Abstract
Magnesium is one of the essential elements for plant growth and cerium is a beneficial element for plant growth. However, the effects of the fact that cerium improves the nitrogen metabolism of plants under magnesium deficiency is poorly understood. The main aim of the study was to determine the role of cerium in the amelioration of magnesium-deficiency effects in spinach plants. Spinach plants were cultivated in Hoagland’s solution. They were subjected to magnesium deficiency and to cerium chloride administered in the magnesium-present media and magnesium-deficient media. Spinach plants grown in the magnesium-present media and magnesium-deficient media were measured for key enzyme activities involved in nitrogen metabolism such as nitrate reductase, nitrite reductase, glutamate dehydrogenase, glutamate synthase, urease, glutamic–pyruvic transaminase, and glutamic–oxaloace protease transaminase. As the nitrogen metabolism in spinach was significantly inhibited by magnesium deficiency, it caused a significant reduction of spinach plant weight, leaf turning chlorosis. However, cerium treatment grown in magnesium-deficiency media significantly promoted the activities of the key enzymes as well as the contents of the free amino acids, chlorophyll, soluble protein, and spinach growth. It implied that Ce3+ could partly substitute for magnesium to facilitate the transformation from inorganic nitrogen to organic nitrogen, leading to the improvement of spinach growth, although the metabolism needs to be investigated further.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the essential nutrient macroelements for plant growth, magnesium (Mg) plays very important roles in plants, i.e., Mg is the central atom of the chlorophyll (chl) molecule, and fluctuations in its levels in the chloroplast regulate the activity of key photosynthetic enzymes such as Rubisco, fructose-1,6-bisphosphatase, and phosphoribulokinase [1, 2]. Mg is the most abundant free divalent cation in the plant cytosol. Deficiency of Mg has been linked to its functions as a cofactor for enzymes related to cell respiration, glycolysis, and ion transport (e.g., Na–K-ATPase) [3]. In fact, the central position of Mg in its role in energy storage, transfer, and utilization is mediated through its function in the formation of Mg–ATP, the ultimate form of stored energy in biological systems [4–8]. In addition, Mg has functions related to protein synthesis through its action on nucleic acid polymerization, binding of ribosomes to RNA, and the synthesis and degradation of DNA [1, 2]. Mg is also an integral player in calcium biology via its ability to maintain low resting concentrations of intracellular calcium ions. It competes with calcium for membrane-binding sites and, as such, has been described as a “calcium channel blocker” [2]. However, the effects of Mg deficiency on nitrogen metabolism of plants have rarely reported.
At present, researches related to the mechanism of rare earths to plants are rare outside China, but it is continuously active in China. Hong et al. discovered that La3+ or Ce3+ could obviously promote growth and increase chlorophyll contents and photosynthetic rate in spinach [9–11]. Moreover, La3+ or Ce3+ could substitute Mg2+ for chlorophyll formation of spinach under Mg2+ deficiency. Hong and Liu found that Rubisco, Rubisco activase contents, and their activities in spinach were significantly increased by La3+, Ce3+, and Nd3+ treatments [12, 13]. On the other hand, we also speculate that the improvements of plant growth caused by rare earths might be closely related to the promotion of nitrogen metabolism, especially, the effects of rare earths on the nitrogen metabolism of plants under magnesium deficiency is poorly understood.
The effects of magnesium deficiency and cerium treatment on the growth of spinach plants were studied in this paper. The results showed that spinach old leaves developed distinct magnesium-deficient symptoms, and plant growth was significantly inhibited by magnesium deprivation; cerium-treated groups under the same conditions did not develop magnesium-deficient symptoms. Magnesium deprivation inhibited nitrogen metabolism and photosynthesis in spinach plants, and cerium treatment under magnesium-deficient media could improve nitrogen metabolism and photosynthesis and increase plant weight. This is viewed as evidence that cerium added to magnesium-deficient media in the spinach plants could partly substitute for magnesium and improve spinach growth.
