Abstract
Zygosaccharomyces rouxii V19 was grown in YPG medium (yeast extract, 0.5%, peptone, 1.0%, glucose, 10%). Fermented broth was purified through a series of ion-exchange columns and ODS column and the purified sample was TMS-esterified. Malic and succinic acids were identified with GC-MS analysis. The yeast was cultivated under various cultural conditions and quantitative determination of the organic acids was carried out with HPLC on Shodex column. Glucose concentration of 30%, initial pH 5.0, and 25 °C incubation temperature were the optimum conditions. Inclusion of glutamic, malic, and succinic acid precursors in the medium increased the production of malic acid. On the other hand, only addition of malic acid enhanced the production of succinic acid. Maximum amount of malic acid produced was 74.9 g/L (32.8% yield, based on glucose consumption) in the medium with 0.5% glutamic acid supplement, and that of succinic acid was 7.7 g/L (8.1% yield) when 0.3% malic acid was added in the medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malic and succinic acids, along with other organic acids, are widely used as acidulant in soft drinks, and as chelating agents in a variety of other products. Radler [1] had reviewed the possibility of production of many organic acids by yeasts. There were some reports on formation of malic acid from S. cerevisiae [2, 3] and from S. bayanus [4], and that of succinic acid from S. cerevisiae [5–7]. Burden and Evenleigh [8] proposed that yeasts have potential for commercial production of a wide range of organic acids. However, with malic and succinic acids, Radler [1] concluded in his work that the quantities produced were so low that industrial utilization was not practical. On the other hand, there were some works on transport of malic and succinic acids, together with other dicarboxylic acids, in Hansenula anomala [9], Schizosaccharomyces pombe [10, 11], and S. bailii [12]. With S. cerevisiae, Albers et al. [7] had studied the influence of precursor glutamic acid on product formation. Camarasa et al. [13] also explored the tricarboxylic acid pathway in this yeast and concluded that assimilation of precursor aspartate led to the formation of malate and succinate. Sousa et al. [11] mentioned that succinic acid and malic acid itself, among other precursors, were competitive inhibitors of malic acid transport at pH 5.0. Pines et al. [14] reported that l-malic acid was synthesized from its precursor pyruvic acid via oxaloacetic acid.
Z. rouxii is an osmo-tolerant yeast associated with foods of low water-activity, such as soy paste, soy sauce, and high-sugar foods. It has been known that salt-tolerant strains of Z. rouxii produced considerable amount of polyalcohols such as glycerol and/or d-arabitol [15–18]. Young et al. [19] reported on the formation of acetic acid from glucose during anaerobic fermentation with a strain of salt-tolerant soy yeast. Apart from this information, little has been known about formation of organic acids by this yeast. Nowadays it is generally regarded that salt-tolerance and sugar-tolerance are different aspects of osmo-tolerance; salt-tolerant strains were not necessarily tolerant to sugar concentrations of same water activity, and vice versa [20, 21]. Although there has been considerable work on salt-tolerant strains of Z. rouxii, little has been done with its sugar-tolerant counterpart. In our previous work, we isolated, identified, and characterized many strains of Z. rouxii from high-sugar fermented foods [21]. During our investigation of antibacterial activity of these strains, we found out that some strains produced organic acids in such a considerable amount that bacteria were killed when they were co-incubated with the yeasts.
Our preliminary results and lack of information so far prompted us to investigate the potential application of Z. rouxii in production of organic acids. This paper deals with identification and quantitative determination of organic acids produced by a selected strain of Z. rouxii. The most suitable culture medium was chosen and the cultural conditions were optimized. Effects of precursors and their possible metabolic pathways were also investigated.
Materials and methods
Yeast strain and medium
The strain used in this work was Z. rouxii V19, which was isolated from high-sugar fermented food and identified according to standard taxonomy [21]. Unless otherwise expressed, the yeast was grown under control conditions, in YPG control medium (0.5% yeast extract, 1% peptone, 10% glucose; all% w/v, initial pH unadjusted), at 25 °C, for 140 h. All of the media used in our work were filter-sterilized to avoid formation of Maillard reaction products during autoclaving.
Optimization of cultural conditions
With many variables to be determined for optimization, we varied one by one, keeping the rest at control conditions. Cultural conditions were varied as follows: glucose concentration, from 10 to 50%; initial pH, from 4.0 to 8.0; incubation temperature, from 20 to 37 °C. The yeast was cultured in 5 mL medium, inside 15 mm-test tubes, for 140 h. Overnight grown yeast (0.1 mL) containing approximately 4×106 cells/mL was inoculated to each test tube, and the yeast was incubated semiaerobically, by gentle shaking each day. Growth was determined either by measuring optical density (OD) at 660 nm or wet cell weight.
