Abstract
Gastric gland mucin secreted from gland mucous cells located in lower portions of the gastric mucosa contains unique O-linked oligosaccharides displaying terminal α1,4-linked N-acetylglucosamine (αGlcNAc). αGlcNAc inhibits growth and motility of Helicobacter pylori, a microbe causing gastric cancer, by inhibiting biosynthesis of its cell wall component cholesteryl-α-D-glucopyranoside. In addition, αGlcNAc serves as a tumor suppressor for gastric differentiated-type adenocarcinoma, and its loss in gastric cancer cells is associated with progression and poor prognosis of patients with this subtype of gastric cancer. This chapter summarizes protective functions of αGlcNAc against gastric cancer development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- α1,4-N-acetylglucosaminyltransferase
- Gastric cancer
- Helicobacter pylori
- Knockout mouse
- Mucin
- Patient prognosis
- Terminal α1,4-linked N-acetylglucosamine residue
1 Introduction
Gastric cancer ranks fourth in the most commonly diagnosed cancers and second in the most common causes of cancer-related death worldwide and thus remains one of the most common malignancies (Ferlay et al. 2010). On the other hand, gastric mucins play important roles in forming the surface mucous gel layer, which protects tissues from the external environment (Ota and Katsuyama 1992). However, how gastric mucins alter gastric cancer pathogenesis remains unknown. Gastric mucins are divided into surface and gland mucins (Ota et al. 1991). The first are secreted from surface mucous cells lining the gastric mucosa and contain surface mucin-specific glycans such as Lewis-related blood group carbohydrates attached to the mucin core protein MUC5AC (Nordman et al. 2002) (Fig. 7.1a). By contrast, the latter are secreted from gland mucous cells such as pyloric gland cells and mucous neck cells located in the lower layer of the gastric mucosa and contain gland mucin-specific O-glycans exhibiting terminal α1,4-linked N-acetylglucosamine residues (hereafter termed αGlcNAc) attached to MUC6 (Ishihara et al. 1996; Zhang et al. 2001; Ferreira et al. 2006). αGlcNAc is a unique O-glycan, as its distribution is limited to gastric gland mucous cells and Brunner’s glands of the duodenal mucosa (Nakamura et al. 1998).
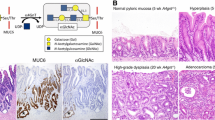
Expression of MUC5AC, MUC6, and αGlcNAc in gastric mucosa and the αGlcNAc biosynthetic pathway. (a) MUC5AC is expressed in surface mucous cells, while MUC6 is detected in pyloric gland cells of human gastric mucosa. Note that αGlcNAc is coexpressed with MUC6 in pyloric gland cells. Hematoxylin and eosin (HE) staining (upper left) and immunohistochemistry using CLH2 antibody for MUC5AC (upper right), CLH5 antibody for MUC6 (lower left), and HIK1083 antibody for αGlcNAc (lower right). Bar indicates 200 μm. (b) α1,4-N-Acetylglucosaminyltransferase (α4GnT) forms αGlcNAc primarily attached to MUC6
αGlcNAc biosynthesis is catalyzed by α1,4-N-acetylglucosaminyltransferase (α4GnT), which transfers N-acetylglucosamine (GlcNAc) from UDP-GlcNAc to terminal β-linked galactose (Gal) residues present in O-glycans with an α1,4-linkage (Fig. 7.1b). Previously, we used an expression cloning to isolate α4GnT cDNA from a human stomach cDNA library (Nakayama et al. 1999). Then, using α4GnT cDNA as a molecular tool, we investigated αGlcNAc function in the pathogenesis of gastric cancer. In this chapter, I describe the roles of αGlcNAc in the gastric mucosa and focus in particular on its protective function against Helicobacter pylori (H. pylori) infection and gastric cancer development.
