Abstract
Colletotrichum gloeosporioides is the causal agent of anthracnose in mango. Burkholderia cepacia XXVI, isolated from mango rhizosphere and identified by 16S rDNA sequencing as a member of B. cepacia complex, was more effective than 6 other mango rhizosphere bacteria in inhibiting the model mango pathogen, C. gloeosporioides ATCC MYA 456. Biocontrol of this pathogen was demonstrated on Petri-dishes containing PDA by > 90 % reduction of surface colonization. The nature of the biocontrol metabolite(s) was characterized via a variety of tests. The inhibition was almost exclusively due to production of agar-diffusible, not volatile, metabolite(s). The diffusible metabolite(s) underwent thermal degradation at 70 and 121 °C (1 atm). Tests for indole acetic acid production and lytic enzyme activities (cellulase, glucanase and chitinase) by B. cepacia XXVI were negative, indicating that these metabolites were not involved in the biocontrol effect. Based on halo formation and growth inhibition of the pathogen on the diagnostic medium, CAS-agar, as well as colorimetric tests we surmised that strain XXVI produced a hydroxamate siderophore involved in the biocontrol effect observed. The minimal inhibitory concentration test showed that 0.64 μg ml−1 of siderophore (Deferoxamine mesylate salt-equivalent) was sufficient to achieve 91.1 % inhibition of the pathogen growth on Petri-dishes containing PDA. The biocontrol capacity against C. gloeosporioides ATCC MYA 456 correlated directly with the siderophore production by B. cepacia XXVI: the highest concentration of siderophore production in PDB on day 7, 1.7 μg ml−1 (Deferoxamine mesylate salt-equivalent), promoted a pathogen growth inhibition of 94.9 %. The growth of 5 additional strains of C. gloeosporioides (isolated from mango “Ataulfo” orchards located in the municipality of Chahuites, State of Oaxaca in Mexico) was also inhibited when confronted with B. cepacia XXVI. Results indicate that B. cepacia XXVI or its siderophore have the potential to be used as a biological control agent against C. gloeosporioides; thus diminishing environmental problems caused by the current practices to control this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthracnose, caused by Colletotrichum gloeosporioides, is the main disease in mango production, causing up to 60 % of losses in world-wide in pre- and post-harvest when climatic conditions are optimal to its development, particularly, high relative humidity (Ann et al. 1997; Thahir Basha et al. 2010). This disease has been controlled by excessive applications of fungicides, generating disadvantages such as environmental contamination, resistance development of pathogens and residual contamination in fruits (de los Santos-Villalobos et al. 2011).
Recently, many antagonistic microorganisms have been studied to control diseases of agricultural importance, i.e. Burkholderia cepacia complex (Bcc), a group of remarkably versatile bacteria that have been found naturally in a wide diversity of clinical and environmental habitats (Parke and Gurian-Sherman 2001; Bevivino et al. 2002; Mahenthiralingam et al. 2008). This complex includes plant and human pathogens, plant growth promoting bacteria, bioremediation agents and strains capable of biological control of plant diseases (Hebbar et al. 1998; Okoh et al. 2001; Hwang et al. 2002; LiPuma 2003; Stoyanova et al. 2007). Several secondary volatile or non-volatile metabolites from Bcc with the capacity for pathogen biocontrol have been reported, such as antibiotics, alkaloids and siderophores (Roitman et al. 1990; Cartwright et al. 1995; Xi et al. 1996). Siderophores have gained attention as the major metabolites involved in this biocontrol activity (Cartwright and Benson 1995; Páez et al. 2005).
Siderophores are relatively low molecular weight, ferric ion-specific chelating agents, biosynthesized by bacteria and fungi growing under low iron stress (Neilands 1995). Iron is one of the most important nutrients of organisms because it has an essential metabolic role, i.e. transport, storage and activation of molecular oxygen, amino acid syntheses, respiration, DNA biosynthesis, nitrogen fixation, methanogenesis, reduction of ribonucleotides and dinitrogen, activation and decomposition of peroxides and electron transport (Faraldo-Gómez and Sansom 2003; Katiyar and Goel 2004; Miethke and Marahiel 2007; Sandy and Butler 2009).
Under iron limiting conditions, many microorganisms biosynthesize siderophores in order to solubilize, capture and transport inorganic iron to the cell (O’Sullivan and O’Gara 1992; Carrillo-Castañeda et al. 2005; Sandy and Butler 2009); once the iron is depleted in this environment, other microorganisms that need this element for their growth cannot grow, leading to growth inhibition. Therefore, microorganisms that capture iron via siderophores can effect biological control (Wong et al. 1996; Sritharan 2000).
From an agricultural-management perspective, the control of anthracnose in mango would best be achieved at least two ways: (1) pre- harvest control through the use of biocontrol agents native to mango orchards [involving inoculation/re-inoculation of these microorganisms or increasing their populations using specific agricultural practices (weeds and/or fertilization managements)] and (2) post-harvest control, focusing on the application of metabolites responsible for effectiveness of the biocontrol agents—thus diminishing the hazards inherent in the use of intact microbial cells (and the associated potential risk to human health). Key steps toward the above-described biological control of anthracnose include: obtaining a potential biological control agent and exploring mechanistic details. Here we characterize a metabolite conferring biological control of the causal agent of anthracnose (C. gloeosporioides). The metabolite was produced by a bacterium (B. cepacia XXVI) isolated from mango tree rhizosphere.
