Abstract
Differences in population growth and physiological characters of Nilaparvata lugens between the populations of the tropics and of temperate East Asia are discussed. Fundamental differences in population dynamics seem to be related to the origin of initial immigrants and activity of natural enemies. In tropical fields, initial immigrants originate from nearby paddy fields, resulting usually in high immigrant densities. On the other hand, few immigrants after seasonal long-distance migration initiate population in temperate paddy fields. In the tropics, N. lugens exhibit various population growth patterns depending on the interaction with the natural enemies. While in the temperate areas, populations tend to increase gradually due to paucity of natural enemies probably due to collapse of natural enemies during cold winter. N. lugens in subtropical and temperate East Asia, compared to tropical Asia, produce more macropters, and have longer pre-ovipositional period and more starvation tolerance. Thus, the East Asian population of N. lugens is more adapted to migration, while tropical populations in southeast Asia are adapted to multiplication. Biotype compositions and insecticide resistance in N. lugens populations in time and space are quite similar within East Asia (subtropical and temperate areas), while they tended to slightly differ depending on locations in the tropics. It is considered that these characters in East Asian N. lugens population are genetically maintained by a migration system mediated by seasonal monsoon wind. Strong population suppression by natural enemies in the tropics implies the possibility that escape from natural enemies was a driving force for evolution of migration in N. lugens.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Status of planthoppers as rice pests have changed depending on time in the tropics. Before the Green Revolution , the brown planthopper (BPH), Nilaparvata lugens, was one of the minor insect pests in the tropical paddy fields (Dyck and Thomas 1979; Heinrichs and Mochida 1984). International Rice Research Institute (IRRI) was founded in the Philippines in 1960. In fact, little description of N. lugens was found in IRRI Reports published in early 1960s (Kisimoto 1981). The first outbreak of BPH was observed at IRRI fields in 1964 (Iida 1972). In 1966, IR8 , a miracle rice, was released, and the green revolution began. In early 1970s, BPH outbreaks frequently occurred in the Southeast Asian countries where high-yielding rice varieties originated from IRRI were introduced (Dyck and Thomas 1979; Kisimoto 1981). IRRI released the varieties such as IR26 and IR32, which were resistant to BPH. However, the BPH overcome the resistance soon by the appearances of new biotypes (Saxena and Barrion 1985). Repeating events of releasing resistant varieties and overcoming their resistance by the appearances of new BPH biotypes have continued until now. Another problem with the BPH management is the development of insecticide resistance in BPH populations. Reduction of susceptibility to organophosphorus or carbamate insecticides appeared in late 1970s (Nagata et al. 1979; Kilin et al. 1981). Recently, the BPH populations showing high resistance to chloronicotinyl insecticides is a serious problem in Asian paddy fields (Matsumura et al. 2008).
The causes for BPH outbreaks in tropical paddy fields after the late 1960s are generally considered to be: (i) introduction of nitrogen responsive high-yielding varieties, which favors BPH multiplication through increased fecundity and low mortality; (ii) improvement of irrigation system, which facilitated intensive and successive rice planting throughout the year favored generation continuity of N. lugens; (iii) resurgence induced by abuse of insecticide applications: non-selective insecticide sprays destroy natural enemy fauna and possibly also increase N. lugens fecundity to a certain extent.
On the other hand, rice planthoppers, N. lugens and Sogatella furcifera, were serious pests of rice in East Asia from long time. The oldest record of planthopper outbreak was in 697 AD in Japan (Mochida and Okada 1979). Korean records show planthopper outbreaks since eighteenth century. During the early modern period of Edo era (1600–1867) in Japan, many records are found describing serious famines caused by planthopper damage together with cool and longtime rainy summer (Nagata 1982; Miyashita 1961). One of the great famines occurred in West Japan in 1732, when rice yields were decreased to only 10 % of normal yields. Recent advancements in understanding of wind-assisted long-distance planthopper migration into West Japan (Kisimoto 1976; Seino et al. 1987) explain why serious outbreaks occurred in cool rainy summers. There were many guardian deities of children (jizo), which console dead people on great famines of Edo era in all over Japan (Fig. 4.1a). In Amakusa, Kyushu, West Japan, where planthopper immigrant density is usually higher, farmers have performed an annual festival since Edo era in mid-July (“mushi-oi sai” that means driving away insects) in which they imagine control of rice insect pests (Fig. 4.1b). These outbreak events of planthoppers in Japan happened in the periods much before creations of modern high-yielding varieties and synthetic insecticides. In Edo era, farmers sometimes used whale oil or rape seed oil to control planthoppers (Nagata 1982; Tateishi 1981). They tapped or immersed plants and dropped planthoppers onto oil-spread water surface. Planthoppers died from drowning or asphyxiation. Unlike in the tropics, N. lugens have been a serious insect pest of rice since history in the temperate regions.
