Abstract
Aluminum (Al), cerium (Ce), cobalt (Co), iodine (I), lanthanum (La), sodium (Na), selenium (Se), silicon (Si), titanium (Ti), and vanadium (V) are emerging as novel biostimulants that may enhance crop productivity and nutritional quality while improving responses to environmental stimuli and stressors in some plant species. These beneficial elements are not essential for most plants, but when supplied at low dosages, they help improve their growth, development, and yield quality by stimulating different molecular, biochemical, and physiological mechanisms triggering adaptive responses to challenging environments. When plants are exposed to environmental cues such as drought, heavy metal toxicity, low temperatures, saline soils, pest insects, or pathogens, beneficial elements may induce tolerance, resistance, or defense responses that allow plants to achieve acclimation to such stressors. Enhancement of nutrient uptake, synthesis of antioxidants and osmoprotectants, stimulation of secondary metabolism and signaling cascades, and reduction of senescence are among the responses boosted by beneficial elements when applied at low dosages. Nevertheless, beneficial elements may trigger hormesis in plants, a biphasic dose response with at low-dose stimulation or beneficial effect and a high-dose inhibitory or toxic effect. Thus, when properly applied, beneficial elements may have great potential to cope with some of the most daunting challenges facing humanity, such as climate change and food production under restrictive conditions for the growing human population. In this chapter, we mainly focus on the positive effects of beneficial elements on plant performance in restrictive environments and discuss some of the challenges of using these elements as biostimulants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Climate change
- Environmental stressors
- Plant nutrition
- Nonessential elements
- Biostimulants
- Hormesis
- Innovation
- Beneficial elements
1 Introduction
Plants need essential elements to ensure successful growth and development during both vegetative and reproductive stages. Essential elements are classified as macronutrients and micronutrients, depending on the amounts contained in plant tissues. Macronutrients are represented by elements which are generally found in plants at concentrations greater than 0.1% of dry matter weight (DMW, >1000 mg kg−1), consisting of nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), and magnesium (Mg). Micronutrients are represented by chlorine (Cl), boron (B), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), and zinc (Zn); these nutrients are typically found at concentrations lower than 0.01% DMW, (<100 mg kg−1DMW) (Pilon-Smits et al. 2009; Alcántar-González et al. 2016). These 14 nutrients, along with the elements carbon (C), hydrogen (H), and oxygen (O), are broadly accepted as essential for all plant species (Kirkby 2012; Alcántar-González et al. 2016).
Beneficial elements are not essential for most plants, but they can promote growth and be essential for some plant species under specific conditions (Pilon-Smits et al. 2009). When supplied at low dosages, they have a favorable impact on some vital processes and can also stimulate the mechanisms of resistance to biotic and abiotic stresses or promote the uptake of other nutrients (Trejo-Téllez et al. 2016). Additionally, beneficial elements can compensate for or remedy the toxic effects of other elements, and they can also, in some cases, provide certain functions of essential nutrients, such as the maintenance of osmotic pressure (Trejo-Téllez and Gómez-Merino 2012), or induce adaptive plant responses to adverse environmental phenomena (Pilon-Smits et al. 2009).
This chapter describes the effects of the beneficial elements identified so far, that is, aluminum (Al), cerium (Ce), cobalt (Co), iodine (I), lanthanum (La), sodium (Na), selenium (Se), silicon (Si), titanium (Ti), and vanadium (V), on the physiology of plants, with special emphasis on the induction of adaptive responses to challenging environments.
2 The Ten Beneficial Elements
The ten beneficial elements recognized until now have been shown to improve plant growth, production, and yield quality, as well as ameliorate the responses of plant to different environmental stress factors. A summary of the main functions of these elements in plants is displayed in Fig. 1.
Overview of the mechanisms responsible for the stimulating effects of the ten beneficial elements Al, Ce, Co, I, La, Na, Se, Si, Ti, and V on plant growth and stress responses. Main groups of plants on which beneficial effects of these elements have been documented are displayed at the heading of each box text
2.1 Aluminum (Al)
Aluminum is the most abundant metal in the Earth’s crust (comprising about 7% of its mass), and its solubility increases with decreasing soil pH (Dong et al. 2002). While in acidic soils (pH less than 5) Al may inhibit root growth and display toxic effects to plants, it can be a beneficial element for some plant taxa under certain conditions (Moreno-Alvarado et al. 2017). One of the best-known examples of the beneficial effect of Al is observed in hydrangea (Hydrangea macrophylla Thunb. Ser.), since supplying them with different concentrations of Al turns them from pink (50 mg kg−1 DBW) to blue (4000 mg kg−1 DBW), which is attributed to the formation of a colloidal complex or to the combination of Al with a pigment called delphinidin (Trejo-Téllez et al. 2016), an anthocyanin responsible for the pigments in the plant’s epidermal or subepidermal cells.
In rhododendron (Melastoma malabathricum L.), supplying Al in the complete nutrient solution enhances root development and plant growth (Watanabe et al. 2005). In rose (Rosa spp.) cv. “Cherry Brandy,” supplying Al2(SO4)3 significantly increased vase life and improved postharvest quality, because it may maintain the flower’s fresh matter weight (FMW) and may increase the chlorophyll content in leaves (Jowkar et al. 2012). According to Seyf et al. (2012a), the application of 0, 150, and 300 mg L−1 Al2(SO4)3 in “Boeing” roses increased vase life from 9 to 12 and 12.3 days, respectively, and increased flower diameter compared to control plants. In lisianthus (Eustoma grandiflorum Raf. Shinn.), the application of 150 mg L−1Al sulfate to flowers prolonged vase life from 8 to 15 days, and FMW continued to increase up to 8 days after the start of the experiment (Li-Jen et al. 2001). In tuberose (Polianthes tuberosa L.) cv.”Single,” the application of 50 and 100 mg L−1aluminum sulfate extends the vase life to 11.5 and 12 days, respectively (Mohammadi et al. 2012a). Furthermore, Al increased protein content and reduced FMW losses. The foliar application of 0.5, 1.0, and 1.5 g L−1potassium aluminum sulfate [KAl(SO4)2] in sampaguita (Jasminum sambac L.) twice a day increased vessel life and FMW (Acero et al. 2016). The application of aluminum sulfate and 8% sucrose increases the quality and durability of rose cv. “Maroussia” stems in postharvest (Basaki et al. 2013). Likewise, De la Cruz-Guzmán et al. (2007) found that treatment with 0.6 g L−1 Al2(SO4)3, in rose cv. “Royalty,” reduces FMW loss during the vase period. Therefore, Al has great potential as a senescence retardant in cut flowers and also enhances their quality.
In medicinal plants such as chamomile (Matricaria chamomilla.), 60 μM Al increases soluble phenol and flavonoid contents in shoots and free amino acids in roots (Kováčik et al. 2010), which are antioxidant compounds that help plants overcome some stress factors. In silver birch (Betula pendula Roth.), the application of 2 and 5 mg L−1 Al enhances leaf growth (Kidd and Proctor 2000). In soybean (Glycine max L. Merr.) plants exposed to 1.0 μM Cd and 150 μM Al at pH 4.0, the malondialdehyde (MDA, a lipid peroxidation marker) content and superoxide dismutase (SOD) and peroxidase (POD) enzyme activities increased, which shows that Cd and Al are synergistic and stimulate antioxidant mechanisms (Shamsi et al. 2008). In maize (Zea mays L.), the application of 48 μM Al increased leaf growth rates, as a consequence of an increase in protein synthesis and a reduction in ubiquitin-mediated proteasomal degradation of growth-repressing proteins, such as DELLA in plants, and consequently promoted growth (Conti et al. 2014; Wang et al. 2015).
2.2 Cerium (Ce)
Cerium levels in soil range from 2 to 150 ppm and average 50 ppm (Trejo-Téllez et al. 2016). As a beneficial element, Morales et al. (2013) reported that adding 125 mg kg−1CeO2 nanoparticles (nCeO2) to cilantro (Coriandrum sativum L.) plants produces longer roots and increases catalase (CAT) enzyme activity in shoots and that of POD in roots.
Tomato (Solanum lycopersicum L.) seeds treated with CeO2 nanoparticles (<10 mg L−1) develop seedlings with more extensive root hairs than the control. However, substantially higher Ce concentrations were detected in the fruits exposed to 10 mg nCeO2L−1, compared with controls (Wang et al. 2012), shedding light on the long-term impact of nCeO2on plant health and its implications for our food safety and security. Importantly, second-generation seedlings grown from seeds collected from treated parent plants with nCeO2 (treated second-generation seedlings) were generally smaller and weaker, as indicated by their smaller biomass, lower water transpiration, and slightly higher reactive oxygen species content (Wang et al. 2013a).
In spinach (Spinacia oleracea L.) plants grown in Mg-deficient medium and treated with CeCl3, Ce stimulated the activity of nitrate reductase, nitrite reductase, glutamate dehydrogenase, glutamate synthase, urease, and glutamic-pyruvic transaminase, which are key for N metabolism, suggesting that Ce may partially replace Mg functions to transform inorganic N to organic N, but the mechanisms underlying such responses need further study (Yin et al. 2009).