Materials and methods
Sampling site
Soils of ten “Ataulfo” mango trees were sampled according to Avilán (2008) from orchards located in the municipality of Apatzingan, State of Michoacan, an important mango-producing state in Mexico (SAGARPA 2007). The collected samples were transferred to moist chambers and transported in a cooler at 4 °C.
Isolation of microorganisms
Baz agar medium (g L−1) had the following composition: solution 1 (0.4 K2HPO4, 0.4 KH2PO4, 0.2 MgSO4, 0.02 CaCl2, 0.01 FeCl3, 0.002 Na2MoO4, 0.5 yeast extract and 15 agar) and solution 2 (5 arabinose), both solutions were adjusted with HCl to pH 5.7 and autoclaved separately at 121 °C (1 atm) for 15 min. Later solutions were combined-mixed and filter-sterilized cycloheximide (80 mg ml−1) was then added (Estrada-de los Santos et al. 2001). This medium was used to isolate bacteria with biocontrol capacity against C. gloeosporioides ATCC MYA 456. This isolation was performed using the direct method as well as by serial dilutions. Thus, 1 g of soil was placed onto Petri-dishes containing Baz agar. Also 10 g of soil was homogenized with 90 ml of 10 mM MgSO4 × 7H2O (autoclaved at 121 °C and 1 atm for 15 min) during 1 h in a rotary shaker at 100 rpm. Next, serial dilutions were prepared up to 10−3 and 1 ml of these dilutions was spread onto the surface of Petri-dishes containing Baz agar and incubated at 28 °C for 7 days. All experiments were performed in triplicate.
Biocontrol of C. gloeosporioides by isolated bacteria
As reported by Silveira Mello et al. (2004) and Cuervo-Parra et al. (2011), confrontation assays were performed on Petri-dishes containing Potato Dextrose Agar (PDA) inoculating, in the center, 1 × 106 spores of C. gloeosporioides ATCC MYA 456 and around the pathogen, in two equidistant points, 1 × 104 CFU of each bacterium isolated. These Petri-dishes were incubated at 28 °C for 7 days. The percentage of growth inhibition of the pathogen (as determined by surface colonization) was calculated using the following equation: \( \% \,{\text{of}}\,{\text{growth}}\,{\text{inhibition}} = \frac{{{\text{Ac}} - {\text{Ab}}}}{\text{Ac}}*100, \) where Ac: control mycelial area and Ab: mycelial area in treatment (Schmidt et al. 2009). This assay was completed using three independents replicates.
The bacterial strain presenting the highest biocontrol capability against C. gloeosporioides ATCC MYA 456 was used, under same conditions mentioned above, against 5 strains of C. gloeosporioides (VI, X, XI, XII and XIV). These isolates were obtained from fruits collected at mango orchards located in the municipality of Chahuites, State of Oaxaca in Mexico.
Molecular identification
Bacterial DNA was extracted from the isolate that presented the major biocontrol against the photogenic fungi studied using the Bacterial/Fungal DNA kit (Cat D6005) Zymo Research and used for the 16S ribosomal DNA gene amplification, according to Weisburg et al. (1991). The product generated was purified using the GFX PCR DNA kit and Gel Band Purification (Cat. 28-9034-70) Illustra, sequenced and compared with sequences deposited in the NCBI GenBank.
Biocontrol of C. gloeosporioides ATCC MYA 456 by volatile metabolites of B. cepacia XXVI
1 × 106 spores of C. gloeosporioides ATCC MYA 456 was inoculated in the center of Petri-dishes containing PDA and another was spread with 1 × 104 CFU of B. cepacia XXVI, both plates were placed face to face and they were sealed to prevent the loss of potential volatile metabolites. This experiment was carried out at 28 °C for 7 days. The inhibition of the pathogen was determined according to Schmidt et al. 2009. This assay was performed using three independent replicates.
Biocontrol of C. gloeosporioides ATCC MYA 456 by diffusible metabolites of B. cepacia XXVI
1 × 104 CFU of B. cepacia XXVI was inoculated in 250 ml of Potato Dextrose Broth (PDB) at 28 °C for 4 days in a rotary shaker at 100 rpm. 1 ml of culture was centrifuged at 10,000 rpm for 10 min and filtered through hydrophilic Millipore membrane with pore size 0.45 μm. Petri-dishes containing PDA were inoculated, in the center, with 1 × 106 spores of C. gloeosporioides ATCC MYA 456 and 300 μl of the filtered supernatant was placed in each of three points around the pathogen. The inoculated Petri-dishes were incubated at 28 °C for 7 days. The inhibition of the pathogen was determined according to Schmidt et al. 2009. This assay was performed using three independent replicates.
Characterization of metabolite(s) causing inhibition of C. gloeosporioides
In order to characterize the nature of the inhibitory metabolite(s), 1 × 104 CFU of B. cepacia XXVI was inoculated in 100 ml of PDB and incubated at 28 °C for 4 days in rotary shaker at 100 rpm. The culture was centrifuged at 10,000 rpm for 10 min and filtered through hydrophilic Millipore membrane, with pore size 0.45 μm to obtain a stock of supernatant containing the biocontrol metabolite(s) for further experiments.