Historically, why is there difference in pest status of N. lugens between tropical Asia and temperate East Asia? In order to understand the reason, differences in biology of N. lugens between the tropics and temperate East Asia are focused on in this chapter.
4.2 Population Dynamic
Several reports (Kuno and Dyck 1985; Perfect and Cook 1994; Sawada et al. 1993; Wada and Nik 1992) focused on the difference in population dynamic of N. lugens between tropical regions and temperate East Asia. Detailed population study was first done in temperate paddy fields in South Japan by Kuno (1968), and Kuno and Hokyo (1970), followed by Watanabe (1996). In temperate Japan, where N. lugens cannot overwinter, population dynamics are generally characterized by low initial immigrant population which invades from overseas with the assist of monsoon wind, and high-population growth rate throughout the crop season, resulting in rather monotonous increase through three consecutive generations. Populations of the initial densities finally reach 1,500 times on average (Kuno 1968). The populations in tropical fields, however, show entirely different features. Populations in tropical paddy fields are generally characterized by (i) high initial immigrant density, (ii) low population growth rate, (iii) earlier population peak: The peak occurs in the 2nd generation as compared to the 3rd in temperate Japan, (iv) difficult to predict latter population density from the initial population . General feature of the both temperate and the tropical populations is summarized in Table 4.1.
However, the situations are rather complicated in the tropical fields. For examples, as for initial immigrants, the densities were low in the fields of the specific conditions such as rain-fed fields in the Philippine, where rice were planted more synchronously (Cook and Perfect 1989), the first crop of the wet season after the fallow period in Indonesia (Sawada et al. 1993), and the fields just after the fallow period, which was settled in the dry season in Malaysia (Wada and Nik 1992). Additionally, in these fields, populations tended to increase gradually with high-population growth rates, showing rather “temperate type.” Thus, various population growth patterns seem to exist in the tropical fields (Cook and Perfect 1989; Wada and Nik 1992).
What are the fundamental differences between temperate and tropics? According to vigorous studies since the 1970s (Kisimoto 1976; Kisimoto and Sogawa 1995; Otuka et al. 2006), the source of immigrants of N. lugens and S. furcifera in temperate fields is long-distance (over 1,500 km) migrants, thus the densities of initial immigrants are usually very low. However, N. lugens in the tropic are considered to be much less mobile (Riley et al. 1987; Perfect and Cook 1987). Origins of initial immigrants were estimated less than from 30 km. Therefore, immigrant densities are usually high in the tropics where rice is more or less staggered planted. But the densities are low even in tropical fields around which there are no or very few paddy fields in the later growth stages from which planthoppers are expected to emigrate (Kisimoto and Rosenberg 1994). In fact, after the fallow period, initial immigrant densities were very low but populations increased gradually as of temperate areas (Wada and Nik 1992; Sawada et al. 1993).
The other fundamental difference seems to lie in the factor, which determines the population growth pattern. In temperate fields, populations basically increase through generations although the situation is not so simple due to variation in the proportions of macropters to brachypters in the population (Suzuki 2002). Thus, the later populations are predictable from initial immigrant densities. On the other hand, tropical N. lugens exhibit various population growth patterns. Some determinants, probably within-field factors, regulate population growth. One of the important determinants, which many authors pointed out, is natural enemy activity. Kuno and Dyck (1985) described importance of Microvelia, due to the close relationship between population change rates of N. lugens and Microvelia densities. Cook and Perfect (1989) suggested abundance of natural enemies over the first 20 days after transplant was a critical factor in determining later population size. Kenmore et al. (1984) showed physical exclusion of predators or reduction of predators, in particular, spiders and veliids, caused outbreak of N. lugens. Sawada et al. (1993) concluded that high N. lugens densities in synchronous planting areas were caused by paucity of natural enemy activity. Wada and Nick (1992) concluded that interaction between planthopper and natural enemies was a major factor, which determines population growth patterns of planthoppers. Thus, natural enemy activity fluctuates by specific situations in the tropics, which seems to cause the variation in population growth patterns. Importance of natural enemy activity as a factor, which regulates N. lugens population in the tropics, has been verified by frequent occurrences of hopperburn s (resurgence) caused by abuse of insecticides (Heinrichs and Mochida 1984; Heong 2009).
