saxitoxin by-products were not elucidated, the acute toxicity as determined by mouse bioassay was eliminated.
Chlorine dioxide reacts with tertiary amine and activated aromatic systems and does not promote brominated by-products [71]. Although chlorine dioxide does not promote a brominated by-product, it does produce chlorite and chlorate, which are regulated inorganic compounds. This creates a balancing act between inactivation of the contaminant and the production of regulated contaminants. The apparent rate constant for microcystin LR was determined by Kull et al. [72] to be about 1 M−1 s−1 at pH 8. This reaction has slight pH dependence. The reactivity of cylindrospermopsin with chlorine dioxide was also about 1 M−1 s−1 at pH 8, but decreased by about 30% at pH 6 [66]. The reactivity of anatoxin-a with chlorine dioxide was not measurable [66].
Chloroamines are poor oxidants, but they are frequently used to provide residual disinfectant to minimize the formation of regulated chlorinated by-products such as trihalomethanes. Chloroamine and chlorine dioxide are not effective treatment barriers for microcystin, cylindrospermopsin, anatoxin-a, and saxitoxins. Treatment utilities using chlorine dioxide and chloramines to reduce disinfection by-products may not have an oxidant treatment barrier for cyanotoxin inactivation.
Potassium permanganate mainly oxidizes double bonds to diols and very little is known about its reaction with amines. The reactivity of potassium permanganate with microcystin LR was not dependent on pH. The apparent rate constant for microcystins LR, RR, and YR ranged between 350 and 420 M−1 s−1 [66], which was consistent with the findings of Chen and Yeh [24]. Potassium permanganate is not very reactive with cylindrospermopsin [64, 66]. From pH 6 to 8, the reactivity of anatoxin-a with permanganate was constant at 2,100 M−1 s−1. A pH dependence was observed for an apparent rate constant between 8 and 10, which is consistent with the pK a of 9.4 for the protonated secondary amine in anatoxin-a. The apparent rate constant doubles between pH 8 and 10. Ho et al. [22] recently reported that saxitoxin is not oxidized by potassium permanganate.
Ozonolysis has two mechanisms of oxidation, ozone and hydroxyl radical. Ozone reacts with alkene groups, activated aromatic and neutral amine functional groups, whereas the hydroxyl radical randomly attacks carbon-hydrogen bonds in organic molecules [73]. Onstad et al. [63] added several valuable pieces of information regarding the ozonation of cyanotoxins by systematically looking the each of the cyanotoxins in both deionized and natural water matrices. Through the use of model compounds and adjustment of pH, they determined the ozone reactive functional groups in the cyanotoxins and the secondary rate constant; the information was then evaluated relative to natural waters. Ozone reacts with the conjugated double bond and single double bond in the Adda and the Mdha group, respectively, of microcystin. In contrast to the pH-independent ozone oxidation of microcystins, anatoxin-a is pH-dependent from 7 to 10 and cylindrospermopsin is pH-dependent between pH 4 and 10. This is consistent with the pK a values of the anatoxin-a amine and the cylindrospermopsin uracil moiety. At pH 8 the cyanotoxins react with ozone in the order microcystins (3.4 × 105 M−1 s−1) > cylindrospermopsin (3.4 × 105 M−1 s−1) > anatoxin-a (6.4 × 104 M−1 s−1) [63].
Advanced oxidation processes
Advanced oxidation processes are processes that generate and use the hydroxyl radical. The hydroxyl radical is a relatively nonselective oxidant. The hydroxyl radical can be derived by both photochemical and nonphotochemical means. The hydroxyl radical reacts in the order microcystins (1.1 × 1010 M−1 s−1) > cylindrospermopsin (5.5 × 109 M−1 s−1) > anatoxin-a (3.0 × 109 M−1 s−1) [63]. The findings of radiolysis studies by Song et al. [81] are in agreement with Onstad et al. [63] reporting the rate constant for the reaction of the hydroxyl radical with microcystin LR to be 2.3 × 1010 M−1 s−1. No rate constants for the reaction of the hydroxyl radical with saxitoxin variants were found. With a rate constant several orders of magnitude larger than for other oxidants, developing a viable advanced oxidation treatment process for the inactivation of cyanotoxins would be advantageous.
