Abstract
The cotton bollworm Helicoverpa armigera is a destructive pest that affects a variety of crop plants. Because of its polyphagous feeding habit, mobility as adults, and high fecundity, the expanding infestations of H. armigera in different crops have caused economic losses and difficulties for pest population management. In Brazil, a sequence of different crop systems in the same area and crop rotation during the year can create a spatio-temporal mosaic of crops where H. armigera can persist. However, the consequences of the simultaneous and/or alternating presence of host plants for H. armigera populations through generations are unknown. In this study, we simulated, in the laboratory, hypothetical situations for the availability of soybean and cotton crops in the landscape. We evaluated the effects of: (1) the number of generations during which a population feeds on a host-plant species; (2) the succession of host-plant species on which populations have fed for two generations; and (3) the parental host plant on the fitness of H. armigera populations. Only the current host plant on which larvae fed affected the performance of the H. armigera populations. Decrease of mortality rates during the immature period was slowed when the larvae fed on soybean. The lowest value of reproductive potential (R 0) was found for individuals originating from mating between females and males reared in cotton. Our results indicated that pest-management and biological-control plans for H. armigera should be developed on a regional scale rather than for just a specific crop area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
At heterogeneous agricultural landscapes, the pool of migrant adults of Helicoverpa armigera, a polyphagous pest, consists of individuals with different degrees of fitness, resulting from their development on different host plants.
-
The consequences of soybean and cotton available simultaneous or alternately over two generations were analyzed at laboratory conditions, aiming to evaluate the consequences of host-plant succession on populations’ fitness.
-
The fitness was lowest for populations reared successively for two generations in cotton.
Introduction
The cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), one of the most serious insect pests in Europe, Africa, Asia, and Australasia (Fitt 1989; Zalucki et al. 1994), was recently reported to damage several crops in South America (Czepak et al. 2013; Murúa et al. 2014; Tay et al. 2013; Kriticos et al. 2015). Because of its polyphagous feeding habit, mobility as adults, and high fecundity, the expansion of infestations of H. armigera in a variety of agricultural crops has caused economic losses and difficulties for pest population management (Fitt 1989; Zalucki et al. 1994; Tay et al. 2013; Kriticos et al. 2015).
In Brazil, since an outbreak of H. armigera in 2013–2014, studies have examined the possible causes for its rapid spread and damage caused to a variety of crop systems in the country (Mastrangelo et al. 2014; Reigada et al. 2016; Sosa-Gómez et al. 2014). The agricultural landscape in Brazil is characterized mainly by annual cropping systems. The sequence of crop varieties in the same region creates a spatio-temporal mosaic of crops where H. armigera can survive and persist, exploiting different host plants during the year, in a metapopulation dynamic at a landscape scale (Hanski 1999). Populations of H. armigera on neighboring crops can colonize recently planted areas, enabling H. armigera to persist year-round by using different host plants grown in different periods (Kennedy and Storer 2000; Feng et al. 2010).
The host-plant species and the period when they are available for pest populations are important factors in triggering population increases and outbreaks (Lu and Xu 1998). Host plants have direct impacts on H. armigera development, survivorship and reproduction (Liu et al. 2004; Reigada et al. 2016; Naseri et al. 2014). The relative fitness of H. armigera populations on different host plants can lead to different local population dynamics, since the longevity, fecundity and survival rates can differ according to the host plant on which the larvae feed (Liu et al. 2004; Reigada et al. 2016).
Studies based on life tables of H. armigera on different host plants have demonstrated the relative contribution of host plants to individual fitness and to population bio-demographic parameters (Liu et al. 2004; Naseri et al. 2014). However, in these studies, interpretations of the effects of host plants were based on the responses of a group of individuals reared on the same host-plant species, and the evaluations were made for only one generation. Because adults of H. armigera can move hundreds of kilometers between different areas and crop host plants, and the moths may invade from distant or nearby fields (Fitt 1989), it is more realistic to consider the effects of different host plants on a single population. The pool of migrant adults in the landscape and the risk of outbreaks are consequences of the moth population composed of individuals with different degrees of fitness, resulting from their development on different host plants. The effects of simultaneous temporal presence or successive availability (i.e. crop rotation) of host plants on the fitness of H. armigera populations and the consequences over generations have been neglected in earlier studies.