In maize and mung bean (Vigna radiata L. Wilczek), Ce shows favorable effects on the absorption of other nutrients when applied at concentrations below 0.2 μM (Diatloff et al. 2008). In Arabidopsis thaliana L. Heynh., the concentration of Ca2+in protoplasts increased by applying 0.1 mmol Ce3+, showing that this beneficial element can regulate metabolism, growth, and development through changes in the concentration of Ca2+ (Liu et al. 2011). Furthermore, Ce stimulates growth of lettuce (Lactuca sativa L.) cv. “Regina” seedlings (Barbieri et al. 2013), while in cowpea (Vigna unguiculata L. Walp.), the application of 0.713–17.841 μM cerium nitrate [Ce(NO3)3] increases chlorophyll content, relative yield, and nitrate reductase activity (Shyam and Aery 2012).
In wheat (Triticum aestivum L.), the application of 125, 250, and 500 mg nCeO2 per kg soil improved plant growth, shoot biomass, and grain yield by 9.0%, 12.7%, and 36.6%, respectively, in comparison to the control. As well, Ce nanoparticles increased linolenic acid by up to 6.17% but decreased linoleic acid by up to 1.63%, compared to the other treatments. The findings suggest the potential of nanocerium to modify crop physiology and food quality with unknown consequences for living organisms (Rico et al. 2014). On the other hand, Wu et al. (2014) reported that the alleviation of Cd toxicity by cerium in rice (Oryza sativa L.) seedlings is related to improved photosynthesis, elevated antioxidant enzymes, and decreased oxidative stress.
In barley (Hordeum vulgare L.), the application of 500 mg kg−1 nCeO2 promoted plant development resulting in a 331% increase in shoot biomass compared with the control, though these plants did not form grains. Moreover, 250 mg kg−1 nCeO2 enhanced grain Ce accumulation by as much as 294%, with a remarkable increases in P, K, Ca, Mg, S, Fe, Zn, Cu, and Al (Rico et al. 2015).
In turfgrass (Poa pratensis L.) seedlings, pretreatment with Ce(NO3)3 decreased the MDA content and electrolyte leakage and increased the FMW and DMW under Cu stress. Furthermore, Ce alleviated oxidative damage by regulating the metabolism of ascorbate and glutathione under Cu stress, and Ce had an important role in the acquisition of Cu tolerance in this species (Liu et al. 2016).
2.3 Cobalt (Co)
The Co concentration in plants normally ranges between 0.1 and 10 ppm considering DMW, although hyperaccumulator plants of the families Lamiaceae, Scrophulariaceae, Asteraceae, and Fabaceae can accumulate more than 1000 ppm of this element in leaves (Pilon-Smits et al. 2009). In higher plants, Co adheres strongly to the roots and is absorbed from the soil solution through passive transport. Since Co shows chemical similarity with nickel (Ni), it is possible that the two elements enter the cell through the same types of membrane transporter proteins (Chen et al. 2009).
Cobalt concentrations may increase under Fe deficiency, since Co can compete with Fe for the active sites of the transporter IRT1 (Baxter et al. 2008). In fact, Gad (2012) proved that a sufficient supply of Co significantly decreases the Fe content in groundnut (Arachis hypogaea L.) seeds, and therefore both elements are antagonists and compete for the same transporters.
At low concentrations, Co can have beneficial effects, especially in legumes. In pea (Pisum sativum L.), applying 8 ppm Co to the soil increased growth, nodule number and weight, plant nutrient levels, and yield and seed quality, which can be attributed to the importance of Co for Rhizobium populations living in the roots of these plants (Gad 2006). Co is a component of cobalamin (vitamin B12), which is required to activate enzymes related to N fixation in symbiotic microorganisms (Palit et al. 1994).
Likewise, the application of 8 ppm Co significantly increased nitrogenase activity and nodule number and weight, especially when N was applied at 75% and 100% in groundnut (Gad 2012). In the plant, Co improved growth and yield indicators and the contents of N, P, K, Mn, and Zn. Cobalt contents in seeds ranged between 2.3 and 3.5 ppm, which is within the range reported for other plants. The application of Co can contribute to a more efficient use of N, with a savings of up to 25% of the N applied (Gad 2012).
In lily (Lilium spp.) cv. “Star Fighter,” floral stems treated with preservative solutions including 0.1 and 0.2 mM Co increased floral longevity in 61.1% and 44%, respectively, while in the cv. “Star Gazer,” the application of 0.1 mM Co enhanced this variable by 19.7% (Mandujano-Piña et al. 2012). In cut marguerite (Argyranthemum sp.) flowers, applying 1 and 2 mM Co increased vase life by 5 days compared to the control containing only distilled water (Kazemi 2012). Cobalt and nickel (2.5 mM Co + 2 mM Ni + 2 mM salicylic acid with 2.5% sucrose) increased the vase life of lily cv. “Prato” due to improved membrane stability and reduced oxidative stress damage during flower senescence. In addition, these elements reduce the loss of anthocyanins (Kazemi and Ameri 2012). In carnation (Dianthus caryophyllus L.), Co retards senescence since it reduces ethylene production, an effect very similar to that shown with the application of Ni in this species (Jamali and Rahemi 2011).
In tuberose, application of 300 mg L−1 cobalt chloride (CoCl2) stimulated vase life (10.66 days) and water uptake (1.53 mL g−1 FMW) and reduced FMW losses (19.99 g). Moreover, the application of 400 mg L−1 increased the content of carotenoids (0.40 g) and proteins (31.10%) in petals (Mohammadi et al. 2012b). In tomato, the application of 50 mg kg−1 Co to the soil increased the contents of N, P, K, Cu, Fe, Mn, and Zn in leaf tissue (Jayakumar et al. 2013).
In cut roses, cobalt chloride inhibited vascular blockage in the stem and maintained a high water flow rate through stems, leading to significant water uptake by flowers. The best effects were observed with 200 mg L−1 CoCl2 in the vessel solution (Aslmoshtaghi et al. 2014).
In gladiola (Gladiolus grandiflorus Hort.) cv. Borrega Roja, the application of Co (0, 0.3 and 0.6 mM) significantly increased water absorption in flower stems, while the lowest percentage of weight loss was recorded in fresh rods treated with 0.3 mM Co. Furthermore, the low concentration of Co significantly increased N content in stems and leaf concentration of chlorophyll. The total dry weights of leaves and stems were higher with the treatment of 0.3 mM Co (Trejo-Téllez et al. 2014).
2.4 Iodine (I)
In terms of plant nutrition, iodine has been little studied in crop species (Smolen and Sady 2011b), though it can be transported from the underground (roots) to the aboveground (shoots) parts of the plants (Ashworth 2009). As a beneficial element, iodine can promote growth, induce tolerance mechanisms to cope with stress, and trigger antioxidant capacity of the plant (Medrano-Macias et al. 2016). Iodine has the ability to advance the flowering process in fruit tree species, as a result of increased photosynthetic activity, producing a greater accumulation of sugars (Landini et al. 2012).
Blasco et al. (2011) determined that applications of 40 μM of iodate significantly improve nitrogen-use efficiency and nitrogen metabolism, which increase lettuce productivity and quality. The application of 0.05% (w/v) iodine salts in the nutrient solution caused the potato (Solanum tuberosum L.) tubers to absorb 272 mg 100 g−1 FMW and tomato fruits 527 mg 100 g−1 FMW of IO3−, respectively (Caffagni et al. 2011).
After the foliar application of 25 g L−1 I or soil application of 90 g kg−1 I in crops grown in the open field, the maximum iodine content ranged between 9.5 and 14.3 μg 100 g−1 for plum and nectarine fruits, to 89.4 and 144.0 μg 100 g−1 for potato tubers and tomato fruits, respectively (Caffagni et al. 2012). In hydroponic culture, fresh fruits managed to accumulate up to 2423 μg 100 g−1 of iodine. In all cases, iodine was mainly accumulated in the leaves.
In spinach, the application of 1–2 mg dm−3 iodine and 1 g dm−3 sucrose significantly increased the content of this element, the N and the oxalate content in leaves (Smolen and Sady 2011a). Iodine synergistically improves the uptake of Mg, Na, and Ce, as well as of Fe, and reduces chromium (Cr) uptake. After the application of 2 mg dm−3 I in the soil, a greater accumulation of Na, Fe, Zn, and Al and a reduced concentration of P, S, Cu, and barium (Ba) were observed (Smolen and Sady 2011b).
The beneficial effect of foliar-applied iodine was also observed in “Golden Delicious” apple (Malus domestica Borkh.) trees grafted on M.9 by applying an organic-mineral liquid fertilizer (Biojodis) at a concentration of 5 L ha−1 diluted in water (600 L ha−1), which improved fruit yield, diameter, and uniformity of fruits (Szwonek 2009).
In soybean seeds, the application of 20 μM IO3− enhanced the expression of more proteins in comparison to the control. Furthermore, when iodine (20, 40, and 80 μM IO3−) was applied to the seeds, the activity of antioxidant enzymes such as SOD, ascorbate peroxidase (APX), and glutathione reductase (GR) was boosted, counteracting the toxic effects of 100 mM Cd (Gupta et al. 2015).
No negative effects of iodine fertilization (5 kg ha−1 I, either as KI or as KIO3) were noted with respect to carrot (Daucus carota L.) yield, while higher accumulation and uptake by leaves and storage roots of iodine were obtained after the application of KI than KIO3, which improved the biofortification of carrot storage roots (Smolen et al. 2016).
A recent review on the use of iodine to biofortify and promote growth and stress tolerance in crops published by Medrano-Macias et al. (2016) stated that this element has strong interactions with Fe, Mn, Cu, and V, either directly in plant metabolism or indirectly through the microbiome of the plant. In general, good results are obtained regarding biofortification when applied to the soil as KIO3 in concentrations of 7.5 kg ha−1, 10 mg kg−1 soil in pots, or 10−6–10−5 M in the nutrient solution. Leaf spray with KI at 0.5 kg ha−1gave good results. With higher concentrations, the response is variable: negative, neutral, or positive, depending on the plant species (Medrano-Macias et al. 2016).