Thermostability of metabolite(s)
Three supernatant aliquots of 25 ml each were treated for 15 min to three temperature conditions: 28, 70 °C, or autoclaved to 121 °C (1 atm). Then, each supernatant aliquot was evaluated to determine its biocontrol capability, as described above.
Assay for lytic enzyme activity
Supernatant aliquots obtained above were used to quantify cellulase, chitinase and glucanase activities. These were measured according to the method described by Ghose (1987), Suresh and Chandrasekaran (1998) and Kulminskaya et al. (2001), respectively. One unit of activity was defined as the amount of enzyme that releases one micromole equivalent of GlcNAc or glucose per minute under the specified assay conditions. Enzyme yield was expressed as μmol g−1 sust min−1. In relation to chitinase and glucasase/cellulose activities, the reducing sugar released was measured at 565 nm by the 3,5-dinitrosalicylic acid (DNS) modified method (Miller 1959) using N-acetyl-D-glucosamine and glucose as standard, respectively.
Assaying for production of indole acetic acid (IAA)
This test was carried out by inoculating 5 × 103 CFU of B. cepacia XXVI in 50 ml of PDB supplemented with 100 ppm of tryptophan and incubated at 28 °C for 6 days at 100 rpm. After incubation, 1 ml of culture was centrifuged at 13,000 rpm for 10 min, then 2 ml of Salkowski reagent was added to the supernatant and incubated for 20 min at room temperature (Glickmann and Dessaux 1995). The samples were measured at 540 nm in a TECAN A-5082 spectrophotometer, model Genius. The production of IAA was performed using three independent replicates.
Assaying for siderophore production
As established by Schwyn and Neilands (1987), the Chrome Azurol S (CAS)-agar assay was used to determine siderophore production by B. cepacia XXVI. 1 × 104 CFU of this strain were added to Petri-dishes containing CAS-agar, incubated during 7 days at 28 °C. [CAS-agar was prepared by combining four sterile solutions. The Fe-CAS indicator solution (solution 1) was prepared with 10 ml of 1 mM FeCl3 (dissolved in 1 mM HCl) and 50 ml of CAS (1.21 mg ml−1), to the resulting blue solution were added 40 ml of CTAB (1.82 mg ml−1). The buffer solution (solution 2) was prepared by dissolving 30.24 g of PIPES in 750 ml salt solution containing 0.3 g KH2PO4, 0.5 g NaCl and 1 g NH4Cl, the pH was adjusted to 6.8 with KOH at 50 %, the volume was adjusted to 800 ml and 15 g agar was added. Solution 3 was prepared by dissolving 2 g glucose, 2 g mannitol, 493 mg MgSO4, 11 mg CaCl2, 1.17 mg MnSO4, 1.4 mg H3BO3, 0.04 mg CuSO4, 1.2 mg ZnSO4 and 1 mg Na2MoO4 in 70 ml of water. A solution of 30 ml of 10 % casamino acids was sterilized by filtration (solution 4). All the solutions were mixed carefully adding at the end the solution 1 (Alexander and Zuberer 1991).] The experiments to explore the production of siderophores were performed in triplicate.
Characterizing the siderophore produced by B. cepacia XXVI
Hydroxamate
FeCl3 test
2 ml of 2 % FeCl3 were added to 1 ml of the filtered supernatant. The presence of a peak between 400 and 450 nm indicates the nature of hydroxamate (Neilands 1981). The samples were analyzed in a spectrophotometer UV visible VARIAN CARY 3E model.
Tetrazolium test
A pinch of tetrazolium salt and 1–2 drops of 2 N NaOH were added to 1 ml of the filtered supernatant. The instant appearance of a deep red color is indicative of hydroxamate siderophores (Snow 1954).
Catecholate
FeCl3 test
1 ml of 2 % FeCl3 was added to 1 ml of the filtered supernatant. The presence of a peak at 495 nm indicates the presence of catecholate (Neilands 1981). The samples were analyzed in a spectrophotometer UV visible VARIAN CARY 3E model.
Arnow’s test
To 1 ml of culture filtrate was added 0.1 ml of 5 N HCl, 0.5 ml of reagent containing 10 g each of NaNO2 and Na2MoO4 × 2H2O in 50 ml water. After the formation of yellow color at this point, 0.1 ml of 10 N NaOH (a red color resulted) and enough distilled water was added to reach a volume of 5 ml. Absorbance was read at 515 nm (Arnow 1937) using a spectrophotometer UV visible VARIAN CARY 3E model.
Carboxylate
1 ml of 250 μM CuSO4 and 2 ml of acetate buffer pH 4 were added to 1 ml of the filtered supernatant. The copper complex solution was observed in a UV visible spectrophotometer VARIAN model CARY 3E between 190 and 280 nm (Shenker et al. 1992).