2 Role of αGlcNAc in H. pylori Infection
2.1 αGlcNAc Acts as a Natural Antibiotic in Antagonizing H. pylori
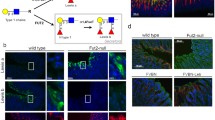
H. pylori is a gram-negative bacteria first isolated from gastric mucosa by Marshall and Warren (1984). This microbe is known to cause various gastric diseases, including chronic active gastritis, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (Peek and Blaser 2002). Although H. pylori infects over half the world’s human population, only a fraction of infected patients develop severe gastric disease. Interestingly, H. pylori largely colonizes surface mucins, while it is rarely found in gland mucins (Hidaka et al. 2001) (Fig. 7.2a), suggesting that αGlcNAc protects the gastric mucosa against H. pylori infection. To test the hypothesis, we cultured H. pylori in the presence of various levels of recombinant soluble CD43 (sCD43) carrying αGlcNAc (hereafter termed αGlcNAc (+)) (Kawakubo et al. 2004), which were secreted from Lec2 cells, a mutant CHO cell line defective in a sialic acid transporter (Deutscher et al. 1984). That line had been cotransfected with three expression vectors encoding α4GnT, core2 β1,6-N-acetylglucosaminyltransferase (C2GnT) (Bierhuizen and Fukuda 1992), and sCD43, respectively. In this assay, sCD43 serves as a glycan scaffold, as it contains 80 O-glycosylation sites in its extracellular domain (Fukuda 1992). Surprisingly, H. pylori growth was suppressed in a dose-dependent manner in the presence of αGlcNAc (+) (Fig. 7.2b). In addition, we observed significantly reduced motility and abnormal morphology, such as elongation and bending in H. pylori cultured with αGlcNAc (+). By contrast, when the microbe was incubated with control sCD43 lacking αGlcNAc (hereafter termed αGlcNAc (−)) secreted from cotransfected Lec2 cells with C2GnT and sCD43 expression vectors, we did not observe these effects, indicating that αGlcNAc antagonizes H. pylori growth like a natural antibiotic. Similar antibiotic effects were also obtained when H. pylori was incubated with p-nitrophenyl-α-N-acetylglucosamine (GlcNAcα–pNP), recombinant soluble CD34 carrying αGlcNAc, or αGlcNAc-containing gland mucin prepared from human gastric mucosa (Kawakubo et al. 2004).
Antimicrobial activity of αGlcNAc against H. pylori infection. (a) Histopathology of chronic active gastritis of human gastric mucosa caused by H. pylori infection. The microbe is rarely found in gland mucin expressing αGlcNAc (*). Hematoxylin and eosin (HE) staining (left), immunohistochemistry using anti-H. pylori antibody (middle), and HIK1083 antibody for αGlcNAc (right). Bar indicates 100 μm. (b) Growth curves of H. pylori cultured in the presence of sCD43 carrying αGlcNAc (αGlcNAc (+)) or sCD43 lacking αGlcNAc (αGlcNAc (−)). One milliunit of αGlcNAc (+) corresponds to 1 μg GlcNAcα–pNP. A600: absorbance at 600 nm. (c) Scanning electron micrographs showing H. pylori incubated with 31.2 mU/ml αGlcNAc (+) or the same concentration of αGlcNAc (−) protein for 3 days. Bar indicates 1 μm (Panels b and c are from Kawakubo et al. 2004; Copyright 2004 American Association for the Advancement of Science)
2.2 αGlcNAc Inhibits Cholesterol α-Glucosyltransferase Activity
To define the molecular mechanism underlying αGlcNAc antimicrobial activity, we focused in particular on morphological changes seen in H. pylori cultured in the presence of αGlcNAc (Fig. 7.2c) (Kawakubo et al. 2004). We noted that those changes were similar to those seen in H. pylori cultured in the presence of β-lactamase inhibitors (Enroth et al. 1999). Thus, we speculated that treatment with αGlcNAc had an effect on the H. pylori cell wall. Hirai et al. (1995) previously demonstrated that the H. pylori cell wall contains a unique glycolipid, cholesteryl-α-D-glucopyranoside (CGL), as well as its derivatives. CGL biosynthesis is catalyzed by cholesterol α-glucosyltransferase (αCgT), which transfers glucose (Glc) from UDP-Glc to cholesterol with an α1,3-linkage. Molecular mimicking between α1,4-linked GlcNAc in gland mucin and α1,3-linked Glc in CGL suggested that αGlcNAc suppressed αCgT enzymatic activity by an end-product inhibitory mechanism. Thus, we analyzed glycolipid fractions isolated from H. pylori cultured in the presence of αGlcNAc (+) or αGlcNAc (−) using mass spectrometry (Kawakubo et al. 2004). We found that CGL levels in H. pylori cultured with αGlcNAc (+) were significantly lower than those seen in H. pylori cultured with αGlcNAc (−), suggesting that αGlcNAc directly inhibits CGL biosynthesis by H. pylori in vivo. Subsequently, we used expression cloning to isolate αCgT gene from H. pylori (Lee et al. 2006) and proved that αCgT enzymatic activity is inhibited by core2-branched O-glycans displaying αGlcNAc in vitro (Lee et al. 2008). We also showed that an active form of αCgT is present in the H. pylori membrane fraction, suggesting that bacterial αCgT is likely accessible to αGlcNAc in gland mucin (Hoshino et al. 2011).
