Abstract
Gastric gland mucin secreted from pyloric gland cells, mucous neck cells, and cardiac gland cells of the gastric mucosa harbors unique O-glycans carrying terminal α1,4-linked N-acetylglucosamine residues (αGlcNAc), which are primarily attached to the scaffold mucin core protein MUC6. αGlcNAc acts as an antibiotic against Helicobacter pylori (H. pylori), a microbe causing gastric cancer. In addition, mice deficient in A4gnt, which encodes the enzyme α1,4-N-acetylglucosaminyltransferase (α4GnT) that catalyzes αGlcNAc biosynthesis, spontaneously develop gastric differentiated-type adenocarcinoma, even if not infected by H. pylori. Thus, αGlcNAc prevents gastric cancer as both an antibiotic and a tumor suppressor (Nakayama in Acta Histochem Cytochem 47:1–9, 2014b). Indeed, in humans αGlcNAc loss on MUC6 in differentiated-type adenocarcinoma is closely associated with poor patient prognosis (Shiratsu et al. in Cancer Sci 105:126–133, 2014). Recently, we reported reduced αGlcNAc expression on MUC6 in both pyloric gland adenoma of the stomach and chronic atrophic gastritis, in Barrett’s esophagus, and in pancreatic intraductal papillary-mucinous neoplasm (IPMN)/pancreatic intraepithelial neoplasia (PanIN), all potentially premalignant conditions. This review discusses whether relatively reduced levels of αGlcNAc in these lesions could serve as a biomarker to predict malignant potential and cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric mucin consists of heavily glycosylated glycoproteins and protects the gastric mucosa from extracellular environments such as acidity by forming a mucous gel layer (Ota et al. 1991; Ota and Katsuyama 1992). In particular, gland mucin secreted from gland mucous cells, including pyloric gland cells, mucous neck cells, and cardiac gland cells located in the lower layer of the gastric mucosa contains unique O-glycans carrying terminal α1,4-linked N-acetylglucosamine (αGlcNAc) residues primarily attached to the scaffold mucin core protein MUC6 (Ishihara et al. 1996; Zhang et al. 2001; Ferreira et al. 2006) (Fig. 1a). We previously isolated cDNA encoding α1,4-N-acetylglucosaminyltransferase (α4GnT) by expression cloning and showed that α4GnT is the sole enzyme that catalyzes αGlcNAc biosynthesis (Nakayama et al. 1999; Nakayama 2014a). We then demonstrated that αGlcNAc acts as an antibiotic against Helicobacter pylori (H. pylori), a microbe causing gastric cancer, by inhibiting biosynthesis of its cell wall component cholesteryl-α-d-glucopylanoside (Kawakubo et al. 2004). We have since generated mutant mice deficient in A4gnt, which encodes α4GnT, and found that these mice completely lack αGlcNAc in gland mucin (Karasawa et al. 2012). Surprisingly, differentiated-type adenocarcinoma develops spontaneously in pyloric mucosa of A4gnt-deficient mice through a hyperplasia–dysplasia–carcinoma sequence (Fig. 1b). These observations indicate that αGlcNAc protects gastric mucosa from cancer development as both an antibiotic against H. pylori and a tumor suppressor of gastric cancer (Nakayama 2014b).

[b is From Nakayama (2014b); Copyright 2014 The Japanese Society of Histochemistry and Cytochemistry]
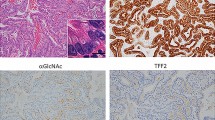
a αGlcNAc biosynthesis is catalyzed by α1,4-N-acetylglucosaminytransferase (α4GnT), which transfers GlcNAc from UDP-GlcNAc to βGal residues of O-glycans linked to MUC6 Ser/Thr residues via an α1,4-linkage. b Gastric pathology of A4gnt-deficient mice, showing hyperplasia at 5 weeks of age (5 wk A4gnt−/−), high-grade dysplasia at 20 weeks (20 wk A4gnt−/−), and differentiated-type adenocarcinoma at 50 weeks (50 wk A4gnt−/−) in the pyloric mucosa. Pyloric mucosa of a wild-type mouse is shown for comparison (5 wk A4gnt+/+). Scale bar 100µm. (HE) c Early gastric differentiated-type adenocarcinoma in a patient specimen. Panels show HE staining (left), and immunohistochemistry for αGlcNAc (right; clone HIK1083) and MUC6 (middle; clone CLH5). Note that αGlcNAc is absent in MUC6-positive adenocarcinoma cells. Adjacent to carcinoma cells are normal pyloric glands (indicated by asterisks), which are positive for αGlcNAc and MUC6. Scale bar 200µm.