Effect of precursors
To YPG control medium (10% glucose), 0.3% each of malic, succinic, fumaric, aspartic, or glutamic acid was added and pH was adjusted to 5.0. With each type (group) of medium, the yeast was cultured for 30 days in a number of test tubes. From each group, two test tubes were taken out occasionally for analyses. Values were expressed as average of two.
Next, in order to study the effect of concentration of each precursor, 0.3–0.7% each of malic, succinic, or glutamic acid precursor was added to YPG medium with 30% glucose, and initial pH was adjusted to 5.0. Inoculation was carried out as described above. Incubation was carried out at 25 °C for 15 days. For chemical analyses, two test tubes were taken out each time at prescribed interval. Values were expressed as average of two.
Analytical methods
pH was measured by Horiba pH meter. Remaining glucose in the broth was determined by AOAC method [22]. Glucose consumed was calculated by subtracting glucose remaining from the initial added amount.
Identification and quantitative determination of organic acids
Sample preparation
After incubation, the cultural suspension was centrifuged (9,000×g, 15 min) at 4 °C, followed by filtering through a membrane filter of 0.5 μm pore size. The filtered broth was kept frozen at −18 °C until use. One mL of this sample was ion-exchanged with Amberlite IR-120 [H+] and Amberlite IR-45 [OH−] resins in two successive columns. The latter was eluted with 2 N NH3 solution followed by washing with distilled water. Combined eluates were evaporated to dryness under vacuum at 30 °C. The solid residue was dissolved in 10 mL of distilled water, and the solution, after purification through ODS filter (syringe type), was injected to HPLC.
HPLC analysis
Quantitative determination of succinic and malic acids were carried out with HPLC, Shimadzu LC-10AS liquid chromatograph, equipped with Toyosoda UV-8 model II spectrophotometer and Shimadzu C-R4A Chromatopac integrator. The column was of 50 cm×4.6 mm i.d., packed with Shodex HC-125S resin. Mobile phase was 0.1% v/v phosphoric acid. Analysis conditions were: constant pressure of 43 Kg/cm2; temperature, 55 °C; flow rate, 0.3 mL/min; detector, UV 210 nm.
GC-MS analysis
ODS-purified sample was TMS-esterified and analyzed for mass spectra. GC-MS analysis was carried out with Hitachi M-80B gas chromatograph, equipped with Chrompack capillary column (CP-Sil SCB, 25 m×0.25 mm i.d.; 1.2 mm coating) and coupled to Hitachi M-80B mass selective detector. The analysis conditions were ionization potential, 70 eV; source temperature, 200 °C; GC-MS interface temperature, 220 °C. Oven temperature was increased from 80 to 250 °C at 5 °C/min.
Results and discussion
Optimization of cultural conditions
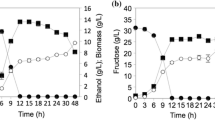
With each variable, we determined best values both for growth and production of the organic acids, keeping the others at control conditions. Figure 1 shows the effect of glucose concentration on growth and acid production. Although 20% glucose resulted in a maximum growth, 30% was optimum for production of both acids. Figure 2 shows the effect of incubation temperature. Here again, while the optimum temperature for growth was around 32 °C; that for acid formation was about 26 °C. Acidic medium favored growth of V19, as can be seen in Fig. 3 that growth was maximum at pH 4.0. However, production of acids peaked at pH 5.0. These results helped us choose the best combination of cultural conditions for getting maximum concentration of the two organic acids in the later part of our work.
Effect of added precursors
Figure 4 shows growth curves of strain V19 in media with various added precursors. Growth (rate as well as yield) was in ascending order for fumaric-, malic-, succinic-, glutamic- acid containing media; it was clearly repressed by addition of precursors. Maximum growth was observed with control medium. However, except fumaric acid, production of malic acid was greatly enhanced by all precursors added (Fig. 5). In particular, addition of glutamic acid and malic acid precursors increased malic acid concentration in the fermenting mass by about four and five times, respectively, that of control. In Fig. 5, malic acid concentration was expressed as net amount, which was obtained by subtracting the initial added amount from the final value. Hence it was clear that addition of malic acid enhanced remarkably the production of itself. However, this effect was transitory, as the concentration decreased beyond about 15 days. Based on this finding, we chose the cultural time of 15 days for further experiments. On the other hand, only malic acid precursor showed a moderate positive effect on succinic acid production (Fig. 6); it increased the production by about 30%, compared to control. All other precursors had either adverse or insignificant effect. Addition of succinic acid did not appreciably increase the net production of itself.
