Abstract
Background and aims
Rice plants (Oryza sativa L.) contain large quantities of silicon (Si) in form of phytoliths, which increase their resistance to abiotic and biotic stresses. The Si cycle through rice fields is hardly studied. We tested how increasing Si availability affects rice growth and the decomposability of the straw. Secondly we tested the role of straw recycling for Si availability.
Methods
In a field experiment, we applied three levels of silica gel during one rice cropping season. In a follow-up laboratory experiment, we used straw produced in the field experiment, having different Si concentrations, and studied straw decomposition, straw Si release, and Si uptake by plants.
Results
Silicon fertilization increased Si contents, biomass production, and grain yield of rice plants. Increased Si uptake by rice decreased concentrations of C and some essential nutrients (N, P, K, Ca, and Mg) in the straw, and increased straw decomposability and Si release.
Conclusions
Fertilization with silica gel is an option to improve Si supply to rice plants growing on weathered soils with low levels of plant-available Si. Phytoliths from fresh rice straw dissolve fast in soil, thus, recycling of rice straw is an important source of plant-available Si.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the staple food for more than half of the world’s population and the demand for rice is continuously increasing (De Datta 1981; IRRI 1997). Silicon (Si) is a beneficial element for rice plants, usually taken up in larger amounts than essential nutrients, such as nitrogen (N), phosphorus (P), potassium (K), and calcium (Ca) (Ma and Takahashi 2002). Recent research revealed that Si increases plant resistance to biotic stresses, such as fungal and insect pests, as well as to abiotic stresses, such as strong rain, wind, and salinity (Guntzer et al. 2012). Rice plants take up Si in the form of monosilicic acid from the soil solution, involving active and passive transport (Ma et al. 2006; Ma et al. 2007; Yamaji and Ma 2009). Within the plant, Si is transported with the transpiration stream and precipitates near evaporating surfaces in cell walls, cell lumina, or intercellular spaces, forming amorphous SiO2 bodies, called phytoliths (Jones and Handreck 1967; Epstein 1999). Plant-available monosilicic acid in paddy soils originates from irrigation water, desorption from the soil matrix and weathering of Si-containing soil minerals (Klotzbücher et al. 2015). Rice straw contains about 86 % of Si taken up by rice plants (Klotzbücher et al. 2015). Some rice farmers remove part of the straw after harvest permanently from the field; others leave it in the field (Klotzbücher et al. 2014). Deposition of crop residues is a crucial factor for Si balance of rice fields (Klotzbücher et al. 2015). The effects of rice straw input on Si availability in soil should strongly depend on the solubility of the phytoliths, a process that is still not well understood.
Phytoliths have been extracted from archaeological sites and used to date back land-use changes (Santos et al. 2010; Piperno 2014). This suggests that phytoliths cycle slowly in soils and can be preserved for centuries. Phytoliths can contain considerable quantities of carbon (C) (e.g., 1–13 %; Parr and Sullivan 2011). Phytolith cycling is, thus, discussed as mechanism for long-term sequestration of C in soils (Parr and Sullivan 2011). By contrast, a number of recent studies suggest that fresh phytoliths are among the most important sources of Si in soil solution. Fraysse et al. (2009) showed in laboratory experiments that phytoliths extracted from different plants (larch, elm, fern, horsetail) are 100–10,000 times more soluble than clay minerals, primary mafic silicates, or feldspars. Few data are available on the Si release during rice straw decomposition. They suggest that decomposition produces considerable amounts of plant-available Si (Hossain et al. 2001; Ma and Takahashi 2002; Watanabe et al. 2013), implying that phytoliths are relatively soluble. It is not clear how the contrasting findings can be explained. Possibly, the solubility of phytoliths decreases during litter decomposition in soils by yet unknown mechanisms.
In regions with soils of low Si availability, Si fertilization might be an alternative or additional option to recycling of rice straw. Various authors reported positive effects of the addition of Si sources on rice growth and grain yields (reviewed by Guntzer et al. 2012). The consequences of Si fertilization for the ecosystem are, however, not yet well explored. Changing Si availability may, for instance, affect the cycling of C and nutrients by altering (i) biomass production, (ii) plant nutrient uptake (Guntzer et al. 2012) and (iii) re-mineralization of nutrients during litter decomposition.
