Abstract
Foot rot disease is a very destructive disease in black pepper in Malaysia. It is caused by Phytophthora capsici Leonian, which is a soilborne pathogenic protist (phylum, Oomycota) that infects aerial and subterranean structures of many host plants. This pathogen is a polycyclic, such that multiple cycles of infection and inoculum production occur in a single growing season. It is more prevalent in the tropics because of the favourable environmental conditions. The utilization of plant growth-promoting rhizobacteria (PGPR) as a biological control agent has been successfully implemented in controlling many plant pathogens. Many studies on the exploration of beneficial organisms have been carried out such as Pseudomonas fluorescens, which is one of the best examples used for the control of Fusarium wilt in tomato. Similarly, P. fluorescens is found to be an effective biocontrol agent against the foot rot disease in black pepper. Nowadays there is tremendous novel increase in the species of Burkholderia with either mutualistic or antagonistic interactions in the environment. Burkholderia sp. is an indigenous PGPR capable of producing a large number of commercially important hydrolytic enzymes and bioactive substances that promote plant growth and health; are eco-friendly, biodegradable and specific in their actions; and have a broad spectrum of antimicrobial activity in keeping down the population of phytopathogens, thus playing a great role in promoting sustainable agriculture today. Hence, in this book chapter, the potential applications of Burkholderia sp. to control foot rot disease of black pepper in Malaysia, their control mechanisms, plant growth promotion, commercial potentials and the future prospects as indigenous PGPR were discussed in relation to sustainable agriculture.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Black pepper (Piper nigrum L.) known as “the king of spices” is a historic, traditional spice and one of the most important agricultural produce in Malaysia (Anon 2003). The ~80% of pepper is processed as black pepper, and the remaining ~20% is processed as white pepper. Despite the substantial contribution made by this crop to the socio-economy of Malaysia and other parts of the world, production potentials of the crop are on the trend of decline due to the activities of pests and diseases. For example, a disease known as foot rot caused by Phytophthora capsici Leonian is a major obstacle in black pepper production in Malaysia and worldwide. The pathogen was first isolated and identified under P. palmivora (Holiday and Mowat 1963) in Malaysia, but later further investigations by Kuch and Khuthubutheen (1985) identified the pathogen as P. capsici Leonian (Fig. 12.1a–d). Foot rot is considered as the most serious disease of black pepper causing yield reduction that ranges from ~20 to 80 and up to 95% for individual farmers (Manohara et al. 2004). The fungus is a soilborne pathogen that causes infections on roots, leaves and fruits of black pepper (Fig. 12.2a–c) and other crops (Fig. 12.3a–c) in most of the tropical countries. Efforts have been made to check these incessant problems caused by this fungus. Today, the primary means of controlling the disease is through synthetic fungicides applications which have been effective but found to be associated with some drawbacks. Among the drawbacks are their high cost, carcinogenicity, teratogenicity, high and acute residual toxicity, long degradation period, environmental pollution and possible side-effects on human health through the food (Wang et al. 2011; Meena et al. 2013a, 2016a; Bahadur et al. 2014; Maurya et al. 2014; Jat et al. 2015; Kumar et al. 2015, 2016b; Ahmad et al. 2016; Parewa et al. 2014).
These drawbacks coupled with public concern have increased interest in developing further alternative control methods, particularly those that are eco-friendly, biodegradable, feasible to the farmers, non-toxic to human and animals, specific in their actions and have a broad spectrum of antimicrobial activity (Abhishek et al. 2013). Thus, indigenous plant growth-promoting rhizobacteria (PGPR) have been found to play a major role in keeping down the population of pathogen to a low level and can therefore be used as an alternative to synthetic chemicals. Some PGPR, such as Burkholderia sp., Pseudomonas sp. and Bacillus sp., have been found to perform these functions by inducing systemic resistance in plants and showing biological traits like antibiosis and lysis (Eberl and Vandamme 2016; Rahamat Bivi et al. 2010; Prakash and Verma 2016; Priyadharsini and Muthukumar 2016; Kumar et al. 2017; Meena et al. 2015a, 2016b; Jaiswal et al. 2016, 2016a; Jha and Subramanian 2016).
The soil system is a natural body called “pedosphere” that served as a habitat for a quantum of endophytic and rhizospheric microorganisms which in turn modify the complex matrices of the soil especially in the root zone. Recent studies on microbial plant-related interactions revealed that bacterial communities called PGPR belonging to the genus Burkholderia are associated with the development of plants and are responsible for a range of physiological activities. In addition to their beneficial features as promoters of plant growth, they also protect plants against pests and pathogens (biocontrol agents) and increase plant fitness by nitrogen fixation, production of phytohormones and antimicrobial substances and induction of systemic resistance (Eberl and Vandamme 2016; Lodewyckx et al. 2002). Additionally, they are involved directly in the plant growth through biofertilization, stimulation of root growth, control of plant stress through host adaptation to environmental stress, sequestration of iron, phosphate solubilization (Raghavendra et al. 2016; Zahedi 2016; Meena et al. 2015b, 2015f, 2016c; Rawat et al. 2016; Yasin et al. 2016; Saha et al. 2016a; Dominguez-Nunez et al. 2016; Dotaniya et al. 2016), ACC deaminase activities and quinolinate phosphoribosyltransferase activity (Barrett and Parker 2006; Janssen 2006; Balandreau and Mavingui 2007; Compant et al. 2008) without conferring pathogenicity (Lugtenberg and Kamilova 2009; Compant et al. 2010; Eberl and Vandamme 2016).
These efficient bacteria are found in the “rhizosphere” which is defined as any volume of soil specifically influenced by plant roots and/or in association with root hairs and plant-produced materials (Dessaux et al. 2009; Silveira et al. 2012; Ahemad and Kibret 2014). The rhizosphere has been identified to consist of three major separates but interacting components that include rhizosphere (soil), the rhizoplane and the root itself. The rhizosphere is the soil zone influenced by roots through the release of substrates that affect microbial activity. The rhizoplane is the root surface that consists of strongly adhering soil particles, and the root itself is a component of the system, where the tissues are colonize by many microorganisms (like endophytes) (Barea et al. 2005; Ahemad and Kibret 2014).
Colonization of the rhizoplane and rhizosphere differs from one another (Kloepper et al. 1991), in that microbial colonization of rhizoplane is termed as root colonization, whereas rhizosphere colonization is microbial colonization of the adjacent volume of soil under the influence of the root (Kloepper 1994; Barea et al. 2005). Hiltner (1904) discovered that the rhizosphere is much richer in bacteria than the surrounding bulk soils, with composition of 10–1000 times higher than that in bulk soil.
The bacteria covered a small part of the root surface (Rovira 1956), and the most popular sites for bacterial growth are junctions between epidermal cells and areas where side roots appear. The effect of rhizosphere is caused by substantial amount of the carbon fixed by the plant, ~5–21% (Marschner 1995), which is secreted mainly as root exudates. In addition to facilitating water and nutrient uptake and providing mechanical support to the plants, a diverse array of compounds is synthesized, accumulated and secreted by plant roots (Walker et al. 2003).
The compounds secreted by the roots are generally referred to as root exudates. They act as attractants on diverse number of active microorganisms in the soil. In addition, they change the physical features and chemical compositions of the soil, therefore, restructuring the microorganisms in the area of the root (Eberl and Vandamme 2016; Dakora and Phillips 2002). They also repel microorganisms, promote symbiosis and control the growth of other unwanted plant species (Nardi et al. 2000). Kang et al. (2010) reported that the compositions of these exudates depend on the species of plants, their physiological status and microorganisms present.
PGPR are species of bacteria collectively found growing around plant tissues in the rhizosphere that enhanced the growth of plant by a number of mechanisms (Vessey 2003; Lemaire et al. 2015). They are distinctively characterized by some inherent features that include the following: they must (i) colonize the surface of the root effectively; (ii) promote plant growth; (iii) be able to survive and multiply, at least for sometimes to exert their protection and growth-promoting activities; and (iv) be able to compete well with other rhizosphere microbes for nutrients secreted by the root and for sites that can be occupied on the root (Kloepper 1994; Lugtenberg and Kamilova 2009). Certain species in this extremely versatile group are capable of causing disease in humans and plants (Eberl and Vandamme 2016), while others are very effective as biological control agents, bioremediation and promotion of plant growth (Perin et al. 2006).
Nowadays, rigorous research are carried out globally with greater aim to explore a vast number of PGPR having novel characteristics that could serve as biocontrol agents (Eberl and Vandamme 2016; Hynes et al. 2008; Joo et al. 2005; Russo et al. 2008) alongside with normal growth promotion characteristics like biofertilization (Tank and Saraf 2010; Ahemad and Khan 2012b), ACC deaminase (1-aminocyclopropane-1-carboxylate), production of ammonia and nitrogenase activities (Khan 2005; Glick 2012), siderophore (Tian et al. 2009; Jahanian et al. 2012), solubilization of phosphate and potentials in heavy metal detoxification (Ma et al. 2011; Ahemad and Khan 2012b), salinity tolerance (Tank and Saraf 2010; Mayak et al. 2004) and pesticide degradation (Ahemad and Khan 2012a). Typical examples of rhizobacteria that showed marvellous plant growth beneficial traits and potential as biological control agents against various root pathogenic microbes that are today used globally as bioinoculants in promoting growth and development of plant under different stresses such as heavy metals (Wani and Khan 2010), herbicides (Ahemad and Khan 2010, 2011a), insecticides and fungicides (Ahemad and Khan 2011b, 2011c, 2012c) and salinity (Mayak et al. 2004) include Agrobacterium sp., Arthrobacter sp., Azotobacter sp., Azospirillum sp., Azomonas sp., Bacillus sp., Caulobacter sp., Chromobacterium sp., Erwinia sp., Flavobacterium sp., Micrococcus sp., Pseudomonas sp., Serratia sp., Allorhizobium sp., Azorhizobium sp., Bradyrhizobium sp., Mesorhizobium sp., Rhizobium sp., Micromonospora sp., Streptomyces sp., Streptosporangium sp., Thermobifida sp., Klebsiella sp. and Burkholderia sp.