Soybeans and cotton are economically important crops in Brazilian agribusiness, comprising, respectively, 31,504 and 1075 million ha of cultivated area in the country (MAPA 2016). The simultaneous presence or succession of these two host plants in a common area is frequent in Brazilian agricultural landscapes. It is expected that populations of H. armigera feeding on both host plants contribute to the pool of migrant adults, with important consequences for survivorship, fecundity and dispersal potential of the insect pest.
Aiming to understand the consequences of simultaneous temporal presence or temporal succession of two host plants in a crop rotation practice for H. armigera populations, we evaluated the effects of soybean and cotton on H. armigera populations over two generations. Based on the hypothetical situations for soybean and cotton availability in the landscape shown in Fig. 1, we evaluated the effects of: (1) the number of generations in which a population had fed on a host-plant species; (2) the succession of host-plant species on which populations had fed during two generations; and (3) the host plant of the parental generation.
Hypothetical crop arrangements simulated in laboratory experiments to evaluate the cross-crop effects of soybean (S)-cotton (C) on bio-demographic factors of Helicoverpa armigera populations. a Only one host plant contributes to the pool of migrant moths in the first generation. b The pool of migrant moths (first generation) is composed of individuals that were reared on two host-plant species available simultaneously in the landscape. In (a, b) the moths have the same or a different (crop rotation) host plant available to develop their offspring (second generation)
Materials and methods
Insect colony and plant sources
The host plants cotton (Gossypium hirsutum, ‘FM993′) and soybean (Glycine max, ‘99R01′) were grown under field conditions at the University of São Paulo—USP/ESALQ, without pesticides.
A laboratory colony of H. armigera was established with pupae collected from cowpea crop farms in western Bahia State (12°5′58″S, 045°47′54″W) from January through December 2015. The adult moths were reared in cages made of PVC tubes (14 × 20 cm) closed at the top with a fine-mesh net, and were allowed to mate. The adult moths were provided with a 10% honey solution. The eggs were collected from the net every 48 h. After hatching, the larvae were reared on an artificial diet (Greene et al. 1976) at 25 °C with a photoperiod of 14 L:10 D. The insects to be tested on the different host plants were reared for at least five generations on the artificial diet, to prevent any influence of the host used for the source colony (i.e., pre-imaginal conditioning).
Populations studied
The populations were defined by groups composed of offspring from different combinations of matings between individuals reared on soybean and/or cotton. Thus, the groups of larvae obtained from the laboratory colony reared on soybean and cotton were the parental (F 0) populations S and C, respectively. These larvae were reared on the respective host plants, and after adult emergence the moths were mated in different combinations to obtain the F 1 populations.
The first (F 1) generation was composed of “pure” populations, i.e. those derived from mating between females and males reared on the same host plant, and of “mixed” populations, i.e. those derived from mating between females and males reared on different host plants. The experimental design is shown in Fig. 2. The characteristics of each population were as follows.
Experimental diagram showing how the different populations of Helicoverpa armigera were obtained in this study: (I) S and C populations represented larvae (F 0) obtained from the laboratory colony and reared on soybean or cotton, respectively; (II) After adult emergence, female and male moths (F 0) were paired in different mating combinations. Pure populations were established from matings between males and females that were reared on the same host plant (S × S or C × C). Mixed populations were established from matings between males and females that were reared on different host plants (S × C or C × S). (III) Neonate larvae (F 1) were reared on soybean (pure: SSS, CCS and mixed: SCS, CSS) or cotton (pure: SSC, CCC and mixed: SCC, CSC)
Parental populations—S and C (F 0)
Initially, 2000 neonate larvae from the laboratory colony were divided into two groups (Fig. 2-I): (1) population S: 1000 larvae reared on soybean, and (2) population C: 1000 larvae reared on cotton. Neonate larvae were reared in groups of 50 in Petri dishes (15 × 2 cm) until the third instar, when they were placed individually in smaller Petri dishes (6 × 2 cm) to prevent cannibalism. Leaves and pods were offered for larvae reared on soybean; and for those reared on cotton, leaves and cotton bolls were offered. The leaves and plant structures were changed each day until pupation. After 24 h, the pupae were weighed and separated by sex (Butt and Cantu 1962). Individual insects were checked daily, and the survival, pupal weight, duration of the immature stages, and pupal period were recorded.