Materials and Methods
Material Treatment and Culture
Seeds of Spinacia oleracea were scarified in 85% H2SO4, rinsed in running water, and sterilized in 0.2% HgCl2 for 10–15 min. Seeds were then planted in a perlite-containing pot and placed in porcelain dishes, which were respectively added with 500 ml of the following culture solutions: (1) magnesium-present Hoagland’s nutrient solution; (2) 15 μM CeCl3 + magnesium-present Hoagland’s nutrient solution; (3) magnesium-deficient Hoagland’s nutrient solution; (4) 15 μM CeCl3 + magnesium-deficient Hoagland’s nutrient solution. Magnesium-present Hoagland’s nutrient solution and magnesium-deficiency Hoagland’s nutrient solution were prepared as described in Ref. [14]. In preparation of magnesium-present Hoagland’s nutrient solution, the macronutrient concentrations were (mM): KNO3, 0.5; Ca(NO3)2, 0.5; MgSO4, 0.2; KH2PO4, 0.017; and NaH2PO4, 0.008. Micronutrient concentrations were (mM): H3BO3, 1.25; MnSO4, 0.1; ZnSO4, 0.025; CuSO4, 0.025; Na2MoO4, 0.025; and NiSO4, 0.04; Fe–EDTA, 10. And in preparation of magnesium-deficiency Hoagland’s nutrient solution, the macronutrient concentrations were (mM): KNO3, 0.5; Ca(NO3)2, 0.5; KH2PO4, 0.017; and Na2SO4, 0.008; micronutrient concentrations were same as magnesium-present Hoagland’s nutrient solution. Plants were grown at 20°C using a 16/8 h light/dark cycle in a growth chamber under 400 µmol⋅m−2 s−1 of cool fluorescent light for 5 weeks. The nutrient solution was renewed every week. The physiological and biochemical indexes of strain and leaves were measured 5 weeks after germination.
Growth Measurement
The fresh weight and dry weight of spinach were weighted at 35th day. The chlorophyll contents were determined by Arnon’s method [15].
Nitrogen Content Assay
Total nitrogen concentration was determined by the H2SO4–H2O2 digestion method of Kjeldahl [16]. Nitrogen content was determined as the product of nitrogen concentration (on a dry weight basis) and dry weight.
Assay of Free Amino Acid and Soluble Protein Content
Spinach leaves (0.5 g) were used for the extraction and analysis of amino acid and soluble protein content. The sample was homogenized at 4°C in 5 ml cold water (Milli Q reagent grade), and centrifuged at 800 ×g for 5 min. The supernatant was stored on ice, and the pellet resuspended in 3 ml cold water prior to re-centrifugation (800 ×g) for a further 5 min. The supernatants from both centrifugations were pooled and stored on ice, the pellet was resuspended in a further 2 ml of cold water, and centrifuged at 800 ×g again. The supernatant was pooled for analysis. The total free amino acid content was determined by ninhydrin assay [17] and the absorbency reading was converted to mg amino acid g−1 spinach fresh weight using glycine standard curve. Soluble protein content was precipitated with 20% trichloroacetic acid (TCA) and determined by the method of Lowry et al. [18].
Nitrate Reductase (NR) and Nitrite Reductase (NiR)
Spinach leaves (1 g) were hand homogenized in 10 ml cold 0.15 M phosphate buffer (pH 7.5) containing 1 mM cysteine and 1% (w/v) casein in a previously chilled mortar using acid-washed sand as an abrasive. The homogenate was centrifuged at 10,000 ×g for 30 min in a refrigerated centrifuge at 0 to −4°C. The resultant supernatant was referred as the enzyme extract and stored in a refrigerator for enzyme assays and soluble protein estimation.
NR activity was assayed by the method suggested by Hageman and Flesher [19]. The assay mixture in a final volume of 2 ml contained 200 μM phosphate buffer (pH 7.5), 20 μM KNO3, and 0.4 μM NADH. The enzymatic reaction was initiated by addition of 0.5 ml of enzyme extract. A blank without NADH was also run simultaneously. After incubation at 30°C for 15 min, the reaction was terminated by adding rapidly 0.1 ml of 1 M zinc acetate and 1.9 ml of 70% ethanol. The contents were mixed thoroughly and centrifuged at 3,000 ×g for 15 min. Two milliliters of supernatant was then removed and transferred to a test tube. One milliliter of 1% sulfanilamide reagent (prepared in 1 M HCl) followed by 1 ml of 0.02% N-(1-naphthyl ethylene diamine dihydrochloride) was added. After 30 min, the absorbance of violet color was measured at 540 nm on a dual-beam spectrophotometer (UV-3010, Hitachi Co., Japan). The enzyme activity was measured as μmol of nitrite produced h−1 mg−1 protein.