2.5 Lanthanum (La)
Lanthanum is considered a beneficial element as it enhances the uptake of essential nutrients such as K, Ca, and Mg (Wahid et al. 2000). In rice, the application of 0.1 mM La(NO3)3 increased germination rate and biomass accumulation in plantlets (Liu et al. 2012a). Liu et al. (2013) found that applying 0.05 mmol La promotes root growth and that this element tends to accumulate in the cell wall of the root. In addition, La treatments affect the accumulation of K, Mg, Ca, Na, Fe, Mn, Zn, Cu, and Mo in the root and thus plant growth.
The application of 5 up to 50 μM La in the nutrient solution stimulates the growth of maize, mung bean (Vigna radiata L. Wilczek), and black gram (V. mungo L. Wilczek) plants and the germination percentage, root length, shoot length, as well as FMW and DMW in all three crops (Chaturvedi et al. 2014). In tobacco (Nicotiana tabacum L.), the application of 5–20 mg L−1 LaCl3 in the Hoagland solution gradually increased dry matter accumulation and chlorophyll content, which decreased when the concentration of LaCl3 exceeded 50 mg L−1 (Chen et al. 2001). Best results were observed with 20 mg L−1, since the synthesis and activity of choline, Mg2+-ATPase, and phosphorylation were significantly activated. Diatloff et al. (2008) reported that concentrations of La below 0.2 μM produced positive effects on maize and mung bean, although at higher concentrations it decreases absorption of Ca, Na, Zn, and Mn.
In cucumber (Cucumis sativus L.), La3+ reduces the levels of Na, Mg, Cl, K, and Ca while increasing Mn and Fe levels. Also, the effects of La3+ on ion absorption show similarity to those of Ca2+, indicating that La affects physiological mechanisms in the plant by regulating the levels of Ca; optimal growth occurred at a concentration of 0.02 mM La3 + (Zeng et al. 2000).
In cucumber plants, La has been found to be involved in ion transport and also modifies the absorption and distribution of Se, Co, V, and technetium (Tc), affecting growth and absorption of other elements that influence the physiology of cells and biochemical functions in this plant (Huang et al. 2003). In addition, Shi et al. (2005) reported that low concentrations of La (0.002 and 0.02 mM LaCl3) promote growth of cucumber plantlets and increase chlorophyll and carotenoid contents, while La was found to be involved in activating antioxidant enzymes such as POD, CAT, and SOD, as well as in reducing MDA contents.
According to Liu et al. (2012b), the Ca level decreases slightly with 0.2 mM La3+; with 1.0 mMLa3+, oscillations of Ca2+ were observed; and at 2.0 mMLa3+, there was an increase in Ca2+, indicating that La3+ participates in signal transduction networks mediated by calmodulin (CaM) and that it can enter the root through the cell membrane and intracellular Ca2+ channels.
In tulip (Tulipa gesneriana L.) cv. “Ile de France,” the diameter and length of the floral stem were higher when plants were ferti-irrigated with 10 μM La (Ramírez-Martínez et al. 2009). In the same ornamental species, Ramírez-Martínez et al. (2012) observed that La stimulates the accumulation of Ca, K, and La itself at concentrations of 10 and 20 μM.
Yan et al. (2007) found that adding 20 mg L−1 La+3 decreased cell membrane permeability and the contents of MDA, H2O2, and proline in soybean plants exposed to UV-B radiation (280–320 nm; 0.15–0.45 W cm−2). Moreover, the activity of the enzymes CAT and POD was higher in La-treated plants, demonstrating the activation of antioxidant mechanisms.
In a study aimed at evaluating the bioaccumulation of La and its effects on growth and mitotic index of soybean, plants were exposed to increasing concentrations of La (0, 5, 10, 20, 40, 80, and 160 μM) in the nutrient solution for 28 days. Roots accumulated 60-fold more La than shoots. La deposition occurred mainly in cell walls and in crystals dispersed in the root cortex and in the mesophyll. The application of La resulted in increased contents of essential nutrients such as Ca, P, K, and Mn, whereas Cu and Fe levels decreased. Furthermore, low La concentrations (i.e., 5–10 μM La) stimulated the photosynthetic rate and total chlorophyll content and led to a higher incidence of binucleate cells, resulting in a slight increase in root and shoot biomass. At higher La levels (i.e., 20–160 μM La), soybean growth was reduced, as a result of ultrastructural modifications in the cell wall, thylakoids, and chloroplasts and the appearance of c-metaphases (de Oliveira et al. 2015).
In rangpur lime (Citrus limonia Osbeck), 50 mg LaCl3 increased mass and height of plants with a consequent increase of dry matter, suggesting the use of La as fertilizer in citrus, especially when used at low concentrations (Turra et al. 2015).
Recently, García-Jiménez et al. (2017) reported that the application of 10 μM to four sweet pepper (Capsicum annuum L.) varieties significantly increased seedling height, shoot diameter, number of flower buds, number of leaves, and leaf area, though it did not affect dry biomass accumulation. Furthermore, La stimulated the biosynthesis of chlorophyll a and b and total chlorophylls, total soluble sugars, and soluble protein concentration.
2.6 Selenium (Se)
In seleniferous soils, most plant species contain from 1 to 10 ppm Se, while hyperaccumulators (such as those belonging to the genera Stanleya and Astragalus) can accumulate from 1000 to 15,000 ppm Se (0.1–1.5% Se) (Pilon-Smits et al. 2009).
The application of Se at low concentrations can increase tolerance to oxidative stress induced by UV radiation, retard senescence, and promote growth. In addition, Se can regulate water content under drought conditions (Germ et al. 2007). In ryegrass (Lolium perenne L.), the application of 0.1 and 1 mg kg−1 Se activated antioxidant mechanisms and increased glutathione peroxidase (GPX) activity while reducing senescence processes and promoting plant growth (Hartikainen et al. 2000).
In canola (Brassica napus L.), Se can increase seed yield and enhance its nutritional value (Hajiboland and Keivanfar 2012). In carrot, the combination of KI and Na2SeO4 stimulated uptake, accumulation, and storage of both I and Se, and the consumption of 100 g FMW carrot produced by plants fertilized with KI + Na2SeO3 and KIO3 + Na2SeO3 can provide 100% of the I and Se levels recommended for human nutrition (Smolen et al. 2016).
Because Se increases the absorption of heavy metals like lead (Pb) in common coleus (Coleus blumei Benth.), this beneficial element can be useful in stimulating phytoremediation mechanisms in environments contaminated by heavy metals (Yuan et al. 2013).
The foliar application of 10 mg L−1Se as Na2SeO4 to soybean cv. “Olna” plants increased respiration potential, especially in young plants (Mechora and Germ 2010). In melon (Cucumis meloL.) seedlings under salt stress, supplying 2–8 mM Se improved growth and triggered antioxidant mechanisms by inhibiting lipid peroxidation and increasing the enzymatic activity of SOD and POD (KeLing et al. 2013). Likewise, exogenous Se alleviates salt stress in maize via the improvement of photosynthetic capacity, the activities of antioxidant enzymes, and the regulation of Na+ homeostasis (Jian et al. 2017).
In maize, applying 5 μM dm−3 Se stimulated growth and root elongation (Hawrylak-Nowak 2008). Furthermore, applications of up to 15 μM Se (as selenite or selenate) in lettuce improved plant growth, while no major changes in oxidative state, pigment concentration, and sulfur accumulation were observed (Hawrylak-Nowak 2013). Moreover, the application of Na2SeO4 (0, 5 and 10 μM) in cucumber plants treated with Cd (0, 25 and 50 μM) reduced Cd uptake and lipid peroxidation as well, while plasma membrane was more stable, suggesting a beneficial effect of Se in plants exposed to Cd (Hawrylak-Nowak et al. 2014).
In the grass Stylosanthes humilis Kunth. grown in acidic soils with high concentrations of toxic aluminum (Al3+), applying up to 1 μM Se activated antioxidant mechanisms, and Se itself contributed to remove reactive oxygen species (Ribeiro et al. 2011).
Selenium has also been associated with fruit maturation. In peach cv. “Suncrest” grafted onto GF 677 (Prunus persica L. Batsch. × Prunus amygdalus Stokes) and pear (Pyrus communis L.) cv. “Conference” grafted onto BA 29 (Cydonia oblonga Mill.) receiving 0.1 and 1 mg L−1 Na2SeO4 in leaves or 1 mg L−1 Na2SeO4 in fruits, Se kept firmness and delayed fruit maturation, which prolonged fruit storage (Pezzarossa et al. 2012). Similar effects have been reported in tomato, since the application of 1 mg L−1 Na2SeO4 reduced lipid peroxidation, increased the activity of antioxidant enzymes such as SOD and GPX, and improved fruit quality during storage (Zhu et al. 2016).
2.7 Silicon (Si)
Silicon constitutes between 0.1% and 10% of the DMW of higher plants and its accumulation can vary significantly among species. Importantly, Si-deficient plants are brittle and susceptible to fungal infections (Ma and Yamaji 2006).
Silicon can counteract the toxic effects of elements such as Al and Mn, confer resistance against pests and diseases, and even allow the formation of nanostructures using organic compounds, enzymes, or organisms as catalysts (Raya and Aguirre 2009). Silicon is absorbed in a pH range of 2–9, being taken up by the roots in the solution as monosilicic acid (Si(OH)4) to be accumulated in the epidermal cells of leaves (Borda et al. 2007).