After the adults emerged, S and C males and females were mating according to experimental design described in Fig. 2-II. The moths were placed in plastic containers supplied with a 10% honey solution (one pair per container). For each mating combinations, there were 25 moth couples. Eggs were collected from each cage daily, until the females died. For each mating combination (i.e. SS, SC, CC, CS), the hatched larvae obtained from the second laying of eggs were placed in groups in a common Petri dish. The larvae were reared in groups of 50 in Petri dishes (15 × 2 cm) until the third instar, when they were separated in individual small Petri dishes (6 × 2 cm) to prevent cannibalism. The group of newly hatched larvae from second laying of eggs of each mate population was used to initiate F 1 studied populations, as described in the next two sections. The mortality, longevity, and fecundity of each mating pair were determined. To ensure that the female moths had mated, after they died, they were dissected and examined for the presence of spermatophores in the bursa copulatrix (Liu et al. 2004). The eggs were maintained in plastic containers at 25 °C for 5 days to evaluate the hatching rates. Egg viability was estimated from the proportion of hatched larvae out of the total number of eggs laid during the female’s lifespan.
Pure populations-SSS, SSC, CCC, CCS (F 1)
Newly hatched larvae (F 1) resulting from mating between females and males from the parental population (F 0) reared on soybean (S) were separated into two groups (Fig. 2-III): (1) SSS (n = 303), in which the larvae continued to feed on soybean; and (2) SSC (n = 402), in which the larvae fed on cotton. Similarly, newly hatched larvae (F 1) resulting from matings between females and males from the parental population (F 0) reared on cotton (C) were separated into two groups: (1) CCC (n = 348), in which the larvae continued to feed on cotton; and (2) CCS (n = 300), in which the larvae fed on soybean. Individual insects were observed daily, and the survival, pupal weight, and durations of the immature stages and the pupal period were recorded.
Mixed populations-SCS, SCC, CSC, CSS (F1)
Newly hatched larvae (F 1) resulting from matings between females and males of both parental populations S and C (F 0) comprised the mixed populations (F 1). Two groups of mixed populations, one composed by mating S females with C males, and another group composed by mating C females with S males (Fig. 2-III).
Two subgroups of the F 1 mixed populations that received females from S populations (S female × C male parental population–F 0) were defined: (1) SCS (n = 302), in which the larvae continued to feed on soybean; and (2) SCC (n = 445), in which the larvae fed on cotton. Analogously, two subgroups of the F 1 mixed populations that received females from C populations (C female × S male parental populations–F 0) were also defined: (1) CSC (n = 449), in which the larvae continued to feed on cotton; and (2) CSS (n = 300), in which the larvae fed on soybean.
Data analyses
Survivorship of immatures and development time of H. armigera over two generations reared on successive/alternate host plants
The development times of the larval and pupal phases were analyzed using survival curves, where the means and the standard errors were estimated from the Kaplan–Meier estimators (Mantel 1966) of the corresponding functions for survival and duration for each populations: SSS, SSC, CCC, CCS, SCS, SCC, CSC and CSS. The means were then compared using log-rank tests, with the respective p values corrected using the Bonferroni method (R software, package: survival).
The survivorship curves (proportion of individuals that were alive) were analyzed by fitting generalized linear models with binomial error distribution corrected for overdispersion, including the effects of larval stage (continuous) and population (8-level factor: SSS, CCC, SSC, CCS, SCS, CSC, SCC, CSS). Goodness-of-fit was assessed using half-normal plots with simulation envelopes (Moral et al. 2016). Reduced models were also fitted to the data in order to compare model coefficients among populations. F tests were used to test full versus reduced models. The pupal weight was analyzed by fitting a linear model considering the effects of populations (8-level factor: SSS, CCC, SSC, CCS, SCS, CSC, SCC, CSS) and sex (2-level factor: male, female) and an analysis of variance table was produced.