NiR activity was measured following the method of Sawhney and Naik [20]. The assay mixture in a final volume of 2 ml contained 100 μM phosphate buffer (pH 7.5), 1.0 μM NaNO2, 0.4 μM methylviologen, and 0.5 ml of enzyme extract. The reaction was started with the addition of 0.1 ml of sodium dithionite solution (prepared by dissolving 10 mg sodium dithionite in 10 ml of 0.29 M sodium bicarbonate). A blank without sodium dithionite was also run simultaneously. After incubation for 30 min at 30°C, the reaction was stopped by shaking vigorously. Supernatant (0.1 ml) was then pipetted in a test tube followed by 1.9 ml of distilled water. One milliliter of 1% sulfanilamide reagent (prepared in 1 M HCl) followed by 1 ml of 0.02% N-(1-naphthyl ethylene diamine dihydrochloride) were added. After 30 min, the absorbance of violet color was measured at 540 nm on a dual-beam spectrophotometer (UV-3010, Hitachi). The enzyme activity was measured as μmol of nitrite reduced h−1 mg−1 protein.
Glutamate Dehydrogenase (GDH), Glutamate Synthase (GS), Urease
Spinach leaves (1 g) were hand homogenized in 10 ml of cold 0.1 M phosphate buffer (pH 7.6) containing 2% polyvinylpyrrolidone (PVP), 1% β-mercaptoethanol, and 10 mM dithiothreitol (DTT) in previously chilled mortar using acid-washed sand as an abrasive [21]. The homogenate was centrifuged at 10,000 ×g for 30 min at 0 to −4°C in a refrigerated centrifuge. The resultant supernatant was referred as the enzyme extract and stored in a refrigerator for enzyme assays and soluble protein estimation.
GS activity assayed by the method of O’Neal and Joy [22]. The reaction mixture (4 ml) contained 0.1 M Tris–maleate buffer (pH 7.5), 1 M hydroxylamine (pH 7.0), 100 mM glutamate (pH 7.2), 10 mM ATP (pH 7.2), 1 M MgSO4, and 0.2 ml of properly diluted enzyme extract. The reaction was started by adding hydroxylamine and the mixture was incubated for 20 min at 30°C. The reaction was stopped by the addition of 1 ml of FeCl3 reagent, prepared by mixing equal volumes of 10% FeCl3·6H2O in 0.2 M HCl, 24% TCA, and 5% HCl. After 10 min, the protein precipitate was removed by centrifugation. The hydroxamic acid formed in the supernatant was measured at 540 nm on a dual-beam spectrophotometer (UV-3010, Hitachi) and its concentration computed using λ-glutamyl monohydroxamate (λ-GMH) as standard. The results were expressed as μmol of λ-GMH formed h−1 mg−1 protein.
GDH activity was measured following the method of Boland et al. [21]. The assay mixture (2 ml) contained 14 mM 2-oxoglutarate, 80 mM imidazole–HCl (pH 7.9), 200 mM ammonium acetate, 60 mM NADH, 2 mM ADP, and 0.1 ml enzyme extract. Rate of the reaction was followed by recording the change in absorbance at 340 nm on a dual-beam spectrophotometer (UV-3010, Hitachi). Background rates were also measured in the absence of ammonium acetate. The enzyme activity was expressed as μmol NAD+ formed h−1 mg−1 protein.
Urease activity was determined as per the method of Malhotra and Indira [23]. Incubation mixture contained 4 ml of 0.05 M Tris–acetate buffer (pH 7.3), 12 mg urea, and 1 ml of suitably diluted enzyme extract. The mixture was incubated for 10 min at 35°C. Two milliliters of aliquot was removed and transferred to other tubes containing 1 ml of 2 M trichloroacetic acid (TCA) to stop the reaction. One milliliter of water was added to each tube and the contents were centrifuged for 15 min at 2,000 ×g. Clear supernatant (0.5 ml) was then pipetted into 20 ml of water in 25 ml measuring flask. One milliliter of the Nessler’s reagent was added to each flask and volume made up to the mark. The absorbance of yellow color so produced was measured at 405 nm after 30 min using a dual-beam spectrophotometer (UV-3010, Hitachi). To correct non-specific colors, a blank containing boiled enzyme extract was run simultaneously. The values of ammonia concentration were obtained by comparing the absorbance of test solutions with the absorbance of standard ammonium chloride solution. The enzyme activity was measured as nmol of ammonia formed min−1 mg−1 protein.