The beneficial effects of Si are associated with its high deposition in plant tissues, improving their strength and rigidity (Ma and Yamaji 2006). It is also possible that Si plays an active role in resistance to plant diseases by stimulating defense mechanisms. Also, Si can play an important role in resistance to abiotic stress factors such as heavy metal toxicity, salinity, and drought and can reduce the generation of reactive oxygen species, due to the increased activity of antioxidant enzymes (Balakhnina and Borkowska 2013).
In forage oat (Avena sativa L.), applying 100 mg kg−1 (116 g per pot) of monosilicic acid in the pre-sowing period increased height and dry matter production as a result of better nutritional absorption promoted by Si (Borda et al. 2007). Moreover, Si stimulated cell elongation and turgor and improved the conversion of assimilates. In bitter gourd (Momordica charantia L.) plants under saline stress (50 mM NaCl), the application of increasing concentrations of Si (1–5 mM) stimulated the germination rate and index, seedling vitality, and antioxidant enzyme activities of SOD, POD, and CAT (Wang et al. 2010).
By adding 100, 150, and 200 mg L−1 potassium silicate (K2SiO3), carnation flower cv. “Harlem” improved vase life as a result of a significant reduction in ethylene production (Jamali and Rahemi 2011). The application of 2.5 mM Si together with 3 mM acetylsalicylic acid reduces wilting in carnation flowers, retards chlorophyll and carbohydrate degradation, and reduces the activity of oxidase enzymes (Kazemi et al. 2012).
The application of Si increases the amount of O2 in leaves, stems, and roots, which causes the rhizosphere to oxidize. Thus, the elements Fe and Mn are oxidized, which prevents excessive uptake of these elements by the plant (Furcal-Beriguete and Herrera-Barrantes 2013). In common borage(Borago officinalis L.), Si plays a detoxifying role when the plant is under aluminum stress because it stimulates the synthesis of phenolic compounds and proline (Shahnaz et al. 2011).
In maize, application of Si by seed priming improved growth of stressed plants (exposed to alkaline stress induced by 0, 25, 50, and 75 mM Na2CO3) while enhancing the leaf relative water content and levels of photosynthetic pigments, soluble sugars, soluble proteins, total free amino acids, and K+, as well as activities of SOD, CAT, and POD enzymes. Moreover, Si supplement resulted in a decrease in the contents of proline, MDA, and Na+, which together with an enhanced K+ level led to a favorable adjustment of K+/Na+ ratio, in stressed plants relative to plants treated with alkaline stress alone. These findings confirm that Si plays a pivotal role in alleviating the negative effects of alkaline stress on maize (Abdel Latef and Tran 2016). Similarly, Marxen et al. (2016) showed that application of 0.4 and 17.3 t ha−1Si (as silica gel) to rice plants cv. “Khang Dan 18” increased Si contents in plant tissues, as well as biomass production and grain yield.
2.8 Sodium (Na)
Sodium is one of the most studied ions in plant biology due to its toxic effects, although at low concentrations its beneficial effect has also been proved. In fact, in some plants with C4 photosynthetic metabolism, Na is considered an essential element (Kronzucker et al. 2013), and its benefits are more evident in conditions of potassium deficiency (Schulze et al. 2012). Sodium also increases the biosynthesis of amino acids, especially proline (Jouyban 2012).
Salt stress can promote growth in some crops such as wheat, while in rice the low yield caused by salinity is mainly associated with the reduction in tillers and an increase in sterile spikelets in some cultivars (Läuchli and Grattan 2007).
According to Lee and van Iersel (2008), salinity induced by NaCl has the potential to act as a growth regulator. In fact, the application of 60–120 mM NaCl increases the height of faba bean (Vicia faba L.) plants (Abdul Qados 2011). Importantly, salinity may lead to toughening of tomato fruit skin. Accordingly, Silva et al. (2015) reported a linear correlation between thickness of the subepidermis and salinity of the irrigation water (up to 12.61 dS m−1). Interestingly, the tougher tomato skin obtained under conditions of salinity is attributed to increased number of hypodermal cell layers rather than to changes in cell wall composition.
In a proteomic study using two genotypes of Indian mustard (Brassica juncea L. Czern.) displaying contrasting sensitivity to salt stress, Yousuf et al. (2016) reported differential expression of 21 salt stress-responsive proteins associated with various functional processes, including osmoregulation, photosynthesis, carbohydrate metabolism, ion homeostasis, protein synthesis and stabilization, energy metabolism, and antioxidant defense system. Salt-tolerant genotype (CS-52) showed a relatively higher expression of proteins involved in turgor regulation, stabilization of photosystems and proteins, and salt compartmentalization, as compared to salt-sensitive genotype (Pusa Varuna). These results suggest that modulating the expression of salt-responsive proteins can pave the way for developing salt tolerance in the Indian mustard plants.
According to Lee and van Iersel (2008), the quality of Chrysanthemum x morifolium Ramat. cut flower is improved by applying 1 g L−1NaCl in irrigation water. In cut roses cv. “Avalanche,” the application of 250 mg L−1 sodium benzoate improved vase life by reducing ethylene production (Imani et al. 2012). Similarly, applying 20 μM of sodium nitroprusside (SNP) in rose and 60 μM in sunflower (Helianthus annuus L.) increased vase life (Nazirimoghaddam et al. 2014). In cut rose cv. “Utopia” treated with 50 μM SNP, a higher soluble protein content, an increased solution uptake rate by the flower stems, and an improved FMW ratio were observed, while vase life increased from 11 to 13.3 days compared to the control (Seyf et al. 2012b). Moreover, in giant chincherinchee (Ornithogalum saundersiae Bak.) plants grown in pots receiving either 100 or 200 mM NaCl weekly, Na increased chlorophylls and carotenoids contents, as well as N, K, Na, and Cl concentrations in leaves (Salachna et al. 2016). In purpletop vervain (Verbena bonariensis L.), the application of 200 mM NaCl enhanced Mn contents in leaves, whereas neither P, K, Mg, Cu, Zn, and Fe contents nor the initiation of flowering was affected (Salachna and Piechocki 2016).
2.9 Titanium (Ti)
Titanium began to gain importance in plant biology studies in the 1930s and is now considered a beneficial element (Carvajal and Alcaraz 1998). This element is not toxic to animals or to humans, and at low concentrations it is beneficial for plants since it triggers physiological mechanisms leading to better growth and development under certain environmental conditions (Jaberzadeh et al. 2013). So far, limited information is available regarding critical levels of Ti in plant toxicity.
Apart from the application of Ti as conventional reagent, the use of Ti nanoparticles (nTi) is currently gaining more importance. A recent review of Ti as a beneficial element described that seeds treated with nTi suspensions exhibited increased germination rates, enhanced root lengths, or improved seedling growth in different plant species. Furthermore, the application of nTi increased plant tolerance to abiotic and biotic stresses, including cold, drought, Cd toxicity, and bacterial spot disease caused by Xanthomonas perforans (Lyu et al. 2017).
In oat plants (Avena sativa L.)cv. “Zlat’ák,” the effect of Ti is considerably weaker if it is applied on leaves than if being added to the nutrient solution (Kuzel et al. 2003). Importantly, the action of Ti on plant physiology can be explained by a hormetic effect. The application of 960 g ha−1 Ti to tomato increases the content of N, P, Ca y Mg, and that of K with 80 g ha−1 Ti,which demonstrated the importance of Ti in plant nutrition and the quality of this vegetable (Kleiber and Markiewicz 2013).
Under drought stress, application of 0.02% of titanium dioxide nanoparticles in wheat increases gluten and starch content (Jaberzadeh et al. 2013). Furthermore, the application of 1200 and 1500 mg L−1Ti in canola seedlings produced greater root development and bud growth (Mahmoodzadeh et al. 2013). In addition, TiO2 has the potential to be used as an alternative for the control of bacterial blight caused by Xanthomonas in zonal geranium (Pelargonium x hortorum L. H. Bailey) and leaf spot in poinsettia (Euphorbia pulcherrima Willd. ex Klotzsch), since treatments using this compound at concentrations of 25 and 75 mM showed a reduction in lesions of 85% and 93%, respectively (Norman and Chen 2011).
Two applications of TiO2 at 125 cm3 ha−1 in cowpea in the 1st and 2nd year of production improved development and yield and reduced the severity of foliar and pod diseases compared to a single application at lower concentration. Application of TiO2 increased cowpea yield from 8.74% to 36.11% and from 10.33% to 51.31%, respectively, in the 2 years of evaluation (Owolade and Ogunleti 2008).
The response of potato, barley, and wheat plants to titanium application is almost negligible under N deficiency. However, when there is sufficient N, responses are more evident (Tlustos et al. 2005). In mung bean, foliar application of 10 mg L−1 TiO2 increased shoot (17.0%) and root (49.6%) lengths, chlorophylls, as well as total protein contents in leaves. Furthermore, Ti application increased microbial populations in the rhizosphere and the activity of enzymes involved in the P nutrient cycle, such as acid phosphatase, alkaline phosphatase, phytase, and dehydrogenase (Raliya et al. 2015). Foliar application of Ti-ascorbate at concentrations of 25, 50, 75, and 100 mg L−1 improved height of geranium cv. “Elite Cherry,” petunia (Petunia x hybrida hort. ex E.Vilm.) cv. “Celebrity White,” pansy (Viola x wittrockiana Gams.) cv. “Delta Premium Marina,” and snapdragon (Antirrhinum majus L.) cv. “Montego Purple” (Whitted-Haag et al. 2014).