The name of the genus Burkholderia was derived from “Walter H. Burkholder” who described Phytomonas caryophylli (Burkholder 1942) as the first Burkholderia sp. which was later known as Pseudomonas caryophylli. Burkholder (1950) again described another species named “cepacia” named after onion, which was later called Pseudomonas cepacia. Species of Burkholderia were included for years in the genus of Pseudomonas, but with the advent of molecular rRNA-DNA hybridization analysis, considerable diversity in the genotype was noticed between the genus members (Compant et al. 2008), and as a result they were grouped into five rRNA groups (Palleroni et al. 1973). Later, genomic analysis had shown that five groups are related to one another. Recently, considerable numbers of species are included in the genus of Burkholderia (Coenye and Vandamme 2003) known as Burkholderia cepacia and representing complex of closely related genotypic species as confirmed by numerous taxonomic studies (Coenye et al. 2001; Vandamme et al. 2003; Vermis et al. 2004; Eberl and Vandamme 2016). The group is called as the Burkholderia cepacia complex and recently consists of a total of nine species that include Burkholderia cepacia (genomovar I), Burkholderia multivorans (genomovar II), Burkholderia cenocepacia (genomovar III), Burkholderia stabilis (genomovar IV), Burkholderia vietnamiensis (genomovar V), Burkholderia dolosa (genomovar VI), Burkholderia ambifaria (genomovar VII), Burkholderia anthina (genomovar VIII) and Burkholderia pyrrocinia (genomovar IX). The first discovery of B. cepacia by W.H. Burkholder had today led to the identification of many other species of Burkholderia.
Currently, the genus Burkholderia includes more than 50 species that are found in various ecological niches, rather than in bulk soil (Coenye and Vandamme 2003; Luvizotto et al. 2010), most of which interact with plants in different ways resulting in beneficial effects to the intimate associating hosts. Finally, the potentials of PGPR should not be overemphasized as their application under both normal and stressed conditions has increased the health and productivity of different plant species and decreased global huge reliance on synthetic chemical pesticides that pollute the ecosystem (Yadav and Sidhu 2016; Saha et al. 2016b; Verma et al. 2014, 2015b; Masood and Bano 2016;Teotia et al. 2016; Meena et al. 2015e, 2016d, 2016e; Bahadur et al. 2016b; Das and Pradhan 2016. Therefore, in this book chapter, the potential application of indigenous PGPR (Burkholderia sp.) to control foot rot disease of black pepper in Malaysia, their control mechanism and plant growth promotion, the commercial potential application and the future prospects for sustainable agriculture were discussed.
2 General Mechanisms of Action for PGPR as a Biological Control Agent
These PGPR generally mediated plant growth promotions in rhizosphere as biocontrol agents by reducing the inhibitory effects of various pathogenic microbes on plant growth and development (Glick 2012), and their utilization to control diseases as biocontrol agents is an eco-friendly approach (Lugtenberg and Kamilova 2009). The following are the mechanisms that can be distinguished in PGPR as a biocontrol agent.
2.1 Competition for Nutrients
The first step in pathogenesis of soilborne microbes is the colonization of rhizosphere and rhizoplane (Lugtenberg et al. 2001; Compant et al. 2010; Eberl and Vandamme 2016). As it is widely believed that root colonization is an important aspect of biocontrol, therefore, PGPR have to be highly competitive to successfully colonize the narrow root zone of the plant to be protected and also be able to exhaust the available nutrients against other microorganisms (Lugtenberg and Kamilova 2009; Compant et al. 2010; Shehata et al. 2016). The roots produce what is known as root exudates which consist of food nutrients that are essentially required by rhizosphere microbes that include sugars, amino acids, organic acids and numerous compounds including enzymes, sterols, vitamins, fatty acids, putrescine, nucleotides, osmoprotectants and signal molecules. In general, PGPR acted by displacing and suppressing the growth and development of pathogens through competition for the nutrients, space and essential elements ( Sharma et al. 2016; Verma et al. 2015a; Meena et al. 2013b, 2013c, 2014a, 2015d; Shrivastava et al. 2016;Singh et al. 2015; Bahadur et al. 2016a).
As a mechanism, some of the PGPR secreted siderophores and lytic enzymes that deter the growth of the phytopathogens present in the rhizosphere and rhizoplane. However, some secreted antibiotics that offer them a better chance for rhizosphere and rhizoplane colonization (van Loon and Bakker 2006; Shehata et al. 2016) and typical examples of the antibiotics secreted include 2,4-diacetylphloroglucinol (DAPG), rhamnolipids, hydrogen cyanide, zwittermicin A, oligomycin A, oomycin A, phenazine, pyoluteorin, pyrrolnitrin, thiotropocin, tropolone, cyclic lipopeptides, kanosamine and xanthobaccin, as well as many others (Takeshita et al. 2015; Nielsen et al. 2002; Raaijmakers et al. 2002; de Souza et al. 2003; Compant et al. 2010). Fan et al. (2011) reported that successful colonization of seedlings root was achieved via root dipping in the suspension of Bacillus strain (FZB42) before transplanting. The biocontrol ability of Bacillus can be understood by the reports of Chen et al. (2009) and Malfanova et al. (2011) that Bacillus produces cyclic lipopeptides (cLPs) that are involved in the biological control through ISR (Ongena et al. 2007), in a mechanism that requires rhizosphere colonization only (Dekkers et al. 2000).
2.2 Signal Interference
Signal interference is a biocontrol mechanism employed by some PGPR to break the sensing ability of some virulent and/or pathogenic microbes. This is specifically seen in bacteria toward their ability to sense the production level of exoenzymes (cell wall-degrading enzymes) regulated by quorum sensing (QS) molecules such as homoserinelactones (AHLs) (Lugtenberg et al. 2013; Bassler 1999). Inactivation of the molecule called homoserinelactones (AHLs) needed for the production of exoenzyme is one way of controlling the activities of pathogens that can be achieved through signalling interference mechanism (Dong et al. 2004). Lactone ring-hydrolyzing enzymes, AHL lactonases, and the amide linkage-breaking enzymes, AHL acylases, are the two main types of AHL-inactivating enzymes that have been identified (Uroz et al. 2009; Lugtenberg et al. 2013).
Typical example of signal interference mechanism is the production of AHL lactonases by B. thuringiensis strains which hydrolyse the lactone ring and/or AHL acylases that break the amide link in the pathosystem (Lugtenberg et al. 2013). Volatile organic compounds (VOCs) produced by rhizospheric strains P. fluorescens B-4117 and S. plymuthica IC1270 have been demonstrated to be involved in the suppression of crown gall disease in tomato plants caused by Agrobacterium (Dandurishvili et al. 2011). Also VOCs produced these strains, which are capable of causing a noticeable decrease in the transcription of phzI and csaI genes capable of AHL synthesis (Chernin et al. 2011; Velazquez et al. 2016; Sindhu et al. 2016; Meena et al. 2014b, 2015c; Singh et al. 2016).
2.3 Induced Systemic Resistance (ISR)/Systemic Acquired Resistance (SAR)
The phenomenon induced systemic resistance (ISR) is an activated response immunity by plant that is mediated by some rhizobacteria living on or interacting with roots of host plants (Pierterse et al. 2009, 2014), mediated by the signalling pathway of jasmonic acid (JA), salicylic acid (SA) and ethylene (ET) (Van Wees et al. 2000; Pierterse et al. 2014) within the plant resulting in the host plant’s defence responses against a number of bacteria, fungi, viruses, nematodes and insects (Beneduzi et al. 2012; Glick 2012). Labuschagne et al. (2010) showed that PGPR elicited the ISR in the host plants by increasing the mechanical and physical strength of the cell wall of the host plant as well as changing the physiological and biochemical reactions of the host plant. The successes of ISR rely on the plant species or cultivar (van Loon and Bakker 2006) and require only rhizosphere colonization as a competitive mechanism (Dekkers et al. 2000; Lugtenberg et al. 2013).
It is important to note that ISR is not associated with the activation of pathogenesis-related proteins (PRs) as was the case in systemic acquired resistance (SAR). Various individual bacterial-derived compounds were reported to induce ISR, such as bacterial molecules like lipopolysaccharides and salicylic acid; organelles such as flagella; metabolites like siderophores, cyclic lipopeptides and biosurfactants; volatiles such as 2,3-butanediol and acetoin; phenolic compounds; antibiotics; and the signal molecule or quorum sensing molecules (Ahemad and Kibret 2014; Lugtenberg et al. 2013; Beneduzi et al. 2012; Perez-Garcıa et al. 2011).
The term SAR describes a salicylic acid-defendant induced resistance caused by a localized infection (Vleesschauwer and Hofte 2009). Ryals et al. (1996) defined SAR as a defence mechanism activated in the plant following the primary infection by pathogens, mediated through the accumulation of salicylic acid signalling pathway (Beneduzi et al. 2012). The plant resists further attacks after the first infection that predisposes the host to subsequent attacks. The defence capacity is linked with the accumulation of PRs. This, therefore, suggested the relevance of these PRs in their contribution to increased defence ability of the infected or induced tissue (Beneduzi et al. 2012). The most important feature of SAR is the activation of SAR genes especially those encoding the PRs that are usually taken as molecular markers for the state of induced resistance attained (Vleesschauwer and Hofte 2009; Mandal and Ray 2011; Shaikh et al. 2016).
Typical examples of PRs that served as hallmarks in several plant species and which have also shown to contribute in the inducement of resistance are 1,3-glucanases and chitinases that are effective in hydrolyzing fungal cell walls. Pieterse et al. (1996) reported that in an experiment conducted on Arabidopsis plants inoculated with Pseudomonas syringae pv. tomato and/or sprayed with salicylic acid, it developed PRs (PR-1, -2, and -5 mRNAs), and with this conclusion, it could be made that PRs are dominantly associated with induction of SAR (Beneduzi et al. 2012; Meena et al. 2017). Both ISR and SAR can act together in conferring resistance to host against pathogens and exert a protection better than each system alone (Van Wees et al. 2000). Salicylic acid transduction signal needs the activator (regulatory) protein NPR1 which works in the terminal signalling pathway of the SAR, and NPR1 takes part in the defence responses mediated by various signalling ways that act beyond the expression of pathogenesis-related genes, showing ISR and SAR meet at the end of the signalling pathway (Van Loon et al. 1998; Beneduzi et al. 2012). The transduction signal pathways leading to ISR (rhizobacteria) and pathogen-induced SAR in Arabidopsis thaliana are shown below (Fig. 12.4).