Glutamic–Pyruvic Transaminase (GPT) and Glutamic–Oxaloace Transaminase (GOT)
Spinach leaves (0.5 g) were homogenized in buffered medium (0.05 mM Tris–HCl, pH 7.2), and the homogenate was centrifuged at 26,100 ×g for 10 min at 0°C. The supernatant was analyzed for GPT activity. A mixture, including 0.5 ml alanine (0.8 M) in 0.1 M Tris–HCl (pH 7.5), 0.1 ml pyriodoxal phosphate solution (2 mM), 0.2 ml 2-oxoglutarate solution (0.1 M), and 0.2 ml of the enzyme preparation, was used. The reaction mixture was incubated at 37°C for 10 min, then 0.1 ml trichloroacetic acid solution was added to terminate the reaction. The pyruvate with chromogen was converted to pyruvate hydrazone. The color intensity of the hydrazone in water-saturated toluene was measured at 520 nm on a dual-beam spectrophotometer (UV-3010, Hitachi). The GPT activity, in terms of pyruvate production, was calculated from authentic pyruvate standards run simultaneously. The procedure used for assaying the activity of GOT was identical to that described for the GPT assay except that, in the GOT assay, 0.5 ml of a 0.1 M buffered aspartate solution was substituted for 0.5 ml of a 0.8 M alanine in 0.1 M Tris–HCl (pH 7.5) in the reaction mixture. The GOT activity, in terms of oxaloacetate production, was calculated from authentic oxaloacetate standards run simultaneously [24].
Assay of Nitrate in Spinach
The nitrate of spinach leaves (5 g) was extracted with 15 ml of Milli Q water at 80°C in a water bath for exactly 20 min. Then, the content of NO −3 was analyzed by colorimetrical methods using a dual-beam spectrophotometer (UV-3010, Hitachi) [14].
Assay of Oxygen Evolution
The oxygen evolution of spinach leaves was measured with an Oxygraph oxygen electrode (Hansatech Instruments, UK). The assay medium contained 0.5 M sorbitol, 10 mM KCl, 0.5 mM MgCl2, 0.05% (w/v) bovine serum albumin, 10 mM NaHCO3, and 4-2-hydroxyethyl-1-piperazineethanesulfonic acid–KOH (pH 7.6).
Statistical Analysis
Each biochemical indicators was replicated five times. All data were expressed as mean ± standard error and were analyzed by an analysis of variance (ANOVA). If significance was found in ANOVA, group means were compared using Student’s t test. Differences were considered significant when p ≤ 0.05.
Results
The Growth of Spinach Plants
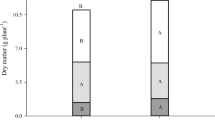
We observe in Fig. 1 (columns 1, 2) that the single fresh and dry weights of the Ce3+-treated groups grown in the Mg2+-present media were increased by 37.17% and 44.75%, respectively. Figure 1 (column 3) presents that the fresh and dry weights of single plant under Mg2+-deficient media were much lower than those grown in the Mg2+-present media, suggesting 37.08% and 40.12% reduction, respectively. However, Ce3+-treated groups grown in the Mg2+-deficient media were decreased by 5.79% and 4.31% in Fig. 1 (column 4), respectively.
The effects of Ce3+ on spinach plant weight under culture of Mg2+-deficient Hoagland’s solution. 1 Mg2+-present Hoagland’s solution; 2 Mg2+-present Hoagland’s solution + Ce3+; 3 Mg2+-deficient Hoagland’s solution; 4 Mg2+-deficient Hoagland’s solution + Ce3+. Bars marked with double stars were different from Hoagland’s solution in that panel at the 1% confidence level. Bars indicate mean and error bars are SE.
The results mentioned above proved that deprivation of Mg2+ inhibited the growth of spinach significantly, and Ce3+ treatment could promote growth of spinach.