Just recently, Andersen et al. (2016) evaluated the application of different concentrations of Ti dioxide nanoparticles (nTiO2) on the germination of ten crop species. They reported that the application of 500 μgmL−1 nTiO2 increased the percentage of germination in cabbage, and root length in onion, while applying 1000 μg mL−1 enhanced root length both in cucumber and onion. In barley the application of 500 or 1000 mg kg−1 nTiO2 to the soil stimulated plant growth and reduced the toxic effects induced by nCeO2 (Marchiol et al. 2016).
It is important to note that Ti and nTi may have neutral or negative effects on plant physiology, which may be attributed to several factors including differences in plant species, physiological status of plants at the time being evaluated, seed quality, nanoparticle sizes and their uniformity, and experimental objectives and methods. Furthermore, attention does need to be given to the fate and consequence of applied nTi within the environment and food chain (Lyu et al. 2017).
2.10 Vanadium (V)
The biological importance of V can be divided into three levels, depending on the daily intakes and tissue contents: nutritional (intakes of μg a day), pharmacological (mg a day), and toxicological (mg kg−1 food DMW) (Antal et al. 2009). Because of its toxic, mutagenic, genotoxic, and even carcinogenic potential, V has been the subject of numerous public health studies worldwide (Rodríguez-Mercado and Altamirano-Lozano 2006), while its beneficial effects on plants have been sparsely addressed. In the field of plant physiology, one of those first reports on this element showed that soybean plants grown in oxisols develop normally even at concentrations of 75 mg kg−1 V in the soil (Wang and Liu 1999).
Vanadium has been considered as either beneficial or as a secondary metabolism elicitor in plants, but the mechanisms involved are not yet fully understood (Saco et al. 2013). This element is a metal widely distributed both in nature and in biological systems and is also one of the trace elements present in fossil fuels (Rodríguez-Mercado and Altamirano-Lozano 2006).
Antal et al. (2009) studied 56 medicinal plant species to determine V contents, finding the highest V contents in flowering aerial parts, with an average of 763 μg kg−1DMW, followed by the leaves (682 μg kg−1 DMW), roots (600 μg kg−1 DMW), flowers (352 μg kg−1 DMW), and fruits (112 μg kg−1 DMW). Of the plants analyzed, lemon thyme (Thymus pulegioides L.) displays a particular capacity to accumulate this element, while other species like Geum urbanum L., Urtica dioica L., Hypericum perforatum L., and Valeriana officinalis. also tend to accumulate this element (Antal et al. 2009).
In soybean, the application of two different sources of V increased chlorophyll contents, as well as fresh and dry biomass (Sozudogru et al. 2001). The application of 240 μM V in common bean (Phaseolus vulgaris L.) cv. “Contender” caused thicker roots, where it accumulated more than in leaves (Saco et al. 2013). In mustard (Brassica campestris L. ssp. chinensis cv. “Parachinensis”) and tomato (Solanum lycopersicum L.), Vachirapatama et al. (2011) showed that the application of 20 mgL−1NH4VO3 improves growth in both species; V accumulates mostly in root in comparison to leaf, stem, or fruit. In wheat, the application of 40 μM V effectively improved the antioxidant defense system to alleviate the oxidative damage induced by Cu (Wang et al. 2013b). In pennyroyal (Mentha pulegiumL.), the application of 10, 20, and 40 mg L−1NH4VO3 increased root DMW, while V concentration was higher in roots than in shoots. Furthermore, V did not affect K, Ca, Mn, and Zn concentrations in roots, while a reduction of Ca, K, Mg, and Mn concentration was observed in leaves at high V application rates (Akoumianaki-Ioannidou et al. 2015).
Root uptake and translocation efficiency of V do not significantly vary with the species, whereas its translocation to plant aerial parts depends on individual plant response. Future studies are necessary to determine the effect of V-status on different taxa, while the accumulation and transfer of vanadium within the food chain remain a daunting task (Qian et al. 2014).
A summary of the levels at which beneficial elements have been applied in various model and cultivated species is presented in Table 1.
3 Effects of Beneficial Elements on Plant Nutrition as a Mean to Overcome Abiotic Stress
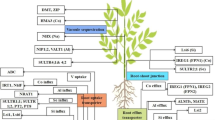
In several modern plant nutrition approaches, beneficial elements are considered part of nutrient management in an increasing number of crop plants. Some of these elements, including Ce, Co, I, Na, Se, Si, and Ti, have been proved to increase crop yield. Some others such as Al and La have been less studied, and their influence on plant yield and nutrient status are little known. Cerium raises P, K, Ca, Mg, S, Fe, Zn, Cu, and Al concentrations in barley leaves and grains (Rico et al. 2015), which boosts plant growth, yield, and grain quality. Cobalt enhances N, P, K, Cu, Fe, Mn, and Zn concentrations in plant tissues not only in tomato (Jayakumar et al. 2013) but also in groundnut (Gad 2012) and pea (Gad 2006), which results in increased yields and harvest quality. Iodine has been found to improve N, Mg, Na, Ce, and Fe uptake in fruits (Szwonek 2009), lettuce (Blasco et al. 2011), spinach (Smolen and Sady 2011a, b), and tomato (Caffagni et al. 2012) while enhancing productivity and crop quality. Lanthane increases Mn and Fe contents in cucumber (Zeng et al. 2000), displays a synergic effect with Ca and K uptake in tulip (Ramírez-Martínez et al. 2012), and increases Ca, P, K, and Mn concentrations in soybean (de Oliveira et al. 2015), which renders better plant growth and productivity. Selenium enhances P and Ca uptake, though it also reduces K absorption in maize (Hawrylak-Nowak 2008). Application of Se increases its concentrations in fruit trees (Pezzarossa et al. 2012), lettuce (Hawrylak-Nowak 2013), and carrot (Smolen et al. 2016), which in turn improves plant growth and development. Under saline stress, Si reduces Na uptake and increases that of K, in order to maintain a better K/Na ratio in maize (Abdel Latef and Tran 2016), while in rice, Si increases P, N, and Mg uptake, though reduces that of K, which dramatically improves plant growth, yield, and grain quality (Marxen et al. 2016). Sodium increases N and K contents in Ornithogalum saundersiae Baker. (Salachna et al. 2016), which improves vessel life and flower quality. Titanium may improve crop performance through stimulating the activity of certain enzymes, enhancing chlorophyll content and photosynthesis, promoting nutrient uptake, strengthening stress tolerance, and improving crop yield and quality. These benefits lie in its interaction with other nutrient elements, especially Fe. Fe and Ti have synergistic and antagonistic relationships, depending on the Fe status of the plant. When plants experience Fe deficiency, Ti may enhance Fe uptake and utilization and subsequently improving plant growth. When Ti concentration is high in plants, Ti competes with Fe for ligands or proteins. The competition could be severe, resulting in Ti phytotoxicity (Lyu et al. 2017). Moreover, Ti has been shown to increase N, P, K, Ca, and Mg contents in tomato (Kleiber and Markiewicz 2013). Vanadium does not affect Ca, K, Mn, and Zn concentrations in roots, though it does reduce Ca, K, Mg, and Mn concentrations in leaves of Mentha pulegium L. (Akoumianaki-Ioannidou et al. 2015). Importantly, its role in productivity, nutrient uptake, and mobility within the plant deserves further investigation. All these effects of beneficial elements ameliorate the responses of plants to environmental stressors. A summary of the main effects of the beneficial elements on plant nutrition and their functions on plant performance is presented in Fig. 2.
4 Conclusions and Perspectives
Aluminum, cerium, cobalt, iodine, lanthanum, sodium, selenium, silicon, titanium, and vanadium have been shown to have beneficial effects in some species of model and cultivated plants. The positive effects of these elements on plants include improved yield and postharvest quality, absorption of other nutrients, and activation of mechanisms of defense against pests and diseases and resistance or tolerance to abiotic stress factors such as heavy metals, drought, and salinity. In some cases, these elements can replace some biological functions of other essential nutrients in plant metabolism.
In every case, the beneficial effects of these elements are always observed when they are used at low concentrations. Since they trigger hormetic effects, at high doses, these elements can disrupt the homeostasis of the plant and cause deleterious effects.
The great challenges facing humanity such as population growth, climate change, pollution, and the depletion and degradation of natural resources make it necessary to find new methods of sustainable crop production. In this context, beneficial elements offer an alternative little explored until now.
To date, well-supported experimental evidence demonstrates the positive effects of beneficial elements in different plant species. Nevertheless, more in-depth research is still needed in order to know their action based on the plant genotypes used, the production systems, the chemical forms in which they should be applied, and, above all, the optimal doses and phenological stages in which their beneficial effects are more evident and their application cost-effective. Importantly, nanotechnology has the potential to positively impact the agrifood sector, minimizing adverse problems of agricultural practices on environment and human health and improving food security and productivity while promoting social and economic equity. However, acquisition of knowledge and developments of methods for risk and life-cycle assessment of nanomaterials, nanopesticides, and nanofertilizers, as well as assessment of the impacts on nontarget organisms (i.e., other plants, soil microbiota, and bees), and the regulations about the use of nanomaterials require further attention (Amenta et al. 2015; Fraceto et al. 2016).
In addition to the ten beneficial elements described herein, recent reports indicate that other elements such as silver (Ag), chromium (Cr), fluorine (F), and tungsten (W) may also have potential benefits in crops, but research on them is still in its infancy.