Minimum inhibitory concentration
Different volumes of supernatant were added to Petri-dishes containing PDA reaching concentrations of 0.04, 0.08, 0.16, 0.32, 0.64, 0.80, 0.96 μg ml−1 of siderophore (Deferoxamine mesylate salt-equivalent). 1 × 106 spores of C. gloeosporioides ATCC MYA 456 was inoculated in the center of these Petri-dishes and incubated at 28 °C during 7 days. After this period, the growth of C. gloeosporioides ATCC MYA 456 was measured. This experiment was performed using three independent replicates.
Growth kinetics and siderophore production by B. cepacia XXVI and growth inhibition against C. gloeosporioides ATCC MYA 456
These tests were carried out incubating 1x104 CFU of B. cepacia XXVI in 30 ml of PDB at 28 °C and 150 rpm determining growth kinetics and siderophore production every 24 h during 7 days. Growth kinetics was carried out using optical density at 600 nm using a Varian Cary 3 E model spectrophotometer. Siderophore production was quantified using an equivalent standard calibration curve of hydroxamate nature (Deferoxamine mesylatem salt, Sigma-Aldrich), in a range between 0.1 and 10 μg ml−1, subjecting to the FeCl3 test and measuring absorbance in UV visible range in a Varian Cary 3E spectrophotometer at 420 nm. These tests were performed using two independent replicates. Growth inhibition of C. gloeosporioides ATCC MYA 456 by siderophore was performed by inoculating 1 × 106 spores of C. gloeosporioides ATCC MYA 456 in 30 ml PDB containing the same siderophore concentration produced by B. cepacia XXVI every 24 h during its growth kinetics, centrifuged at 10,000 rpm for 10 min and filtered through hydrophilic Millipore membrane, with pore size 0.45 μm. The growth inhibition of the pathogen was calculated using its mycelia dry weight at 70 °C and expressed as a percentage.
Statistical analysis
Data were analyzed by one-way analyses of variance (ANOVA) test and Tukey–Kramer method (P = 0.05) using JMP-SAS software v. 8.0.2.
Results and discussion
Growth inhibition of C. gloeosporioides by B. cepacia XXVI
Anthracnose, the main disease of world-wide mango production, has been controlled by several alternatives: (1) physical methods such as pruning, ultraviolet light, modified atmosphere, etc. (Stevens et al. 1997; Karabulut and Baykal 2004) and (2) chemical methods as fungicides, copper, ergosterol inhibitor, etc. (Ker 2001; Arias and Carrizales 2007). These alternatives present disadvantages mainly in regard to efficiency of disease control (physics methods) and environmental hazards (chemical methods) as well as high economical costs (de los Santos-Villalobos et al. 2011).
Therefore the discovery and optimization of alternatives to control efficiently this disease is necessary. We studied a biological control using bacterial strains isolated from mango orchards or its metabolite(s) produced. Seven bacterial isolates were obtained in this study, one of them, B4, was identified as a member of Bcc and named strain XXVI. According to Coenye et al. (2001), members of Bcc show similarities in the 16S ribosomal DNA higher than 97.7 %. B. cepacia XXVI showed a 99 % similarity with sequences of these strains deposited in the NCBI GenBank. Therefore, we can refer strain XXVI as a member of the Bcc.
This isolate showed the highest growth inhibition against C. gloeosporioides ATCC MYA 456, 91.5 ± 0.3 %, when they were co-inoculated onto Petri-dishes containing PDA in confrontation assays (Fig. 1a, b). In addition, B. cepacia XXVI was evaluated against five strains of C. gloeosporioides VI, X, XI, XII, XIV, which were isolated from mango “Ataulfo” orchards located in the municipality of Chahuites, State of Oaxaca in Mexico. B. cepacia XXVI was able to inhibit the growth of these strains, observing 73.1 ± 4.2, 64.2 ± 3.4, 58.2 ± 1.7, 50.6 ± 2.3, 48.2 ± 5.6 %, respectively, suggesting the potential use of this strain or its metabolite(s) as a biocontrol agent against at least C. gloeosporioides, the causal agent of anthracnose. Similar results have been reported for others strains of B. cepacia showing biological control against several phytopathogenic fungi such as Botrytis cinerea, Schizophyllum commune, Fusarium oxysporum, Phytium, Rhizoctonia solani, causal agents of blue molt of apple, seed rot of oil palm, wilt of tomato, root rot of pea, root rot of Poinsettia, respectively (Kang et al. 1998; Kamaruzaman and Dikin 2005).
Confrontation assays a of the seven isolates obtained in this study, (b1) B. cepacia XXVI (arrowhead) against C. gloeosporioides ATCC MYA 456 and (b2) only pathogen inoculated, onto Petri-dishes containing PDA incubated at 24 °C during 7 days. Values with the same letter are not significantly different according to ANOVA test (P = 0.05)
With regard to the potentially volatile metabolites that may contribute to the inhibition of C. gloeosporioides ATCC MYA 456, the results of our plate-colonization assays did not indicate that volatile metabolites were significantly involved, 0.3 ± 0.1 % of inhibition. That was dominated by non-volatile, diffusible metabolites reaching 93.2 ± 1.6 %. A similar tendency for both classes of metabolites has been reported by Rahman et al. (2007), observing that the contribution of diffusible metabolites was more significant than by volatile metabolites corresponding to 100 and 26.61 % of inhibition of radial growth of C. gloeosporioides using a B. cepacia strain as antagonist microorganism.