Transduction signal pathways leading to rhizobacteria-mediated induced systemic resistance (ISR) and pathogen-induced systemic acquired resistance (SAR) in A. thaliana (Source: Van Loon et al. 1998)
2.4 Siderophores Production to Compete for Ferric Ions
The term siderophores is a ferric ion (Fe3+)-chelating compound produced by many rhizobacteria in an attempt to overcome the conditions under Fe3+ limitations (Lugtenberg et al. 2013). Virtually, all living organisms essentially need Fe3+ for a variety of functions such as synthesis of ATP, formation of heme, reduction of ribotide precursors of DNA and for growth (Saraf et al. 2011; Lugtenberg et al. 2013; Sermwan et al. 2015). The need for iron to support the growth of organism became a challenge to the organisms in a situation of shortage supply. Therefore, survival of the fittest became the rule to survive. As a mechanism of biocontrol agents, siderophore-Fe3+ complex is formed by continuous binding to Fe3+ limitation receptors, and the Fe3+ ion is subsequently conveyed into the cell of bacterial where it becomes active as Fe2+. Those bacteria that secrete siderophores effectively good enough to bind Fe3+ to a level that fungal pathogens can no longer grow anymore under iron limitation can act as biological control agents (Leong 1986). Pyoverdine is a good example of a siderophore (Lugtenberg et al. 2013), and examples of bacteria that produced siderophore include P. fluorescens strains, Bacillus, Alcaligenes, Bradyrhizobium, Rhizobium and Enterobacter (Shaikh et al. 2014; Shaikh and Sayyed 2015). Burkholderia cepacia was reported to produce siderophore called deferoxamine mesylate salt equivalent. A concentration of 0.64 μg mL−1 is sufficient to inhibit 91.1 ± 0.5% of phytopathogen growth on mango (Santos Villalobos et al. 2012). In short, increased concentration siderophore production by the PGPR bacteria could trigger inhibition of phytopathogens due to the starvation of iron.
2.5 Antibiosis
The term antibiosis is an antagonistic association between organisms and is the productions of metabolic substances by one organism which is detrimental to the other. In addition to siderophore production, majority of rhizosphere bacteria produced metabolites with antifungal properties which are known in controlling fungal diseases (Shehata et al. 2016; Opelt et al. 2007). These AFMs are also known as antibiotics which are compounds that deter the metabolic processes or growth of other microorganisms (Beneduzi et al. 2012; Duffy et al. 2003). Generally, PGPR produced one or more antibiotic as a mechanism which gave them ability to play the role of antagonism against pathogens (Beneduzi et al. 2012; Glick et al. 2007). Better understanding of the phenomenon of antibiosis as the activity of biocontrol has come to the domain of its peak in the last two decades (Lugtenberg and Kamilova 2009).
The possible mechanisms of action for most of these compounds are discussed by Haas and Defago (2005). Majority of the antibiotics have been isolated and studied, and a great diversity has been observed in their mechanisms to prevent synthesis of pathogen cell walls and inhibit the formation of initiated complexes on the small subunit of the ribosome (Maksimov et al. 2011). The antibiotics best known to involve in biological control by PGPR include bacillomycin D, phenazines, pyocyanin, pyrroles, pyoluteorin, pyrrolnitrin, volatile hydrogen cyanide (HCN), oomycin A, iturins, fengycins, surfactin, mupirocin, bacillomycin, zwittermicin, 2-hexyl-5-propyl resorcinol (Sindhu et al. 2009; Akhtar and Siddiqui 2010; Ahanger et al. 2014; Mabood et al. 2014; Shaikh and Sayyed 2015), volatiles 2,3-butanediol (Ryu et al. 2003), d-gluconic acid (Kaur et al. 2006), 2-hexyl-5-propyl resorcinol (Cazorla et al. 2006), 6-pentyl-α-pyrone (Lorito et al. 2010) and polymyxin, circulin and colistin (Maksimov et al. 2011). Some researchers have proved this through analysis (mutational) followed by studies like complementation studies (Lugtenberg et al. 2013). Majority of these antibiotics were produced by the group of bacteria known as Bacillus sp. These antibiotics are found to be effective against phytopathogenic fungi Aspergillus flavus, Fusarium oxysporum, Alternaria solani, Botryosphaeria ribis, Phomopsis gossypii, Helminthosporium maydis, Colletotrichum gloeosporioides, etc. (Maksimov et al. 2011).
Nowadays, a detailed investigation has been carried out on the class of antibiotics secreted by numerous species of bacteria, including Bacillus known as cyclic lipopeptides (cLPs). The cLPs consist of three major families, namely, the iturins, surfactins and the fengycins. Their mechanisms of beneficial action depend on direct antibiosis of phytopathogens (Borriss 2011; Perez-Garcıa et al. 2011). Several reports have been presented as evidences for the involvement of cLPs in biocontrol activity as exemplified by the fengycins’ activity in biological control of B. cinerea on apple which was traced in the infected parts of apple at some level of concentrations (Toure et al. 2004). Zeriouh et al. (2011) recently proved the involvement of iturins in the control of Xanthomonas campestris and Pectobacterium carotovorum. Similarly, Yanez-Mendizabal et al. (2012) observed and reported the involvement of fengycins in the inhibition of peach brown rot disease with mutational analysis. Henry et al. (2011) also enumerated that fengycins combined with surfactins affect defence pathways in tomato and bean. Furthermore, cLPs are involved in biofilm formation, cell differentiation and cannibalism (Lopez et al. 2009).
2.6 Bacteriocins Production
Bacteriocins are proteinaceous toxins produced by some bacteria to inhibit the growth of similar or closely related bacterial strains which were first discovered and called colicine in 1925 by A. Gratia because it killed Escherichia coli (Gratia 2000). Bacteriocins are narrow in their action and toxic mostly to bacteria related to the producing species, and this is the main difference between bacteriocins and antibiotics (Riley and Wertz 2002). Typical examples of bacteriocins secreted by some bacteria especially gram negative that are lethal to related strains include cloacins derived from Enterobacter cloacae, pyocins from P. pyogenes, colicin from E. coli, megacins from B. megaterium and marcescens from Serratia marcescens (Beneduzi et al. 2012; Cascales et al. 2007). Abriouel et al. (2011) reported that bacteriocins from Bacillus sp. have a broad spectrum against gram-positive species, gram-negative bacteria and fungi or yeast.
2.7 Interference with the Activity in Survival, Multiplication, Germination, Sporulation and Spread of the Pathogen
Many bacterial strains have been harnessed and used as biocontrol agents to interfere with growth of some soilborne fungal pathogens. Majority of these strains are from fluorescent pseudomonads including P. fluorescens, P. putida, P. aeruginosa and P. aureofaciens that suppressed soilborne pathogens through antibiosis, rhizosphere competition and iron chelation by siderophores production (Jianbin et al. 2010). Pseudomonas strains, P. fluorescens WCS365 and P. putida PCL1760, have been reported to suppress tomato foot and root rot (TFRR) in stone wool, and their characteristics are well known and documented (Kamilova et al. 2006; Validov et al. 2009). Studies on the control of tomato Fusarium root rot disease with the biological control agent P. fluorescens strain WCS365 have indicated a positive result through a series of activities that interfere with the cyclic events in the growth of the pathogen including germination, sporulation, multiplication, survival and spread of the pathogen (Lugtenberg et al. 2013).
In the process of biocontrol, the hyphae of the fungus secreted fusaric acid (FA) which is believed to attract the cells of the strain P. fluorescens WCS365 with subsequent extensive colonization of hyphae, leading to the formation of biofilms or microcolonies (Lugtenberg et al. 2013; de Weert et al. 2004). Colonization of hyphae and subsequent formation of biofilms make the fungus ineffective and inhibit its growth, reproduction and survival. In a situation where there is nutrient scarcity (nutrient deprivation), biocontrol strain P. fluorescens WCS365 used the hyphae as a food source through hyphal colonization with subsequent spore germination inhibition (Kamilova et al. 2008). This conclusively showed that in the presence of P. fluorescens WCS365, spore formation will be reduced, and, therefore, this will also reduce pathogen spread, thus, serving as a biocontrol agent (Kamilova et al. 2008; Validov et al. 2009).
2.8 Cell Lysis and Degradation
Most of the PGPR produce enzymes such as chitinases, cellulases, glucanases and proteases that hydrolyse polymeric compounds like chitin, cellulose, proteins, hemicellulose and DNA. This will help in the inhibition of phytopathogens (Shaikh et al. 2016). Mabood et al. (2014) reported that these enzymes are known to cause degradation and lysis of cell walls which help in the control of phytopathogens. For example, chitinases and β-1,3-glucanase-producing PGPR such as B. subtilis BSK17, B. suly, Paenibacillus illinoisensis, P. illinoisensis KJA-424, Pseudomonas sp., Enterobacter ammrenus, Pantoea dispersa and Pythium ultimum are reported to demonstrate some potentials in biocontrol activity (Shaikh et al. 2016). Dubbey et al. (2014) reported that chitinases and β-1,3-glucanase are produced by B. subtilis BSK17 that assist in their root zone competition and antagonistic activity. Similarly, severity of Fusarium infections produced under greenhouse conditions is reduced through chitinase production by B. suly (Hariprasad et al. 2011). Biocontrol activity by Paenibacillus illinoisensis has also been demonstrated against Phytophthora capsici causing blight in pepper by the secretion of chitinase (Jung et al. 2005).
3 Biological Control Mechanisms in Burkholderia sp. Against Phytopathogens
Burkholderia species are considered beneficial in the ecosystem in that they can be used for biological control of diseases caused by fungi in plants, plant growth promotion and bioremediation (Perin et al. 2006; Compant et al. 2008). Several Burkholderia species have shown the ability to use different mechanisms such as competition and secretion of allelochemicals, including antibiotics and siderophores known with antimicrobial activity, competition for nutrients, induced systemic resistance (ISR), antagonism as well as hyphal colonization. All these are good features of potential biocontrol agents against phytopathogenic fungi (Baldani et al. 2000; Welbaum et al. 2004; Compant et al. 2005b; Kang et al. 1998; Hu and Young 1998). The efficacy of these Burkholderia species as biocontrol agents has been shown by B. cepacia, B. ambifaria, B. pyrrocinia, B. cenocepacia, B. vietnamiensis and B. phytofirmans strains against Fusarium sp., P. capsici, Pythium ultimum, P. aphanidermatum, B. cinerea and R. solani (Compant et al. 2008; Ait Barka et al. 2002; Cain et al. 2000; Parke and Gurian-Sherman 2001; Singh et al. 2006). Several reports have proved these potentials as exemplified by the report of Cuong et al. (2011) by the colonization activity of hyphae-colonizing Burkholderia sp. against R. solani causing sheath blight in rice. Some traits of Burkholderia sp. strains have been shown to encompass antifungal genes which enable members of the group to produce a wide range of secondary metabolites active against R. solani. Examples of the metabolites are pyrrolnitrin, phenazine, cepaciamide A (Cartwright et al. 1995; Rosales et al. 1995; El-Banna and Winklemann 1998; Jiao et al. 1996; Mao et al. 2006) and some unknown compounds (Mao et al. 2006).