Rates of litter decomposition depend on climate, litter quality, and the structure of the decomposer community (e.g., Cornwell et al. 2008; Strickland et al. 2008). Schaller and Struyf (2013) showed for the common reed (Phragmites australis) that an increased Si availability during plant growth affects nutrient composition of the plant tissue, which in turn caused changes in decomposability. In addition, Si concentrations in litter can affect fungal growth (Schaller et al. 2014) and the activity of invertebrate decomposers (Schaller and Struyf 2013), which play a crucial role in controlling rice straw decomposition rates (Schmidt et al. 2015a). The phytoliths may also act as a physical barrier hindering fungal hyphae to penetrate the litter surface and insects to feed on the litter (Schaller and Struyf 2013; Schaller et al. 2014). The mechanisms linking Si in the litter to growth and activity of decomposers have, however, not yet been studied in detail. In particular, effects of Si concentrations on decomposition and recycling of nutrients in rice fields have not been tested so far.
Using a combined field and laboratory experiment, we tested the effects of increasing Si availability on rice growth and decomposition of the resulting straw. In addition, we tested the role of straw recycling for Si supply to rice plants (Fig. 1). Finally, the study also examined some of the consequences of increasing Si availability for the system.
Following questions were addressed:
-
Q1
Does Si fertilization increase Si uptake, biomass production, and yield of rice in soils with low levels of plant-available Si?
-
Q2
How do increased Si concentrations in rice straw affect its decomposition by microorganisms and mesofauna?
-
Q3
Are phytoliths readily dissolvable and does therefore recycling of rice straw increase Si availability in soil and Si uptake by rice plants?
Fertilization effects (Q1) were tested in the field experiment. Straw with different Si concentrations produced in the field experiment was then used in the follow-up laboratory experiment. Here, straw poor in Si and straw rich in Si were used to test for decomposition effects (Q2), as well as for Si release and Si uptake by rice plants (Q3).
Material and methods
Field experiment
The field plot experiment was conducted in the Northern Vietnamese Vinh Phuc province (21°21’8.33”N 105°42’26.90”E), where plant-available Si concentrations in soils are low (Klotzbücher et al. 2014). The experiment was conducted during the dry season in 2013 (February-June) on a hydragric Anthrosol (Dystric, Siltic); basic soil parameters are given in Table 1. We used silica gel for Si fertilization, applying Si at three levels: 17.3 Mg Si ha−1, 0.4 Mg Si ha−1, and a control without Si application. The very high Si application rate of 17.3 Mg Si ha−1 was applied to produce Si-rich straw for the laboratory experiment. The low Si application rate of 0.4 Mg Si ha−1 was used for testing the effects of an application rate that would be feasible for farmers to apply in economic terms. Five neighbouring paddies (farmers’ fields) were selected, and one replicate of each treatment was established in each paddy. The control plot was established at the edge of water inflow; next to it the plot with the low Si application rate, followed by the plot with the high Si application rate. This arrangement of plots was chosen in order to minimize redistribution of applied Si with the inflowing water. Plot size was 2 m × 2 m; plot areas were marked with bamboo sticks at the corners. Industrial-grade silica gel of 2–3 mm grain size (TTL Phú Lương, Hanoi, Vietnam) was homogeneously distributed in the plots and mixed into the topsoil using a spade before flooding the fields. Soil tillage, fertilization with N and K, and pesticide application were carried out by the farmers according to their common practice. Soil was ploughed by a machine 8 to 12 days after Si application (dates varied between the five fields); 12 to 14 days after Si application, soil was ploughed again by buffalo directly before rice (Oryza sativa L. cv. Khang Dan 18) seedlings were transplanted. Four months later, rice plants from the core of each plot (inner 1.5 m × 1.5 m) were harvested in two steps: first, the upper part of all plants in a core plot was cut (one third to a half of the plant’s height), then the lower part was cut directly above the soil surface. Grains were separated from straw (stems and leaves). Afterwards, hulls were separated from grains using forceps.
Laboratory pot experiment
Straw produced in the field experiment was used in the laboratory experiment. Therefore, the lower part of straw from the control plots and from the 17.3 Mg Si ha−1 plots of the field experiment were pooled, respectively. These samples are referred to as Si-poor straw (control in the field experiment) and Si-rich straw (17.3 Mg Si ha−1 in the field experiment).