Determinants other than natural enemy interaction, Sawada et al. (1993) pointed out water availability in the field causing big fluctuation of population growth rate s in dry season in Indonesia. Kuno and Dyck (1985) suggested climate and rice varieties also influence population growth of N. lugens.
4.3 Natural Enemy Abundance
Difference in the N. lugens population growth in fields between temperate and tropics is mainly attributed to natural enemy activities. Since N. lugens are not able to overwinter in temperate regions, specific natural enemies hardly exist. In addition, cold winter is thought to significantly destroy natural enemy fauna. Immigrants, which invade young paddy fields, are able to increase their populations through a few generations with advantage of paucity of natural enemies. Although some predators (Cyrtorhinus) and parasitoids (Drynids) invade fields, accompanying planthopper migration (Kisimoto and Rosenberg 1994; Kitamura and Nishikata 1987), there is likely to be an establishment time lag that often precludes their effectiveness (Perfect and Cook 1994). In other word, N. lugens succeed to escape from natural enemies by long-distance migration and explode their population in temperate habitats.
On the other hand, natural enemy activities in the tropics without winter seem to be maintained, more or less, continuously throughout the year. Before the green revolution , N. lugens populations were effectively suppressed into low level by local natural enemies. However, after the green revolution, introduction of high-yielding varieties favored multiplication of N. lugens (Lu and Heong 2009) and resulted in an environmental shift. When natural enemy activities are disturbed by some reasons, outbreaks of N. lugens quickly occur. Abuse of insecticides is a typical case in which natural enemy activities are disturbed. Insecticide applications disorganize predator–prey interaction and the food web structure, thus favoring N. lugens, an r-strategist pest with high fecundity and short life span (Heong 2009). There are factors besides insecticide applications, which disturb natural enemy activities in the tropics. The dry season allowing most insects living in paddy fields to face shortage of hosts seems one of them. The crop-free fallow period is sometimes considered for planthopper management (Nozaki et al. 1984). However, N. lugens population levels were ironically higher in the cops after the fallow period than in the other crop season with asynchronous plantings (Wada and Nik 1992; Sawada et al. 1993; Way and Heong 1994). It is quite probable that eradication of N. lugens in a certain area by the fallow period destroys residential natural enemies and favors a few new planthopper immigrants from other areas or possibly within the area. The crop-free fallow period in the dry seasons sometimes provided more serious impact on biodiversity . Hopperburn frequencies relative to planting area were extremely high in the paddy fields, which were seeded just after the fallow period in the Muda Area of Malaysia in 1990 (Wada and Nik 1992). Climate and farmer practices profoundly influence predator–prey interactions, and thus, N. lugens seem to exhibit various population growth patterns in the tropics.
4.4 Physiological Characters
Nagata and Masuda (1980) first found the genetic difference of the physiological character between tropical and temperate N. lugens. They found that Japanese BPH populations produce higher proportion of female macropters compared to tropical population (Thailand and Philippine), which dominates brachypters at same rearing densities. Iwanaga et al. (1985, 1987) reported that majority of the populations collected from various locations of Japan and coastal areas of China produced more female macropters as compared to the populations collected in tropical Philippine, Indonesia, and Malaysia. However, they found that a few populations in Japan exhibited a similar trend of the wing-form production as tropical populations. This was explained by the difference in the sources of immigrants, i.e., Japanese N. lugens were originated from South China in most cases, but sometimes also from other tropical countries.