Bevivino et al. (1994) reported that Burkholderia sp. produced very efficient low-molecular-weight iron-chelating compounds known as siderophores which are shown to be involved in antibiosis mechanism against plant pathogens through iron competition under iron-limiting conditions. Ornibactins, cepaciacheline and cepabactine are the predominant siderophores produced by Burkholderia strains (Meyer et al. 1995; De Meyer et al. 2015). Recently, it has been reported that 1-aminocyclopropane-1-carboxylate (ACC) deaminase-containing endophyte belonging to Burkholderia sp. exhibited antagonistic activity against R. solani and Sclerotinia sclerotiorum (Pandey et al. 2005).
4 PGPR as a Plant Growth Promoter
Generally, plant growth-promoting mechanisms exhibited by PGPR were categorized into two main groups, i.e. direct and indirect mechanisms. In the past, more emphasis has been laid on direct interaction rather than indirect interaction. Direct mechanism may involve nitrogen fixation, phosphate solubilization ability, siderophore production and production of plant growth regulators. On the other hand, indirect mechanisms may include suppression of phytopathogens and enhancement of mutualisms between host plants and other symbionts (Kloepper et al. 1989).
4.1 Nitrogen Fixation
Nitrogen-fixing microbes are generally categorized into two main groups (a) symbiotic N2-fixing bacteria and (b) non-symbiotic bacteria. Diazotrophs are a PGPR that fix N2 in nonleguminous plants (Glick et al. 1999). Basically, biological nitrogen fixation (BNF) is restricted to prokaryotic organisms. Currently, hundreds of bacterial species were identified, covering most of the different biotrophic energy systems such as photosynthetic bacteria (e.g. Rhodospirillum rubrum), anaerobic bacteria (e.g. Clostridium sp.), microaerobic (Burkholderia sp.) and aerobic bacteria (e.g. Azotobacter). Biological nitrogen fixation usually takes place at mild temperatures (Raymond et al. 2004), so that the fixation process can occur everywhere on the earth (Table 12.1). The genus Burkholderia was documented as one of the richest N2-fixing bacteria. Among them B. vietnamiensis was the first known N2-fixing species of this genus and was isolated from the rhizosphere of rice plants in Vietnam. This bacterium has attracted interest of many researchers because of its abilities to fix N2, promote rice plant growth and enhance grain yield.
4.2 Phosphate Solubilization
The search for an ecologically safe and economically reasonable option for improving crop production in low-phosphorus soils becomes the ultimate outcome in soil fertility research. In this context, phosphate-solubilizing bacteria (PSB) are considered as promising biofertilizers since they can supply plants with phosphate from sources otherwise poorly available by various mechanisms (Zaidi et al. 2009). Excellence examples of phosphate-solubilizing bacteria are Azotobacter, Bacillus, Beijerinckia, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Microbacterium, Pseudomonas, Rhizobium and Serratia (Bhattacharyya and Jha 2012). These bacteria were reported to solubilize inorganic phosphorus through synthesization of the low-molecular-weight organic acids in the soil (Zaidi et al. 2009). The mineralization of organic phosphorus occurs through the synthesis of a variety of different phosphatases, catalysing the hydrolysis of phosphoric esters (Glick 2012). Most importantly, both phosphate solubilization and mineralization can coexist in the same bacterial strain (Tao et al. 2008).
4.3 Phytohormone Production
Microbial synthesis of the phytohormone, namely, auxin (indole-3-acetic acid/indole acetic acid/IAA), was reported a long time ago. Apart from IAA, PGPR are also capable of synthesizing other plant hormones, such as gibberellins (GAs) and cytokinins (CKs) or affecting plant hormone biosynthesis (homeostasis) in planta (Kurepin et al. 2014). IAA plays crucial role in bacteria-host interactions (Spaepen and Vanderleyden 2011). It is well known that IAA affects plant physiological processes such as cell division, extension and differentiation; stimulates seed and tuber germination; increases the rate of xylem and root development; controls processes of vegetative growth; initiates lateral and adventitious root formation; mediates responses to light, gravity and florescence; and affects photosynthesis, pigment formation, biosynthesis of various metabolites and resistance to stressful conditions. Burkholderia phytofirmans strain PsJN was reported capable of inducing biomass growth of several crops including potato. This report showed massive root growth increases after inoculation, and this was associated with a twofold to threefold increase in IAA and CK (trans-zeatin or tZ) levels (Kurepin et al. 2015).
4.4 Harmonizing Ethylene Production
Many studies show ethylene gas is a crucial growth regulator of numerous aspects of plant development and physiology (Merchante et al. 2013) such as germination, seedling growth and morphology, fruit ripening, organ senescence and stress/defence response (Khalid et al. 2006; Broekgaarden et al. 2015). However, under usual condition the ethylene gas production is always in low concentration. This is due to the biosynthesis of this compound which depends on transcriptional and post-translational mechanisms that regulate the activity levels of the biosynthetic enzymes (Booker and DeLong, 2015). On the other hand, if ethylene present is in high concentration, it may inhibit physiological activities in plant-like root elongation. In this case, PGPR are needed in harmonizing the level of ethylene in plant by converting 1-aminocyclopropane-1-carboxylate (ACC) into ammonia and α-ketobutyrate (Nascimento et al. 2014). Currently, Achromobacter, Agrobacterium, Alcaligenes, Acinetobacter, Azospirillum, Bacillus, Burkholderia, Enterobacter, Pseudomonas, Ralstonia, Serratia and Rhizobium were reported to have ability to harmonize ethylene gas production in plant (Kang et al. 2010; Zahir et al. 2008, 2009). Burkholderia phytofirmans PsJN is one of the best-studied Burkholderia. This strain was reported to inhabit the rhizosphere and endosphere of plant, thus promoting growth and enhancing stress adaptation in selected herbaceous and woody plant species (Da et al. 2012; Fernandez et al. 2012; Kim et al. 2012; Naveed et al. 2014). According to Poupin et al. (2013) and Zuniga et al. (2013), B. phytofirmans PsJN showed excellent capability of promoting growth and accelerating the whole life cycle of Arabidopsis thaliana. Moreover, this strain also induces primary root growth and root hair development and promotes aerial growth increasing the epidermal cell size (Poupin et al. 2013) and induces salt stress tolerance (Pinedo et al. 2015) in A. thaliana.
5 Commercial Potentials of PGPR in Malaysia
Malaysia was the largest pepper-producing country in the world. However, after 1980, Malaysia lost its top position to India and Indonesia (Azmil 1993). Currently, Malaysia is ranked sixth in terms of world pepper production (IPC 2012). Approximately 45,000 families and more than 115,000 workers are involved in the pepper industry in Malaysia. This crop generates about one third of Sarawak’s agriculture export earnings, and Sarawak is the main black pepper export producer in Malaysia. Currently, production of black pepper in Sarawak showed a declining trend. One of the main factors is due to pests and diseases infestation. Foot rot disease is considered the most devastating disease in black pepper.
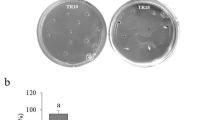
At present, no effective control measure is available to effectively manage this disease in the world. Application of PGPR might be one of the alternative solutions to chemical control of the disease in the field. Attempt was made to find potential indigenous PGPR strain to control foot rot disease in vitro and in vivo. We found promising PGPR strains that are able to induce systemic resistance in black pepper as well as showing biological control traits like producing antibiotics which caused lysis of the mycelial cells of P. capsici. The tested PGPR strains were also found to promote the growth of the treated plants. The use of PGPR should be a preferable method as they are internal colonizers and more efficient to compete in the vascular systems. Thus, this will certainly deprive P. capsici in terms of nutrient uptake and space for their proliferation. Based on dual culture test, these three PGPR strains, BPA011, BPA040 and BPA025, exhibited high percentage of inhibition on radial growth with recorded PIRG values as ~81, 83 and 81%, respectively. Furthermore, in culture filtrate test, all the three strains exhibited 100% PIRG (Fig. 12.5 and Table 12.2). These potential strains were successfully identified using GC-FAME as B. cepacia, B. cenocepacia and Bacillus alcalophilus, respectively.
The effect of endophytic bacteria on mycelial growth of P. capsici in dual culture and cultural filtrate tests at 7 days after incubation. Pure culture of P. capsici in control plate (a), BPA011 (b), BPA040 (c), BPA025 in dual culture test (d) and BPA011 and BPA040 in culture filtrate test (e and f), respectively
Results from in vivo test demonstrated that application of PGPR resulted in disease suppression and delayed disease onset on treated plant. The present study showed that there were significant differences in terms of disease incidence (DI) and disease severity index (DSI) as compared with control treatment. Our findings showed treated plant with B. cenocepacia showed the lowest DSI (1.67%) in the first month, and the severity index was increased gradually in the second month (5.85%), and finally the DSI remained steady at ~10% along the assessment period. A similar trend was observed at B. cepacia and B. alcalophilus treatments.
Assessment on production of inducible compounds by the host plant was also conducted. Our findings revealed an increased in enzymatic activity of peroxidase (PO), total phenolic content (TPC) and hydrogen peroxidase (H2O2) in the treated plants. Significant amount of inducible compounds was expressed in root, stem and leaf parts of the treated plants. Our findings indicated that the systemic protection was offered to the host plant by the tested PGPR strains. This event resulted in limiting and preventing the phytopathogens activities, even at foliar infection by the P. capsici. Moreover, the positive effects of PGPR on plant growth are always correlated with a remarkable increase in the root morphology such as lateral root length, root hair number and also shoot length and yield. In our study, we found root, stem and leaf biomass were significantly increased in the treated plants, and this is generally assumed that these developmental responses are triggered by phytohormones such as auxins, cytokinins and gibberellins produced by the PGPR strains.