2.3 CGL Is Indispensible for H. pylori Survival
H. pylori requires exogenous cholesterol for CGL biosynthesis. Thus to further define CGL function in H. pylori, we created H. pylori lacking CGL by culturing the microbe in the absence of cholesterol (Kawakubo et al. 2004). Resultant H. pylori exhibited reduced growth and motility, and all microbes died following prolonged (21-day) incubation in cholesterol-free media, indicating that CGL is indispensable for H. pylori survival. Overall, these results show that αGlcNAc inhibits CGL biosynthesis by H. pylori by suppressing αCgT, thus protecting the gastric mucosa from infection. In fact, αGlcNAc does not exhibit antimicrobial activity against bacteria lacking CGL such as E. coli and S. aureus (Kawakubo et al. 2004). Most recently, we reported that αCgT enzymatic activity in H. pylori was highly correlated with the degree of glandular atrophy in gastric mucosa infected by the bacteria and that the monoacyled form of cholesteryl-6-O-phosphatidyl-α-D-glucopyranoside (CPG), a minor constituent of CGL derivatives in the H. pylori cell wall, is the most potent antigen for invariant natural killer T cells, thus eliciting an immune response in gastric mucosa (Ito et al. 2013).
3 αGlcNAc Serves as a Tumor Suppressor for Gastric Cancer
3.1 αGlcNAc Suppresses Tumorigenesis of Gastric Differentiated-Type Adenocarcinoma
We then asked whether αGlcNAc had a more general or broader protective function in the gastric mucosa. To address to this question, we generated mice deficient in α4GnT by disrupting the A4gnt gene and analyzed αGlcNAc function in vivo (Karasawa et al. 2012). Immunohistochemistry using the αGlcNAc-specific antibody HIK1083 and MALDI-TOF-MS analyses revealed that A4gnt-deficient mice showed a complete lack of αGlcNAc expression in gastric gland mucin and duodenal Brunner’s gland, formally establishing that α4GnT is the sole enzyme catalyzing addition of αGlcNAc to O-glycans in vivo (Fig. 7.3). Surprisingly, A4gnt-deficient mice, even in the absence of H. pylori infection, spontaneously developed tumor in the antrum as early as 5 weeks of age, and tumor size gradually increased as mice aged (Fig. 7.4a). Histopathology of tumors revealed that the mutant mice exhibited hyperplasia by 5 weeks of age, low-grade dysplasia by 10 weeks, and high-grade dysplasia by 20 weeks in the glandular stomach (Fig. 7.4b). In 30-week-old mice, gastric adenocarcinoma developed in 2 of 6 A4gnt-deficient mice, and adenocarcinoma incidence increased by 50 weeks of age. Furthermore, all 50- and 60-week-old mice exhibited gastric adenocarcinoma. These pathologies were consistently seen in the antrum of the glandular stomach, and cancer cells were mostly restricted to the mucosa. No sign of distant metastasis was noted up to 60 weeks of age. Gastric adenocarcinoma is largely classified into differentiated (or intestinal) or undifferentiated (or diffuse) types, based on tumor cell morphology and histogenesis background (Lauren 1965; Nakamura et al. 1968). Interestingly, the gastric adenocarcinoma seen in A4gnt-deficient mice was only of the differentiated type, while undifferentiated-type adenocarcinoma, such as signet ring cell carcinoma, never arose. This indicates that mutant mice develop gastric differentiated-type adenocarcinoma through a hyperplasia-dysplasia-carcinoma sequence in the absence of H. pylori infection. Intestinal metaplasia was rarely detected in gastric mucosa of either A4gnt-deficient or wild-type mice during the 60-week observation period, indicating that metaplasia is not associated with gastric tumorigenesis in this model. These results establish that αGlcNAc serves as a tumor suppressor for gastric differentiated-type adenocarcinoma.