Earlier studies demonstrated inactivation of microcystin LR by UV/H2O2 [74], Fenton and photo-Fenton processes [75], and TiO2/UV[54, 76, 77]. Choi et al. [78] focused on the fabrication of mesoporous nitrogen-doped TiO2 photocatalysts and their ability to inactivate microcystin LR under UV and visible light. Acidic conditions were more favorable for adsorption and photocatalytic degradation of microcystin LR, making this process currently not applicable to water treatment. Liang et al. [79] investigated the application of electrochemical oxidation using a Ti/RuO2 anode to inactivate microcystin LR. Total removal of microcystin LR was achieved at a current density of 5 mA/cm2. The advantage to this system is that it works on natural waters without pH adjustment.
In a study by Zhang et al. [80] the inactivation of microcystin RR on a boron-doped diamond electrode was investigated. The supporting electrolyte and the applied current density were varied to determine their effects on inactivation. They determined that the optimum conditions were pH 3 and 20 mM sodium chloride. The major disadvantage with this technology is the low pH coupled with the sodium chloride concentration above the 7 mM sodium chloride US secondary drinking water standard.
Song et al. [81] demonstrated the inactivation of the microcystin LR by ultrasonic irradiation at 40 kHz. Inactivation occurred from three chemical processes; hydroxyl radical, hydrolysis, and pyrolysis. Later studies identified that the degradation products were due to a hydroxyl radical attack on the phenyl and diene present in the Adda group and cleavage of the Mdha-Ala peptide bond [81]. As in the TiO2 system, degradation of microcystin LR is much greater at acidic pH. The major disadvantage to this technology is that it is most effective at pH 3, which is not practical for drinking water treatment. These emerging technologies need to expand their investigations to cylindrospermopsin, anatoxin-a, and saxitoxins.
Conclusion
Toxic cyanobacteria are an emerging and increasing threat to public water supplies across the world. Protection of the source water is the best approach. However, as society deals with climate change, invasive species and increasing nutrient load to our water supplies, source water protection alone is insufficient to ensure safe drinking water. As the threats from cyanobacteria and their toxins are multifaceted, so too must be the treatment approach of the drinking water utilities. It is essential to minimize risk from toxic cyanobacteria and their metabolites while complying with existing regulations, particularly with respect to proper disinfection and control of disinfection by-products. The key in this case is to be vigilant and monitor the water throughout the treatment plant and, in particular, the source water. More research needs to be done on the ecology, conditions that lead to cyanotoxin production, and diurnal patterns of cyanobacteria. The general rules that emerged from this review are that it is imperative to try and remove intact cyanobacterial cells and that auxiliary treatment barriers must be implemented. These two principals should guide treatment decisions. The multibarrier concept should be extended to cyanotoxins and critical decisions such as the oxidant type and location of oxidant addition must be based on recent reports of investigations of the underlying chemical mechanisms of cyanotoxin inactivation. The emerging threat of cyanobacteria and their toxins must be considered when retrofitting old plants or designing new plants. Furthermore, plants should provide multiple points at which auxiliary treatment can be provided. Finally, it is imperative that the industry recognizes that cyanotoxins pose significant threats to their operations and that they must monitor and respond proactively.