Nitrogen Contents in Spinach
To demonstrate the effect of Mg2+ deficiency and Ce3+on the nitrogen metabolism of spinach under Mg2+-deficient media, the experiments assayed the contents of total nitrogen in spinach. The results, in Fig. 2, show that the content of total nitrogen of spinach with Ce3+ treatment was 8.3% higher than that of the control, under culture with Mg2+-present Hoagland solution. However, the content of total nitrogen caused by deficiency of Mg2+ was decreased by 21.87% as compared to the control grown in the Mg2+-present Hoagland media, and Ce3+-treated groups grown in the Mg2+-deficient media were reduced by 10.0% as compared to the control grown in the Mg2+-present media (p > 0.05), implying that deficiency of Mg2+ decreased the accumulation of nitrogen, and Ce3+ treatment might promote the accumulation of organic nitrogen, such as amino acid, soluble protein, and chlorophyll, under Mg2+-deficiency stress.
The effects of Ce3+ on total nitrogen contents of spinach under culture of Mg2+-deficient Hoagland’s solution. 1 Mg2+-present Hoagland’s solution; 2 Mg2+-present Hoagland’s solution + Ce3+; 3 Mg2+-deficient Hoagland’s solution; 4 Mg2+-deficient Hoagland’s solution + Ce3+. Bars marked with double stars were different from Hoagland’s solution in that panel at the 1% confidence level. Bars indicate mean and error bars are SE.
The Contents of Free Amino Acid, Chlorophyll, and Soluble Protein in Spinach
The changes of the contents of spinach free amino acid, chlorophyll, and soluble protein were very similar to that of the total nitrogen content (Fig. 3). In comparison to the control grown in the Mg2+-present media, the contents of free amino acid and soluble protein caused by deficiency of Mg2+ were decreased by 20.51% and 61.97%, respectively, while those of Ce3+-treated group grown in the Mg2+-deficient media were alleviated by 7.69% and 13.36%, and in the Mg2+-present media were increased by 5.13% and 38.03%, respectively. We also observe that deprivation of Mg2+ inhibited chlorophyll formation of spinach significantly, and Ce3+ treatment could promote chlorophyll formation, particularly grown in the Mg2+-deficient media. For example, the chlorophyll content of the Ce3+-treated groups grown in the Mg2+-present media was enhanced by 50.0% compared with the control, Mg2+-deficient treated groups was 57.84% as compared to the Mg2+-present groups, and Ce3+-treated groups grown in the Mg2+-deficient media was 84.31% as compared to in the Mg2+-present Hoagland’s media groups.
The effects of Ce3+ on organic nitrogen contents of spinach under culture of Mg2+-deficient Hoagland’s solution. 1 Mg2+-present Hoagland’s solution; 2 Mg2+-present Hoagland’s solution + Ce3+; 3 Mg2+-deficient Hoagland’s solution; 4 Mg2+-deficient Hoagland’s solution + Ce3+. Bars marked with double stars were different from Hoagland’s solution in that panel at the 1% confidence level. Bars indicate mean and error bars are SE
The results mentioned above suggested that Ce3+ treatment can improve the synthesis of organic nitrogen, especially under Mg2+ deficiency, which might be closely related to the activities of key enzymes involving nitrogen assimilation.
Enzyme Activities of Spinach
The key enzyme activities of nitrogen metabolism in spinach are listed Table 1. We can see that NR and NiR activities caused by Ce3+ adding to the Mg2+-present media were significantly higher than the control, suggesting 48.47% and 63.22% increase, respectively, but in the Mg2+-deficient group were obviously inhibited, having 63.91% and 65.23% reduction, and by Ce3+ adding to the Mg2+-deficient media were reduced by 38.78% and 27.59%, respectively, as compared to the Mg2+-present media. According to Table 1, the activities of GDH, GS, urease, GPT, and GOT in spinach caused by treatment of Ce3+ under Mg2+-present media were significantly higher than those of the control of Mg2+-present media, showing 27.31%, 44.49%, 21.82%, 41.28%, and 21.61% enhancement, respectively; the enzyme activities from Mg2+-deficient group were obviously inhibited, having 50.81%, 42.73%, 47.05%, 72.41%, and 72.61% reduction, respectively. But the reduction of the enzyme activities caused by deficiency of Mg2+ was significantly decreased by treatment of Ce3+, suggesting 12.25%, 20.26%, 22.49%, and 47.9% descent, respectively. The results suggested that deficiency of Mg2+ inhibited the key enzyme activities of nitrogen metabolism and treatment of Ce3+ could activate GDH, GS, urease, GPT, and GOT in spinach.