αGlcNAc loss in A4gnt-deficient mice. αGlcNAc is expressed in mucous neck cells of the gastric corpus, pyloric gland cells of the gastric antrum, and Brunner’s glands of the duodenum of wild-type mouse (+/+), while it is completely absent in these mucous cells of A4gnt-deficient mouse (−/−). Shown is immunohistochemistry of gastroduodenal mucosa from 1-week-old mice with αGlcNAc-specific HIK1083 antibody. Bar indicates 100 μm
Gastric pathology of A4gnt-deficient mice. (a) Macroscopic appearance of the stomach removed from wild-type mice (+/+) and A4gnt-deficient mice (−/−). Bar indicates 5 mm. (b) Representative histopathology showing hyperplasia at 5 weeks, low-grade dysplasia at 10 weeks, high-grade dysplasia at 20 weeks, and differentiated-type adenocarcinoma at 40 and 50 weeks in the antral mucosa of A4gnt-deficient mice. For comparison, pyloric mucosa from a 5-week-old wild-type mouse is shown (upper left). Bar indicates 100 μm (Panel a is from Karasawa et al. 2012; Copyright 2012 The American Society for Clinical Investigation)
3.2 αGlcNAc Suppresses Tumor-Promoting Inflammation in A4gnt-Deficient Mice
It remained unclear why A4gnt-deficient mice develop only differentiated-type adenocarcinoma in the gastric mucosa. To clarify molecular mechanisms underlying such a specific tumor suppression function by αGlcNAc, we carried out microarray analysis followed by quantitative RT-PCR using mRNA derived from gastric mucosa of A4gnt–deficient and wild-type mice at 5, 10, and 50 weeks of age (Karasawa et al. 2012). Our analysis identified eight genes upregulated in A4gnt-deficient mice compared with wild-type mice (Fig. 7.5). Among these genes significantly upregulated in the gastric mucosa of mutant mice older than 10 weeks were those encoding inflammatory chemokine ligands such as Ccl2, Cxcl1, and Cxcl5; proinflammatory cytokines such as Il-11 and Il-1β; and growth factors such as Hgf and Fgf7. In addition, Hgf was upregulated even in 5-week-old mutant mice, indicating that altered gene expression patterns are apparent even at low-grade dysplasia stages, prior to gastric cancer development. Of the altered factors, Ccl2 is of particular interest as it attracts tumor-associated macrophages, which exert pro-tumorigenic immune responses and promote tumor angiogenesis (Grivennikov et al. 2010; Mantovani et al. 2010). In fact, both infiltration of inflammatory cells such as mononuclear cells and neutrophils and angiogenesis increased progressively in the gastric mucosa as A4gnt-deficient mice aged. IL-11 is also noteworthy because it functions in progression of inflammation to gastric tumorigenesis via gp130 signaling, followed by STAT3 phosphorylation (Ernst et al. 2008; Howlett et al. 2009). Taken together, our results indicate that αGlcNAc loss triggers gastric carcinogenesis through inflammation-associated pathways in vivo.