Using the HIK1083 monoclonal antibody, which specifically recognizes αGlcNAc (Ishihara et al. 1996), we revealed that αGlcNAc but not MUC6 expression is frequently lost in differentiated-type adenocarcinoma of human gastric cancer and that αGlcNAc loss is significantly associated with poor patient prognosis (Fig. 1c) (Shiratsu et al. 2014). Reduced αGlcNAc expression on MUC6 is also observed in pyloric gland adenoma of the stomach and chronic atrophic gastritis, both potential risk factors for gastric cancer (Yamanoi et al. 2015b; Yamada et al. 2015). We also observed relatively decreased αGlcNAc expression in Barrett’s esophagus (Iwaya et al. 2014) and intraductal papillary–mucinous neoplasm (IPMN)/pancreatic intraepithelial neoplasia (PanIN) of the pancreas (Ohya et al. 2017), which are potentially premalignant conditions for Barrett’s adenocarcinoma and pancreatic cancer, respectively. In this review, we discuss whether relative decreases in αGlcNAc expression could serve as a biomarker of malignant potential of these respective lesions.
Reduced αGlcNAc expression is a biomarker of pyloric gland adenoma of the stomach
Pyloric gland adenoma of the stomach is a gastric neoplasia exhibiting closely packed pyloric gland-like structures that express MUC6, a marker of pyloric gland differentiation (Vieth et al. 2003; Kushima et al. 2006; Chen et al. 2009). Pyloric gland adenoma often shows high-grade dysplasia and is thought to be associated with a high risk of malignant transformation.
To assess whether αGlcNAc reduction in pyloric gland adenoma is associated with malignant potential, we examined αGlcNAc and MUC6 expression status in 18 specimens of pyloric gland adenomas including 15 cases of low-grade dysplasia and 3 cases of high-grade dysplasia (Yamanoi et al. 2015b). αGlcNAc and MUC6 were equally co-expressed in 12 of the 18, indicating pyloric gland differentiation with fully glycosylated MUC6 (Fig. 2a). However, reduced αGlcNAc expression relative to that of MUC6 was observed in other 6 cases. Notably, such αGlcNAc reduction was found in all 3 cases of pyloric gland adenoma with high-grade dysplasia. Because epithelial cells in the pyloric mucosa of A4gnt-deficient mice proliferate more actively than do their counterparts in wild-type mice (Karasawa et al. 2012), we asked whether reduced αGlcNAc expression in pyloric gland adenoma was associated with increased tumor cell proliferation, as assessed by the MIB-1 labeling index (LI) (Fig. 2b). Analysis showed that the MIB-1 LI significantly increased in pyloric gland adenoma exhibiting reduced αGlcNAc expression [33 ± 8.1% (mean ± SEM)] relative to pyloric gland adenoma with unchanged αGlcNAc expression (7.5 ± 1.9%).

[From Yamanoi et al. (2015b); Copyright 2015 John Wiley & Sons Ltd.]
a Immunohistochemistry showing expression of αGlcNAc, MUC6 and MIB-1 in pyloric gland adenoma of the stomach in low-grade (left) and high-grade (right) dysplasia. Insets show enlarged view of the HE-stained section. In low-grade dysplasia, MUC6 and αGlcNAc are coexpressed in tumor cells at significant levels. However, in high-grade dysplasia, expression of αGlcNAc decreases relative to MUC6. Scale bar 200 µm. Inset scale bar 50 µm. b Calculation of the MIB-1 labeling index (LI) in pyloric gland adenoma (PGA) samples showing reduced versus unchanged αGlcNAc expression. The LI of the former is significantly higher than that of the latter.