Nitrate Content in Spinach
To further evaluate deprivation of Mg2+ and Ce3+ treatment on nitrogen metabolism in spinach, we measured NO −3 content for four experimental groups, and the results are shown in Fig. 4. The NO −3 content of spinach grown in the Mg2+-deficient media (Fig. 4, column 3) was 43.96% as that in the Mg2+-present media (Fig. 5, column 1); Ce3+-treated groups grown in the Mg2+-present media (Fig. 4, column 2) and Ce3+-deficient media (Fig. 4 column 4) were 126.05% and 80.65% as that grown in the Mg2+-present media (Fig. 4, column 1). The results suggested that deprivation of Mg2+ significantly inhibited nitrate uptake and Ce3+ treatment improved nitrate uptake of spinach from Hoagland’s media.
The effects of Ce3+ on spinach NO −3 contents under culture of Mg2+-deficient Hoagland’s solution. 1 Mg2+-present Hoagland’s solution; 2 Mg2+-present Hoagland’s solution + Ce3+; 3 Mg2+-deficient Hoagland’s solution; 4 Mg2+-deficient Hoagland’s solution + Ce3+. Bars marked with a star and double stars were different from Hoagland’s solution in that panel at the 5% confidence level and at the 1% confidence level, respectively. Bars indicate mean and error bars are SE
The effects of Ce3+ on oxygen evolution of spinach under culture of Mg2+-deficient Hoagland’s solution. 1 Mg2+-present Hoagland’s solution; 2 Mg2+-present Hoagland’s solution + Ce3+; 3 Mg2+-deficient Hoagland’s solution; 4 Mg2+-deficient Hoagland’s solution + Ce3+. Bars marked with a star and double stars were different from Hoagland’s solution in that panel at the 5% confidence level and at the 1% confidence level, respectively. Bars indicate mean and error bars are SE
Oxygen Evolution of Spinach
It is well known that nitrogen metabolism is closely related to carbon fixation and reducing power in plants. In the experimental study, we also measured a photosynthesis parameter—the rate of oxygen evolution in spinach (see Fig. 5). The rate of oxygen evolution of Ce3+-treated spinach grown in the Mg2+-present media increased by 18.25%, Mg2+-deficient group decreased by 59.18%, and Ce3+-treated group grown in the Mg2+-deficient media decreased by 23.69% as compared to the control grown in the Mg2+-present media, suggesting that deficiency of Mg2+ inhibited photosynthesis and Ce3+ treatment promoted photosynthesis of spinach.
Discussion
The results of the experimental study showed that deprivation of Mg2+ inhibited the chlorophyll, free amino acids, and protein synthesis, which resulted in the old leaf chlorosis and the reduction of total nitrogen and the growth of spinach significantly, but the significant increases of total nitrogen and the growth caused by Ce3+ treatment were observed; this is viewed as evidence that Ce3+ treatment can improve the synthesis of organic nitrogen, especially promote to the synthesis of protein and chlorophyll in spinach under Mg2+ deficiency, which might be closely associated with the activities of key enzymes involving nitrogen assimilation. Polle and Anza observed that Mg deficiency induced a decrease in chlorophyll and leaf protein content in spruce trees and pepper, respectively [25, 26]. Cakmak also found that with increasing plant age Mg-deficient leaves developed severe interveinal chlorosis and, accordingly, chlorophyll concentrations were reduced in bean [5]. The enhancement of free amino acids caused by La3+ in cucumber has been made by Dong et al. [27]. Hong et al. observed that La3+ added to Mg2+-deficient media could relieve Mg2+-deficient symptoms of old leaves and improve spinach growth and chlorophyll synthesis; however, the nitrogen metabolism had been not concerned under Mg2+-deficient stress [9]. In the paper, we measured the activities of key enzymes involving nitrogen assimilation, such as nitrate reductase (NR), nitrite reductase (NiR), glutamate dehydrogenase (GDH), glutamate synthase (GS), urease, glutamic–pyruvic transaminase (GPT), and glutamic–oxaloace protease transaminase (GOT), in spinach plants grown in the Mg2+-present Hoagland’s media and Mg2+-deficient Hoagland’s media.