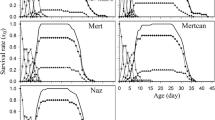
Wada et al. (2007) demonstrated that N. lugens populations collected from subtropical and temperate East Asia (Northern Vietnam, Central China, and Kyushu, Japan) had longer adult immature stage before oviposition, compared to tropical populations (Southern Vietnam, Thailand, and Malaysia). The periods required for 50 % female to begin oviposition at 25 °C were 4.7 days for tropical populations and 7.6 days for East Asian populations (Fig. 4.2). Further, Wada et al. (2009) reported that macropters originated from East Asia were more tolerant to starvation than those from tropical countries. Macropters feed actively on rice for the first 2 or 3 days after eclosion (Tanaka 1999). Macropters of East Asia populations possess increased starvation tolerance after the post-eclosion feeding (live 2.6 times longer without feeding than newly emerged adults) relative to the tropical populations (1.7 times). Accordingly, the periods required for 50 % macropters to die without feeding after 2-day post-eclosion feeding were 11.5 days for East Asia populations and 7.0 for topical populations. These results also suggest the timing of takeoff by N. lugens: macropters emigrate from a paddy field after two- or three-day post-eclosion feeding when they maximize starvation tolerance. Figure 4.3 illustrates a typical example showing the difference in longevity of macropters between temperate and tropical populations, in relation to post-eclosion feeding. Taken pre-ovipositional periods into consideration, Wada et al. (2009) suggested the difference in resource allocation (vitellogenesis or stored resources) between East Asia and tropical populations: Macropters of East Asia populations invest energy intake from feeding mainly on reserves, which enhance starvation tolerance , while those of tropical populations invest on ovary development as well as stored reserves.
These differences in characters, which are closely related to dispersal or migration , provide the evidence that N. lugens populations which are adapted to pre-ovipositional migration are distributed in subtropical and temperate East Asia. On the other hand, N. lugens populations in the tropics are adapted to multiplications with higher brachypter production and shorter pre-oviposition period . These differences influence population dynamic in fields, in particular, numbers of eggs laid relative to adult density. But information on the difference in oviposition performance between populations in tropics and East Asia is not yet available.
Other characters that are not directly related to migration also indicate genetic difference between N. lugens populations in East Asia and in the tropics. The study of biotypes (individual or population that shows virulence to different cultivars) was widely carried out in Asia (Saxena and Barrion 1985). Sogawa (1992) suggested that the change from biotype 1 to biotype 2 capable of feeding on rice with Bph 1 resistance gene had simultaneously occurred in Northern Vietnam, China, and South Japan. Due to the capability of feeding on ASD7 with bph2 resistance gene, similarity of the biotype composition in local populations throughout subtropical and temperate East Asia again reported by Wada et al. (1994) and Takahashi et al. (1994). Tanaka and Matsumura (2000) reported that increase of N. lugens capable of feeding on ASD7 in Japan occurred after wide spread of varieties with bph2 gene in China and Northern Vietnam. As a whole, biotype compositions in time and space are quite similar within East Asia, while they tended to slightly differ depending on locations in the tropics.
Development of chloronicotinyl insecticide resistance in N. lugens which appeared from the mid-2000s in Asian countries has been a serious problem until now. Geographical differences in resistance development against various insecticides also suggest genetic similarity of N. lugens in East Asia. N. lugens in Northern Vietnam, China, and Japan had high resistance to imidacloprid (Matsumura et al. 2008; Matsumura and Sanada-Morimura 2010). The populations in Southern Vietnam also exhibited high resistance, but the populations in the Philippines were still susceptible to this chemical. This fact suggests insecticide resistance occurred simultaneously over the countries in East Asia, but the status of resistance depended on the local situations in the tropics.
4.5 Some Implications of Evolution for Planthopper Migration
Studies of physiological characters demonstrated that there is a so-called East Asian population of N. lugens (Sogawa 1993), which are migratory and are isolated to some extent from tropical populations. Studies of long-distance migration have indicated genetic exchange within East Asia. N. lugens overwinter in subtropical East Asia (Northern Vietnam and southern end of China) (Chen et al. 1982; Cheng et al. 1979). In spring, the first northeastward migration occurs from overwintering sites to early crops in South China (Otuka et al. 2008). After population multiplications through one or two generations in South China, another step of northeastward migrations occur, which led to initial immigrants in newly planted rice in Japan, Korea, and Central China (Sogawa and Watanabe 1992; Otuka et al. 2006). Although migration events are not clearly observed compared to early summer migration (Kisimoto and Rosenberg 1994), southwestward return migration from temperate regions toward overwintering sites has been demonstrated (Cheng et al. 1979; Riley et al. 1994; Qi et al. 2010). Destinations of N. lugens migration depend entirely on wind directions. Wind-dependent migration simulations can predict long-distance immigrations (Seino et al. 1987; Watanabe et al. 1991; Otuka et al. 2005). So, the migration system of N. lugens in East Asia is mediated by seasonal monsoon winds, allowing northeastward expansion during spring and early summer, and southwestward return movement in autumn. This system maintains N. lugens having longer adult immaturity genetically for long years in spite of disadvantage of prolonged oviposition for multiplication.