These results indicate that decreased glycosylation of MUC6 with αGlcNAc is associated with high mitotic activity of tumor cells and indicates malignant potential of pyloric gland adenoma.
Reduced αGlcNAc expression in chronic gastric atrophy may be a risk factor for differentiated-type gastric carcinoma
Chronic gastric atrophy is histologically characterized by chronic inflammation of the gastric mucosa and reduced number of gastric glandular cells. This condition is closely associated with H. pylori infection and considered a premalignant lesion (Morris and Nicholson 1987). However, it is not fully understand why gastric atrophy is linked to gastric cancer risk.
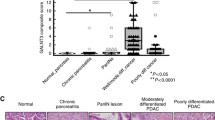
The presence of αGlcNAc on gastric gland mucins antagonizes gastric cancer development (Nakayama 2014b). Thus, to test whether reduced αGlcNAc expression in chronic atrophic gastritis is associated with malignant potential, we quantitatively analyzed αGlcNAc expression relative to that of MUC6 in non-neoplastic pyloric glands. To do so, we performed immunohistochemistry of specimens from patients from one of the following groups: normal gastric mucosa without H. pylori infection, chronic atrophic gastritis with H. pylori infection, intramucosal differentiated-type adenocarcinoma, and intramucosal undifferentiated-type adenocarcinoma (Yamada et al. 2015) (Fig. 3A). Semiquantitative analysis revealed co-expression of αGlcNAc with MUC6 in pyloric glands of normal gastric mucosa (Fig. 3B). In contrast, in pyloric glands of chronic atrophic gastritis, αGlcNAc expression was significantly reduced relative to that of MUC6. In intramucosal gastric cancer, αGlcNAc expression in non-neoplastic pyloric glands located just beneath differentiated-type adenocarcinoma cells was also reduced relative to MUC6. However, αGlcNAc expression did not decrease in non-neoplastic pyloric glands found just beneath undifferentiated-type adenocarcinoma. These results are consistent with gastric phenotypes of A4gnt-deficient mice, which spontaneously develop differentiated-type but not undifferentiated-type adenocarcinomas (Karasawa et al. 2012). In addition, the MIB-1 LI evaluated in gastric epithelial cells of chronic atrophic gastritis lacking αGlcNAc expression was significantly increased compared to normal gastric mucosa (Yamada et al. 2015). Considering that αGlcNAc protects gastric mucosa from H. pylori infection as an antibiotic (Kawakubo et al. 2004), reduced αGlcNAc expression in pyloric glands seen in chronic atrophic gastritis could account for why these cases are at risk for differentiated-type gastric adenocarcinoma.

[From Yamada et al. (2015); Copyright 2015 Journal of Gastroenterology and Hepatology Foundation and Wiley Publishing Asia Pty Ltd.]
A Immunohistochemical expression of MUC6 and αGlcNAc in non-neoplastic pyloric glands in indicated types of patient specimens. Scale bar 200 μm. B Quantification of the number of MUC6-positive and αGlcNAc-positive pyloric glands in specimens shown in A. There was no significant difference between the number of MUC6-positive and αGlcNAc-positive pyloric glands in normal gastric mucosa (a) or in intramucosal undifferentiated-type adenocarcinoma (d). In contrast, there was significant reduction in the number of αGlcNAc-positive relative to MUC6-positive pyloric glands in chronic atrophic gastritis (b) and in differentiated-type adenocarcinoma (c). (*** P < 0.001).