NR is the first enzyme in the process of nitrate reduction to amino form. It is affected by factors such as plant development stages [28] as well as environmental conditions [29]. We observed that deprivation of Mg2+ inhibited NR and NiR activities in spinach significantly, which either is due to reduced carbon fixation or low nitrate translocation and subsequently low nitrate availability in spinach or reduced uptake of nitrate by roots or limitations in reducing power. Our data proved that deprivation of Mg2+ caused to the reduction of nitrate uptake and photosynthesis of spinach, which might be due to decreased root activity and chlorophyll content. However, Ce3+ treatment could decrease the inhibition of deprivation of Mg2+ on NR and NiR activities, nitrate uptake, and photosynthesis of spinach, which might be closely associated with the increased of root activity and chlorophyll content. Dong et al. reported that La3+ could relieve the reduction of cucumber root and NR activity caused by deficiency of Ca2+ [30].
As we all know, GS and GDH are two important enzymes in the assimilation of ammonia, and GPT and GOT are two important amino transferases of the plant, and urease is an important enzyme in prevention of protein degradation [1]. Therefore, the activities of the enzymes are closely related to the rate of ammonia assimilation and other nitrogen metabolism. The present study demonstrated that deprivation of Mg2+ significantly inhibited the activities of GS, GDH, GPT, GOT, and urease, which resulted in the reduction of synthesis of organic nitrogen. However, Ce3+ treatment could activate the enzymes, accelerate the rate of ammonia assimilation, and promote the synthesis of amino acid, chlorophyll, protein, etc., and resulting in the improvement of spinach growth under Mg2+-deficient stress. The decreased availability of ATP, NADPH, and Mg2+ ions which act as cofactor in various metabolic processes may be the possible reason for the observed decrease in GS, GDH activities under Mg2+-deficient stress [31, 32]. Ce3+ significantly promoted GS and GDH activity of spinach under Mg2+-present Hoagland’s media and Mg2+-deficient stress; this might also be due to the increase of ATP production which improved photophosphorylation by Ce3+. Huang et al. also observed that ATP production was increased as a result of cerium application [33].
Conclusion
The activities of key enzymes of nitrogen metabolism, such as NR, NiR, GS, GDH, urease, GPT, and GOT, in spinach decreased by deprivation of Mg2+, which resulted in the obvious reduction of organic nitrogen and plant growth. From the results, it is obvious that deprivation of Mg2+ adversely affected the nitrogen metabolism by inhibiting the activity of NR, NiR, GS, GDH, urease, GPT, and GOT. Further, deprivation of Mg2+ inhibits both GS/GOGAT and GDH pathway for ammonium assimilation. So, it can be concluded that deprivation of Mg2+ adversely affects the growth of spinach as a result of its interference with photosynthetic pigments and key enzymes of nitrogen metabolism. But activities of these enzymes, organic nitrogen, and plant weight increased and photosynthesis improved by Ce3+ treatment under Mg2+-deficient stress. This is viewed as evidence that Ce3+ treatment can partly be substituted for the role of Mg2+ under Mg2+-deficient stress and improve nitrogen metabolism in spinach. But the mechanisms need further study.
References
Buchanan BB, Gruissem W, Johones RL (2002) Biochemistry and molecular biology of plants. American Society of Plant Physiology, Science Press, Beijing, pp 568–628
Wu WH (2003) Plant physiology. Science Press, Beijing, pp 93, 105–108, 134–135 (in Chinese)
Briskin DP, Pooler J (1983) Role of magnesium in plasma membrane ATPase of red beet. Plant Physiol 71:969–971
Fisher ES, Bream EE (1993) Influence of magnesium deficiency on rates of leaf expansion, starch and sucrose accumulation, and net assimilation in Phaseolus vulgaris. Physiol Planta 89:271–276
Cakma KI (1994) Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium and potassium deficient leaves, but not in phosphorus-deficient leaves. J Exp Bot 45(278):1259–1266
Cakma KI, Hengeler C, Marschner H (1994) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45:1245–1250
Cakma KI, Hengeler C, Marschner H (1994) Changes in phloem export of sucrose in leaves in response to phosphorous, potassium and magnesium deficiency in bean plant. J Exp Bot 45:1251–1257