The experiment was conducted in a climate chamber with 11/13 h light/dark cycle at air temperature of 28 °C/25 °C, air humidity of 70 %, and light intensity of 350 μmol m−2 s−1.
The experiment had three factors:
-
(i)
Kind of Si source: We used straw with different Si concentrations (Si-poor straw and Si-rich straw) to study the effect of increased Si uptake by rice plants on their decomposability, Si release and availability. We also applied a control without Si application and a treatment with silica gel application to compare Si release and availability to the treatments with straw.
-
(ii)
Addition of mesofauna: For both types of straw (Si-poor straw and Si-rich straw), we applied treatments with (+Fauna) and without (−Fauna) the addition of individuals of three decomposer groups (see below) in order to study the effect of increased Si concentration in rice straw on decomposition by those invertebrates.
-
(iii)
Rice growth: Rice seedlings (Oryza sativa L. cv. IR 64) were transplanted to all treatments with straw to study Si uptake. The treatment with silica gel and the control without Si application were run with (+Plant) and without (−Plant) rice plants to study the effect of Si uptake by plants on Si release and availability.
The approach resulted in eight treatments (Fig. 2) replicated five times. The experiment was divided in two phases: a litterbag incubation phase of 33 days and a rice growth phase of 30 days, resulting in an entire experimental period of 63 days.
Pots of 2 l volume were filled with 1700 g of topsoil from Vinh Phuc province; basic soil parameters are given in Table 1. For the treatments with straw, 2 g of straw, out of 12 g per treatment in total were packed in litterbags and the remaining 10 g were mixed with the soil (straw was cut in pieces of 2–7 cm length). The straw application equalled about 20 Mg ha−1, roughly the equivalent of straw produced in five cropping seasons. We decided to use such a large amount of straw to (i) achieve a relatively large ratio of straw mixed with soil to straw in the litterbags, thus, to keep the portion of the straw removed with litterbags after 33 days small, and (ii) to maintain a sufficient amount of straw residues after 33 days in the litterbags for nutrient analyses. Litterbags had a size of 10 cm × 10 cm and were made of nylon net of 5 mm × 5 mm mesh size. Litterbags were retrieved on day 33. For the treatments with silica gel, soil was mixed with 2.85 g of silica gel of 70–200 μm grain size (Fluorochem Ltd, Hadfield, UK). This corresponded to the amount of Si added with the Si-rich straw based on the initial analyses of straw samples from the field plots using XRF spectroscopy (see analysis below).
Fauna was added to the particular treatments at the beginning of the experiment, directly after submergence of the soils. Around 200 individuals (0.6 g dry weight) of Lumbriculus variegatus (Annelida, Lumbricidae), around 2000 individuals (0.6 g of dry weight) of Tubifex tubifex (Annelida, Naididae), and around 900 larvae (0.7 g dry weight) of Chironomus plumosus (Arthropoda, Chironomidae) were added. Individuals of the same species were packed in bags containing 90 ml of nutrient solution and were ordered from www.interaquaristik.de. We have chosen those species as annelids and chironomid larvae are among the most abundant groups of invertebrate decomposers in flooded rice fields (Schmidt et al. 2015b). Most earthworm species, like Lumbriculus variegatus are edaphic litter-dwelling organisms feeding on dead organic matter. Chironomus plumosus and Tubifex tubifex rather indirectly contribute to the decomposition of rice straw, e.g., by grazing on the straw surface which promotes microbial reproduction and colonization rates and therefore their decomposition activity.
At the beginning of the second phase (day 33), immediately after litterbag retrieval, rice seedlings (Oryza sativa L. cv. IR 64) were transplanted to the pots. Seeds had been incubated 15 days for germination, 2 days in an oven at 58 °C, 1 day at room temperature, 1 day soaking in distilled water, 3 days in a moist tissue in an incubator with 14/10 h light/dark cycle at air temperature of 30 °C, and 8 days on vermiculite in the incubator. After transplanting to the pots, plants grew for 30 days, and then rice shoots were cut above the soil surface. We kept the soils submerged with distilled water during the entire experiment. Supernatant water was only allowed to evaporate at the end of both experimental phases, before litterbag retrieval and before plant harvest.