Emergence time, adult longevity, and reproductive potential of H. armigera adults in parental populations (F 0)
The effects of the host plant of parental populations (4-level factor: SS, CC, SC, CS) and sex (2-level factor: male and female) on the adult longevity were compared using log-rank tests, with the respective p values corrected using the Bonferroni method. The number of eggs and egg viability (proportion of viable eggs) data were analyzed by fitting a generalized linear model with Poisson and binomial error distributions corrected for overdispersion, respectively, including the effects of the host plant of parental populations (4-level factor: SS, SC, CC, CS) in the linear predictor. The significance of the host plant of parental populations effect was assessed through analysis of deviance tables. Multiple comparisons were performed by obtaining the 95% confidence intervals for the linear predictors. The proportion of mated females for the mating combinations of parental populations was analyzed by fitting a Bernoulli model with the effect of host plant of parental populations (4-level factor: SS, CC, SC, CS) in the linear predictor, with its significance assessed through an analysis of deviance table.
Considering each day as a categorized ordinal variable, proportional-odds logistic regression models were fitted to the emergence data, including the effects of sex (2-level factor: males and females) and host plant (2-level factor: soybean and cotton) in linear predictor, as well as the interaction between the factors. The number of days ranged from 28 to 42, consisting of 15 categories. The significance of this effect was assessed through likelihood-ratio tests between the model that included this effect and the null model, which included only a common intercept. All statistical analyses were performed in R version 3.3.1 (R Core Team 2015).
Effects of host plants on fertility life table of H. armigera parental populations (F 0)
We evaluated host-plant effects on the reproductive potential of H. armigera populations considering the populations originated from the mating combinations: SS, SC, CC and CS (Fig. 1-II). The reproductive capacity of these populations was evaluated using fertility life-tables (Liu et al. 2004). For each studied population, there were 25 moth couples, as described previously. Eggs were collected from each cage daily, until the females died. The net reproductive rate (R 0) was calculated as the product of the survivorship of the immature stages, the proportion of adults that were female, and the number of eggs laid per female in each population.
Results
Survivalship of immatures and development time of H. armigera over two generations reared on successive/alternate host plants
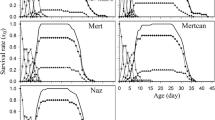
The mortality rates of immature stages were high in all populations (ranged from 100% in SSS, CCS and SCC to 71.6% in S populations). Because of the incremental mortality rates during development, few adults of all populations were produced (Fig. 3; Table 4 in Appendix). The survivorship of the parental populations (F 0) was higher than that of the F 1 populations (χ 2 = 76.97, df = 1, p < 0.001). For F 1 populations, the decrease of survivorship of individuals fed on soybean was slower during the immature developmental period (Fig. 3). After performed statistical analysis to compared the survival curve of populations, we looked at the confidence intervals for the model coefficients and then compared the populations by fitting a reduced model, merging populations whose coefficients were similar, and testing the full and reduced model. In this case, we grouped the CCC and SSC populations, SSS and CSS populations, and CSC and SCC populations, which yielded F = 1.60 on 6 and 32 df, p = 0.1801, which means that the full model does not provide a significantly better model fit to the data and hence we can group the populations as such. By this form, the analysis showed that decrease in curves of immature survivorship was slower in SSS and CSS, followed by CCS and SCS. Faster decrease of survivorship long to immature stage was observed in CSC and SCC followed by CCC and SSC population (F 7,32 = 9.33, p < 0.01).
Decrease in curves of immature survivorship of Helicoverpa armigera populations reared on different host plants. Populations resulting from mating between males and females that were reared on the same host plant (pure populations) are represented by circles, and populations resulting from mating between males and females reared on different host plants (mixed populations) are represented by triangles. The parental populations (F 0) are represented by squares (black for soybean and gray for cotton). Filled symbols represent pure populations (SSS, CCC, SCS and CSC), and open symbols represent populations reared on different host plants (SSC, CCS, SCC and CSS) over two generations
The larval and pupal development times varied from 20 to 26.8 and from 13 to 15.3 days, respectively. The mean total development time of immature stages varied from 33.9 to 42 days (Table 1). Populations reared for only one generation on soybean (parental populations- S, F 0) developed faster (χ 2 = 55.1, df = 1, p < 0.001) than those fed on cotton for one generation (parental population C, F 0) and host plants over two generations (F 1 populations) (Table 1). For the individuals that fed on host plants over two generations no statistical difference in development time was found (Table 1).