H. pylori infection potently induces methylation of CpG islands in affected tissues, and that modification is potentially associated with gastric cancer risk (Maekita et al. 2006). More recently, we showed that altered DNA methylation profiles seen in chronic atrophic gastritis are inherited by gastric cancer cells and are significantly correlated with tumor aggressiveness and patient outcomes (Yamanoi et al. 2015a). These results overall suggest that in cases of chronic gastric atrophy, altered glycosylation or DNA methylation patterns should be considered in predicting cancer risk.
Reduced αGlcNAc expression in Barrett’s esophagus could predict Barrett’s adenocarcinoma
In Barrett’s esophagus, squamous epithelium in the lower esophagus adjacent to the esophago-gastric junction is replaced by columnar metaplastic epithelium (Wang and Samliner 2008). The condition is caused by healing reflux esophagitis and occasionally progresses to dysplasia and then to Barrett’s adenocarcinoma (Reid et al. 1992). Thus, Barrett’s esophagus is considered a premalignant lesion of Barrett’s adenocarcinoma. In fact, aberrant p53 expression is reportedly an independent predictor of neoplastic progression from Barrett’s esophagus (Janmaat et al. 2017).
Relatively reduced αGlcNAc expression in Barrett’s esophagus could also predict the risk of adenocarcinoma (Iwaya et al. 2014): αGlcNAc and MUC6 are co-expressed in Barrett’s esophagus in the absence of Barrett’s adenocarcinoma (Fig. 4A), while αGlcNAc expression in Barrett’s esophagus adjacent to Barrett’s adenocarcinoma decreases relative to that of MUC6 (Fig. 4B, C). This decrease is particularly significant in patients who exhibit both smaller tumor sizes (< 20 cm) and minimally invasion by tumor cells to the lamina propria mucosa or muscularis mucosa. These observations indicate that decreased αGlcNAc expression relative to MUC6 in Barrett’s esophagus could mark Barrett’s adenocarcinoma development.

[From Iwaya et al. (2014) with modification; Copyright 2014 John Wiley & Sons Ltd.]
A Barrett’s esophagus without Barrett’s adenocarcinoma. Both a squamous island (arrow in a) lies distal to columnar epithelium and an esophageal gland proper (EGP) under that columnar epithelium are characteristics of Barrett’s esophagus (a HE, b higher magnification image of boxed area in a). Immunohistochemistry shows that MUC6 (c) and αGlcNAc (d) are co-expressed in mucous glands under the columnar epithelium. Bar in a 500 µm, and bar in d 200 µm. B Barrett’s esophagus adjacent to Barrett’s adenocarcinoma (a HE, b higher magnification image of boxed area in a). In Barrett’s esophagus adjacent to Barrett’s adenocarcinoma (BAC), immunohistochemistry shows that MUC6 (c) is positive in mucous glands under the columnar epithelium but αGlcNAc (d) is negative. Bar in a 500 µm, and bar in d 200 µm. C Relative expression of αGlcNAc to MUC6 in Barrett’s esophagus (BE) adjacent to Barrett’s adenocarcinoma (BAC) (left) or in BE without BAC (control BE). In the former, the ratio of αGlcNAc to MUC6 expression is significantly reduced relative to control BE (**P < 0.01).
The relative risk of adenocarcinoma among patients with Barrett’s esophagus is reportedly 11.3 in the general population (Frederik et al. 2011). However, the absolute annual risk that a patient with Barrett’s esophagus will develop Barrett’s adenocarcinoma is 0.12% (Frederik et al. 2011). Given that the assumed risk as reported in current surveillance guidelines is 0.5% (Wang and Samliner 2008), the absolute annual risk is much lower. It is difficult to detect early Barrett’s adenocarcinoma in Barrett’s esophagus, and to do so requires gastric endoscopy using iodine staining and/or narrow banding imaging. However, since most patients with Barrett’s esophagus will not develop adenocarcinoma over their lifetime, this procedure is considered a costly burden for patients. Our results suggest that immunohistochemistry of biopsy tissue from Barrett’s esophagus for αGlcNAc and MUC6 could be an alternative screen for malignant potential.