Because the responses that trigger beneficial elements differ among families, genera, and species of plants, it is crucial to explore the underlying genetic and molecular foundations that explain the positive effect of these elements, which constitute an area of great interest for future studies. Food security, sustainability, and efficient use of current inputs eminently justify such an approach.
References
Abdel Latef AA, Tran LSP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci 7:243. https://doi.org/10.3389/fpls.2016.00243
Abdul Qados AMS (2011) Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.) J Saudi Soc Agric Sci 10:7–15. https://doi.org/10.1016/j.jssas.2010.06.002
Acero LH, Tuy FS, Virgino JS (2016) Potassium aluminum sulfate solution on the vase life of sampaguita (Jasminum sambac) flowers. J Med Bioeng 5:33–36. https://doi.org/10.12720/jomb.5.1.33-36
Akoumianaki-Ioannidou A, Barouchas PE, Kyramariou A et al (2015) Effect of vanadium on dry matter and nutrient concentration in pennyroyal (Mentha pulegium L.) Bull UASVM Hortic 72:295–298. https://doi.org/10.15835/buasvmcn-hort:11348
Alcántar-González G, Trejo-Téllez LI, Fernández-Pavía L et al (2016) Elementos esenciales. In: Alcántar-González G, Trejo-Téllez LI, Gómez-Merino FC (eds) Nutrición de cultivos, 2nd edn. Colegio de Postgraduados, Mexico City, pp 23–55
Amenta V, Aschberger K, Arena M et al (2015) Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul Toxicol Pharmacol 73:463–476. https://doi.org/10.1016/j.yrtph.2015.06.016
Andersen CP, King G, Plocher M et al (2016) Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ Toxicol Chem 35:1–7. https://doi.org/10.1002/etc.3374
Antal DS, Dehelean CA, Canciu CM et al (2009) Vanadium in medicinal plants: new data on the occurrence of an element both essential and toxic to plants and man. An Univ Oradea Fasc Biol 2:5–10
Ashworth DJ (2009) Transfers of iodine in the soil–plant–air system: solid-liquid partitioning, migration, plant uptake and volatilization. In: Preedy VR, Burrow GN, Watson R (eds) Comprehensive handbook of iodine. Oxford Academic Press, San Diego, pp 107–118
Aslmoshtaghi E, Jafari M, Rahemi M (2014) Effects of daffodil flowers and cobalt chloride on vase life of cut rose. J Chem Health Risks 4:1–6
Balakhnina T, Borkowska A (2013) Effects of silicon on plant resistance to environmental stresses: review. Int Agrophys 27:225–232. https://doi.org/10.2478/v10247-012-0089-4
Barbieri APP, Espíndola GMC, de Menezes NL et al (2013) Lettuce seeds treated with cerium and lanthanum aqueous solutions. Pesq Agropec Trop 43:104–109. https://doi.org/10.1590/S1983-40632013000100013
Basaki T, Faraji S, Nejat MA, Azimi MH (2013) The effect of chemical treatments on cut flower longevity of Rosa hybrid cultivar Maroussia. Int J Agron Plant Prod 4:450–453
Baxter IR, Vitek O, Lahner B et al (2008) The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc Natl Acad Sci U S A 105:12081–12086. https://doi.org/10.1073/pnas.0804175105
Blasco B, Rios JJ, Cervilla LM et al (2011) Iodine application affects nitrogen-use efficiency of lettuce plants (Lactuca sativa L.) Soil Plant Sci 61:378–383. https://doi.org/10.1080/09064710.2010.492782
Borda OA, Barón FH, Gómez MI (2007) Silicon as a beneficial element in forage oat (Avena sativa L.): physiological responses of growth and management. Agron Colomb 25:273–279
Caffagni A, Arru L, Meriggi P et al (2011) Iodine fortification plant screening process and accumulation in tomato fruits and potato tubers. Commun Soil Sci Plant Anal 42:706–718. https://doi.org/10.1080/00103624.2011.550372
Caffagni A, Pecchioni N, Meriggi P et al (2012) Iodine uptake and distribution in horticultural and fruit tree species. Ital J Agron 7:229–236. https://doi.org/10.4081/ija.2012.e32
Carvajal M, Alcaraz CF (1998) Why titanium is a beneficial element for plants. J Plant Nutr 21:655–664. https://doi.org/10.1080/01904169809365433
Chaturvedi N, Gannavarapu R, Dhal NK (2014) Effect of lanthanum on the growth and physiological activities of Zea mays, Vigna radiata and Vigna mungo. Int J Environ Sci 4:653–659. https://doi.org/10.6088/ijes.2014040404505
Chen WJ, Tao Y, Gu YH et al (2001) Effect of lanthanide chloride on photosynthesis and dry matter accumulation in tobacco seedlings. Biol Trace Elem Res 79:169–176. https://doi.org/10.1385/BTER:79:2:169
Chen Z, Watanabe T, Shinano T et al (2009) Rapid characterization of plant mutants with altered ion-profile: a case study using Lotus japonicus. New Phytol 181:795–801. https://doi.org/10.1111/j.1469-8137.2008.02730.x
Conti L, Nelis S, Zhang C et al (2014) Small ubiquitin-like modifier protein SUMO enables plants to control growth independently of the phytohormone gibberellins. Dev Cell 28:102–110. https://doi.org/10.1016/j.devcel.2013.12.004
de la Cruz-Guzmán GH, Arriaga-Frías A, Mandujano-Piña M et al (2007) Effect of three longevity preservatives on post-harvest life of Rosa cv. Royalty. Rev Chapingo Ser Hortic 13:109–113
de Oliveira C, Ramos SJ, Siqueira JO et al (2015) Bioaccumulation and effects of lanthanum on growth and mitotic index in soybean plants. Ecotoxicol Environ Saf 122:136–144. https://doi.org/10.1016/j.ecoenv.2015.07.020
Diatloff E, Smith FW, Asher CJ (2008) Effects of lanthanum and cerium on the growth and mineral nutrition of corn and mungbean. Ann Bot 101:971–982. https://doi.org/10.1093/aob/mcn021
Dong B, Sang WL, Jiang X et al (2002) Effects of aluminum on physiological metabolism and antioxidant system of wheat (Triticum aestivum L.) Chemosphere 47:87–92. https://doi.org/10.1016/S0045-6535(01)00210-7
Fraceto LF, Grillo R, de Medeiros GA et al (2016) Nanotechnology in agriculture: which innovation potential does it have? Front Environ Sci 4:20. https://doi.org/10.3389/fenvs.2016.00020
Furcal-Beriguete P, Herrera-Barrantes A (2013) Efecto del silicio y plaguicidas en la fertilidad del suelo y rendimiento del arroz. Agron Mesoam 24:365–378
Gad N (2006) Increasing the efficiency of nitrogen fertilization through cobalt application to pea plants. Res J Agric Biol Sci 2:433–442
Gad N (2012) Role and importance of cobalt nutrition on groundnut (Arachis hypogaea) production. World Appl Sci J 20:359–367. https://doi.org/10.5829/idosi.wasj.2012.20.03.2819
García-Jiménez A, Gómez-Merino FC, Tejeda-Sartorius O, Trejo-Téllez LI (2017) Lanthanum affects bell pepper seedling quality depending on the genotype and time of exposure by differentially modifying plant height, stem diameter and concentrations of chlorophylls, sugars, amino acids, and proteins. Front Plant Sci 8:308. https://doi.org/10.3389/fpls.2017.00308
Germ M, Stibilj V, Kreft I (2007) Metabolic importance of selenium for plants. Eur J Plant Sci Biotech 1:91–97
Gupta N, Bajpai MS, Majumdar RS et al (2015) Response of iodine on antioxidant levels of Glycine max L. grown under Cd2+ stress. Adv Biol Res 9:40–48. https://doi.org/10.5829/idosi.abr.2015.9.1.9183
Hajiboland R, Keivanfar N (2012) Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants. Acta Agric Slov 99:13–19. https://doi.org/10.2478/v10014-012-0002-7
Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200. https://doi.org/10.1023/A:1026512921026
Hawrylak-Nowak B (2008) Effect of selenium on selected macronutrients in maize plants. J Elem 13:513–519
Hawrylak-Nowak B (2013) Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul 70:149–157. https://doi.org/10.1007/s10725-013-9788-5
Hawrylak-Nowak B, Dresler S, Wójcik M (2014) Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci Hortic 172:10–18. https://doi.org/10.1016/j.scienta.2015.12.002
Huang Z, Chen G, Du J (2003) Influence of lanthanum on the uptake of trace elements in cucumber plant. Biol Trace Elem Res 95:185–195. https://doi.org/10.1385/BTER:95:2:185
Imani MH, Hashemabadi D, Kaviani B et al (2012) Effect of sodium benzoate on longevity and ethylene production in cut rose (Rosa hybrida L. cv. Avalanche). Eur J Exp Biol 2:2485–2488
Jaberzadeh A, Moaveni P, Tohidimoghadam HR et al (2013) Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not Bot Hortic Agrobo 41:201–207
Jamali B, Rahemi M (2011) Carnation flowers senescence as influenced by nickel, cobalt and silicon. J Biol Environ Sci 5:147–152
Jayakumar K, Rajesh M, Baskaran L, Vijayarengan P (2013) Changes in nutritional metabolism of tomato (Lycopersicon esculantum Mill.) plants exposed to increasing concentration of cobalt chloride. Int J Food Nutr Saf 4:62–69
Jian C, Zu C, Lu D et al (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Jouyban Z (2012) The effects of salt stress on plant growth. Tech J Eng App Sci 2:7–10