The pupal weight was influenced by the sex of individuals (F 10,488 = 9.49, p < 0.001). Overall, male pupae were heavier than female pupae (Table 1). For the F 1 populations fed on cotton, the pupae from pure populations were heavier than the pupae from mixed populations (Table 1). Because of the absence of data for F 1 pure populations and the overall high mortality of immature stages, the comparison between pure and mixed population should be considered with caution.
Emergence time, adult longevity, and reproductive potential of H. armigera adults in parental populations (F 0)
The host plant affected the emergence times of both male and female moths (interaction between sex and host plant effect: χ 2 = 2.18, df = 1, p = 0.1397; sex main effect: χ 2 = 72.91, df = 1, p < 0.0001; host plant main effect: χ 2 = 80.85, df = 1, p < 0.0001). Moths reared on soybean emerged faster than those reared on cotton (χ 2 = 63.04, df = 1, p < 0.0001) (Fig. 4). On each host plant, however, the emergence times differed between females and males: females emerged faster than males [34 and 38 days for soybean (χ 2 = 65.21, df = 1, p < 0.0001), and 39 and 42 days for cotton (χ 2 = 11.98, df = 1, p < 0.0001)].
Cumulative probability of emergence of female and male adults of Helicoverpa armigera reared on soybean and cotton. The cumulative probabilities are obtained from the cumulative sums of the probabilities: \(\pi_{j} = \frac{{\hat{\epsilon}^{\eta j}}}{{\mathop \prod \nolimits_{i = 1}^{j} \left({1 + {\hat{e}}^{{\eta_{i}}}} \right)}}\), where \(\hat{\epsilon}_{j}\), j = 1,2, k is the estimate of the linear predictor for each of the k categories (days)
Host plants and sex had effects on adult longevities (χ 2 = 15.00, df = 7, p = 0.036). Male (χ 2 = 5.94, df = 3, p = 0.12) and female (χ 2 = 4.75, df = 3, p = 0.191) adults had similar longevities in all F 0 populations (four mating groups: SS, SC, CC, CS) (Table 2).
The host plant on which males and females fed as larvae did not affect (F 3,34 = 1.76, p = 0.169) the number of eggs produced by females (Table 2). However, the egg viability was affected by parental host plants (F 3,56 = 3.73, p = 0.0177), that was highest for S × S couples. The C × C and C × S couples showed intermediate egg viability, and the S × C couples showed the lowest egg viability (Table 3). The proportion of mated females was not different among populations (Deviance = 0.35, df = 3, p = 0.95) (Table 3). The pre-oviposition period was around 4 days for all populations (Table 2).
Effects of host plants on fertility life table of H. armigera parental populations (F 0)
Life tables were constructed from the survival and fecundity data for H.armigera populations that fed on soybean and cotton over one generation (parental populations: F 0 in Table 2 and Appendix Table 4). The net reproductive rate (R 0) of populations originating from mating between males and females reared on soybean (SS) was highest, but was not significantly different from populations originated from the mating between females and males originating from different host plants SC and CS. The lowest value for R 0 found for the population originating from females and males from cotton was significantly different form SS population (Table 3).
The intrinsic rate of increase (r m) and the finite rate of increase (λ) obtained for all populations indicated that both host species, soybean and cotton, can increase the H. armigera population sizes (r m > 0 and λ > 1; Table 3). The results indicated that individuals originating from parental population in which mating occurred between males and females reared on soybean (S × S) can double the most rapidly (T and D t ; Table 3).
Discussion
The populations were subject to the same founder effects, since the parental individuals (S and C) were obtained from the progeny of moths from the laboratory colony, reared exclusively on an artificial diet for over 5 generations. Therefore, we can consider that the observed differences in the bio-demographics of the immature stages were mainly due to the effects of feeding on different host plants, reflecting the plasticity in the responses of a polyphagous insect to its potential host plants. Because we evaluated the fitness of H. armigera over only two generations, the probability that these insects adapted to feed on these host plants was minimal, although genetic variability effects that were not evaluated in this study may also have influenced our results.
The host plant significantly affected the immature life stages of the parental (F 0) and F 1 populations. However, the host plant did not influence the number of produced eggs and longevity of individuals that reached the adult stage in F 0 populations. Although the adult life-table parameters of F 1 populations could not be estimated due to the high mortality of immatures, the results for the parental populations SS, SC, CC and CS showed that the host plants mainly affected the immature stages, with direct consequences for the adult stage by limiting the number of adult moths and their emergence time.