Once the diffusible metabolites were identified as the main fraction with biological control against C. gloeosporioides ATCC MYA 456, the impact of a variety of heat treatments on their biocontrol efficiency was evaluated, observing the loss of activity when they were subjected, during 15 min, at 70 °C and autoclaving at 121 °C (1 atm) compared when they were incubated at 28 °C for the same time period; the latter showed 93.2 ± 1.6 % of inhibition of the pathogen. This thermal degradation of diffusible metabolites produced by B. cepacia XXVI suggests that these have a different chemical nature compared with cyclic antibiotic lipopeptides such as iturins and pyrrolnitrin produced by Bacillus sp. and B. cepacia, which resist these thermal degradations (Bernal et al. 2002; Kadir et al. 2008).
Characterization of metabolites involved in biocontrol
With the aim to characterize the nature of diffusible metabolites involved in the biocontrol against C. gloeosporioides ATCC MYA 456 produced for B. cepacia XXVI, the activity of lytic enzyme produced by this strain was evaluated, observing no activity of cellulase, glucanase and chitinase contained in the supernatant presenting biocontrol capacity; this eliminated the possible role of lytic enzymes studied in the biocontrol activity of B. cepacia XXVI, in contrast to reports by several authors indicating that B. cepacia produces beta 1, 3, glucanases, chitinases which are involved in the degradation of cell wall in phytopathogenic fungi (Fridlender et al. 1993; Compant et al. 2005).
We considered the possibility that the inhibitory factor may be IAA. Our rationale was that, in addition to its role as a phytohormone, IAA has been reported as antifungal metabolite against Phymatotrichum omnivorum, Penicillium herquei, Fusarium nivale, Thielavia terricola, and Cunninghamella echinulata in high concentration up to 400 ppm, evidenced by early studies (Leonian and Lilly 1937; Leelavathy 1969). Results for IAA assays were negative for B. cepacia XXVI, indicating non-role of this phytohormone on the inhibition of C. gloeosporioides ATCC MYA 456.
Assays for siderophore production by B. cepacia XXVI were conducted using CAS-agar. We observed that strain XXVI is able to produce siderophore as indicated by the presence of an orange halo (Fig. 2a) (Schwyn and Neilands (1987). To explore the role of this siderophore in the biological control against C. gloeosporioides ATCC MYA 456, a confrontation assay on CAS-agar between the pathogen and B. cepacia XXVI was conducted. We observed that this metabolite is involved in the biocontrol capacity against C. gloeosporioides ATCC MYA 456, as shown by its growth inhibition in the orange halo zone, with the iron limiting condition of less than 0.56 μg ml−1 of iron (Fig. 2b). C. gloeosporioides is dependent on iron availability, thus, in an environment with iron limitation imposed by siderophore action, C. gloeosporioides’ growth is inhibited, and it is restored when this element is added (Santoyo et al. 2010). Several authors have reported the role of siderophores in biocontrol of a wide diversity of bacteria genera such as Pseudomonas, Streptomyces, Ochrobacterium, Rhizobium and Burkholderia as biocontrol metabolite against Pyricularia, Fusarium, Alternaria, Macrophomina, Sclerotium and Phytophotora (Arora et al. 2001; Díaz de Villegas et al. 2002; Ezziyyani et al. 2004; Chaiharn et al. 2009).
Identification of siderophore production by CAS-agar indicated by an orange halo at 28 °C, a B. cepacia XXVI after 3 days of inoculation and b confrontation assays of co-inoculated B. cepacia XXVI and C. gloeosporioides ATCC MYA 456 after 3 days of inoculation (the pathogen was inoculated 3 days prior to B. cepacia XXVI), the dotted line represents the expected radial growth of the pathogen in absence of B. cepacia XVVI
The class of siderophore produced by B. cepacia XXVI, involved in its biocontrol capability, was identified using colorimetric test (Tetrazolium test) and spectrophotometric assays (FeCl3), showing that this metabolite belongs to hydroxamate class, due to the appearance of a deep red color and the presence of an only peak at 420 nm, respectively.
Minimum inhibitory concentration
Inhibition of C. gloeosporioides ATCC MYA 456 was quantitatively correlated with siderophore concentration. We found that a concentration of 0.64 μg ml−1 of siderophore (Deferoxamine mesylate salt-equivalent) is sufficient to inhibit 91.1 ± 0.5 % of pathogen growth and this percent did not increase significantly when a siderophore concentration of 0.96 μg ml−1 was added to Petri-dishes containing PDA (Fig. 3). These results suggest that 0.64 μg ml−1 of siderophore is sufficient to chelate and decrease the major iron concentration in the medium. Similarly, Santoyo et al. (2010) had reported that siderophore concentration is inversely related to the amount of iron available. These data are consistent with the notion that the increased siderophore production by the biocontrol strain triggers inhibition of pathogen fungi due to the starvation of iron. This inhibition can be restored when iron is added to the medium.