Soil solution was sampled every 2–5 days, using permanently installed suction cups (Rhizon SMS, Rhizosphere research products, Wageningen, The Netherlands) that consisted of a 5 cm long porous part with an outside diameter of 2.5 mm, a pore size of 0.12–0.18 μm, and a 60 cm PE/PVC tube. Up to 2 ml of soil solution were sampled per pot at a suction of 40 kPa for determination of Si; sample vials contained 40 μl of HNO3 (65 %) to prevent co-precipitation of dissolved Si and Fe oxides and sorption of dissolved Si onto Fe oxide-surfaces (Sauer et al. 2006). Additional samples were taken at the same dates for pH measurement using empty vials.
For continuous measurement of the soil redox-potential, one pot of each treatment was equipped with a redox electrode and a reference electrode (Ag/AgCl) connected to a logger. The reference electrode was in contact with the soil solution via a salt bridge constructed according to Ackermann et al. (2008) to avoid ion transfer to the soil solution.
Analyses
For determination of Si, P, K, Ca, and Mg in plant samples of the field experiment, XRF spectroscopy (S4 PIONEER, Bruker-AXS, Karlsruhe, Germany) was applied. To do so, three grams of ground and dried (85 °C) samples were mixed with 650 mg wax; then 32 mm pellets were prepared by pressing with a force of 12 Mg. The XRF measurements were performed using a wavelength-dispersive XRF spectrometer (S4 PIONEER, Bruker-AXS, Karlsruhe, Germany), equipped with a 4 kW-Rh X-ray tube (75 μm Be window), 60 kV generator, and an eight-position crystal changer. The spectrometer operating conditions were vacuum, 23-mm collimator mask and 0.46° collimator in conjunction with the analyzing crystal PET, and 30 kV at current of 80 mA. Calibration was performed using a plant matrix standard addition method: a dried and ground grass sample was mixed with different amounts of SiO2 (2–14 % with an increment of 2 %), pressed to pellets and measured under above described conditions.
For determination of Si in plant samples of the laboratory experiment (Si-poor straw, Si-rich straw, straw residues from the litterbags, and harvested rice shoots), samples were dried at 65 °C for 48 h, ground, and extracted using a microwave-assisted digestion method (Haysom and Ostatek‐Boczynski 2006). To do so, 100 mg of a sample (two repetitions) were subjected to nitric acid/peroxide oxidation in a low-pressure microwave digestion system (MARS 5 Xpress, CEM, Kamp-Lintfort, Germany). Then, the residue was dissolved in 10 % sodium hydroxide at 180 °C with maximum energy input of 800 W using a microwave-system (MARS 5 Xpress, CEM, Kamp-Lintfort, Germany). The extracts were neutralized and filtered (0.45 μm) before Si was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) (Ultima 2, Horiba Jobin-Yvon, Longjumeau, France). Silicon in soil solutions was also determined by ICP-OES.
We used different methods for determining Si in plant samples of both experiments, because sample masses were too low to allow XRF spectroscopy in the laboratory experiment. XRF spectroscopy requires lower effort (time and chemicals), but more sample material (3 g of dry matter) than alkaline digestion; it was used in the field experiment as well as in a field survey (Klotzbücher et al. 2014, 2015). We tested the comparability of the two methods by analysing 13 plant samples (originating from different studies) with Si concentrations covering the range of our samples. Results were 1.4 ± 0.4 times higher when samples were analysed by XRF spectroscopy.
For determination of P, K, Ca, and Mg in Si-poor straw, Si-rich straw and straw residues from the litterbags, samples were subjected to digestion with HNO3 in a pressure unit; the extracts were analyzed by ICP-OES. Concentrations of C and N were measured using a dry combustion analyzer (Vario EL, Elementar, Hanau, Germany).
Data analysis
Analyses of variance (ANOVA) were performed using SigmaPlot software version 12.0 (Systat Software Inc.). If required, Tukey’s HSD test was performed as post-hoc analysis. Results are given in the tables and figures presenting the analysed data.
Results
Field experiment
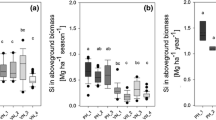
Silicon concentrations in rice straw increased upon the application of Si gel in the field (Fig. 3a). The increase averaged 28 % (addition of 0.4 Mg Si ha−1) and 120 % (addition of 17.3 Mg Si ha−1) compared to the control, respectively. Likewise, Si concentrations in rice hulls increased by 34 % (0.4 Mg Si ha−1) and 72 % (17.3 Mg Si ha−1), respectively (Fig. 3b). Silicon concentration in rice grains was ≤ 0.1 in all treatments (data not shown).