Mortality in the larval phases of H. armigera was high for all populations. The population mortality rates varied over the larval stages, influenced by the host plants on which the larvae fed. Previous studies have found high mortality in early larval stages of H. armigera populations (Zalucki et al. 2002; Liu et al. 2004; Reigada et al. 2016). In our study, however, all populations (pure and mixed) that fed on soybean showed a delay in the reduction of the number of individuals in the larval period. In agreement with the study by Reigada et al. (2016), soybean was a more favorable host plant for H. armigera than cotton.
The morphological and physiological attributes of host plants, such as the integument, surface waxes, hardness of the cuticle, and nutritional value, are important factors affecting the survivorship of larvae (Zalucki et al. 2002). First- and second-instar larvae commonly feed on leaves of cotton and soybean, avoiding wear and tear on their mandibles from feeding on the fruits (Zalucki et al. 2002; Liu et al. 2004). The leaves of the two host plants differed in the hardness of the cuticles, and mainly in the presence of chemicals. Our results suggest that first and second larval instars of H. armigera digest soybean more efficiently than cotton, resulting in lower mortality during the early larval stages.
Since Helicoverpa armigera was reported in Brazil, it has damaged a variety of crop systems in the country, resulting in much research that has tried to understand the causes for its rapid spread in many crops. Life-table studies have been carried out in many host plants and rearing methods have been developed to study the biology of the species. However, many researchers in Brazil have reported the higher mortality of immature stages (in the field and the laboratory) compared with he results found in places which the species is native or has already had a long period of adaptation. These facts suggest that the species can be incompletely adapted to the new conditions of the country, exhibiting strains with different performances for different regions of country in which the species has been reported. The presence of strains of H. armigera with poor fitness in he field and their usage in laboratory colonies can be a possible explanation for he high mortality rates in laboratory conditions reported by researchers.
Many life-table studies have evaluated the fitness of Helicoverpa armigera for one generation and have called attention to the high potential of polyphagia exhibited by the species (Zalucki et al. 2002; Liu et al. 2004; Reigada et al. 2016). In this study, however, we showed that, after two generations, the fitness of individuals reared on different host plants could be dramatically reduced. Evaluations considering fitness data for more than one generation and different availability of host plant species (i.e. successional or simultaneously) can improve the estimation of the infestation potential of pests and pest management in different types of crop mosaics.
The host plant on which previous generations (parent moths) fed did not affect the performance of the F 1 generation immatures. However, this result can be affected by the high mortality rates of F 1 immature stages. The low survival rates of F 1 immatures did not allowed the evaluation of the effects of parental feeding on the fecundity and longevity of F 1 adults to be assessed. Further studies should be considered to infer the effects of parental host plants in the performance of H. armigera immatures.
We found that the net reproductive rate (R 0) and other life-table parameters of offspring from S × S mating were highest, followed bythe S × C, C × S and C × C mating combinations. However, the potential for the increase of populations originating from S × S and S × C should be evaluated with caution, since the analyses of their progeny (Fig. 3; Appendix Table 4) revealed that the survival rates were affected by the host plant on which they fed, with important consequences for population sizes. The evaluation of the contributions of groups of individuals reared on different host plants for overall populations present in an area should consider the effects of host plants on the immature phase of the adults (F 0) present, as well as on their progeny (F 1). The findings suggest that the effects of parental diet can have some influence on offspring performance, despite the high mortality rates which did not allow the correct evaluation of this effect. This indicates that the effect of host plants on a polyphagous pest such as H. armigera cannot be expressed in a linear manner as some previous studies (Liu et al. 2004; Naseri et al. 2014; Reigada et al. 2016) have reported.
Although F 1 population survival rates were very low, our results suggest that the parental diet can indirectly affect the offspring in the larval stage. Other studies have found evidence that the maternal diet affects the size and survivorship of insects (Fox et al. 1995, 1997a, b; Tariq et al. 2010; Newcombe et al. 2015). Furthermore, non-genetic influences of the parental host plants can increase the fitness of generalist species in spatio-temporally variable environments (Tariq et al. 2010; Newcombe et al. 2015; Spitzer 2004). For instance, the mother can allocate nutrients to the eggs, altering the egg composition and increasing the success of immatures when the environment is poor or variable (Leather and Burnand 1987; Rossiter 1991; Fox et al. 1997a, b).