Growth kinetics, siderophore production by B. cepacia XXVI and its growth inhibition against C. gloeosporioides ATCC MYA 456
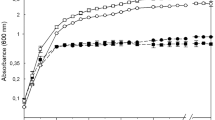
As shown in Fig. 4, B. cepacia XXVI reached its exponential phase at 4th day of incubation, observing a minimal production of siderophore, 0.2 ± 0.0 μg ml−1 (Deferoxamine mesylate salt-equivalent), probably due to background iron concentration in the medium, leading to low inhibition of the pathogen, 9.8 ± 0.1 %, suggesting that siderophore is a secondary metabolite as previously reported by Drechsel and Jung (1998) and Fischbach et al. (2006). Siderophore production increased, during the stationary phase (at 4th day of incubation) correlating with the inhibition of C. gloeosporioides ATCC MYA 456, observing a high production of this metabolite, 1.7 ± 0.1 μg ml−1 (Deferoxamine mesylate salt-equivalent), and inhibition of the pathogen, 94.9 ± 4.3 %, at 7th day of incubation. Data suggest that in the stationary phase, the amount of siderophore was increased due to a state of iron starvation attributed to the consumption of this element in the culture medium as a result of bacterial multiplication (Crosa 1997; Lim et al. 1998; Cowart 2002; González-Carreró et al. 2002), resulting in the pathogen inhibition when this siderophore was added to the culture medium. These complex iron-siderophores present a high dissociation constant (~pKa = 29) generating a strong starvation of iron in the environment when the concentration of this element is low (Chen et al. 1994), helping to capture and supply this element to siderophore-producing strains under that condition (Loper and Henkels 1999).
Siderophore production (Deferoxamine mesylate salt-equivalent) during the growth of B. cepacia XXVI measured every 24 h over 7 days, contrasting this siderophore production with growth inhibition of C. gloeosporioides ATCC MYA 456 in broth culture by suspension of C. gloeosporioides spores in PDB. Means with the same letter (growth inhibition) and asterisk numbers (siderophore production) are not significantly different according to ANOVA test (P = 0.05)
This capability has great environmental significance, during the interaction (colonization and establishment) of siderophore-producing strains/plants, conferring competitive advantages for space and nutrients by these strains (Harrison et al. 2008; Eberl and Collinson 2009).
Our results suggest the role of siderophore produced by B. cepacia XXVI (isolated from mango orchards) as an alternative to biocontrol the pathogenic fungus causing anthracnose in mango, C. gloeosporioides.
It is important to mention that B. cepacia strains have been reported as human or plant pathogens, therefore, the potential application of this biocontrol alternative requires further studies focusing mainly on the characterization of these pathogenic traits. Alternatively, the optimization of siderophore production in large scale to apply this metabolite, alone, against anthracnose in pre and/or post-harvest opens an attractive alternative.
Conclusions
Burkholderia cepacia XXVI is a promising biological control agent against the causal agent of anthracnose, C. gloeosporioides, through the production of hydroxamate siderophore. The siderophore is produced in high concentration at the stationary phase, when the iron concentration in the medium was decreased by bacterial growth, reaching 1.7 ± 0.1 μg ml−1 (Deferoxamine mesylate salt-equivalent), and showing a growth inhibition of the pathogen of 94.9 ± 4.3 % at 7th day of incubation.
These results support the potential use of B. cepacia XXVI or its siderophore as a microbial alternative to control pathogens involved in high losses of agricultural production, diminishing the environmental problems caused by current practices.
References
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 45:12–39. doi:10.1007/BF00369386
Ann PJ, Chen MF, Hwang RC (1997) Effects of environmental factors on disease incidence of mango anthracnose and bacterial black spot. In: Tu CC, Yang CM (eds) Proceedings of the symposium on climatic effects on the occurrence of plant diseases and insects. Society of Agrometereology, Wufeng, Taichung, Taiwan, R.O.C, pp 29–40
Arias B, Carrizales L (2007) Control químico de la antracnosis del mango (Mangifera indica L.) en pre y postcosecha en el municipio Cedeño, estado Monagas, Venezuela. Bioagro 19(1):19–25
Arnow LE (1937) Colorimetric determination of the components of 3,4-dihydroxyphenylalanine tyrosine mixtures. J Biol Chem 118:531–537
Arora NK, Kang SC, Maheshwari DK (2001) Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81:673–677
Avilán RL (2008) Nutrición y fertilización del mango. Int Plant Nutr Inst 33:44
Bernal G, Illanes A, Ciampi L (2002) Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp. with antibiotic activity against plant pathogenic agents. EJB 5:12–20
Bevivino A, Dalmastri C, Tabacchioni S, Chiarini L, Belli ML, Piana S, Materazzo A, Vandamme P, Manno G (2002) Burkholderia cepacia complex bacteria from clinical and environmental sources in Italy: genomovar status and distribution of traits related to virulence and transmissibility. J Clin Microbiol 40:846–851. doi:10.1128/JCM.40.3.846-851