Reduced αGlcNAc expression is a biomarker of pancreatic epithelial neoplasia
Pancreatic cancer is lethal, as it is often detected at late stages. Until recently, computed tomography and ultrasound (US) studies were the only methods used to diagnose pancreatic tumors prior to surgical operation; now, however, biopsy specimens from pancreatic tumors have become more easily obtainable by endoscopic US-guided fine needle aspiration methods. Thus, application of novel biomarkers of precursor lesions could enable histological diagnosis of pancreatic neoplasms at earlier stages.
Recently, two major precursor lesions to invasive pancreatic carcinoma were defined histologically: pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN) (Basturk et al. 2015). PanIN is a microscopic, flat or papillary, noninvasive epithelial neoplasm characterized by varying amounts of mucin and varying degrees of cytologic and architectural atypia. IPMN, on the other hand, is a grossly visible, predominantly papillary and rarely flat, noninvasive mucin-producing epithelial neoplasm arising in the main pancreatic duct or branch ducts. Both lesions types follow distinct carcinogenic pathways in becoming invasive, namely, PanIN to invasive ductal adenocarcinoma (IDAC) (the PanIN-IDAC sequence) and IPMN–IPMN associated with invasive carcinoma (IPMNAIC) (the IPMN–IPMNAIC sequence) (Basturk et al. 2015).
MUC6 and αGlcNAc are co-expressed in normal periductal glands of the main pancreatic duct (Kobayashi et al. 2014), and αGlcNAc is expressed in IPMN (Kobayashi et al. 2014; Matsuzawa et al. 1992). We compared αGlcNAc and MUC6 expression levels in both PanIN and IMPN pathways and found that decreased αGlcNAc expression relative to that of MUC6 is also associated with pancreatic neoplasm progression in both (Ohya et al. 2017): in the PanIN–IDAC sequence, relative expression of αGlcNAc to MUC6 was significantly reduced at each histological grade, namely, low-grade PanIN, high-grade PanIN/intraductal spread of IDAC (high-grade PanIN/IDC), and IDAC (Fig. 5a, b). On the other hand, in the IPMN–IPMNAIC sequence, relative expression of αGlcNAc to MUC6 was significantly decreased in low-grade IPMN (P < 0.05) but not in high-grade IPMN or IPMNAIC (Fig. 5c, d). These observations suggest that αGlcNAc expression relative to MUC6 has already begun to decrease early in pancreatic tumor progression in both the PanIN–IDAC and IPMN–IPMNAIC sequences. Thus, routine pathological examinations could include αGlcNAc and MUC6 immunohistochemistry in biopsy tissues of pancreatic tumors as a means of detecting disease progression.

[From Ohya et al. (2017) with modification]
a Immunohistochemical expression of MUC5AC, MUC6, and αGlcNAc in the PanIN-IDAC sequence during pancreatic carcinogenesis. MUC6 is expressed in tumor cells showing pyloric gland phenotypes in low- and high-grade PanIN/IDS, and αGlcNAc expression coincides with that of MUC6 in low-grade PanIN. In contrast, in high-grade PanIN/IDS and IDAC, αGlcNAc is not expressed in MUC6-positive tumor cells. MUC5AC is expressed in each lesion. Scale Bar 100 µm. b Semi-quantitation of data shown in a. Note that MUC6 expression levels exceed those of αGlcNAc, with significant differences, in each lesion (*P < 0.05). c Immunohistochemistry showing expression of MUC5AC, MUC6, and αGlcNAc in the IPMN-IPMNAIC sequence during pancreatic carcinogenesis. MUC6 is expressed in tumor cells showing pyloric gland phenotypes in low- IPMN and high-grade IPMN. αGlcNAc expression coincides with that of MUC6 in low-grade IMPN. In contrast, in high-grade IPMN and IPMNAIC, αGlcNAc is not expressed in MUC6-positive tumor cells. MUC5AC is expressed in each lesion. Scale Bar 100 µm. d Semi-quantitation of data shown in c. MUC6 expression levels exceed those of αGlcNAc, with significant differences, in low-grade IPMN (*P < 0.05).