The time-limited presence of host plants and the sequence of crops available to individuals have important consequences for the population density, range expansion, and spatial distribution pattern in the agricultural landscape (Wardhaugh et al. 1980). The moths originating from larvae reared on soybean emerged earlier than those reared on cotton. In the growing season, the availability of different host plants for H. armigera can lengthen the period of adult moth encounters and consequently increase the chances of mating. Together with the high dispersal potential of the adult moths, the asynchronous emergence (see Fig. 4) of adults on neighboring crops can increase the number of mated females and consequently increase the population growth rates in a particular area. This suggests that, for a polyphagous insect such as H. armigera, pest-management and biological-control plans should be developed on a regional scale rather than for only a specific crop area.
Although our results are based on laboratory observations, this study provides insights into the dynamics of a polyphagous pest in an agricultural mosaic of annual crops. Our findings showed that the simultaneous presence of different host plants can increase the number of mating encounters. The reason is that the reduction of mating probability between the members of the same population, caused by the asynchrony between male and female emergence, can be offset by the longer period when the moths are present in the landscape, due to the variations in the frequency of moth emergence on different host crops. Consequently, more matings involving moths from soybean can contribute to increase the offspring fitness, even when the larvae then feed on other host plants (i.e., benefit from the parental diet).
Based on this information, we believe that establishing an appropriate and effective control program to manage H. armigera, mainly in areas occupied by soybean, can help to reduce pest infestations at the landscape scale. Further studies should analyze the performance and genetic variability of H. armigera populations in the field, testing the relationship between the diversity of host plants in the landscape and the infestation potential of this polyphagous pest.
Authors contribution
CR and JRPP conceived and designed research. CR conducted the experiments. CR, CGBD and RAM analyzed the data. CR and JRPP wrote the manuscript. All authors have read and approved the manuscript.
References
Butt BA, Cantu E (1962) Sex determination of lepidopterous pupae. USDA, Washington, DC
Czepak C, Albernaz KC, Vivan LM, Guimaraes HO, Carvalhais T (2013) First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Pesqui Agropec Trop 43: 110–113
Feng H, Gould F, Huang Y, Jiang Y, Wu K (2010) Modeling the population dynamics of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) over a wide area in northern China. Ecol Model 221:1819–1830
Fitt GP (1989) The ecology of Heliothis species in relation to agroecosystems. Annu Rev Entomol 34:17–52
Fox CW, Waddell KJ, Mousseau TA (1995) Parental host plant affects offspring life histories in a seed beetle. Ecology 76:402–411
Fox CW, Nilsson J, Mousseau T (1997a) The ecology of diet expansion in a seed-feeding beetle: pre-existing variation, rapid adaptation and maternal effects? Evol Ecol 11:183–194
Fox CW, Thakar MS, Mousseau TA (1997b) Egg size plasticity in a seed beetle: an adaptive maternal effect. Am Nat 149:149–163
Greene GL, Leppla NC, Dickerson WA (1976) Velvetbean caterpillar: a rearing procedure and artificial medium. J Econ Entomol 69(4):487–488
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Kennedy GG, Storer NP (2000) Life systems of poplyphagous arthropod pests in temporally unstable cropping systems. Annu Rev Entomol 45:467–493
Kriticos DJ, Ota N, Hutchinson WD, Beddow J, Walsh T, Tay WT, Borchert DM, Paula-Moreas SV, Czepak C, Zalucki MP (2015) The potential distribution of invading Helicoverpa armigera in North America: is it just a matter of time? PLoS ONE 10:e0119618
Leather SR, Burnand AC (1987) Factors affecting life history parameters of the pine beauty moth, Panolis flammea (D&S): the hidden costs of reproduction. Funct Ecol 1:331338