Impact of fertilization with silica gel on Si concentrations a in rice straw and b in rice hulls at harvest stage, and on c straw biomass production and d grain and hull biomass production in the field experiment; error bars represent standard errors; results of one way ANOVA are given in the legends, results of Tukey’s HSD test (P <0.05) are given by small letters
Biomass production of straw was not affected by Si application (Fig. 3c), but grain yield increased by 35 % upon the high Si application rate (17.3 Mg Si ha−1) (Fig. 3d). Total Si uptake by rice shoots (including straw, hulls, and grains) increased by 37 % (application of 0.4 Mg Si ha−1) and 126 % (application of 17.3 Mg Si ha−1) compared to the control (Table 2); the increased uptake corresponds to 10 % (application of 0.4 Mg Si ha−1) and 1 % (application of 17.3 Mg Si ha−1) of the applied Si. Total uptake of P was increased and total uptake of Ca was decreased due to the high Si application rate (17.3 Mg Si ha−1) (Table 2); N, K, and Mg uptake were not affected by Si application (Table 2).
Laboratory pot experiment
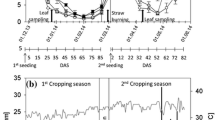
Initial nutrient composition of the straw samples used for incubation is given in Table 3; initial C/N ratios were the same for Si-poor straw and Si-rich straw. During 33 days of incubation, relative C loss was larger for Si-rich straw than for Si-poor straw (Fig. 4a). Also, relative Si loss was larger from Si-rich straw than from Si-poor straw (Fig. 4b), thus absolute Si release increased with Si concentration in the straw. Fauna affected neither relative C loss nor relative Si release from straw.
Redox potential in all pots decreased within the first 6 days to ~ −160 mV (data not shown). Soil solution Si concentration reflected application of silica gel already at the first sampling time (day 1; Fig. 5a). Without plants, Si concentration in soil solution of the silica gel treatment (Silica gel -Plant) increased steadily, while it was constant in the control treatment (Control -Plant). In the treatments with straw (Si-poor straw -Fauna and Si-rich straw -Fauna; Fig. 5b), Si concentration was initially similar to the control treatments (Control -Plant and Control + Plant). After a few days, Si concentration started to increase at a faster rate than in the treatments with silica gel (Silica gel -Plant and Silica gel + Plant). The concentrations levelled off after around 25 days; the plateau was on a higher level for Si-rich straw (Si-rich straw -Fauna) than for Si-poor straw (Si-poor straw -Fauna). In all treatments with plants, Si concentration started to decrease a few days after transplanting; the decreases were faster and ended up at a lower level in the treatments with straw than in the treatment with silica gel.
Discussion
Effect of Si fertilization on Si uptake and growth of rice (Q1)
Increasing Si availability in the soil resulted in increased Si uptake by plants. Hence, Si uptake is limited by Si availability in soil. Straw from the control plots had 5.4 % Si in the dry matter (measured by XRF spectroscopy), thus only little more than the critical value of 5 % proposed by Dobermann and Fairhurst (2000). A lower concentration is thought to indicate Si limitation to rice growth, meaning that the plants’ leaves are soft and droopy, and the plants’ resistance against abiotic and biotic stresses is reduced. ‘Optimal’ Si concentrations of > 8 % (Dobermann and Fairhurst 2000) were only achieved by a very high application rate of silica gel (17.3 Mg ha−1). In this treatment, also biomass production and grain yield increased, which suggests that Si availability is indeed a limiting factor to plant growth in the study region.
Application of Si fertilizers may, thus, be an option for farmers to increase rice yields. We found that the additional Si uptake equalled 10 % of the applied Si in the treatment with the low application rate of silica gel (0.4 Mg Si ha−1) and only 1 % of the applied Si in the treatment with the high application rate of silica gel (17.3 Mg Si ha−1). These data suggest that dissolution of silica gel limited Si uptake by rice in the treatment with the low Si application rate, while for the treatment with the high Si application rate, the availability of Si was likely higher than the plants’ demand. Si concentration in the soil solution of the 17.3 Mg Si ha−1 treatment increased faster and to a greater extent than in the 0.4 Mg Si ha−1 treatment. Therefore leaching of Si might be more important in the treatment with the high Si application rate.