Perspectives
As discussed in this review, reduced αGlcNAc expression on MUC6 in the gastric gland mucin is an initial event marking not only the early phase of gastric and pancreatic tumor progression but also premalignant lesions in the stomach and in Barrett’s esophagus. GNAS mutations are reported often in pyloric gland adenoma and IPMN (Matsubara et al. 2013; Furukawa et al. 2011). In addition, KRAS mutations have been reported in pyloric gland adenoma and PanIN (Furukawa et al. 2011; Matthaei et al. 2014). However, how αGlcNAc reduction is linked with GNAS and/or KRAS mutation is yet unknown. Further studies are needed to define mechanisms regulating αGlcNAc expression to better understand carcinogenesis of tumors marked by gastric gland mucin expression.
Conclusions
Decreased expression of αGlcNAc on MUC6 is closely associated with malignant potentials of pyloric gland adenoma of the stomach, chronic atrophic gastritis, Barrett’s esophagus, and IPMN/PanIN of the pancreas. Thus, combined use of immunohistochemistry for αGlcNAc and MUC6 could be useful to evaluate malignant potentials of these lesions in routine pathological examinations.
References
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Klöppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T (2015) A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursors lesions in the pancreas. Am J Surg Pathol 39:1730–1741
Chen ZM, Scudiere JR, Abraham SC, Montgomery E (2009) Pyloric gland adenoma: an entity distinct from gastric foveolar type adenoma. Am J Surg Pathol 33:186–193
Ferreira B, Marcos NT, David L, Nakayama J, Reis CA (2006) Terminal α1,4-linked N-acetylglucosamine in Helicobacter pylori-associated intestinal metaplasia of the human stomach and gastric carcinoma cell lines. J Histochem Cytochem 54:585–591
Frederik HJ, Lars P, Asbjørn MD, Henrik TS, Peter FJ (2011) Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Eng J Med 365:1375–1383
Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K (2011) Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 1:161–167
Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K (1996) Peripheral α-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 318(Pt 2):409–416
Iwaya Y, Hasebe O, Koide N, Kitahara K, Suga T, Shinji A, Muraki T, Yokosawa S, Yamada S, Arakura N, Tanaka E, Nakayama J (2014) Reduced expression of αGlcNAc in Barrett’s oesophagus adjacent to Barrett’s adenocarcinoma—a possible biomarker to predict the malignant potential of Barrett’s oesophagus. Histopathology 64:536–546
Janmaat VT, van Olphen SH, Biermann KE, Looijenga LHJ, Bruno MB, Spaander MCW (2017) Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s oesophagus surveillance: a systematic review and meta-analysis. PLoS One 12:e0186305
Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S, Ueda M, Fukuda MN, Fukuda M, Ishihara K, Nakayama J (2012) Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest 122:923–934
Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda M, Katsuyama T, Nakayama J (2004) Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003–1006
Kobayashi M, Fujinaga Y, Ota H (2014) Reappraisal of the immunophenotype of pancreatic intraductal papillary mucinous neoplasms (IPMNs)—gastric pyloric and small intestinal immunophenotype expression in gastric and intestinal type IPMNs—. Acta Histochem Cytochem 47:45–57
Kushima R, Vieth M, Borchard F, Stolte M, Mukaisho K, Hattori T (2006) Gastric-type well-differentiated adenocarcinoma and pyloric gland adenoma of the stomach. Gastric Cancer 9:177–184
Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, Tamura G, Saito D, Sugimura T, Ichinose M, Ushijima T (2006) High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res 12:989–995
Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y (2013) Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol 229:579–587