References
Dittmann E, Neilan BA, Erhard M, von Dèohren H, Bèorner T (1997) Mol Microbiol 26:779–787
Borner T, Dittmann E (2005) In: Huisman J, Matthijs HCP, Visser PM (eds) Harmful cyanobacteria. Springer, Berlin, pp 25–40
Yoo RS, Carmichael WW, Hoehn RC, Hurdley SE (1995) Cyanobacterial (blue-green algal) toxins: a resource guide. AWWA Research Foundation and American Water Works Association, Denver
Chorus I, Bartram JF (eds) (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. Spon, London
Oehrle SA, Westrick J (2003) LCGC North Am 21:634–639
Svrcek C, Smith D (2004) J Environ Eng Sci 3:31
Newcombe G, Nicholson B (2004) J Water Supply Res Technol AQUA 53:227–239
Falconer IR (2005) Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. CRC, Boca Raton
Westrick JA (2008) Adv Exp Med Biol 619:16
Hudnell HK (2008) Adv Exp Med Biol 619:980
Hrudey S, Burch M, Drikas M, Gregory R (1999) In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. Spon, London
EPA US (2006) Toxicological reviews of cyanobacterial toxins: cylindrospermopsin (external review draft). United States Environmental Protection Agency, Washington
Fawell JK, Mitchell RE, Everett DJ, Hill RE (1999) Hum Exp Toxicol 18:162
Knappe DRU, Belk RC, Briley DS, Gandy SR, Rastogi N, Rike AH, Glasgow H, Hannon E, Frazier WD, Kohl P, Pugsley S (2004) Algae detection and removal strategies for drinking water treatment plants. AWWA Research Foundation, Denver
Griffiths DJ, Saker ML (2003) Environ Toxicol 18:16
Werblow S (2008) J AWWA 100:48–50, 52
Newcombe G (2002) Removal of algal toxins from drinking water using ozone and GAC. AWWA Research Foundation, Denver, p 133
Jolley RL, Gorchev H, Heyward Hamiliton JD (1977) Water chlorination: environmental impact and health effects. Ann Arbor Science Publishers, Ann Arbor
Miao H, Tao W (2007) Sep Purif Technol 66:7
Huang W-J, Cheng Y-L, Cheng B-L (2008) Environ Eng Sci 25:139–152
Cheng X, Shi H, Adams CD, Timmons T, Ma Y (2009) Water Sci Technol 60:689–698
Ho L, Tanis-Plant P, Kayal N, Slyman N, Newcombe G (2009) J Water Health 7:544–556
Schmidt W, Willmitzer H, Bornmann K, Pietsch J (2002) Environ Toxicol 17:11
Chen JJ, Yeh HH (2005) Water Res 39:4420–4428
Teixeira MR, Rosa MJ (2006) Sep Purif Technol 53:9
Teixeira MR, Rosa MJ (2006) Water Res 40:2837–2846
Drikas M, Chow CWK, House J, Burch MD (2001) J AWWA 93:12
Lam A-Y, Prepas E, Spink D, Hrudey S (1995) Water Res 29:1845–1854
Heng L, Yanling Y, Weijia G, Xing L, Guibai L (2008) Desalination 222:7
Lee JW, Walker H (2006) Environ Sci Technol 40:7
Qin J-J, Oo MH, Kekre KA, Knops F, Miller P (2006) Desalination 193:6
Kwon B, Park N, Cho J (2005) Desalination 179:12
Huang H, Schwab K, Jacangelo JG (2009) Environ Sci Technol 43:3011–3019
Chow CWK, Panglisch S, House J, Drikas M, Burch MD, Gimbel R (1997) Aqua 46:11
Gijsbertsen-Abrahamse AJ, Schmidt W, Chorus I, Heijman SGJ (2006) J Membr Sci 276:252–259
Zhou H, Smith D (2001) J Environ Eng Sci 1:18
Ho L, Slyman N, Kaeding U, Newcombe G (2008) J AWWA 100:88–96
Orr PT, Jones GJ, Hamilton GR (2004) Water Res 38:4455–4461
Fawell JK, Hart J, James HA, Parr W (1993) Water Supply 11:109–122
Muntisov M, Trimboli P (1996) Water 23:34
Neumann U, Weckesser J (1998) Environ Toxicol Water Qual 13:6
Vuori E, Pelander A, Himberg K, Waris M (1997) Water Res 31:2922