Decomposability of rice straw as a function of straw Si concentration (Q2)
Increased Si availability during rice growth increased the decomposability of the produced straw (i.e., increased the relative loss of organic C during 33 days; Fig. 4a). The same observation was made in similar experiments using litter of the common reed (Phragmites australis) (Schaller and Struyf 2013; Schaller et al. 2014). The reason for an altered decomposability might be changes in the quality of the organic matrix of the straw upon increased Si availability. Raven (1983) proposed that the formation of structural C compounds, such as cellulose, lignin, or polyphenols decreases upon increased Si availability during plant growth. Silicon seems to perform similar functions in plants than structural C compounds, including cell strength and tissue support, defence against insects and diseases, and alleviation of abiotic stresses (Cooke and Leishman 2011). Hence, phytolith formation following Si uptake could be an energetically cheaper alternative to the synthesis of structural C compounds (Raven 1983). In line with this hypothesis, Schoelynck et al. (2010) found negative relationships between lignin and Si concentration for wetland species. Lignin is generally assumed to be among the most degradation-resistant organic litter components; lignin concentrations are frequently negatively related to litter decomposition rates (Aerts 1997; Cornwell et al. 2008). Increasing Si uptake, however, changed also other potential controls on straw decomposition rates such as the concentrations of essential nutrients in straw (Table 3). In addition, altered Si and phytolith concentrations in straw could affect its decomposability. The current opinion, however, suggests that increased phytolith concentration might rather decrease straw decomposition by the microbial community (Schaller et al. 2014), a view that is not supported by our data. In summary, the mechanisms linking changes in Si availability and straw decomposability are currently uncertain. Detailed studies on this issue are necessary, as Si concentration in the straw can affect a number of decomposition controls.
Added fauna affected neither decomposability of straw nor Si release from straw (Fig. 4). An explanation for this finding might be that microbial processes were dominant in straw decomposition in our experiment. Based on field data, we used three decomposer species representing abundant groups of secondary decomposers in rice fields which stimulate the decomposition process by grazing on the microflora on pre-decomposed organic material. Often, their effect is less obvious than the effect of primary decomposers (which ingest litter material directly). Further, even though the soil was dried before the experiment, a number of indigenous mesofauna emerged from the substrate in all treatments (mainly ostracods) which also may have alleviated treatment differences. Other studies demonstrated positive effects of invertebrate grazers on decomposition in aquatic environments but also could not show a mediating effect of Si concentration in litter (Schaller 2013).
Release of Si during straw decomposition (Q3)
We found particularly fast losses of Si in the straw decomposition experiment. The increased Si uptake of rice plants in the treatments with straw addition compared to the control (Table 4) clearly shows that Si released during straw decomposition is plant-available. The amounts of Si taken up by plants were much larger than the decrease of dissolved Si in the soil solution (Table 4); Si concentration in the soil solution of the control without plant remained nearly constant during the whole experimental period, indicating that these systems were in equilibrium. These data suggest that the plant-Si-uptake accelerated the release of dissolved Si by weathering of phytoliths in straw and soil minerals (i.e., phytoliths, primary and secondary silicates); decreased Si concentrations in soil solutions might be the reason for the accelerated weathering.
The temporal changes in Si concentrations in the soil solution show that Si release rate and pattern for rice straw and silica gel differed. In the treatments with silica gel, Si was rapidly released and Si concentration in the soil solution was already increased 1 day after start of the experiment (Fig. 5). Decomposing rice straw started to release Si after 5 days of incubation. We assume that Si concentrations in the soil solution increased only when the organic matrix surrounding the phytoliths was decomposed and the surface of the phytoliths became exposed to soil solution. The finding that Si concentrations in the straw residues were similar to the initial Si concentrations (Fig. 4) suggests high rates of phytolith losses during the first 33 days (as high as the organic C losses). We calculated that, on average, 2–2.5 % of the added phytoliths dissolved per day during the first 33 days of the experiment (pH in the soil solutions varied between 6.6 and 7.0). Assuming constant dissolution rates, it would take around 50 days until the phytoliths added with the straw completely dissolved. Fraysse et al. (2009) found comparably high dissolution rates in a laboratory experiment with phytoliths extracted from horsetail; the dissolution rates at 25 °C were ~0.6 % of phytolith-Si per day at pH 6 and ~3 % of phytolith-Si per day at pH 8.6.