6 Future Prospect
Currently, majority of black pepper farmers in Malaysia rely extensively on chemical fungicides to control foot rot disease in black pepper. Heavy reliance on chemical fungicides may lead to numerous biohazards such as environmental pollutions, residual effect in food and pathogen resistance and may be hazardous to beneficial microorganisms. From crop management perspective for sustainable agriculture, the control of foot rot disease in black pepper using PGPR (Burkholderia) would best be achieved by combining these two techniques: (i) disease control through the use of biocontrol agents native to black pepper farms involving continuously inoculation of PGPR inoculum to increase their populations and (ii) disease control through application of antifungal metabolites responsible for effectiveness of the biocontrol agent-developed product usually more effective and easier to be used by farmers. Meanwhile, diminishing the biohazards is inherent in the use of intact microbial cells (and the associated potential risk to human health). Application of green technology in agriculture in Malaysia has become more evident in recent year. Implementation of National Green Technology Policy since 2009 contributed huge impact in research and development as well as in agriculture practices in Malaysia. One of the biggest impacts is the ability to achieve reduction in the greenhouse gas intensity of gross domestic product (GDP) of 35% in 2015. Even though many incentives and funds were given by Malaysian government in developing new and effective formulations for effective delivery of PGPR, the process is still very slow. Formulation of biopesticides with PGPR-like Burkholderia sp. is a big challenge in practical agriculture especially in the tropical regions where the environmental conditions are favourable for the pathogen to grow. Hence, improvement in the formulation of biopesticides is the key to the success in the development of sustainable agriculture.
In plant protection perspective, integrated pest management (IPM) programme is now adopted widely by commercial planters and farmers. With this regard, PGPR strains tested in this study are showing promising outcomes to be used for sustainable and environmentally friendly horticultural production system. The prospect and potential of manipulating PGPR by direct cell inoculation to increase crop yield and reduce disease pressure have shown considerable promise in laboratory and greenhouse studies. However, this technique is not really successful under field conditions. This might be due to climatic variations, and the soil itself is an unpredictable environment, and an intended result is sometimes difficult to achieve. Hence, development of new formulation biofungicide is urgently needed to overcome the above-mentioned limitations as well as to effectively control phytopathogens in field condition. As reported by many authors, biofungicides are safe or have very small residues and harmless to beneficial organisms, and the most important biofungicides are cost-effective to control many pests and diseases in the field.
7 Conclusions
Burkholderia sp. are promising biological control agents against the causal agent of foot rot disease, P. capsici Leonian, through the production of antifungal metabolites, induction of disease resistance and promoting plant growth. These results support the potential use of B. cepacia or its antifungal metabolites as a microbial alternative to control phytopathogens involved in high losses of agricultural production, diminishing the environmental problems caused by current practices. Government involvement by introducing specific policies or long-term programmes which is associated with “green technology” in order to monitor and protect clean environment is highly recommended for sustainable agriculture.
References
Abhishek T, Sharma N, Sharma V, Afroz A (2013) A review on conventional and non-conventional methods to manage postharvest diseases of perishables. Researcher 5(6):6–19
Abriouel H, Franz CM, Ben Omar N, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35:201–232
Ahemad M, Khan MS (2010) Growth promotion and protection of lentil (Lens esculenta) against herbicide stress by Rhizobium species. Ann Microbiol 60:735–745
Ahemad M, Khan MS (2011a) Insecticide-tolerant and plant growth promoting Bradyrhizobium sp. (Vigna) improves the growth and yield of greengram [Vigna radiata (L.) Wilczek] in insecticide stressed soils. Symbiosis 54:7–27
Ahemad M, Khan MS (2011b) Plant growth promoting fungicide tolerant rhizobium improves growth and symbiotic characteristics of lentil (Lens esculentus) in fungicide-applied soil. J Plant Growth Regul 30:334–342
Ahemad M, Khan MS (2011c) Response of greengram [Vigna radiata (L.) Wilczek] grown in herbicide-amended soil to quizalofop-p-ethyl and clodinafop tolerant plant growth promoting Bradyrhizobium sp. (Vigna) MRM6. J Agric Sci Technol 13:1209–1222
Ahemad M, Khan MS (2012a) Ecological assessment of biotoxicity of pesticides towards plant growth promoting activities of pea (Pisum sativum)-specific Rhizobium sp. strain MRP1. Emirates J Food Agric 24:334–343
Ahemad M, Khan MS (2012b) Evaluation of plant growth promoting activities of rhizobacterium Pseudomonas putida under herbicide-stress. Ann Microbiol 62:1531–1540
Ahemad M, Khan MS (2012c) Productivity of greengram in tebuconazole-stressed soil, by using a tolerant and plant growth promoting Bradyrhizobium sp. MRM6 strain. Acta Physiol. Plant 34:245–254
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26(1):1–20
Ahmad M, Nadeem SM, Naveed M, Zahir ZA (2016) Potassium-solubilizing bacteria and their application in agriculture. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 293–313. doi:10.1007/978-81-322-2776-2_21
Ait Barka E, Gognies S, Nowak J, Audran JC, Belarbi A (2002) Inhibitory effect of bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol Control 24:135–142
Akhtar MS, Siddiqui Z (2010) Role of plant growth promoting rhizobacteria in biocontrol of plant diseases and sustainable agriculture. In: Maheshwari DK (ed) Plant growth and health promoting bacteria, Microbiology monographs. Springer, Berlin, pp 157–195
Anon (2003) Technical bulletin on paper cultivation. Technical Bulletins No:4. Department of Export Agriculture, 1095, Peradeniya
Azmil ARI (1993) Pengeluaran Lada–Laporan Khas Institut Penyelidikan dan Kemajuan Pertanian Malaysia (MARDI). Kementerian Pertanian Malaysia, Kuala Lumpur
Bahadur I, Meena VS, Kumar S (2014) Importance and application of potassic biofertilizer in Indian agriculture. Int Res J Biol Sci 3:80–85
Bahadur I, Maurya BR, Kumar A, Meena VS, Raghuwanshi R (2016a) Towards the soil sustainability and potassium-solubilizing microorganisms. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 225–266. doi:10.1007/978-81-322-2776-2_18
Bahadur I, Maurya BR, Meena VS, Saha M, Kumar A, Aeron A (2016b) Mineral release dynamics of tricalcium phosphate and waste muscovite by mineral-solubilizing rhizobacteria isolated from indo-gangetic plain of India. Geomicrobiol J. doi:10.1080/01490451.2016.1219431
Balandreau J, Mavingui P (2007) In: Vandamme P, Coenye T (eds) Beneficial interactions of Burkholderia spp. with plants. Burkholderia: molecular biology and genomics. Horizon Scientific Press, Norwich, pp 129–151
Baldani VLD, Baldani JI, Dobereiner J (2000) Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol Fertil Soils 30:485–491
Barea JM, Pozo MJ, Azcon R, Aguilar CA (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Barrett CF, Parker MA (2006) Coexistence of Burkholderia, Cupriavidus, and Rhizobium sp. nodule bacteria on two Mimosa spp. in Costa Rica. Appl Environ Microbiol 72:1198–1206
Bassler BL (1999) How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2:582–587
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35(4):1044–1051
Bevivino A, Tabacchioni S, Chiarini L, Carusi MV, Del Gallo M, Visca P (1994) Phenotypic comparison between rhizosphere and clinical isolates of Burkholderia cepacia. Microbiology 140(5):1069–1077
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Booker MA, DeLong A (2015) Producing the ethylene signal: regulation and diversification of ethylene biosynthetic enzymes. Plant Physiol 169(1):42–50
Borriss R (2011) Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. In: Maheshwari DK (ed) Bacteria in agrobiology: plant growth responses. Springer, Berlin, pp 41–76
Broekgaarden C, Caarls L, Vos IA, Pieterse CM, Van Wees SC (2015) Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiol 169:2371–2379. doi:10.1104/pp.15.01020
Burkholder WH (1942) Three bacterial plant pathogens. Phytomonas caryophylli sp.n., Phytomonas alliicola sp.n. and Phytomonas manihotis (Artaud, Berthet and Bondar) Vi´egas. Phytopathology 32:141–149
Burkholder WH (1950) Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115–117
Cain CC, Henry AT, Waldo RH, Casida LJ, Falkinham JO (2000) Identification and characteristics of a novel Burkholderia strain with broad-spectrum antimicrobial activity. Appl Environ Microbiol 66:4139–4141
Cartwright DK, Chilton WS, Benson DM (1995) Pyrrolnitrin and phenazine production by Pseudomonas cepacia, strain 5.5B, a biocontrol agent of Rhizoctonia solani. Appl Microbiol Biotechnol 43:211–216
Cazorla FM, Duckett SB, Bergstom ET, Noreen S, Odijk R, Lugtenberg BJJ, Yhomas-Oates JE, Bloemberg GV (2006) Biocontrol of avocado Dematophora root rot by the antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5propyl resorcinol. Mol Plant Microbe Interact 19:418–428
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Sussmuth R, Piel J, Borriss R (2009) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37
Chernin L, Toklikishvili N, Ovadis M, Kim S, Ben-Ari J, Khmel I, Vainstein A (2011) Quorum sensing quenching by rhizobacterial volatiles. Environ Microbiol rep 3:698–704
Coenye T, LiPuma JJ, Henry D, Hoste B, Vandemeulebroecke K, Gillis M, Speert DP, Vandamme P (2001) Burkholderia cepacia genomovar VI, a new member of the Burkholderia cepacia complex isolated from cystic fibrosis patients. Int J Syst Evol Microbiol 51:271–279
Coenye T, Vandamme P (2003) Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol 5:719–729
Compant S, Reiter B, Sessitch A, Nowak J, Clement C, Ait Barka E (2005b) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71:1685–1693