Bourne DG, Blakeley RL, Riddles P, Jones GJ (2006) Water Res 40:1294–1302
Lahti K, Rapala J, Kivimaki AL, Kukkonen J, Niemela M, Sivonen K (2001) Water Sci Technol 43:225–228
Grutzmacher G, Bottcher G, Chorus I, Bartel H (2002) Environ Toxicol 17:9
Ho L, McDowall B, Wijesundara S, Shaw G, Saint C, Newcombe G (2005) Water 32:64–68
Jones GJ, Orr PT (1994) Water Res 28:871
Cousins IT, Bealing DJ, James HA, Sutton A (1996) Water Res 30:481
Christoffersen K, Lyck S, Winding A (2002) Aquat Microb Ecol 27:125–136
Rapala J, Lahti K, Sivonen K, Niemelae SI (1994) Lett Appl Microbiol 19:423
Kayal N, Newcombe G, Ho L (2008) Environ Toxicol 23:751
Tsuji K, Naito S, Kondo F, Ishikawa N, Watanabe MF (1994) Environ Sci Technol 28:173
Senogles P, Shaw G, Smith M, Norris R, Chiswell R, Mueller J, Sadler R, Eaglesham G (2000) Toxicon 38:1203–1213
Lawton LA, Robertson PKJ (1999) Chem Soc Rev 28:217–224
Jochimsen EM (1998) N Engl J Med 338:6
Acero JL, Rodriguez E, Meriluoto J (2005) Water Res 39:1628–1638
Ho L, Onstad G, v Gunten U, Rinck-Pfeiffer S, Craig K, Newcombe G (2006) Water Res 40:1200–1209
Nicholson BC, Rositano J, Burch MD (1994) Water Res 28:1297
Xagoraraki I, Harrington GW, Zulliger K, Zeier B, Krick W, Karner DA, Standridge JH, Westrick J (2006) J Environ Eng 132:818
Rodriguez E, Sordo A, Metcalf JS, Acero JL (2007) Water Res 41:2048–2056
Nicholson BC, Shaw GR, Morrall J, Senogles PJ, Woods TA, Papageorgiou J, Kapralos C, Wickramasinghe W, Davis BC, Eaglesham GK (2003) Environ Technol 24:1341–1348
Rositano J, Newcombe G, Nicholson B, Sztajnbok P (2001) Water Res 35:23–32
Onstad GD, Strauch S, Meriluoto J, Codd GA, von Gunten U (2007) Environ Sci Technol 41:4397–4404
Banker R, Carmeli S, Werman M, Teltsch B, Porat R, Sukenik A (2001) J Toxicol Environ Health A 62:281–288
Kull TPJ, Sjovall OT, Tammenkoski MK, Backlund PH, Meriluoto JAO (2006) Environ Sci Technol 40:1504–1510
Rodriguez E, Majado ME, Meriluoto J, Acero JL (2007) Water Res 41:102–110
Rodriguez E, Onstad GD, Kull TP, Metcalf JS, Acero JL, von Gunten U (2007) Water Res 41:3381–3393
Hall T, Hart J, Croll B, Gregory R (2000) Water Environ Manag 14:143–149
Rositano J, Nicholson BC, Pieronne P (1998) Ozone Sci Eng 20:16
Senogles-Derham PJ, Seawright A, Shaw G, Wickramisingh W, Shahin M (2003) Toxicon 41:979–988
Hoigne J, Bader H (1994) Water Res 28:45
Kull TPJ, Backlund PH, Karlsson KM, Meriluoto JAO (2004) Environ Sci Technol 38:7
von Gunten U, Hoigne J (1994) Environ Sci Technol 28:1234
Qiao RP, Li N, Qi XH, Wang QS, Zhuang YY (2005) Toxicon 45:745–752
Bandala ER, Martinez D, Martinez E, Dionysiou DD (2004) Toxicon 43:829–832
Liu I, Lawton L, Robertson PKJ (2003) Environ Sci Technol 37:3214–3219
Feitz AJ, Waite TD, Jones GJ, Boyden BH, Orr PT (1999) Environ Sci Technol 33:7
Choi H, Antoniou MG, Pelaez M, de la Cruz AA, Shoemaker JA, Dionysiou DD (2007) Environ Sci Technol 41:6
Liang W, Qu J, Wang K, Wang J, Liu H, Lei P (2008) Environ Eng Sci 25:635–642
Zhang C, Fu D, Gu Z (2009) J Hazard Mater 172:847–853
Song W, de la Cruz AA, Rein K, Shea KE (2006) Environ Sci Technol 40:6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westrick, J.A., Szlag, D.C., Southwell, B.J. et al. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal Bioanal Chem 397, 1705–1714 (2010). https://doi.org/10.1007/s00216-010-3709-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3709-5