The increases in soil solution Si concentrations upon straw addition mainly occurred during the first 25 days; thereafter the concentrations levelled off (Fig. 5). The temporal changes in Si release into soil solution might be linked to the decomposition rates of the organic matrix, which typically decreases over time, and the decrease is particularly strong in the first days (e.g., Klotzbücher et al. 2011). Another explanation might be that the solubility of phytoliths decreases with aging in soil. Processes causing such decreases in solubility have not been studied but deserve further attention, because they should strongly determine phytolith storage in soils and, more generally, the Si cycle and balance in rice production systems. ‘Stabilization’ of at least some of the phytoliths in soil would be in line with the proposed existence of old phytoliths in soils (Santos et al. 2010; Piperno 2014). This could mean that the dissolution rates measured in short-term laboratory experiments are not fully applicable for explaining the long-term fate of phytoliths in soil. In our experiment, initial Si concentrations in the soil solutions were low, promoting phytolith dissolution. High Si concentrations in the soil solution slowing Si release would probably promote phytolith stabilization in soil.
The Si budgets of the pots revealed large gaps. The sum of the increased Si uptake by the plants relative to the control and the Si dissolved in soil solution equalled only 10 % of the Si added with Si-poor straw and 7 % of the Si added with Si-rich straw, respectively. However, assuming the straw mixed with the soil released Si at the same rate as the straw within the litterbags, 67 % of Si from the Si-poor straw and 82 % of Si from the Si-rich straw were released already during 33 days. One explanation for the gap in the budgets might be adsorption of Si to soil particles. Another explanation might be that Si from the soil solution was consumed by algae growing in the pots. At the end of the experiment, some algae were collected and analysed; Si concentrations of about 6 % were measured. However, it was not possible to completely separate algae from the soil, and thus, to quantify algae mass.
Conclusions
Increasing Si availability in a soil with originally low plant-available Si increases Si uptake by rice plants and plant productivity (total aboveground biomass and grain yield) as well as the decomposability of the produced straw. Increased phytolith formation probably substitutes the formation of hardly-degradable cell wall components, thus, more energy is invested in the production of biomass. Consequently, biomass production increases, whereby also the portion of labile components in the biomass and the decomposability of the straw increase. Our study, thus, adds to increasing awareness that Si cycling plays a significant role in cycling of C and essential nutrients through rice production systems.
The loss of Si during straw decomposition equalled the loss of the organic matrix. This suggests that phytolith dissolution occurs rapidly after exposure of phytoliths to soil solution. Thus, phytoliths from fresh rice straw are strongly soluble and might have high turnover rates. However, decreasing Si release rates from the straw suggest that phytolith solubility decreases over time in soil. This together with the fact that geologically old phytoliths occur in soils might be explained by stabilization of (at least some of the) phytoliths during aging in the soil. However, the mechanisms inducing stabilization are still unknown.