Lavon R, Goldshmidt EE, Salomon R (1995) Effect of potassium, magnesium and calcium deficiencies on carbohydrate pools and metabolism in citrus leaves. J Am Soc Hortic Sci 120(1):54–58
Hong FS, Wei ZG, Zhao GW (2002) Mechanism of lanthanum effect on the chlorophyll of spinach. Sci China Ser C 45(2):166–176
Hong FS, Wang L, Meng XX, Wei Z, Zhao GW (2002) The effect of cerium on the chlorophyll formation of spinach. Biol Trace Element Res 89:263–277
Hong FS, Wang XF, Liu C, Su GX, Song WP, Wu K, Tao Y, Zhao GW (2003) Effect of Ce3+ on spectral characteristic of D1/D2/Cytb559 complex from spinach. Sci China Ser B 46(1):42–50
Hong FS, Liu C, Zheng L, Wang XF, Wu K, Song WP, LV SP, Tao Y, Zhao GW (2005) Formation of complexes of rubisco–rubisco activase from La3+, Ce3+ treatment spinach. Sci China Ser B 48(1):67–74
Liu C, Hong FS, Wu K, Ma HB, Zhang XG, Hong CJ, Wu C, Gao FQ, Yang F, Liu T, Xu JH, Xie YN, Li ZR (2006) Effects of Nd3+ ion on carboxylation activity of ribulose-1, 5-bisphosphate carboxylase/oxygenase of spinach. Biochem Biophys Res Comm 342(1):36–43
Meider H (1984) Class experiments in plant physiology. Allen and Unwin, London, pp 72–74
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Richards EJ (1993) Chemical characterization of plant tissue. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis, Boca Raton, pp 115–139
Yemm EW, Cocking EC (1995) Determination of amino acid with ninhydrin. Analyst 80:209–213
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Hageman RH, Flesher D (1960) Nitrate reductase activity in corn seedlings as affected by light and nitrate content of nutrient media. Plant Physiol 35:700–708
Sawhney SK, Naik MS (1973) Role of light in synthesis of nitrate reductase and nitrite reductase in rice seedlings. Biochem J 130:475–485
Boland MJ, Fordyce AM, Greenwood RM (1978) Enzymes of nitrogen metabolism in legume nodules: a comparative study. Aust J Plant Physiol 5:553–559
O’Neal D, Joy KW (1973) Glutamine synthetase of pea leaves I. Purification stabilization and pH optima. Arch Biochem Biophys 159:113–122
Malhotra OP, Indira R (1969) Purification and properties of urease of Cajanus indicus. Indian J Biochem 6:15–20
Tonhazy NE, White NG, Umbriet WW (1950) Colorimetric assay of glutamic–pyruvic transaminase. Arch Biochem Biophys 28:36–38
Polle A, Otter T, Mehne-Jakobs B (1994) Effect of magnesium-deficiency on antioxidative systems in needles of Norway spruce (Picea abies (L.) Karst.) grown with different ratios of nitrate and ammonium as nitrogen sources. New Phytol 128:621–628
Anza M, Riga P (2001) Effect of magnesium deficiency in antioxidant enzymes from pepper plants (Capsicum annuum L.). Acta Horticulturae 559:365–370
Dong B, Wu ZM, Xu L, Gao XX (1992) Effects of LaCl3 on composition of amino acid in plant exudation. Chin Bulletin Bot 9(3):44–46 in Chinese
Eck HV, Wilson GC, Martinez T (1975) Nitrate reductase activity of grain sorghum leaves as related to yields of grain, dry matter and nitrogen. Crop Sci 15:557–560
Srivastava HS (1980) Regulation of nitrate reductase activity in higher plants. Phytochem 19:725–733
Dong B, Wu ZM, Tang YK (1993) The affect of Ce3+ on the physiology of cumber young root under Ca-deficient condition. J Chin Rare Earth 11(1):65–68 in Chinese
Burdygina VS, Kuzin AT (1966) In: Gupta US (ed) Physiological aspects of crop nutrition and resistance. Atma Ram, Delhi
Wellburn AR, Higginson C, Robinson D, Walmsley C (1981) Biochemical explanations of more than additive inhibitory effects of low atmospheric levels of sulphur dioxide plus nitrogen dioxide upon plants. New Phytol 88:223–227
Huang H, Liu XQ, Qu CX, Liu C, Chen L, Hong FS (2008) Influences of calcium deficiency and cerium on the conversion efficiency of light energy of spinach. Biometals 21:553–561
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant no. 30470150, 30800068).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sitao Yin and Yuguan Ze contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yin, S., Ze, Y., Liu, C. et al. Cerium Relieves the Inhibition of Nitrogen Metabolism of Spinach Caused by Magnesium Deficiency. Biol Trace Elem Res 132, 247–258 (2009). https://doi.org/10.1007/s12011-009-8392-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8392-z