Compant S, Nowak J, Coenye T, Clément C, Barka EA (2008) Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol rev 32(4):607–626
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42(5):669–678
Cuong ND, Nicolaisen MH, Srensen J, Olsson S (2011) Hyphae-colonizing Burkholderia sp.—a new source of biological control agents against sheath blight disease (Rhizoctonia solani AG1-IA) in rice. Microb Ecol 62(2):425–434
Da K, Nowak J, Flinn B (2012) Potato cytosine methylation and gene expression changes induced by a beneficial bacterial endophyte, Burkholderia phytofirmans strain PsJN. Plant Physiol Biochem 50:24–34. doi:10.1016/j.plaphy.2011.09.013
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
Dandurishvili N, Toklikishvili N, Ovadis M, Eliashvili P, Giorgobiani N, Keshelava R (2011) Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown-gall tumors on tomato plants. J Appl Microbiol 110:341–352
Das I, Pradhan M (2016) Potassium-solubilizing microorganisms and their role in enhancing soil fertility and health. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 281–291. doi:10.1007/978-81-322-2776-2_20
De Meyer SE, Tian R, Seshadri R (2015) High-quality permanent draft genome sequence of the Lebeckia Ambigua-nodulating Burkholderia sp. strain WSM4176. Stand Genomic Sci 10:79
de Souza JT, de Boer M, de Waard P, van Beek TA, Raaijmakers JM (2003) Biochemical, genetic, and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl Environ Microbiol 69:7161–7172
De Weert S, Kuiper I, Lagendijk EL, Lamers GEM, Lugtenberg BJJ (2004) Role of chemotaxis toward fusaric acid in colonization of hyphae of Fusarium oxysporum f.sp. radicis-lycopersici by Pseudomonas fluorescens WCS365. Mol Plant Microbe Interact 16:1185–1191
Dekkers LC, Mulders CHM, Phoelich CC, Chin-A-Woeng TFC, Wijfjes AHM, Lugtenberg BJJ (2000) The colonization gene of the tomato-Fusarium f.Sp. radicis-lycopersici biocontrol strain Pseudomonas fluorescens WCS365 can improve root colonization of other wild type Pseudomonas spp. bacteria. Mol Plant-Microbe Interact 13:1177–1183
Dessaux Y, Hinsinger P, Lemanceau P (2009) Rhizosphere: so many achievements and even more challenges. Plant Soil 321:1–3
Dominguez-Nunez JA, Benito B, Berrocal-Lobo M, Albanesi A (2016) Mycorrhizal fungi: role in the solubilization of potassium. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 77–98. doi:10.1007/978-81-322-2776-2_6
Dong YH, Zhang XF, Xu JL, Zhang LH (2004) Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl Environ Microbiol 70:954–960
Dotaniya ML, Meena VD, Basak BB, Meena RS (2016) Potassium uptake by crops as well as microorganisms. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 267–280. doi:10.1007/978-81-322-2776-2_19
Dubbey RC, Khare S, Kumar P, Maheshwari DK (2014) Combined effect of chemical fertilizers rhizospherecompetent Bacillus subtilis BSK17 on yield of Cicer arietinum. Arch Phytopathol Plant Protect 47:2305–2318
Duffy B, Schouten A, Raaijmakers JM (2003) Pathogen self-defence: mechanisms to counteract microbial antagonism. Annu Rev Phytopathol 41:501–538
Eberl L, Vandamme P (2016) Members of the genus Burkholderia: good and bad guys. F1000 res 5:1–10
EL-Banna N, Winkelmann G (1998) Pyrrolnitrin from Burkholderia cepacia: antibiotic activity against fungi and novel activities against streptomycetes. J Appl Microbiol 85:69–78
Fan B, Chen XH, Budiharjo A, Bleiss W, Vater J, Borriss R (2011) Efficient colonization of plant roots by the plant growth promoting bacterium, Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J Biotechnol 151:303–311
Fernandez O, Theocharis A, Bordiec S, Feil R, Jacquens L, Clement C (2012) Burkholderia phytofirmans PsJN acclimates grapevine to cold by modulating carbohydrate metabolism. Mol Plant-Microbe Interact 25:496–504. doi:10.1094/mpmi-09-11-0245
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Hindawi Publishing Corporation, Scientifica
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London
Gratia JP (2000) André Gratia: a forerunner in microbial and viral genetics. Genetics 156(2):471–476, PMC 1461273 [free to read].PMID 11014798
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hariprasad P, Divakara ST, Niranjana SR (2011) Isolation and characterization of chitinolytic rhizobacteria for the management of Fusarium wilt of tomato. Crop Prot 36:1606–1612
Henry G, Deleu M, Jourdan E, Thonart P, Ongena M (2011) The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell Microbiol 13:1824–1837
Hiltner L (1904) Uber neuere erfahrungen und probleme auf dem gebiete der bodenbakteriologie unter bessonderer berucksichtigung der grundung und brache. Arb Dtsch Landwirtsch Ges Berl 98:59–78
Holiday P, Mowat WP (1963) Foot rot of Piper nigrum L. (Phytophthora palmivora). Phytopathological paper No. 5, Commonwealth Mycological Institute, Kew
Hu FP, Young JM (1998) Biocidal activity in plant pathogenic Acidovorax, Burkholderia, Herbaspirillum, Ralstonia and Xanthomonas spp. J Appl Microbiol 84:263–271
Hynes RK, Leung GC, Hirkala D.L. and Nelson LM (2008) Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil and chickpea grown in Western Canada. Can J Microbiol 54:248–258.
International Pepper Community (2012) Malaysia: annual export of black pepper and white pepper. Pepper Statistical Year Book 2012. (http://www.ipenet.org/n/psy2012/my.html)
Jahanian A, Chaichi MR, Rezaei K, Rezayazdi K, Khavazi K (2012) The effect of plant growth promoting rhizobacteria (PGPR) on germination and primary growth of artichoke (Cynara scolymus). Int J Agric Crop Sci 4:923–929
Jaiswal DK, Verma JP, Prakash S, Meena VS, Meena RS (2016) Potassium as an important plant nutrient in sustainable agriculture: a state of the art. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 21–29. doi:10.1007/978-81-322-2776-2_2
Janssen PH (2006) Identifying the dominant soil bacteria taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 72:1719–1728
Jat LK, Singh YV, Meena SK, Meena SK, Parihar M, Jatav HS, Meena RK, Meena VS (2015) Does integrated nutrient management enhance agricultural productivity? J Pure Appl Microbiol 9(2):1211–1221
Jha Y, Subramanian RB (2016) Regulation of plant physiology and antioxidant enzymes for alleviating salinity stress by potassium-mobilizing bacteria. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 149–162. doi:10.1007/978-81-322-2776-2_11
Jianbin LI, Gilardi G, Sanna M, GULLINO ML, Garibaldi A (2010) Biocontrol of Fusarium crown and root rot of tomato and growth-promoting effect of bacteria isolated from recycled substrates of soilless crops. Phytopathol Mediterr 49(2):163–171
Jiao Y, Yoshihara T, Ishikuri S, Uchino H, Ichihara A (1996) Structural identification of cepaciamide A, a novel fungitoxic compound from Pseudomonas cepacia D-202. Tetrahedron Lett 37:1039–1042
Joo GJ, Kin YM, Kim JT, Rhee IK, Kim JH, Lee IJ (2005) Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. J Microbiol 43:510–515
Jung WJ, Jin YL, Kim KY, Park RD, Kim TH (2005) Changes in pathogenesis-related proteins in pepper plants with regard to control of Phytophthora blight with Paenibacillus illinoisensis. Biocontrol 50:165–178
Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg BJJ (2006) Effects of the tomato pathogen Fusarium oxysporum f. sp. radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol Plant Microbe Interact 19:1121–1126
Kamilova F, Lamers G, Lugtenberg B (2008) Biocontrol strain Pseudomonas fluorescens WCS365 inhibits germination of Fusarium oxysporum spores in tomato root exudate as well as subsequent formation of new spores. Environ Microbiol 10:2455–2461
Kang BG, Kim WT, Yun HS, Chang SC (2010) Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol 4:179–183
Kang YW, Carlson R, Tharpe W, Schell MA (1998) Characterization of genes involved in biosynthesis of a novel antibiotic from Burkholderia cepacia BC11 and their role in biological control of Rhizoctonia solani. Appl Environ Microbiol 64:3939–3947
Kaur R, Macleod J, Foley W, Nayudu M (2006) Gluconic acid, an antifungal agent produced by Pseudomonas species in biological control of take-all. Phytochemistry 67:595–604
Kennedy IR, Cocking EC (1997, April 8–12) Biological nitrogen fixation: The global challenge & future needs. A position paper, discussed at the Rockefeller Foundation Bellagia Conference Centre, Italy
Khalid A, Akhtar MJ, Mahmood MH, Arshad M (2006) Effect of substrate-dependent microbial ethylene production on plant growth. Microbiology 75:231–236
Khan AG (2005) Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elem Med Biol 18:355–364
Kim S, Lowman S, Hou G, Nowak J, Flinn B, Mei C (2012) Growth promotion and colonization of switchgrass (Panicum virgatum) cv. Alamo by bacterial endophyte Burkholderia phytofirmans strain PsJN. Biotechnol Biofuels 5:37. doi:10.1186/1754-6834-5-37
Kloepper JW (1994) Plant growth-promoting rhizobacteria (other systems). In: Okon Y (ed) Azospirillum/Plant associations. CRC Press, Boca Raton, pp 111–118
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7:39–43
Kloepper JW, Zablotowick RM, Tipping EM, Lifshitz R (1991) Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister DL, Cregan PB (eds) The rhizosphere and plant growth. Kluwer Academic Publishers, Dordrecht, pp 315–326
Kuch TK, Khutubhutheen AJ (1985) Phytophthora foot rot of black pepper. Int Conf Plant Prot Trop 4:238
Kumar A, Bahadur I, Maurya BR, Raghuwanshi R, Meena VS, Singh DK, Dixit J (2015) Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J Pure Appl Microbiol 9:715–724
Kumar A, Meena R, Meena VS, Bisht JK, Pattanayak A (2016a) Towards the stress management and environmental sustainability. J Clean Prod 137:821–822
Kumar A, Patel JS, Bahadur I, Meena VS (2016b) The molecular mechanisms of KSMs for enhancement of crop production under organic farming. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 61–75. doi:10.1007/978-81-322-2776-2_5
Kumar A, Maurya BR, Raghuwanshi R, Meena VS, Islam MT (2017) Co-inoculation with Enterobacter and Rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under indo-gangetic plain of India. J Plant Growth Regul. doi:10.1007/s00344-016-9663-5
Kurepin LV, Zaman M, Pharis RP (2014) Phytohormonal basis for the plant growth promoting action of naturally occurring biostimulators. J Sci Food Agric 94(9):1715–1722. doi:10.1002/jsfa.6545