Rice cultivation began about 10,000 years ago in Central China and about 4–8000 years ago in Japan (Sato 2008). Because rice is the only host plant of N. lugens, East Asian population must be evolved after northern expansion of rice by mankind in East Asia. In the process of forming East Asian population, yearly seasonal monsoon is considered to favor mobile N. lugens and cause differentiation of the East Asian population probably from tropical populations.
The phenomenon of the effective population suppression by natural enemies in the tropics together with successful escape from natural enemies in temperate regions implies significance of migration as one of the strategies for the rice planthoppers. Evolution of migration has often been discussed in relation to heterogeneous or ephemeral environments in time and space (Roff and Fairbairn 2007). Migration is a strategy to avoid impending unfavorable habitat caused by climate or inter- and intraspecific competitions. Seasonal fluctuation and heterogeneous distribution of food resource should be a typical example causing insect migration. But escape from predators and parasites is also a factor, which may favor evolution of migration (Southwood 1978; Pulido 2007).
In the tropics, planthopper populations usually collapse within a crop period, partially due to predation and parasitism by natural enemies. However, the extinction of the populations in a paddy field does not occur only by the function of natural enemy activities. It is partially caused by the appearance of macropters, which emigrate from the paddy field (Kuno and Hokyo 1970). Appearance of macropters increases with advancement of rice growth (Kisimoto and Rosenberg 1994). In addition, the natural enemy pressure on the planthopper population was also the factor, which increases with the advancement of rice plant growth stages (Wada and Nik 1992): egg mortality due to egg parasitoids (Watanabe et al. 1992) and young nymph mortality caused by predators (Wada and Nik 1992) increases with plant ages and planthopper generations in a crop period. Therefore, staying in a field for a few generations seems to lead the planthopper population to take a risk of the high natural enemy pressure and finally to become extinct. The simplest way to avoid natural enemy pressure for planthoppers seems to take off the field even before deterioration of quality of the host plant. Rice planthoppers migrate from field to field, otherwise they may not be able to survive in the tropics. This idea seems to be probable if we consider S. furcifera, which produce high proportions of macropters even in early rice (Kuno 1968; Watanabe 1996). In addition, because N. lugens populations were always low in ancient tropical paddy fields, a risk of population decline caused by natural enemies seems to be more critical than deterioration of rice damaged by planthoppers themselves. The similar causal aspect of migratory flight escaping from natural enemy attacks was demonstrated in the other important migratory agricultural pest, Spodoptera litura (Tojo et al. 2008). Rice planthoppers migrate from fields to fields to escape from natural enemies, exploring new habitats in the tropics, which may be the preadaptation of long-distance migration of East Asian N. lugens.
References
Chen RC, Zhao J, Xu XY. The overwintering temperature index of brown planthopper Nilaparvata lugens Stal. Acta Entomologica Sin. 1982;25:390–6 (Chinese with English summary).
Cheng SN, Chen JC, Si H, Yan LM, Chu TL. Studies on the migration of brown planthopper, Nilaparvata lugens Stal. Acta Entomologica Sin. 1979;22:1–21 (Chinese with English summary).
Cook AG, Perfect TG. The population characteristics of the brown planthopper, Nilaparvata lugens, in the Philippines. Ecol Entomol. 1989;14:1–9.
Dyck VA, Thomas B. The brown planthopper problem. In: IRRI compiled. Brown planthopper: threat to rice production in Asia. Los Banos (Philippines):IRRI; 1979. p. 3–17.
Heinrichs WA, Mochida O. From secondary to major pest status: the case of insecticide-induced rice brown planthopper, Nilaparvata lugens, resurgence. Prot Ecol. 1984;7:201–18.