Genes upregulated in the gastric mucosa of A4gnt-deficient mice compared with those in age-matched wild-type mice, as determined by quantitative RT-PCR analysis. Grem1, Gremlin 1; Cxcl1, Chemokine (C-X-C motif) ligand 1; Ccl2, Chemokine (C-C motif) ligand 2; Cxcl5, Chemokine (C-X-C motif) ligand 5; Il11, IL-11; Hgf, HGF; Il1b, IL-1β; Fgf7, FGF7. *P < 0.05; **P < 0.01 (From Karasawa et al. 2012; Copyright 2012 American Society for Clinical Investigation)
3.3 αGlcNAc Loss in Gland Mucin Is Associated with Progression of Human Gastric Differentiated-Type Adenocarcinoma
Lastly, we asked whether αGlcNAc loss occurred in human gastric adenocarcinoma and whether such loss was associated with tumor progression. To do so, we used immunohistochemistry to assess expression of αGlcNAc and its scaffold MUC6 in 214 surgically resected gastric adenocarcinomas and then compared those expression patterns with clinicopathological parameters such as vessel invasion and stage and cancer-specific survival (Shiratsu et al. 2014). MUC6 was detected in gastric cancer cells in 102 (47.6 %) of 214 patients. In differentiated-type adenocarcinoma, 33 (58.9 %) of 54 MUC6-positive cancer lacked αGlcNAc expression, while in undifferentiated-type adenocarcinoma, 22 (45.8 %) of 48 MUC6-positive cancer lacked αGlcNAc expression, indicating that there was no significant difference between absence of αGlcNAc expression in differentiated and undifferentiated tumor types. However, when the comparison was made between a subtype of undifferentiated-type adenocarcinoma, signet ring cell carcinoma, and differentiated-type adenocarcinoma (Fig. 7.6a), only 6 (26.1 %) of 23 signet ring cell carcinoma patients lacked αGlcNAc expression, significantly at lower frequency compared with differentiated-type adenocarcinoma (P = 0.0049). Notably, αGlcNAc loss was significantly correlated with depth of invasion, stage, venous invasion, and more importantly, poorer patient prognosis in MUC6-positive differentiated-type adenocarcinoma (Fig. 7.6b). On the other hand, no significant correlation between αGlcNAc loss in tumor cells and any clinicopathological variable or cancer-specific survival of patients with undifferentiated-type adenocarcinoma was observed. Thus, αGlcNAc loss in MUC6-positive cancer cells is significantly associated with progression and poor prognosis in differentiated-type but not undifferentiated-type adenocarcinomas of the stomach, consistent with phenotypes seen in A4gnt-deficient mice (Karasawa et al. 2012). As described in Sect. 7.3.2, inflammatory chemokine ligands, proinflammatory cytokines, and growth factors were upregulated in mutant mice, and these molecules are also thought to function in human gastric cancer development. For example, Ohta et al. (2003) reported that CCL2 expression by human gastric carcinoma cells increases with tumor cell invasiveness, and its expression level is positively correlated with angiogenesis and macrophage recruitment. Verbeke et al. (2012) demonstrated that CXC chemokines, including CXCL1/CXCL5, facilitate progression of gastric cancer tumors. Nakayama et al. (2007) observed that IL-11 expression is significantly higher in differentiated compared to undifferentiated types of adenocarcinoma and that IL-11 functions in gastric carcinoma progression. HGF and FGF7 play important roles in gastric epithelial proliferation. Mohri et al. (2012) suggest that HGF expression is an important prognostic factor in gastric cancer. FGF7 is upregulated by IL-1β (Palmieri et al. 2003). Kai et al. (2005) demonstrated that tumor IL-1β expression levels are elevated more than 50-fold over those seen in normal gastric mucosa and significantly higher in nonscirrhous compared with scirrhous carcinomas. Thus, all of these factors likely promote tumor-promoting inflammation. Accordingly, our results suggest that αGlcNAc loss is correlated with gastric cancer progression in inflammation-related pathways in humans. It remains to be determined how αGlcNAc loss in gastric cancer enhances tumor-promoting inflammation in the stomach. Recently, we demonstrated that reduced αGlcNAc in Barrett’s esophagus could also predict its potential to develop into Barrett’s adenocarcinoma (Iwaya et al. 2014).