Kurepin LV, Park JM, Lazarovits G, Bernards MA (2015) Burkholderia phytofirmans-induced shoot and root growth promotion is associated with endogenous changes in plant growth hormone levels. Plant Growth Regul 75:199–207. doi:10.1007/s10725-014-9944-6
Labuschagne N, Pretorius T, Idris AH (2010) Plant growth promoting rhizobacteria as biocontrol agents against soil-borne plant diseases. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Microbiology monographs. Springer, Berlin, pp 211–230
Lemaire B, Dlodlo O, Chimphango S (2015) Symbiotic diversity, specificity and distribution of rhizobia in native legumes of the Core Cape Subregion (South Africa). FEMS Microbiol Ecol 91(2):1–17
Leong J (1986) Siderophores: their biochemistry and possible role in the biocontrol of plant pathogens. Annu Rev Phytopathol 24:187–209
Lodewyckx C, Vangronsveld J, Porteous F, Moore ERB, Taghavi S, Mezgeay M, Der Lelie D (2002) Endophytic bacteria and their potential applications. Crit Rev Plant Sci 21:583–606
Lopez D, Vlamakis H, Losick R, Kolter R (2009) Cannibalism enhances biofilm development in Bacillus subtilis. Mol Microbiol 74:609–618
Lorito M, Woo SL, Harman GE, Monte E (2010) Translational research on Trichoderma: from omics to the field. Annu Rev Phytopathol 48:395–417
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Lugtenberg BJJ, Dekkers L, Bloemberg GB (2001) Molecular determination of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490
Lugtenberg BJJ, Malfanova N, Kamilova F, Berg G (2013) Microbial control of plant root diseases. In: de Bruijin FJ (ed) Molecular, microbiology and ecology of rhizosphere. Wiley, Hoboken
Luvizotto DM, Marcon J, Andreote FD, Dini-Andreote F, Neves AA, Araújo WL, Pizzirani-Kleiner AA (2010) Genetic diversity and plant-growth related features of Burkholderia spp. from sugarcane roots. World J Microbiol Biotechnol 26(10):1829–1836
Ma Y, Rajkumar M, Luo Y, Freitas H (2011) Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J Hazard Mater 195:230–237
Mabood R, Zhou X, Smith DL (2014) Microbial signalling and plant growth promotion. Can J Plant Sci 94:1051–1063
Maksimov IV, Abizgil’dina RR, Pusenkova LI (2011) Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens. Appl Biochem Microbiol 47:333–345
Malfanova N, Kamilova F, Validov S, Shcherbakov A, Chebotar V, Tikhonovich I, Lugtenberg B (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microb Biotechnol 4:523–532
Mandal S, Ray RC (2011) Induced systemic resistance in biocontrol of plant diseases. In: Singh A, Parmar N, Ramesh CK (eds) Bioaugmentation, biostimulation and biocontrol. Springer, Berlin, pp 241–260
Manohara D, Mulya K, Wahyuno D (2004) Phytophthora disease on black pepper and their control measures. Focus Pepper 1:37–49
Mao S, Lee SJ, Hwangbo H, Kim YW, Park KH, Cha GS, Park RD, Kim KY (2006) Isolation and characterization of antifungal substances from Burkholderia sp. culture broth. Curr Microbiol 53:358–364
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Masood S, Bano A (2016) Mechanism of potassium solubilization in the agricultural soils by the help of soil microorganisms. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 137–147. doi:10.1007/978-81-322-2776-2_10
Maurya BR, Meena VS, Meena OP (2014) Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos 27:181–187
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Meena OP, Maurya BR, Meena VS (2013a) Influence of K-solubilizing bacteria on release of potassium from waste mica. Agric Sust Dev 1:53–56
Meena VS, Maurya BR, Bohra JS, Verma R, Meena MD (2013b) Effect of concentrate manure and nutrient levels on enzymatic activities and microbial population under submerged rice in alluvium soil of Varanasi. Crop Res 45(1, 2 & 3):6–12
Meena VS, Maurya BR, Verma R, Meena RS, Jatav GK, Meena SK, Meena SK (2013c) Soil microbial population and selected enzyme activities as influenced by concentrate manure and inorganic fertilizer in alluvium soil of Varanasi. Bioscan 8(3):931–935
Meena VS, Maurya BR, Bahadur I (2014a) Potassium solubilization by bacterial strain in waste mica. Bang J bot 43:235–237
Meena VS, Maurya BR, Verma JP (2014b) Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res 169:337–347
Meena RS, Meena VS, Meena SK, Verma JP (2015a) The needs of healthy soils for a healthy world. J Clean Prod 102:560–561
Meena RS, Meena VS, Meena SK, Verma JP (2015b) Towards the plant stress mitigate the agricultural productivity: a book review. J Clean Prod 102:552–553
Meena VS, Maurya BR, Meena RS (2015c) Residual impact of wellgrow formulation and NPK on growth and yield of wheat (Triticum aestivum L.) Bangladesh J bot 44(1):143–146
Meena VS, Maurya BR, Verma JP, Aeron A, Kumar A, Kim K, Bajpai VK (2015d) Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecol Eng 81:340–347
Meena VS, Meena SK, Verma JP, Meena RS, Ghosh BN (2015e) The needs of nutrient use efficiency for sustainable agriculture. J Clean Prod 102:562–563. doi:10.1016/j.jclepro.2015.04.044
Meena VS, Verma JP, Meena SK (2015f) Towards the current scenario of nutrient use efficiency in crop species. J Clean Prod 102:556–557. doi:10.1016/j.jclepro.2015.04.030
Meena RK, Singh RK, Singh NP, Meena SK, Meena VS (2016a) Isolation of low temperature surviving plant growth-promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal Agric Biotechnol 4:806–811
Meena RS, Bohra JS, Singh SP, Meena VS, Verma JP, Verma SK, Sihag SK (2016b) Towards the prime response of manure to enhance nutrient use efficiency and soil sustainability a current need: a book review. J Clean Prod 112(1):1258–1260
Meena SK, Rakshit A, Meena VS (2016c) Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal Agric Biotechnol 6:68–75
Meena VS, Bahadur I, Maurya BR, Kumar A, Meena RK, Meena SK, Verma JP (2016d) Potassium-solubilizing microorganism in evergreen agriculture: an overview. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 1–20. doi:10.1007/978-81-322-2776-2_1
Meena VS, Meena SK, Bisht JK, Pattanayak A (2016e) Conservation agricultural practices in sustainable food production. J Clean Prod 137:690–691
Meena VS, Maurya BR, Meena SK, Meena RK, Kumar A, Verma JP, Singh NP (2017) Can Bacillus species enhance nutrient availability in agricultural soils? In: Islam MT, Rahman M, Pandey P, Jha CK, Aeron A (eds) Bacilli and agrobiotechnology. Springer, Cham, pp 367–395. doi:10.1007/978-3-319-44409-3_16
Merchante C, Alonso JM, Stepanova AN (2013) Ethylene signalling: simple ligand, complex regulation. Curr Opin Plant Biol 16:554–560. doi:10.1016/j.pbi.2013.08.001
Meyer JM, Van Tran V, Stintzi A, Berge O, Winkelmann G (1995) Ornibactin production and transport properties in strains of Burkholderia vietnamiensis and Burkholderia cepacia (formerly Pseudomonas cepacia). Biometals 8:309–317
Nardi S, Concheri G, Pizzeghello D, Sturaro A, Rella R, Parvoli G (2000) Soil organic matter mobilization by root exudates. Chemosphere 5:653–658
Nascimento FX, Rossi MJ, Soares CR, McConkey BJ, Glick BR (2014) New insight into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecology significant. PLoS One 9:e99168
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp bot 97:30–39. doi:10.1016/j.envexpbot.2013.09.014
Nielsen TH, Srensen D, Tobiasen C, Andersen JB, Christeophersen C, Givskov M, Srensen J (2002) Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl Environ Microbiol 68:3416–3423
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny J-L, Thonart P (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9:1084–1090
Opelt K, Berg C, Berg G (2007) The bryophyte genus Sphagnum is a reservoir for powerful and extraordinary antagonists and potentially facultative human pathogens. FEMS Microbiol Ecol 61:38–53
Palleroni NJ, Kunisawa R, Contopolous R, Doudoroff M (1973) Nucleic acid homologies in the genus Pseudomonas. Int J Syst Bacteriol 23:333–339
Pandey P, Kang SC, Maheshwari DK (2005) Isolation of endophytic plant growth promoting Burkholderia sp. MSSP from root nodules of Mimosa pudica. Curr Sci 89:177–180
Parke JL, Gurian-Sherman D (2001) Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39:225–258
Parewa HP, Yadav J, Rakshit A, Meena VS, Karthikeyan N (2014) Plant growth promoting rhizobacteria enhance growth and nutrient uptake of crops. Agric Sustain Dev 2(2):101–116
Perez-Garcıa A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological application of Bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Perin L, Mart’ınez-Aguilar L, Castro-Gonz’alez P, Estrada-de Los Santos P, Cabellos-Avelar T, Guedes V, Reis VM, Caballero Mellado J (2006) Diazotrophic Burkholderia species associated with field-grown maize and sugarcane. Appl Environ Microbiol 72:3103–3110
Pieterse CMJ, Van Wees SCM, Hoffland E, Van Pelt JA, Van Loon LC (1996) Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicyclic acid accumulation and pathogenesis-related gene expression. Plant Cell 8:1225–1237
Pieterse CMJ, Leon-Reyes A, Vander Ent S, Van Wees SCM (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375
Pinedo I, Ledger T, Greve M, Poupin MJ (2015) Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front Plant Sci 6:466. doi:10.3389/fpls.2015.00466
Poupin MJ, Timmermann T, Vega A, Zuniga A, Gonzalez B (2013) Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS One 8:e69435. doi:10.1371/journal.pone.0069435
Prakash S, Verma JP (2016) Global perspective of potash for fertilizer production. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 327–331. doi:10.1007/978-81-322-2776-2_23
Priyadharsini P, Muthukumar T (2016) Interactions between arbuscular mycorrhizal fungi and potassium-solubilizing microorganisms on agricultural productivity. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 111–125. doi:10.1007/978-81-322-2776-2_8
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81:537–547
Raghavendra MP, Nayaka NC, Nuthan BR (2016) Role of rhizosphere microflora in potassium solubilization. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 43–59. doi:10.1007/978-81-322-2776-2_4
Rahamat Bivi MR, Farhana MSN, Khaiulmazmi A, Idris AS (2010) Control of Ganoderma boninense: a causal agent of basal stem root in oil palm with endophyte bacteria in vivo. Int J Agric Biol 12:833–839
Rawat J, Sanwal P, Saxena J (2016) Potassium and its role in sustainable agriculture. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 235–253. doi:10.1007/978-81-322-2776-2_17