Heong KL. Are planthopper problems caused by a breakdown in ecosystem services? In: Heong KL, Hardy B, editors. Planthoppers: new threats to the sustainability of intensive rice production systems in Asia. Los Banos: IRRI; 2009. p. 221–31.
Iida T. Memory of the International Rice Research Institute. Nouyaku. 1972;19(4):24–5 (in Japanese).
Iwanaga K, Tojo S, Nagata T. Immigration of the brown planthopper, Nilaparvata lugens, exhibiting various responses to density in relation to wing morphism. Entomol Exp Appl. 1985;38:101–8.
Iwanaga K, Nakasuji F, Tojo S. Wing polymorphism in Japanese and foreign strains of the brown planthopper, Nilaparvata lugens. Entomol Exp Appl. 1987;43:3–10.
Kenmore PE, Carino FO, Perez VC, Dyck AP, Cutierrez AP. Population regulation of the rice brown planthopper (Nilaparvata lugens Stal) within rice fields in the Philippine. J Plant Prot Tropics. 1984;1(1):19–37.
Kilin D, Nagata T, Masuda T. Development of carbamate resistance in the brown planthopper, Nilaparvata lugens Stal (Homoptera: Delphacidae). Appl Ent Zool. 1981;16:1–6.
Kitamura K, Nishikata Y. A monitor-trap survey of parasitoids of the leaf- and planthoppers supposedly migrated from the Mainland China (Homoptera: Auchenorrhycha). Bull Fac Agr Shimane Univ. 1987;21:171–7.
Kuno E. Studies on the population dynamics of rice leafhoppers in a paddy field. Bull Kyushu Agric Expt Stn. 1968;14:131–246 (in Japanese with English summary).
Kuno E, Hokyo N. Comparative analysis of the population dynamics of rice leafhoppers, Nephotettix cincticeps Uhler and Nilaparvata lugens Stal, with special reference to natural regulation of their numbers. Res Popul Ecol. 1970;12:151–81.
Kuno E, Dyck VA. Dynamics of Philippine and Japanese populations of the brown planthopper: comparison of basic characteristics. Chin J Entomol. 1985;4(2):1–9.
Kisimoto R. Synoptic weather conditions inducing long-distance immigration of planthoppers, Sogatella furcifera Horvath and Nilaparvata lugens Stal. Ecol Entomol. 1976;1:95–109.
Kisimoto R. Advancement of rice technology and insect pest problems in Asia. In: Ishii S, editor. Resent advancement of entomology. Tokyo: Tokyo University Publisher; 1981. p. 248–78 (in Japanese).
Kisimoto R, Rosenberg J. Long-distance migration in Delphacid planthoppers. In: Denno RF, Perfect TJ, editors. Planthoppers—their ecology and management. New York: Chapman & Hall; 1994. p. 302–22.
Kisimoto R, Sogawa K. Migration of the brown planthopper, Nilaparvata lugens and the white-backed planthopper Sogatella furcifera in East Asia: the role of weather and climate. In: Drake VA, Gatehouse AG, editors. Insect migration: tracking resources through space and time. Cambridge: Cambridge University Press; 1995. p. 67–91.
Lu Z, Heong KL. Effects of nitrogen-enriched rice plants on ecological fitness of planthoppers. In: Heong KL, Hardy B, editors. Planthoppers: new threats to the sustainability of intensive rice production systems in Asia. Los Banos: IRRI; 2009. p. 247–56.
Matsumura M, Takeuchi H, Satoh M, Sanada-Morimura S, Otuka A, Watanabe T, Thanh DV. Species-specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South-east Asia. Pest Manag Sci. 2008;64:1115–21.
Matsumura M, Sanada-Morimura S. Recent status of insecticide resistance in Asian rice planthoppers. JARQ. 2010;44:225–30.
Miyashita K. Historical records of insect pest outbreaks. Shokubutsu-Boeki. 1961;15:75–81 (in Japanese).
Mochida O, Okada T. Taxonomy and biology of Nilaparvata lugens (Hom., Delphacidae). In: IRRI compiled. Brown Planthopper: threat to rice production in Asia. Los Banos: IRRI; 1979. p. 21–43.