Jowkar MM, Khalighi A, Kafi M, Hasanzadeh N (2012) Evaluation of aluminum sulfate as vase solution biocide on postharvest microbial and physiological properties of ‘Cherry Brandy’ rose. Acta Hortic 1012:1132–1144. https://doi.org/10.17660/ActaHortic.2013.1012.83
Kazemi M (2012) Effect of cobalt, silicon, acetylsalicylic acid and sucrose as novel agents to improve vase-life of Argyranthemum flowers. Trends Appl Sci Res 7:579–583. https://doi.org/10.3923/tasr.2012
Kazemi M, Ameri A (2012) Effect of Ni, Co, SA and sucrose on extending the vase-life of lilycut flower. Iranica J Energy Environ 3:162–166. https://doi.org/10.5829/idosi.ijee.2012.03.02.0258
Kazemi M, GHolami M, Bahmanipour F (2012) Effect of silicon and acetylsalicylic acid on antioxidant activity, membrane stability and ACC-oxidase activity in relation to vase life of carnation cut flowers. Biotechnology 11:87–90. https://doi.org/10.3923/biotech.2012.87.90
KeLing H, Ling Z, JiTao W et al (2013) Influence of selenium on growth, lipid peroxidation and antioxidative enzyme activity in melon (Cucumis melo L.) seedlings under salt stress. Acta Soc Bot Pol 82:193–197. https://doi.org/10.5586/asbp.2013.023
Kidd PS, Proctor J (2000) Effects of aluminium on the growth and mineral composition of Betula pendula Roth. J Exp Bot 51:1057–1066. https://doi.org/10.1093/jexbot/51.347.1057
Kim YH, Khan AL, Waqas M et al (2014) Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. Plant Growth Regul 33:137–149. https://doi.org/10.1007/s00344-013-9356-2
Kirkby E (2012) Introduction, definition and classification of nutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants. Elsevier, Amsterdam, pp 3–5
Kleiber T, Markiewicz B (2013) Application of “Tytanit” in greenhouse tomato growing. Acta Sci Pol 12:117–126
Kováčik J, Klejdus B, Hedbavny J (2010) Effect of aluminium uptake on physiology, phenols and amino acids in Matricaria chamomilla plants. J Hazard Mater 178:949–955. https://doi.org/10.1016/j.jhazmat.2010.02.029
Kronzucker HJ, Coskun D, Schulze LM et al (2013) Sodium as nutrient and toxicant. Plant Soil 369:1–23. https://doi.org/10.1007/s11104-013-1801-2
Kuzel S, Hruby M, Cígler P et al (2003) Mechanism of physiological effects of titanium leaf sprays on plants grown on soil. Biol Trace Elem Res 91:179–190
Landini M, Gonzali S, Kiferle C (2012) Metabolic engineering of the iodine content in Arabidopsis. Sci Rep 2:338. https://doi.org/10.1038/srep00338
Läuchli A, Grattan SR (2007) Plant growth and development under salinity stress. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in molecular breeding toward drought and salt tolerant crops. Springer, Dordrecht, pp 1–32. https://doi.org/10.1007/978-1-4020-5578-2_1
Lee MK, van Iersel MW (2008) Sodium chloride effects on growth, morphology, and physiology of chrysanthemum (Chrysanthemum x morifolium). Horticult Sci 43:1888–1891
Li-Jen L, Yu-Han L, Kuang-Liang H et al (2001) Vase life of Eustoma grandiflorum as affected by aluminumsulfate. Bot Bull Acad Sin 42:35–38
Liu D, Wang X, Lin Y et al (2011) Analysis of the effects of cerium on calcium ion in the protoplasts of Arabidopsis thaliana with confocal microscopy. Afr J Biotechnol 10:10781–10785. https://doi.org/10.5897/AJB11.946
Liu D, Lin Y, Wang X (2012a) Effects of lanthanum on growth, element uptake, and oxidative stress in rice seedlings. J Plant Nutr Soil Sci 175:907–911. https://doi.org/10.1002/jpln.201200016
Liu D, Wang X, Chen X et al (2012b) Effects of lanthanum on the change of calcium level in the root cells of rice. Commun Soil Sci Plant Anal 43:1994–2003. https://doi.org/10.1080/00103624.2012.693231
Liu D, Wang X, Zhang X et al (2013) Effect of lanthanum on growth and accumulation of rice seedlings. Plant Soil Environ 59:19–200
Liu R, Shan C, Gao Y et al (2016) Cerium improves the copper tolerance of turf grass Poa pratensis by affecting the regeneration and biosynthesis of ascorbate. Braz J Bot 39:779–785. https://doi.org/10.1007/s40415-015-0246-7
Lyu S, Wei X, Chen J et al (2017) Titanium as a beneficial element for crop production. Front Plant Sci 8:597. https://doi.org/10.3389/fpls.2017.00597
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Mahmoodzadeh H, Nabavi M, Kashefi H (2013) Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J Orn Hort Plants 3:25–32
Mandujano-Piña M, Colinas-León MT, Castillo-González AM et al (2012) Cobalt as senescence retardant in postharvest of oriental hybrid Lilium. Rev Chapingo Ser Hortic 18:239–252. https://doi.org/10.5154/r.rchsh.2010.09.034
Marchiol L, Mattiello A, Poscic F et al (2016) Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int J Environ Res Public Health 13:332. https://doi.org/10.3390/ijerph13030332
Marxen A, Klotzbücher T, Jahn R et al (2016) Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 398:153–163. https://doi.org/10.1007/s11104-015-2645-8
Mechora S, Germ M (2010) Selenium induced lower respiratory potential in Glycine max (L.) Merr. Acta Agric Slov 95:29–34. https://doi.org/10.2478/v10014-010-0004-2
Medrano-Macias J, Leija-Martínez P, González-Morales S et al (2016) Use of iodine to biofortify and promote growth and stress tolerance in crops. Front Plant Sci 7:1146. https://doi.org/10.3389/fpls.2016.01146
Mohammadi M, Hashemabadi D, Kaviani B (2012a) Improvement of vase life of cut tuberose (Polianthes tuberosa cv. ‘Single’) with aluminum sulfate. Ann Biol Res 3:5457–5461
Mohammadi M, Hashemabadi D, Kaviani B (2012b) Effect of cobalt chloride on vase life and postharvest quality of cut tuberose (Polianthes tuberosa L.) Eur J Exp Biol 2:2130–2133
Morales MI, Rico CM, Hernández-Viezcas JA et al (2013) Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J Agric Food Chem 61:6224–6230. https://doi.org/10.1021/jf401628v
Moreno-Alvarado M, García-Morales S, Trejo-Téllez LI et al (2017) Aluminum enhances growth and sugar concentration, alters macronutrient status and regulates the expression of NAC transcription factors in rice. Front Plant Sci 8:73. https://doi.org/10.3389/fpls.2017.00073
Nazirimoghaddam N, Hashemabadi H, Kaviani B (2014) Improvement of vase life of cut rose, sunflower and lisianthus with sodium nitroprusside. Eur J Exp Biol 4:162–165
Norman DJ, Chen J (2011) Effect of foliar application of titanium dioxide on bacterial blight of geranium and Xanthomonas leaf spot of poinsettia. Horticult Sci 46:426–428
Owolade OF, Ogunleti DO (2008) Effects of titanium dioxide on the diseases, development and yield of edible cowpea. J Plant Prot Res 48:329–335. https://doi.org/10.2478/v10045-008-0042-5
Palit S, Sharma A, Talukder G (1994) Effect of cobalt on plants. Bot Rev 60:149–181. https://doi.org/10.1007/BF02856575
Pezzarossa B, Remorini D, Gentile ML et al (2012) Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J Sci Food Agric 92:781–786. https://doi.org/10.1002/jsfa.4644
Pilon-Smits EA, Quinn CF, Tapken W et al (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12:267–274. https://doi.org/10.1016/j.pbi.2009.04.009
Qian Y, Gallagher FJ, Feng H et al (2014) Vanadium uptake and translocation in dominant plant species on an urban coastal brownfield site. Sci Total Environ 476–477:696–704. https://doi.org/10.1016/j.scitotenv.2014.01.049
Radkowski A, Radkowska I, Lemek T (2015) Effects of foliar application of titanium on seed yield in timothy (Phleum pratense L.) Ecol Chem Eng S 22:691–701. https://doi.org/10.1515/eces-2015-0042
Raliya R, Biswas P, Tarafdar JC (2015) TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.) Biotechnol Rep 5:22–26. https://doi.org/10.1016/j.btre.2014.10.009
Ramírez-Martínez M, Gómez-Merino FC, Trejo-Téllez LI et al (2009) Effect of lanthane on quality of tulip flower ‘Ile de France’. Acta Hortic 847:295–300. https://doi.org/10.17660/ActaHortic.2009.847.39
Ramírez-Martínez M, Trejo-Téllez LI, Gómez-Merino FC et al (2012) Bioaccumulation of potassium, calcium and lanthanum in tulip treated with lanthanum. Terra Latin 30:229–238
Raya PJC, Aguirre MCL (2009) Elemental composition of several wild Mexican plants. Rev Chapingo Ser Cien For Ambient 15:95–99
Ribeiro MD, Mapeli AM, Antunes WC, Barros RS (2011) A dual role of selenium in the growth control of seedlings of Stylosanthes humilis. Agric Sci 2:78–85. https://doi.org/10.4236/as.2011.22012
Rico CM, Lee SC, Rubenecia R et al (2014) Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.) J Agric Food Chem 62:9669–9675. https://doi.org/10.1021/jf503526r