Carrillo-Castañeda G, Juárez-Muñoz J, Peralta-Videa J (2005) A spectrophotometric method to determine the siderophore production by strains of fluorescent Pseudomonas in the presence of copper and iron. Microchem J 81:35–40
Cartwright DK, Benson DM (1995) Comparison of Pseudomonas species and application techniques for biocontrol of Rhizoctonia stem rot of poinsettia. Plant Dis 79:309–313. doi:10.1094/PD-79-0309
Cartwright DK, Chilton C, Benson DM (1995) Pyrrolnitrin and phenazine production by Pseudomonas cepacia, strain 5.5B, a biological agent of Rhizoctonia solani. Appl Microbiol Biotechnol 43:211–216. doi:10.1007/BF00172814
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25:1919–1928. doi:10.1007/s11274-009-0090-7
Chen Y, Jurkevitch E, Bar-Ness E, Hadar Y (1994) Stability constants of pseudobactin complexes with transition metals. Soil Sci Soc Am J 58:390–396
Coenye T, Vandamme P, Govan JRW, Lipuma JJ (2001) Taxonomy and identification of the Burkholderia cepacia complex. JCM 39(10):3427–3436
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959. doi:10.1128/AEM
Cowart RE (2002) Reduction of iron by extracellular iron reductases: implications for microbial iron acquisition. Arch Biochem Biophys 400:273–281. doi:10.1016/S0003-9861(02)00012-7
Crosa JH (1997) Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev 61:319–336
Cuervo-Parra JA, Ramírez-Suero M, Sánchez-López V, Ramírez-Lepe M (2011) Antagonistic effect of Trichoderma harzianum VSL291 on phytopathogenic fungi isolated from cocoa (Theobroma cacao L.) fruits. Afr J Biotechnol 10(52):10657–10663
de los Santos-Villalobos S, de-Folter S, Délano-Frier JP, Gómez-Lim MA, Guzmán-Ortiz DA, Sánchez-García P, Peña-Cabriales JJ (2011) Puntos críticos en el manejo integral de mango: floración, antracnosis y residuos industriales. Rev Mex Cienc Agríc 2(2):221–234
Díaz de Villegas ME, Villa P, Frías A (2002) Evaluation of the siderophores production by Pseudomonas aeruginosa PSS. Rev Latinoam Microbiol 44:112–117
Drechsel H, Jung G (1998) Peptide siderophores. J Pept Sci 4:147–181
Eberl HJ, Collinson S (2009) A modeling and simulation study of siderophore mediated antagonism in dual-species biofilms. TBioMed 6(30):1–16
Estrada-de los santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. App Environ Microbiol 67(6):2790–2798
Ezziyyani M, Pérez C, Requena M, Ahmed A, Candela M (2004) Evaluación del biocontrol de Phytophthora capsici en pimiento (Capsicum annuum L.) por tratamiento con Burkholderia cepacia. Anales de Biología 26:61–68
Faraldo-Gómez JD, Sansom MSP (2003) Acquisition of siderophores in Gram-negative bacteria. Nat Rev Mol Cell Biol 4:105–116. doi:10.1038/nrm1015
Fischbach MA, Lin H, Liu DR, Walsh CT (2006) How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol 2:132–138
Fridlender M, Inbar J, Chet I (1993) Biological control of soilborne plant pathogens by a β-1,3 glucanase-producing Pseudomonas cepacia. Soil Biol Biochem 25:1211–1221. doi:10.1016/0038-0717(93)90217
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
González-Carreró MI, Sangari FJ, Agüero J, García-Lobo JM (2002) Brucella abortus strain 2308 produces brucebactin, a highly efficient catecholic siderophore. Microbiol 148:353–360
Harrison F, Paul J, Massey RC, Buckling A (2008) Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa. ISME J 2(1):49–55
Hebbar KP, Martel MH, Heulin T (1998) Suppression of pre and post emergence damping off in corn by Burkholderia cepacia. Euro J Plant Pathol 104:29–36. doi:10.1023/A:1008625511924
Hwang J, Chilton WS, Benson DM (2002) Pyrrolnitrin production by Burkholderia cepacia and biocontrol of rhizoctonia stem rot of poinsettia. Biol Control 25:56–63. doi:10.1016/S1049-9644(02)00044-0
Kadir J, Rahman MA, Mahmud TMM, Abdul Rahman R, Begum MM (2008) Extraction of antifungal substances from Burkholderia cepacia with antibiotic activity against Colletotrichum gloeosporioides on papaya (Carica papaya). Int J Agri Biol 10:15–20
Kamaruzaman S, Dikin A (2005) Biochemical and physiological characterization of Burkholderia cepacia as biological control agent. Int J Agri Biol 3:385–388
Kang Y, Carlson R, Tharpe W, Schell MA (1998) Characterization of genes involved in biosynthesis of a novel antibiotic from Burkholderia cepacia BC11 and their role in biological control of Rhizoctonia solani. Appl Environ Microbiol 64:3939–3947
Karabulut OA, Baykal N (2004) Integrated control of postharvest diseases of peaches with a yeast antagonist, hot water and modified atmosphere packing. Crop Prot 23:431–435
Katiyar V, Goel R (2004) Siderophore mediated plant growth promotion at low temperature by mutant of fluorescent pseudomonad. Plant Growth Regul 42:239–244. doi:10.1023/B:GROW.0000026477.10681.d2
Ker CK (2001) Sensitivity of mango anthracnose pathogen, Colletotrichum gloeosporioides, to the fungicide prochloraz in Taiwan. Proc Natl Sci Counc 25(3):174–179