Optimum concentration of precursors
In optimizing concentration of precursors, we chose the incubation time of 15 days, as the concentrations decreased beyond this time. From Fig. 7, it was noted that optimum concentration of added malic acid for production of malic acid itself was around 0.5%. Net malic acid concentration in the fermenting mass increased from 43 g/L at 0.3% to about 72 g/L at 0.5%. A similar trend was observed with succinic acid precursor. Malic acid production increased from 8 g/L at 0.3% to about 13 g/L at 0.5% added succinic acid (Fig. 8). Glutamic acid showed the strongest impact on malic acid production (Fig. 9). The concentration of malic acid increased from 30 g/L at 0.3% to about 75 g/L at 0.5%. In all cases, succinic acid production was not affected by increase in concentration of added precursors.
Yield
In Table 1, a summary of production values of malic and succinic acids are shown for selected conditions, together with calculated yields based on glucose consumption. Among various cultural conditions, only glucose concentration (optimum value of 30%) had a considerably high impact on production of malic acid. Of the precursors added, glutamic acid was the most effective enhancer for production of malic acid from the medium with 30% glucose. We achieved maximum concentration of 74.9 g/L with 32.8% yield. This value was much higher than the reported value of 175 mM produced by permeabilized cells of S. bayanus from fumaric acid [4]. On the other hand, only addition of malic acid enhanced the production of succinic acid. Maximum amount of succinic acid produced was 7.7 g/L (8.1% yield) when 0.3% malic acid was included, which we noted as not a feasible value for commercial production. From these results, we consider Z. rouxii V19 as a favorable strain for commercial production of malic acid. It is anticipated that improvement in cultural conditions such as divided dosage of glucose and precursor, maintaining fermenting pH, and controlling aeration in an automated fermentor would increase the yield further, without modifying the strain.
References
Radler F (1993) In: Graham H (ed) Yeasts—metabolism of organic acids. Harwood, chur-Switzerland, pp 165–182
Schwartz H, Radler F (1988) Appl Microbiol Biotechnol 27:553–560
Wang X, Gong CS, Tsao GT (1998) Appl Biochem Biotechnol 70–72:845–852
Vrsalovic Presecki A, Vasic-Racki D (2005) Biotechnol Lett 27:1835–1839
Thoukis G, Ueda M, Wright D (1965) Am J Enol Vitic 16:1–8
Heerde E, Radler F (1978) Arch Microbiol 117:269–276
Albers E, Larsson C, Liden G, Niklasson C, Gustafsson L (1996) Appl Environ Microbiol 62:3187–3195
Burden DW, Evenleigh DE (1990) In: Spencer JFT, Spencer DM (eds) Yeast technology. Springer-Verlag, Berlin Heidelberg, pp 204–205
Corte-Real M, Leao C (1990) Appl Environ Microbiol 56:1109–1113
Osothsilp C, Subden RE (1986) J Bacteriol 168:1439–1443
Sousa MJ, Mota M, Leao C (1992) Yeast 8:1025–1031
Kuczynski JT, Radler F (1982) Arch Microbiol 131:266–270
Camarasa C, Grivet JP, Dequin S (2003) Microbiology 149:2669–2678
Pines O, Even Ram S, Elnathan N, Battat E, Aharonov O, Gibson D, Goldberg I (1996) Appl Microbiol Biotechnol 46:393–399
Onishi H (1960) Bull Agric Chem Soc Jpn 24:126–130
Groleau D, Chevalier P, Tse Hing Yuen TLS (1995) Biotech Lett 17:315–320
Yoshikawa S, Mitsui N, Chikara KI, Hashimoto H, Shimosaka M, Okazaki M (1995) J Ferment Bioeng 80:131–135
Zhuge J, Fang HY, Wang ZX, Chen DZ, Jin HR, Gu HL (2001) Appl Microbiol Biotechnol 55:686–692
Yong FM, Lee KH, Wong HA (1980) J Food Technol 15:421–428
Tokuoka K, Ishitani T, Goto S, Komagata K (1985) J Gen Appl Microbiol 31:411–427
Taing O, Hashinaga F (1997) J Gen Appl Microbiol 43:39–47
AOAC (1980) In: Official methods of analysis, 13th edn. Association of Official Analytical Chemists, Washington, DC
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taing, O., Taing, K. Production of malic and succinic acids by sugar-tolerant yeast Zygosaccharomyces rouxii . Eur Food Res Technol 224, 343–347 (2007). https://doi.org/10.1007/s00217-006-0323-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0323-z