Rico CM, Barrios AC, Tan W et al (2015) Physiological and biochemical response of soil-grown barley (Hordeum vulgare L.) to cerium oxide nanoparticles. Environ Sci Pollut Res 22:10551–10558. https://doi.org/10.1007/s11356-015-4243-y
Rodríguez-Mercado JJ, Altamirano-Lozano MA (2006) Vanadio: contaminación, metabolismo y genotoxicidad (Vanadium: pollution, metabolism and genotoxicity). Rev Int Contam Ambient 22:173–189
Saco D, Martín S, San José P (2013) Vanadium distribution in roots and leaves of Phaseolus vulgaris: morphological and ultrastructural effects. Biol Plant 57:128–132. https://doi.org/10.1007/s10535-012-0133-z
Salachna P, Piechocki R (2016) Effects of sodium chloride on growth and mineral nutrition of purpletop vervain. J. Ecol Eng 17:148–152. https://doi.org/10.12911/22998993/62311
Salachna P, Zawadzinska A, Podsiadlo C (2016) Response of Ornithogalum saundersiae Bak. to salinity stress. Acta Sci Pol-Hortoru 15:123–134
Schulze LM, Britto DT, Li M et al (2012) A pharmacological analysis of high-affinity sodium transport in barley (Hordeum vulgare L.): a 24Na+/42K+ study. J Exp Bot 63:2479–2489. https://doi.org/10.1093/jxb/err419
Seyf M, Khalighi A, Mostofi Y et al (2012a) Study on the effect of aluminum sulfate treatment on postharvest life of the cut rose ‘Boeing’ (Rosa hybrida cv. Boeing). J Hortic Biotech 16:128–132
Seyf M, Khalighi A, Mostofi Y et al (2012b) Effect of sodium nitroprusside on vase life and postharvest quality of a cut rose cultivar (Rosa hybrida ‘Utopia’). J Agric Sci 4:174–181. https://doi.org/10.5539/jas.v4n12p174
Shahnaz G, Shekoofeh E, Kourosh D et al (2011) Interactive effects of silicon and aluminum on the malondialdehyde (MDA), proline, protein and phenolic compounds in Borago officinalis L. J Med Plant Res 5:5818–5827
Shamsi IH, Wei K, Zhang GP et al (2008) Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biol Plant 52:165–169. https://doi.org/10.1007/s10535-008-0036-1
Shi P, Chen GC, Huang ZW (2005) Effects of La3+ on the active oxygen-scavenging enzyme activities in cucumber seedling leaves. Russ J Plant Physiol 52:294–297
Shyam R, Aery NC (2012) Effect of cerium on growth, dry matter production, biochemical constituents and enzymatic activities of cowpea plants [Vigna unguiculata (L.) Walp.] J Soil Sci Plant Nutr 12:1–14. https://doi.org/10.4067/S0718-95162012000100001
Silva RM, Yasuor H, Ben-Gal A et al (2015) Salinity induced fruit hypodermis thickening alters the texture of tomato (Solanum lycopersicum Mill.) fruits. Sci Hortic 192:244–249. https://doi.org/10.1016/j.scienta.2015.06.002
Smolen S, Sady W (2011a) Influence of iodine fertilization and soil application of sucrose on the effectiveness of iodine biofortification, yield, nitrogen metabolism and biological quality of spinach. Acta Sci Pol Hortoru 10:51–63
Smolen S, Sady W (2011b) Influence of soil application of iodine and sucrose on mineral composition of spinach plants. Acta Sci Pol Hortoru 10:3–13
Smolen S, Skoczylas Ł, Ledwozyw-Smolen I et al (2016) Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front. Plant Sci 7:730. https://doi.org/10.3389/fpls.2016.00730
Sozudogru S, Kutuk AC, Halilova H (2001) Effects of vanadium on the growth, chlorophyll, and mineral content of soybean (Glycine max (L.) Merr). Ciencia 9:88–95
Szwonek E (2009) Impact of foliar fertilizer containing iodine on “Golden Delicious” apple trees. Proceedings of the International Plant Nutrition Colloquium XVI. University of California at Davis. pp 1–5. Available online at: https://escholarship.org/uc/item/8bp5w7z7
Tlustos P, Cígler P, Hruby M, Kuzel S, Száková J, Balík JS (2005) The role of titanium in biomass production and its influence on essential elements’ contents in field growing crops. Plant Soil Environ 51:19–25
Trejo-Téllez LI, Gómez-Merino FC (2012) Nutrient solutions for hydroponic systems. In: Asao T (ed) Hydroponics – a standard methodology for plant biological researches. InTech, Rijeka, pp 1–22
Trejo-Téllez LI, Gómez-Merino FC, Gómez-Pérez V, Castro-García FA (2014) Cobalt in postharvest of gladiolus (Gladiolus grandiflorus Hort.) Rev Mex Cienc Agríc 9:1575–1587
Trejo-Téllez LI, Gómez-Merino FC, Alcántar-González G (2016) Elementos benéficos: potencialidades y limitantes. In: Alcántar-González G, Trejo-Téllez LI, Gómez-Merino FC (eds) Nutrición de cultivos. Colegio de Postgraduados, Mexico City, pp 59–101
Turra C, Fernandes EAN, Bacchi MA et al (2015) Effects of lanthanum on citrus plant. Int J New Tech Res 1:48–50
Vachirapatama N, Jirakiattikul Y, Dicinoski G et al (2011) Effect of vanadium on plant growth and its accumulation in plant tissues. Songklanakarin J Sci Technol 33:255–261
Wahid PA, Valiathan MS, Kamalam NV et al (2000) Effect of rare earth elements on growth and nutrition of coconut palm and root competition for these elements between the palm and Calotropis gigantean. J Plant Nutr 23:329–338. https://doi.org/10.1080/01904160009382019
Wang JF, Liu Z (1999) Effect of vanadium on the growth of soybean seedlings. Plant Soil 216:47–51. https://doi.org/10.1023/A:1004723509113
Wang XD, Ou-yang C, Fan ZR et al (2010) Effects of exogenous silicon on seed germination and antioxidant enzyme activities of Momordica charantia under salt stress. J Anim Plant Sci 6:700–708
Wang Q, Ma X, Zhang W et al (2012) The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 4:1105–1112. https://doi.org/10.1039/C2MT20149F
Wang Q, Ebbs SD, Chen Y et al (2013a) Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 5:753–759. https://doi.org/10.1039/c3mt00033h
Wang H, Wang T, You L et al (2013b) Effects of vanadate supply on plant growth, Cu accumulation, and antioxidant capacities in Triticum aestivum L. Environ Geochem Health 35:585–592. https://doi.org/10.1007/s10653-013-9541-z
Wang L, Fan XW, Pan JL et al (2015) Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta 242:1391–1403. https://doi.org/10.1007/s00425-015-2376-3
Watanabe T, Jansen S, Osaki M (2005) The beneficial effect of aluminium and the role of citrate in Al accumulation in Melastoma malabathricum. New Phytol 165:773–780. https://doi.org/10.1111/j.1469-8137.2004.01261.x
Whitted-Haag B, Kopsell DE, Kopsell DA et al (2014) Foliar silicon and titanium applications influence growth and quality characteristics of annual bedding plants. Open Hortic J 7:6–15. https://doi.org/10.2174/1874840601407010006
Wu M, Wang PY, Sun LG et al (2014) Alleviation of cadmium toxicity by cerium in rice seedlings is related to improved photosynthesis, elevated antioxidant enzymes and decreased oxidative stress. Plant Growth Regul 74:251–260. https://doi.org/10.1007/s10725-014-9916-x
Yan S, Huang X, Zhou Q (2007) Effect of lanthanum (III) on reactive oxygen metabolism of soybean seedlings under supplemental UV-B irradiation. J Rare Earths 25:352–358. https://doi.org/10.1016/S1002-0721(07)60435-9
Yin S, Ze Y, Liu C et al (2009) Cerium relieves the inhibition of nitrogen metabolism of spinach caused by magnesium deficiency. Biol Trace Elem Res 132:247–258. https://doi.org/10.1007/s12011-009-8392-z
Yousuf PY, Ahmad A, Ganie AH et al (2016) Salt stress-induced modulations in the shoot proteome of Brassica juncea genotypes. Environ Sci Pollut Res 23:2391. https://doi.org/10.1007/s11356-015-5441-3
Yuan J, Hu M, Zhou Z (2013) Selenium treatment mitigates the effect of lead exposure in Coleus blumei Benth. J Environ Anal Toxicol 3:191. https://doi.org/10.4172/2161-0525.1000191
Zeng FL, Shi P, Zhang MF et al (2000) Effect of lanthanum on ion absorption in cucumber seedling leaves. Biol Trace Elem Res 78:265–270. https://doi.org/10.1385/BTER:78:1-3:265
Zhang X, Wang L, Zhou A et al (2016) Alterations in cytosol free calcium in horseradish roots simultaneously exposed to lanthanum (III) and acid rain. Ecotoxicol Environ Saf 126:62–70. https://doi.org/10.1016/j.ecoenv.2015.12.014
Zhu Z, Chen Y, Zhang X et al (2016) Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci Hortic 198:304–310. https://doi.org/10.1016/j.scienta.2015.12.002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gómez-Merino, F.C., Trejo-Téllez, L.I. (2018). The Role of Beneficial Elements in Triggering Adaptive Responses to Environmental Stressors and Improving Plant Performance. In: Vats, S. (eds) Biotic and Abiotic Stress Tolerance in Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-9029-5_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-9029-5_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9028-8
Online ISBN: 978-981-10-9029-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)