Liu Z, Li Z, Gong P, Wu K (2004) Life table of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), on different host plants. Environ Entomol 33:1570–1576
Lu ZQ, Xu YH (1998) The consideration of the incessant outbreak of the cotton bollworm, Helicoverpa armigera. Entomol Knowl 35(132):136
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170
MAPA—Ministério da Agricultura, Pecuária e Abastecimento (2016) Projeções do Agronegócio. http://www.agricultura.gov.br/arq_editor/PROJECOES_DO_AGRONEGOCIO_2025_WEB.pdf. Accessed May 2016
Mastrangelo T, Paulo DF, Bergamo LW, Morais EGF, Silva M, Bezerra-Silva G, Azevedo-Espin AML (2014) Detection and genetic diversity of a Heliothine invader (Lepidoptera: Noctuidae) from North and Northeast of Brazil. J Econ Entomol 107(3):1–5
Moral RA, Hinde J, Demétrio CGB (2016) hnp: Half-normal plots with simulation envelopes. R package version 1.2-2. https://CRAN.R-project.org/package=hnp
Murúa MG, Scalora FS, Navarro FR, Cazado LE, Casmuz A, Villagrán ME et al (2014) First record of Helicoverpa armigera (Lepidoptera: Noctuidae) in Argentina. Florida Entomol 97:854–856
Naseri B, Golparvar Z, Razmjou J, Golizadeh A (2014) Age-stage, two-sex life table of Helicoverpa armigera (Lepidoptera: Noctuidae) on different bean cultivars. J Agric Sci Technol 16:19–32
Newcombe D, Moore PJ, Moore AJ (2015) The role of maternal effects in adaptation to different diets. Biol J Linn Soc 114:202–211
R Development Core Team, version 3.2.1 (2015) R: a language and environ- ment for statistical computing. http://www.R-project.org. R Foundation for Statistical Computing, Vienna, Austria
Reigada C, Guimarães KF, Parra JRP (2016) Relative fitness of Helicoverpa armigera (Lepidoptera: Noctuidae) on seven host plants a perspective for IPM in Brazil. J Insect Sci 16(1):3; 1–5
Rossiter MC (1991) Maternal effects generate variation in life history: consequences of egg weight plasticity in the gypsy moth. Funct Ecol 5:386–393
Sosa-Gómez DR, Specht A, Paula-Moraes SV, Lopes-Lima A, Yano SAC, Micheli A, Morais EGF, Gallo P, Pereira PRVS, Salvadori JR, Botton M, Zenker MM, Azevedo-Filho WS (2014) Timeline and geographical distribution of Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae: Heliothinae) in Brazil. Rev Bras Entomol 60:101–104
Spitzer BW (2004) Maternal effects in the soft scale insect Saisetia coffeae (Hemiptera: Coccidae). Evolution 58:2452–2461
Tariq M, Wright DJ, Staley JT (2010) Maternal host plant effects on aphid performance: contrasts between a generalist and a specialist species on Brussels sprout cultivars. Agric For Entomol 12:107112
Tay WT, Soria MF, Walsh T, Thomazoni D, Silvie P, Behere GT et al (2013) A brave New World for an Old World pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 8:e80134
Wardhaugh KG, Room PM, Greenup LR (1980) The incidence of Heliothis armigera (Hübner) and H. punctigera Wallengren (Lepidoptera; Noctuidae) on cotton and other host-plants in the Namoi Valley of New South Wales. Bull Entomol Res 70:113–131
Zalucki MP, Murray DAH, Gregg PC, Fitt GP, Twine PH, Jones C (1994) Ecology of Helicoverpa armigera (Hubner) and H. punctigera (Wallengren) in the inland of Australia: larval sampling and host plant relationships during winter and spring. Aust J Zool 42:329–346
Zalucki MP, Clarke AR, Malcolm SB (2002) Ecology and behaviour of first instar larval Lepidoptera. Annu Rev Entomol 47:361–393
Acknowlegements
Financial support for this study was provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Programa Nacional de Pós Doutorado (PNPD: 2014.1.93.11.0), by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ: 459969/2015-5) and by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP: 2014/16609-7and 2014/12903-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by V. Gagic.
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Reigada, C., de Andrade Moral, R., Demétrio, C.G.B. et al. Cross-crop effects on larval growth, survivorship and fecundity of Helicoverpa armigera . J Pest Sci 91, 121–131 (2018). https://doi.org/10.1007/s10340-017-0893-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0893-5