Nagata T, Masuda T, Moriya S. Development of insecticide resistance in the brown planthopper, Nilaparvata lugens Stal (Hemiptera: Delphacidae). Appl Ent Zool. 1979;14:264–9.
Nagata T, Masuda T. Insecticide susceptibility and wing-form ratio of the brown planthopper, Nilaparvata lugens (Stal) (Hemiptera: Delphacidae) and the white-backed planthopper, Sogatella furcifera (Horvath) (Hemiptera: Delphacidae) of Southeast Asia. Appl Ent Zool. 1980;15:10–9.
Nagata T. Insecticide resistance and chemical control of the brown planthopper, Nilaparvata lugens Stal (Homoptera: Delphacidae). Bull Kyushu Natl Agric Exp Stat. 1982;22:49–164.
Nozaki M, Wong HS, Ho NK. A new-double cropping system proposed to overcome instability of rice production in the Muda irrigation area of Malaysia. JARQ. 1984;18:60–8.
Otuka A, Watanabe T, Suzuki Y, Matsumura M, Furuno A, Chino M. Real-time prediction system for migration of rice planthoppers Sogatella furcifera (Horvath) and Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Appl Entomol Zool. 2005;40:221–9.
Otuka A, Watanabe T, Suzuki Y, Matsumura M, Furuno A, Chino M, Kondo T, Kamimuro T. A migration analysis of Sogatera furucifera (Horvath) (Homoptera: Delphacidae) using hourly catches and a three-dimensional simulation model. Agricultural and Forestry Entomology. 2006;8:35–47.
Otuka A, Matsumura M, Watanabe T, Thanh VD. A migration analysis for rice planthoppers, Sogatella furcifera (Horvath) and Nilaparvata lugens (Stal) (Homoptera: Delphacidae), emigrating from northern Vietnam from April to May. Appl Entomol Zool. 2008;43:527–34.
Perfect TJ, Cook AG. Dispersal patterns of rice brown hopper, Nilaparvata lugens Stal., in a tropical rice-growing system and their implication for crop protection. Journal of Plant Protection in the Tropics. 1987;4:121–7.
Perfect TJ, Cook AG. Rice planthopper population dynamics: a comparison between temperate and tropical regions. In: Denno RF, Perfect TJ, editors. Planthoppers—their ecology and management. New York: Chapman & Hall; 1994. p. 282–301.
Pulido F. The genetics and evolution of avian migration. Bioscience. 2007;57:165–74.
Qi HH, Zhang YH, Cheng DF, Han EB, Sun JR. Radar observation and trajectory analysis on the autumn return migration of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae) in 2009 in China. Acta Entomologica Sinica. 2010;53:1256–64.
Riley JR, Reynolds DR, Farrow RA. The migration of Nilaparvata lugens (Stal) (Delphacidae) and other Hemiptera associated with rice during the dry season in the Philippines: a study using radar, visual observations, aerial netting and ground trapping. Bull Entomol Res. 1987;77:145–69.
Riley JR, Reynolds DR, Smith AD, Rosenberg LJ, Cheng XN. The long-distance migration of Nilaparvata lugens (Stal) (Delphcidae) in China: radar observations of mass return flight in the autumn. Bull Entomol Res. 1994;84:389–402.
Roff DA, Fairbairn DJ. The evolution and genetics of migration in Insects. Bioscience. 2007;57:155–64.
Sato Y. History of rice. Kyoto: Kyoto University Press; 2008 251p.
Sawada J, Kusmayadi A, Subroto SWG, Suwardiwitaya E, Mustaghfirin. Comparative analysis of population characteristics of the brown planthopper, Nilaparvata lugens Stal, between wet and dry rice cropping seasons in West Java, Indonesia. Res Popul Ecol 1993;35:113–37.
Saxena RC, Barrion AA. Biotypes of the brown planthopper, Nilaparvata lugens (Stal) and strategies in development of host plant resistance. Insect Sci Appl. 1985;6:271–89.
Seino H, Shiotsuki Y, Oya S, Hirai Y. Prediction of long distance migration of rice planthoppers to northern Kyushu considering low-level jet stream. J Agric Meteorol. 1987;43:203–8.
Sogawa K. 1992. A change in biotype property of brown planthopper populations immigrating into Japan and their probable source area. Proc Assoc Plant Prot Kyushu 38:63–8 (Japanese with English summary).