Raymond J, Siefert JL, Staples CR, Blankenship RE (2004) The natural history of nitrogen fixation. Mol Biol Evol 21:541–554
Riley MA, Wertz JE (2002) Bacteriocins: evolution, ecology and application. Ann Rev Microbiol 56:117–137
Rosales AM, Thomashow L, Cook RJ, Mew TW (1995) Isolation and identification of antifungal metabolites produced by riceassociated antagonistic Pseudomonas spp. Phytopathology 85:1028–1032
Rovira AD (1956) A study of the development of the root surface microflora during the initial stages of plant growth. J Appl Bacteriol 19:72–79
Russo A, Vettori L, Felici C, Fiaschi G, Morini S, Toffanin A (2008) Enhanced micropropagation response and biocontrol effect of Azospirillum brasilense Sp245 on Prunus cerasifera L. clone Mr. S 2/5 plants. J Biotechnol 134:312–319
Ryals JA, Neuenschwander UH, Willits MG, Mollina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1808–1819
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932
Saha M, Maurya BR, Bahadur I, Kumar A, Meena VS (2016a) Can potassium-solubilising bacteria mitigate the potassium problems in India? In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 127–136. doi:10.1007/978-81-322-2776-2_9
Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A (2016b) Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic Plains of India. Biocatal Agric Biotechnol 7:202–209
Sanogo S (2003) Chile pepper and the threat of wilt diseases. Plant Health Prog. doi:10.1094/PHP-2003-0430-01-RV
Santos Villalobos de los S, Barrera-Galicia GC, Pen˜a-Cabriales JJ, Miranda-Salcedo MA (2012) Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World J Microbiol Biotechnol 28:2615–2623. doi:10.1007/s11274-012-1071-9
Saraf M, Rajkumar S, Saha T (2011) Perspective of PGPR in agro-ecosystem. In: Maheshwari DK (ed) Bacteria in agrobiology: crop systems. Springer, Berlin, pp 361–385
Sermwan RW, Royros P, Khakhum N (2015) Direct detection of Burkholderia pseudomallei and biological factors in soil. Trans R Soc Trop Med Hyg 109(7):462–468
Shaikh SS, Patel PR, Patel SS, Nikam SD, Rane TU, Sayeed SZ (2014) Production of biocontrol traits by banana field fluorescent Pseudomonads and their comparison with chemical fungicides. Indian J Exp Biol 52:917–920
Shaikh SS, Sayeed SZ (2015) Role of plant growth promoting rhizobacteria and their formulation as biocontrol of plant diseases. In: Arora NK (ed) Plant microbes symbiosis. Applied facets. Springer, New Delhi, pp 337–351
Shaikh SS, Sayyed RZ, Reddy MS (2016) Plant growth promoting rhizobacteria: an eco-friendly approach for sustainable agroecosystem. In: Hakeem KR, Akhtar MS, Abdullah SNA (eds) Plant, soil and microbes, Implications in crop science, vol 1. Springer, Cham, pp 181–201
Sharma A, Shankhdhar D, Shankhdhar SC (2016) Potassium-solubilizing microorganisms: mechanism and their role in potassium solubilization and uptake. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 203–219. doi:10.1007/978-81-322-2776-2_15
Shehata HR, Lyons EM, Jordan KS (2016) Bacterial endophytes from wild and ancient maize are able to suppress the fungal pathogen Sclerotinia homoeocarpa. J Appl Microbiol 120(3):756–769
Shrivastava M, Srivastava PC, D’Souza SF (2016) KSM soil diversity and mineral solubilization, in relation to crop production and molecular mechanism. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 221–234. doi:10.1007/978-81-322-2776-2_16
Silveira ABD, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35(4):1044–1051
Sindhu SS, Rakshiya YS, Sahu G (2009) Biological control of soil borne plant pathogens with rhizosphere bacteria. Pest Technol 3:10–21
Sindhu SS, Parmar P, Phour M, Sehrawat A (2016) Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 171–185. doi:10.1007/978-81-322-2776-2_13
Singh RK, Mishra RPM, Jaiswal HK, Kumar V, Pandey SP, Rao SB, Annapurna K (2006) Isolation and identification of natural endophytic rhizobia from rice (Oryza sativa L.) through rDNA PCR-RFLP and sequence analysis. Curr Microbiol 52:117–122
Singh NP, Singh RK, Meena VS, Meena RK (2015) Can we use maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos Int J Plant Res 28(1):86–99. doi:10.5958/2229-4473.2015.00012.9
Singh M, Dotaniya ML, Mishra A, Dotaniya CK, Regar KL, Lata M (2016) Role of biofertilizers in conservation agriculture. In: Bisht JK, Meena VS, Mishra PK, Pattanayak A (eds) Conservation agriculture: an approach to combat climate change in Indian Himalaya. Springer, Singapore, pp 113–134. doi:10.1007/978-981-10-2558-7_4
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3(4). doi:10.1101/cshperspect.a001438
Takeshita K, Matsuura Y, Itoh H (2015) Burkholderia of plant-beneficial group are symbiotically associated with bordered plant bugs (Heteroptera: Pyrrhocoroidea: Largidae). Microbes Environ 30(4):321–329
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5:51–58
Tao GC, Tian SJ, Cai MY, Xie GH (2008) Phosphate solubilizing and -mineralizing abilities of bacteria isolated from soils. Pedosphere 18:515–523
Teotia P, Kumar V, Kumar M, Shrivastava N, Varma A (2016) Rhizosphere microbes: potassium solubilization and crop productivity-present and future aspects. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 315–325. doi:10.1007/978-81-322-2776-2_22
Tian F, Ding Y, Zhu H, Yao L, Du B (2009) Genetic diversity of siderophore-producing bacteria of tobacco rhizosphere. Braz J Microbiol 40:276–284
Toure Y, Ongena M, Jacques P, Guiro A, Thonart P (2004) Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J Appl Microbiol 96:1151–1160
Uroz S, Dessaux Y, Oger P (2009) Quorum sensing and quorum sensing: the Yin and Yang of bacterial communication. Chembiochem 10:205–216
Validov SZ, Kamilova F, Lugtenberg BJJ (2009) Pseudomonas putida strain PCL1760 controls tomato foot and root rot in stonewool under industrial conditions in a certified greenhouse. Biol Control 48:6–11
Van Loon LC, Bakker PAHM (2006) Root-associated bacteria inducing resistance. In: Gnanamanickam SS (ed) Plant-associated bacteria. Springer, Dordrecht, pp 269–316
Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Vandamme P, Holmes B, Coenye T, Goris J, Mahenthiralingam E, LiPuma JJ, Govan JRW (2003) Burkholderia cenocepacia sp. nov. a new twist to an old story. Res Microbiol 154:91–96
Van Wees SCM, De Swart EAM, Van Pelt JA, Van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci U S A 97:8711–8716
Velazquez E, Silva LR, Ramírez-Bahena MH, Peix A (2016) Diversity of potassium-solubilizing microorganisms and their interactions with plants. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 99–110. doi:10.1007/978-81-322-2776-2_7
Verma R, Maurya BR, Meena VS (2014) Integrated effect of bio-organics with chemical fertilizer on growth, yield and quality of cabbage (Brassica oleracea var capitata). Indian J Agric Sci 84(8):914–919
Verma JP, Jaiswa DK, Meena VS, Meena RS (2015a) Current need of organic farming for enhancing sustainable agriculture. J Clean Prod 102:545–547
Verma JP, Jaiswal DK, Meena VS, Kumar A, Meena RS (2015b) Issues and challenges about sustainable agriculture production for management of natural resources to sustain soil fertility and health. J Clean Prod 107:793–794
Vermis K, Coenye T, Lipuma JJ, Mahenthiralingam E, Nelis HJ, Vandamme P (2004) Proposal to accommodate Burkholderia cepacia genomovar VI as Burkholderia dolosa sp. nov. Int J Syst Evol Microbiol 54:689–691
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vleesschauwer D, Hofte M (2009) Rhizobacteria-induced systemic resistance. Adv Bot Res 51:223–281
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Wang A, Lou B, Xu T, Lin C (2011) Defense responses in tomato fruit induced by oligandrin against Botrytis cinerea. Afr J Biotechnol 10(22):4596–4601. Doi: http://doi.org/10.5897/AJB11.290
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48:3262–3267
Welbaum G, Sturz AV, Dong Z, Nowak J (2004) Fertilizing soil microorganisms to improve productivity of agroecosystems. Crit Rev Plant Sci 23:175–193
Wharton P, Kirk W, Berry D, Snapp S (2007) Rhizoctonia stem canker and black scurf of potato. Ext Bull 2994
Yadav BK, Sidhu AS (2016) Dynamics of potassium and their bioavailability for plant nutrition. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 187–201. doi:10.1007/978-81-322-2776-2_14
Yanez-Mendizabal V, Zeriouh H, Viñas I, Torres R, Usall J, Vicente A, Perez-Garcıa A (2012) Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur J Plant Pathol 132:609–619
Yasin M, Munir I, Faisal M (2016) Can Bacillus spp. enhance K+ uptake in crop species. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 163–170. doi:10.1007/978-81-322-2776-2_12
Zahedi H (2016) Growth-promoting effect of potassium-solubilizing microorganisms on some crop species. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 31–42. doi:10.1007/978-81-322-2776-2_3
Zahir ZA, Munir A, Asghar HN, Shaharoona B, Arshad M (2008) Effectiveness of rhizobacteria containing ACC-deaminase for growth promotion of pea (Pisum sativum) under drought conditions. J Microbiol Biotechnol 18:958–963
Zahir ZA, Ghani U, Naveed M, Nadeem SM, Asghar HN (2009) Comparative effectiveness of Pseudomonas and Serratia spp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch Microbiol 191:415–424
Zaidi A, Khan MS, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung 56:263–284
Zeriouh H, Romero D, Garcıa-Gutierrez L, Cazorla FM, de Vicente A, Perez-Garcıa A (2011) The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of Cucurbits. Mol Plant Microbe Interact 24:1540–1552
Zuniga A, Poupin MJ, Donoso R, Ledger T, Guiliani N, Gutierrez RA (2013) Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol Plant-Microbe Interact 26:546–553. doi:10.1094/mpmi-10-12-0241-r
Acknowledgement
We thank the editors and anonymous reviewers for their constructive comments, which helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ahmad, K., Ahmadu, T. (2017). Prospect and Potential of Burkholderia sp. Against Phytophthora capsici Leonian: A Causative Agent for Foot Rot Disease of Black Pepper. In: Meena, V., Mishra, P., Bisht, J., Pattanayak, A. (eds) Agriculturally Important Microbes for Sustainable Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-10-5343-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-5343-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5342-9
Online ISBN: 978-981-10-5343-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)