αGlcNAc expression in human gastric adenocarcinoma and correlation with cancer-specific survival of gastric cancer patients. (a) Hematoxylin and eosin (HE) staining (left) and αGlcNAc (middle) and MUC6 (left) expression in differentiated-type adenocarcinoma (upper panels) and signet ring cell carcinoma (lower panels). Bar indicates 200 μm. (b) Cancer-specific survival in patients with MUC6-positive gastric cancer. In differentiated-type adenocarcinoma, patients with αGlcNAc-negative tumors had a significant poorer outcome than did patients with αGlcNAc-positive tumors (P = 0.048). By contrast, in undifferentiated-type adenocarcinoma, there was no significant difference in survival rate of patients harboring αGlcNAc-positive or αGlcNAc-negative tumors (P = 0.549) (Modified from Shiratsu et al. 2014)
4 Conclusion
We conclude that gastric gland mucin-specific αGlcNAc has a protective function against gastric cancer development in two ways: first, as a natural antibiotic against H. pylori and second, as a tumor suppressor for gastric differentiated-type adenocarcinoma. Based on these findings, we anticipate future development of new strategies to detect, diagnose, treat, and prevent gastric cancer.
References
Bierhuizen MF, Fukuda M (1992) Expression cloning of a cDNA encoding UDP-GlcNAc:Gal β1-3-GalNAc-R (GlcNAc to GalNAc) β1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci USA 89:9326–9330
Deutscher SL, Nuwayhid N, Stanley P, Briles EI, Hirschberg CB (1984) Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 39:295–299
Enroth H, Wreiber K, Rigo R, Risberg D, Uribe A, Engstrand L (1999) In vitro aging of Helicobacter pylori: changes in morphology, intracellular composition and surface properties. Helicobacter 4:7–16
Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, Marcusson EG, Karras JG, Na S, Sedgwick JD, Hertzog PJ, Jenkins BJ (2008) STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest 118:1727–1738
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Ferreira B, Marcos NT, David L, Nakayama J, Reis CA (2006) Terminal α1,4-linked N-acetylglucosamine in Helicobacter pylori-associated intestinal metaplasia of the human stomach and gastric carcinoma cell lines. J Histochem Cytochem 54:585–591
Fukuda M (1992) Cell surface carbohydrates in hematopoietic cell differentiation and malignancy. In: Fukuda M (ed) Cell surface carbohydrates and cell development. CRC Press, Boca Raton, pp 127–159
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Hidaka E, Ota H, Hidaka H, Hayama M, Matsuzawa K, Akamatsu T, Nakayama J, Katsuyama T (2001) Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut 49:474–480
Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K (1995) Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol 177:5327–5333
Hoshino H, Tsuchida A, Kametani K, Mori M, Nishizawa T, Suzuki T, Nakamura H, Lee H, Ito Y, Kobayashi M, Masumoto J, Fujita M, Fukuda M, Nakayama J (2011) Membrane-associated activation of cholesterol α-glucosyltransferase, an enzyme responsible for biosynthesis of cholesteryl-α-D-glucopyranoside in Helicobacter pylori critical for its survival. J Histochem Cytochem 59:98–105
Howlett M, Giraud AS, Lescesen H, Jackson CB, Kalantzis A, Van Driel IR, Robb L, Van der Hoek M, Ernst M, Minamoto T, Boussioutas A, Oshima H, Oshima M, Judd LM (2009) The interleukin-6 family cytokine interleukin-11 regulates homeostatic epithelial cell turnover and promotes gastric tumor development. Gastroenterology 136:967–977
Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K (1996) Peripheral α-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 318:409–416
Ito Y, Vela JL, Matsumura F, Hoshino H, Tyznik A, Lee H, Girardi E, Zajonc DM, Liddington R, Kobayashi M, Bao X, Bugaytsova J, Borén T, Jin R, Zong Y, Seeberger PH, Nakayama J, Kronenberg M, Fukuda M (2013) Helicobacter pylori cholesteryl α-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PLoS ONE 8, e78191
Iwaya Y, Hasebe O, Koide N, Kitahara K, Suga T, Shinji A, Muraki T, Yokosawa S, Yamada S, Arakura N, Tanaka E, Nakayama J (2014) Reduced expression of αGlcNAc in Barrett’s oesophagus adjacent to Barrett’s adenocarcinoma – a possible biomarker to predict the malignant potential of Barrett's oesophagus. Histopathology 64:536–546
Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, Tanaka S, Ohmoto Y, Chayama K (2005) Involvement of proinflammatory cytokines IL-1β and IL-6 in progression of human gastric carcinoma. Anticancer Res 25:709–713
Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S, Ueda M, Fukuda MN, Fukuda M, Ishihara K, Nakayama J (2012) Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest 122:923–934
Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J (2004) Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003–1006
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Lee H, Kobayashi M, Wang P, Nakayama J, Seeberger PH, Fukuda M (2006) Expression cloning of cholesterol α-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem Biophys Res Commun 349:1235–1241
Lee H, Wang P, Hoshino H, Ito Y, Kobayashi M, Nakayama J, Seeberger PH, Fukuda M (2008) α1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol α-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 18:549–558
Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R (2010) The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev 21:27–39
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 8390:1311–1315
Mohri Y, Miki C, Tanaka K, Kawamoto A, Ohi M, Yokoe T, Kusunoki M (2012) Clinical correlations and prognostic relevance of tissue angiogenic factors in patients with gastric cancer. Clin Oncol 24:610–616
Nakamura K, Sugano H, Takagi K (1968) Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann 59:251–258
Nakamura N, Ota H, Katsuyama T, Akamatsu T, Ishihara K, Kurihara M, Hotta K (1998) Histochemical reactivity of normal, metaplastic, and neoplastic tissues to α-linked N-acetylglucosamine residue-specific monoclonal antibody HIK1083. J Histochem Cytochem 46:793–801
Nakayama J, Yeh J-C, Misra AK, Ito S, Katsuyama T, Fukuda M (1999) Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1 → 4Galβ → R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci USA 96:8991–8996
Nakayama T, Yoshizaki A, Izumida S, Suehiro T, Miura S, Uemura T, Yakata Y, Shichijo K, Yamashita S, Sekine I (2007) Expression of interleukin-11 (IL-11) and IL-11 receptor alpha in human gastric carcinoma and IL-11 upregulates the invasive activity of human gastric carcinoma cells. Int J Oncol 30:825–833
Nordman H, Davies JR, Lindell G, de Bolós C, Real F, Carlstedt I (2002) Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J 364:191–200
Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K (2003) Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol 22:773–778
Ota H, Katsuyama T (1992) Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J 24:86–92
Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y (1991) A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J 23:22–28
Palmieri C, Roberts-Clark D, Assadi-Sabet A, Coope RC, O’Hare M, Sunters A, Hanby A, Slade MJ, Gomm JJ, Lam EW, Coombes RC (2003) Fibroblast growth factor 7, secreted by breast fibroblasts, is an interleukin-1β-induced paracrine growth factor for human breast cells. J Endocrinol 177:65–81
Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2:28–37
Shiratsu K, Higuchi K, Nakayama J (2014) Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer Sci 105:126−133
Verbeke H, Geboes K, Van Damme J, Struyf S (2012) The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta 1825:117–129
Zhang MX, Nakayama J, Hidaka E, Kubota S, Yan J, Ota H, Fukuda M (2001) Immunohistochemical demonstration of α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1,4Galβ residues in human gastrointestinal mucosa. J Histochem Cytochem 49:587–596
Acknowledgments
I am grateful to all collaborators for their contributions to this research. In particular, I wish to thank Dr. Tsutomu Katsuyama, Emeritus Professor of Shinshu University, Dr. Minoru Fukuda, Professor at the Sanford-Burnham Medical Research Institute, and Dr. Michiko N. Fukuda, Professor at the Sanford-Burnham Medical Research Institute, for their encouragement and collaboration, and Dr. Elise Lamar for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Nakayama, J. (2016). Function of Unique O-Glycan Structures in Protecting Gastric Mucosa Against Helicobacter pylori Infection and Gastric Cancer Development. In: Furukawa, K., Fukuda, M. (eds) Glycosignals in Cancer: Mechanisms of Malignant Phenotypes . Springer, Tokyo. https://doi.org/10.1007/978-4-431-55939-9_7
Download citation
DOI: https://doi.org/10.1007/978-4-431-55939-9_7
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55937-5
Online ISBN: 978-4-431-55939-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)