References
Ackermann J, Vetterlein D, Tanneberg H, Neue H-U, Mattusch J, Jahn R (2008) Speciation of arsenic under dynamic conditions. Eng Life Sci 8(6):589–597
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Cooke J, Leishman MR (2011) Silicon concentration and leaf longevity: is silicon a player in the leaf dry mass spectrum? Funct Ecol 25:1181–1188
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
De Datta SK (1981) Principles and practices of rice production. International Rice Research Institute, Los Banos
Dobermann A, Fairhurst T (2000) Rice: nutrient disorders & nutrient management. International Rice Research Institute, Los Banos
Epstein E (1999) Silicon. Annu Rev Plant Biol 50(1):641–664
FAO (2006) Guidelines for soil description. Food and Agriculture Organisation of the United Nations, Rome
Fraysse F, Pokrovsky OS, Schott J, Meunier J-D (2009) Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem Geol 258(3):197–206
Guntzer F, Keller C, Meunier J-D (2012) Benefits of plant silicon for crops: a review. Agron Sustain Dev 32(1):201–213
Haysom MB, Ostatek‐Boczynski ZA (2006) Rapid, wet oxidation procedure for the estimation of silicon in plant tissue. Comm Soil Sci Plant Anal 37(15–20):2299–2306
Hossain KA, Horiuchi T, Miyagawa S (2001) Effects of silicate materials on growth and grain yield of rice plants grown in clay loam and sandy loam soils. J Plant Nutr 24(1):1–13
IRRI (1997) IRRI Rice Facts. International Rice Research Institute, Los Banos
IUSS Working Group (2014) World reference base for soil classification 2014. World soil resources report 106. Food and Agriculture Organisation of the United Nations, Rome
Jones LHP, Handreck KA (1967) Silica in soils, plants, and animals. Adv Agron 19:107–149
Klotzbücher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011) A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92(5):1052–1062
Klotzbücher T, Marxen A, Vetterlein D, Schneiker J, Türke M, Sinh NV, Manh NH, Chien HV, Marquez L, Villareal S, Bustamante JV, Jahn R (2014) Plant-available silicon in paddy soils as a key factor for sustainable rice production in Southeast Asia. Basic Appl Ecol. doi:10.1016/j.baae.2014.08.002
Klotzbücher T, Leuther F, Marxen A, Vetterlein D, Horgan FG, Jahn R (2015) Forms and fluxes of potential plant-available silicon in irrigated lowland rice production (Laguna, the Philippines). Plant Soil. doi:10.1007/s11104-015-2480-y
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier, Amsterdam
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440(7084):688–691
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448(7150):209–212
Parr JF, Sullivan LA (2011) Phytolith occluded carbon and silica variability in wheat cultivars. Plant Soil 342(1–2):165–171
Piperno DR (2014) Phytolyth analysis: an archaeological and geological perspective. Elsevier, Amsterdam
Raven JA (1983) The transport and function of silicon in plants. Biol Rev 58(2):179–207
Santos GM, Alexandre A, Coe HH, Reyerson PE, Southon JR, De Carvalho CN (2010) The phytolith 14C puzzle: a tale of background determinations and accuracy tests. Radiocarbon 52(1):113
Sauer D, Saccone L, Conley DJ, Herrmann L, Sommer M (2006) Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 80(1):89–108
Schaller J (2013) Invertebrate grazers are a crucial factor for grass litter mass loss and nutrient mobilization during aquatic decomposition. Fundam Appl Limnol 183:287–295
Schaller J, Struyf E (2013) Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia 709(1):201–212
Schaller J, Hines J, Brackhage C, Bäucker E, Gessner MO (2014) Silica decouples fungal growth and litter decomposition without changing responses to climate warming and N enrichment. Ecology 95(11):3181–3189
Schmidt A, Auge H, Brandl R, Heong KL, Hotes S, Settele J, Villareal S, Schädler M (2015a) Small-scale variability in the contribution of invertebrates to litter decomposition in tropical rice fields. Basic Appl Ecol. doi:10.1016/j.baae.2015.01.006
Schmidt A, John K, Arida G, Auge H, Brandl R, Horgan FG, Hotes S, Marques L, Radermacher N, Settele J, Wolters V, Schädler (2015b). Effects of residue management on decomposition in irrigated rice fields are not related to changes in the decomposer community. PLoS ONE 10(7):e0134402. doi:10.1371/journal.pone.0134402
Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E (2010) Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytol 186(2):385–391
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2008) Litter quality is in the eye of the beholder: initial decomposition rates as function of inoculum characteristics. Funct Ecol 23(3):627–636
Watanabe T, Luu HN, Nguyen NH, Ito O, Inubushi K (2013) Combined effects of the continual application of composted rice straw and chemical fertilizer on rice yield under a double rice cropping system in the Mekong Delta, Vietnam. JARQ 47(4):397–404
Yamaji N, Ma JF (2009) A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21(9):2878–2883
Acknowledgments
This work has been financed by the LEGATO project of the German Ministry for Education and Research (BMBF). We thank the coordinator of the project, Josef Settele, for his support. We thank Nguyen Hung Manh for the field work and Alexandra Boritzki, Aleksey Prays, Susanne Horka, Andreas Rämmler, Jutta Fröhlich, and Bernd Apelt for technical assistance. We thank the farmers for allowing us to establish the experiment on their paddies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Rights and permissions
About this article
Cite this article
Marxen, A., Klotzbücher, T., Jahn, R. et al. Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 398, 153–163 (2016). https://doi.org/10.1007/s11104-015-2645-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2645-8