Sogawa K, Watanabe T. Redistribution of rice planthoppers and its synoptic monitoring in East Asia. Technical Bulletin No. 131. Taipei: Food and Fertilizer Technology Center; 1992. p.1–9.
Sogawa K. 1993. Probable source areas of N. lugens immigrating into Japan, based on biotype property. Kongetsu-no-Noyaku. 1993;4:36–40 (in Japanese).
Southwood TRE. Escape in space and time—concluding remarks. In: Dingle H, editor. Evolution of insect migration and diapause. New York: Springer; 1978. p. 277–9.
Suzuki Y. Historical development of research on and management of migratory rice planthoppers. Shokubutu-Boeki. 2002;56:492–4 (in Japanese).
Takahashi A, Ito K, Tang J, Hu G, Wada T. Biotypical property in the populations of brown plant-hopper, Nilaparvata lugens Stal (Homoptera: Delphacidae) collected in China and Japan. Appl Entomol Zool. 1994;29:461–3.
Tanaka K. Quantitative genetic analysis of biotypes of the brown planthopper Nilaparvata lugens: heritability of virulence to resistant rice varieties. Entomol Exp Appl. 1999;90:279–87.
Tanaka K, Matsumura M. Development of virulence to resistant rice varieties in the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae), immigrating into Japan. Appl Entomol Zool. 2000;35:529–33.
Tateishi K. Methods for controlling planthoppers. Tsukushino (Japan): Fukuoka Prefectural Agriculture Institute;1981. p. 44 (in Japanese).
Tojo S, Mishima H, Kamiwada H, Ngakan PO, Chang KS. Variations in the occurrence patterns of male moths of the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae) among Southeast Asian countries, as detected by sex pheromone trapping. Appl Entomol Zool. 2008;43:569–76.
Wada T, Nik MN. Population growth pattern of the rice planthoppers, Nilaparvata lugens and Sogatella furcifera, in the Muda Area, West Malaysia. JARQ. 1992;26:105–14.
Wada T, Ito K, Takahashi A. Biotype comparisons of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae) collected in Japan and Indochina Peninsula. Appl Entomol Zool. 1994;29:477–84.
Wada T, Ito K, Takahashi A, Tang J. Variation of preovipositional period in the brown planthopper, Nilaparvata lugens, collected in tropical, subtropical and temperate Asia. J Appl Entomol. 2007;131:698–703.
Wada T, Ito K, Takahashi A, Tang J. Starvation tolerance of macropter brown planthopper, Nilaparvata lugens, from temperate, subtropical, and tropical populations in East and South-East Asia. Entomol Exp Appl. 2009;130:73–80.
Watanabe T, Sogawa K, Hirai Y, Tsurumachi M, Fukamachi S, Ogawa Y. Correlation between migratory flight of rice planthoppers and the low-level jet stream in Kyushu, southwestern Japan. Appl Entomol Zool. 1991;26:215–22.
Watanabe T, Wada T, Nik MN. Parasitic activities of egg parasitoids on the rice planthoppers, Nilaparvata lugens (Stal) and Sogatella furcifera (Horvath) (Homoptera: Delphcidae), in the Muda Area, Peninsular Malaysia. Appl Entomol Zool. 1992;27:205–11.
Watanabe T. Population dynamics of long-range migratory rice planthoppers, Nilaparvata lugens Stal and Sogatella furcifera Horvath, in Japan. In Hokyo N, Norton G. editors. Pest Management Strategies in Asian Monsoon Agroecosystems. Kumamoto (Japan): Kyushu National Agriculture Experiment Station; 1996. p. 45–54.
Way MJ, Heong KL. The role of biodiversity in the dynamics and management of insect pests of tropical irrigated rice—a review. Bull Entomol Res. 1994;84:567–87.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Zhejiang University Press, Hangzhou and Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wada, T. (2015). Rice Planthoppers in Tropics and Temperate East Asia: Difference in Their Biology. In: Heong, K., Cheng, J., Escalada, M. (eds) Rice Planthoppers. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9535-7_4
Download citation
DOI: https://doi.org/10.1007/978-94-017-9535-7_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9534-0
Online ISBN: 978-94-017-9535-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)