Kulminskaya AA, Tomsen KK, Shabalin KA, Sidorenko IA, Eneyskaya EV, Savel’ev AN, Neustroev KN (2001) Isolation, enzymatic properties, and mode of action of an exo-1,3-β-glucanase from Trichoderma viride. Euro JBiochem 23:6123–6131
Leelavathy KM (1969) Effects of growth-regulating substances on fungi. Can J Microbiol 15:713–721. doi:10.1139/m69-126
Leonian LH, Lilly VG (1937) Is heteroauxin a growth promoting substance? Am J Bot 24:129–135
Lim Y, Shin SH, Lee SI, Kim IS, Rhee JH (1998) Iron repressibility of siderophore and transferrin-binding protein in Staphylococccus aureus. FEMS Microbiol Lett 163:19–24. doi:10.1016/S0378-1097(98)00143-8
Lipuma JJ (2003) Burkholderia cepacia complex as human pathogens. J Nematol 35:212–217
Loper JE, Henkels MD (1999) Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl Environ Microbiol 65:5357–5363
Mahenthiralingam E, Baldwin A, Dowson CG (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104:1539–1551
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–445
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
O’Sullivan DJ, O’Gara F (1992) Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Okoh IA, Ajisebutu S, Babalola GO, Trejo-Hernandez MR (2001) A study of the potentials of a Burkholderia cepacia strain (RQ1) in the biodegradation of heavy crude oil (Maya). Int Microbiol 4:83–87
Páez M, Martínez-Nieto P, Bernal-Castillo J (2005) Siderophore producing Pseudomonas as pathogenic Rhizoctonia solani and Botrytis cinerea antagonists. Universitas Scientiarum 10:65–74
Parke JL, Gurian-Sherman D (2001) Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39:225–258
Rahman MA, Kadir J, Mahmud TMM, Abdul Rahman R, Begum MM (2007) Screening of antagonistic bacteria for biocontrol activities on Colletotrichum gloeosporioides in papaya. Asian J Plant Sci 6:12–20
Roitman JN, Mahoney NE, Janisiewicz WJ (1990) Production and composition of phenylpyrrole metabolites produced by Pseudomonas cepacia. Appl Microbiol Biotechnol 34:381–386
SAGARPA (2007) Servicio de Información Agroalimentaria y Pesquera. Recuperado el 11 de 06 de 2008, de http://www.siap.gob.mx/
Sandy M, Butler A (2009) Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 109:4580–4595
Santoyo G, Valencia-Cantero E, Orozco-Mosqueda MC, Peña-Cabriales JJ, Farías-Rodríguez R (2010) Papel de los sideróforos en la actividad antagónica de pseudomonas fluorescens zum80 hacia hongos fitopatógenos. Terra Latinoamericana 28:53–60
Schmidt S, Blom JF, Pernthaler J, Berg G, Baldwin A, Mahenthiralingam E, Eberl L (2009) Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex. Environ Microbiol 11:1422–1437
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shenker M, Oliver I, Helmann M, Hadar Y, Chen Y (1992) Utilization by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. J Plant Nutr 15:2173–2182
Silveira Mello AF, Zamboni Machado AC, Bedendo IP (2004) Development of Colletotrichum gloeosporioides isolated from green pepper in different culture media, temperatures, and light regimes. Sci Agric 61(5):542–544
Snow GA (1954) Mycobactin, a growth factor for Mycobacterium johnei: II. Degradation and identification of fragments. J Chem Soc 49:2588–2596
Sritharan M (2000) Iron as a candidate in virulence and pathogenesis in mycobacteria and other microorganisms. World J Microbiol Biotechnol 16:769–780
Stevens CV, Khan A, Lu JY, Wilson CL, Pusey PL, Igwegbe ECK, Kabwe K, Mafolo Y, Chalutz E, Droby S (1997) Integration of ultraviolet (UV-C) light with yeast treatment for control of postharvest storage rots of fruits and vegetables. Biol Control 10:98–103
Stoyanova M, Pavlina I, Moncheva P, Bogatzevska N (2007) Biodiversity and incidence of Burkholderia species. Biotechnol Biotechnol Equip 21:306–310
Suresh PV, Chandrasekaran M (1998) Utilization of prawn waste for chitinase production by the marine fungus Beauveria bassiana by solid state fermentation. World J Microbiol Biotechnol 14:655–660
Thahir Basha S, Suvarna J, Hemalatha TM, Eswara Reddy NP (2010) Compatibility of native potential bioagents with different fungicides against Colletotrichum gloeosporioides penz. causing mango anthracnose. Bioscan 5:19–20
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Wong DK, Gobin J, Horwitz MA, Gibson BW (1996) Characterization of exochelins of Mycobacterium avium: evidence for saturated and unsaturated acid for acid and ester forms. J Bacteriol 178:6394–6398
Xi K, Stephens JHG, Verma R (1996) Application of formulated rhizobacteria against root rot of field pea. Plant Pathol 45:1150–1158
Acknowledgments
We express our thanks to Dr. Eugene L. Madsen for his valuable help in the proper use of the English language. We extend our thanks to the Referees (anonymus) for suggestions which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de los Santos-Villalobos, S., Barrera-Galicia, G.C., Miranda-Salcedo, M.A. et al. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides . World J Microbiol Biotechnol 28, 2615–2623 (2012). https://doi.org/10.1007/s11274-012-1071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1071-9