Matsuzawa K, Akamatsu T, Katsuyama T (1992) Mucin histochemistry of pancreatic duct cell carcinoma, with special reference to organoid differentiation simulating gastric pyloric mucosa. Hum Pathol 23:925–933
Matthaei H, Wu J, Dal Molin M, Shi C, Perner S, Kristiansen G, Lingohr P, Kalff JC, Wolfgang CL, Kinzler KW, Vogelstein B, Maitra A, Hruban RH (2014) GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as “incipient IPMNs”. Am J Surg Pathol 38:360–363
Morris A, Nicholson G (1987) Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol 82:192–199
Nakayama J (2014a) Alpha-1,4-N-acetylglucosaminyltransferase (A4GNT). In: Taniguchi N, Honke K, Fukuda M, Narimatsu H, Yamaguchi Y, Angata T (eds) Handbook of glycosyltransferases and related genes, 2nd edn. Springer, Japan, Tokyo, pp 379–391
Nakayama J (2014b) Dual roles of gastric gland mucin-specific O-glycans in prevention of gastric cancer. Acta Histochem Cytochem 47:1–9
Nakayama J, Yeh J-C, Misra A, Ito S, Katsuyama T, Fukuda M (1999) Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1→4GalβR, a glycan specifically expressed in the gastric gland mucous cell type mucin. Proc Natl Acad Sci USA 96:8991–8996
Ohya A, Yamanoi K, Shimojo H, Fujii C, Nakayama J (2017) Gastric gland mucin-specific O-glycan expression decreases with tumor progression from precursor lesions to pancreatic cancer. Cancer Sci 108:1897–1902
Ota H, Katsuyama T (1992) Alterning laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J 24:86–92
Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y (1991) A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J 23:22–28
Reid BJ, Blount PL, Rubin CE, Levine DS, Haggitt RC, Rabinovitch PS (1992) Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology 102:1212–1219
Shiratsu K, Higuchi K, Nakayama J (2014) Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer Sci 105:126–133
Vieth M, Kushima R, Borchard F, Stolte M (2003) Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch 442:317–321
Wang KK, Samliner RE (2008) Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol 103:788–797
Yamada S, Okamura T, Kobayashi S, Tanaka E, Nakayama J (2015) Reduced gland mucin-specific O-glycan in gastric atrophy: a possible risk factor for differentiated-type adenocarcinoma of the stomach. J Gastroenterol Hepatol 30:1478–1484
Yamanoi K, Arai E, Tian Y, Takahashi Y, Miyata S, Sasaki H, Chiwaki F, Ichikawa H, Sakamoto H, Kushima R, Katai H, Yoshida T, Sakamoto M, Kanai Y (2015a) Epigenetic clustering of gastric carcinomas based on DNA methylation profiles at the precancerous stage: its correlation with tumor aggressiveness and patient outcome. Carcinogenesis 36:509–520
Yamanoi K, Sekine S, Higuchi K, Kushima R, Nakayama J (2015b) Decreased expression of gastric gland mucin-specific glycan α1,4-linked N-acetylglucosamine on its scaffold mucin 6 is associated with malignant potential of pyloric gland adenoma of the stomach. Histopathology 67:898–904
Zhang MX, Nakayama J, Hidaka E, Kubota S, Yan J, Ota H, Fukuda M (2001) Immunohistochemical demonstration of α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1,4Galβ residues in human gastrointestinal mucosa. J Histochem Cytochem 49:587–596
Acknowledgements
The authors are grateful to all collaborators for their contribution to research relevant to the gastric gland mucin-specific O-glycan αGlcNAc. The authors also thank Dr. Elise Lamar for editing the manuscript.
Funding
Grants-in-Aid for Scientific Research 15H04712 and 17K15640 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in association with the present study.
Rights and permissions
About this article
Cite this article
Yamanoi, K., Nakayama, J. Reduced αGlcNAc glycosylation on gastric gland mucin is a biomarker of malignant potential for gastric cancer, Barrett’s adenocarcinoma, and pancreatic cancer. Histochem Cell Biol 149, 569–575 (2018). https://doi.org/10.1007/s00418-018-1667-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-018-1667-8