Abstract
-
Anatomy: Gross examination of the CNS reveals two distinct types of tissue (gray and white matter), which are also easily visible on MRI.
-
Histology: Population of pluripotent neural stem cells (capable at least in vitro of dividing into neurons, astrocytes, and oligodendroglia) exists throughout the brain and spinal cord.
-
Pathophysiology: No discrete lesion is pathognomonic for radiation injury to the CNS, which is classically associated with pathologic changes common to most other mechanisms of CNS injury, including demyelination, malacia (decrease in white and gray matter volume), gliosis (scarring), and vascular damage.
-
Pathogenesis: Damage to endothelial tissue within the CNS appears to play a key role in post-radiation demyelination. This was elegantly demonstrated by a boron neutron capture experiment.
-
Biology: An increase in vascular endothelial growth factor (VEGF) production, triggered by blood–brain barrier (BBB) disruption, appears to play a key role in the pathogenesis of white matter lesions.
-
Clinical Syndromes: Brain radiation has been frequently cited as the major cause of neurocognitive decline in cancer patients. All were treated in a fashion that would significantly increase the risk of late radiation toxicity, i.e., large daily fractions and concurrent radiosensitizer.
-
Special Topics: Exposure of the fetal nervous system to ionizing radiation between the gestational ages of 8–25 weeks is associated with microcephaly and mental retardation. An exposure of 1 Gy at the most sensitive gestational age of 8–15 weeks is associated with an estimated 43 % risk of mental retardation. Neurocognitive and neuropsychologic consequences of brain radiation in children may occur in up to 50 % of children who undergo brain radiation. Radiation-induced dementia associated with diffuse leukoencephalopathy is characterized by depression, emotional lability, and deficits in memory and attention which progress to gait disturbance and incontinence in approximately 80 % of patients.
-
Detection Diagnosis: Magnetic resonance imaging (MRI) is a sensitive imaging modality for white matter lesions. Both the incidence and severity of white matter lesions in the brain are directly related to increasing RT dose and worsen with time.
-
Diagnosis: CNS radionecrosis is a focal, well-circumscribed lesion that develops in regions of the brain near the primary tumor that have received high doses of radiation.
-
Management: Corticosteroids are the mainstay of treatment for many radiation-induced CNS toxicities, including radiation necrosis, cranial nerve palsy, and peripheral neuropathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Trigeminal Neuralgia
- White Matter Lesion
- Radiation Necrosis
- Brain Radiation
- Boron Neutron Capture Therapy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Radiation toxicity in the central nervous system (CNS) is potentially devastating. Recent advances in imaging, treatment planning, and molecular biology have increased our understanding of the pathophysiology of this complex process. This chapter will review the anatomy and biology, pathophysiology, imaging characteristics, and clinical findings characteristic of radiation-induced neurotoxicity. The biocontinuum of radiation-induced acute, subacute, chronic, and late effects is illustrated in Fig. 1.
Biocontinuum of radiation induced acute, subacute, chronic, and late effects in the CNS (with permission from Rubin and Casarett 1968)
2 Functional Anatomy and Histology
2.1 Anatomy
The nervous system is primarily divided into the central nervous system (CNS), which consists of the brain and spinal cord, and the peripheral nervous system (PNS). Gross examination of the CNS reveals two distinct types of tissue (gray and white matter), which are also easily visible on MRI. Gray matter is made up of neuronal cell bodies and their supporting glial cells and is concentrated in the cerebral cortex, cerebellum, and interior of the spinal cord. Within the white matter, clusters of gray matter form islands such as the basal ganglia, thalamus, cranial nerve nuclei, and multiple other critical structures. White matter consists primarily of axons, with glial cells interspersed among the axonal processes, and gains its white color from the axons’ myelin coating.
The brain is further subdivided into the cerebrum, cerebellum, and brainstem (Fig. 2). The cerebrum controls voluntary movement, sensory processing, speech, memory, and cognition, while the cerebellum is involved in the integration of motor and sensory function as well as balance and motor coordination. The brainstem controls involuntary vital functions such as respiration, plays a role in the regulation of consciousness and the sleep/wake cycle, and contains the nuclei of cranial nerves III–XII. All neural pathways between the brain and spinal cord also pass through the brainstem. The spinal cord is divided craniocaudally into 31 spinal nerves which carry motor and sensory innervation to the entire body, with the exception of the territories in the head and neck supplied by the 12 cranial nerves. The spinal cord is also functionally separated in the axial plane into ascending (sensory) and descending (motor) pathways.
Different regions of the brain are shown. a Medial view: Median sagittal section; b Left lateral view (with permission from Tillman 2007)
The peripheral nervous system is comprised of all other neural structures outside the spinal cord and includes the somatic nervous system (supplying voluntary motor control to the muscles and returning sensory information to the spinal cord and brain) and the autonomic nervous system (responsible for regulation of involuntary functions such as heart rate, respiration, blood pressure, and digestion).
2.2 Histology
The neuron is well established as the primary structural and functional cell of the nervous system. A variety of glial cells exist as “support cells” in both the central and peripheral nervous system. Oligodendrocytes and Schwann cells are the myelinating cells of the CNS and the PNS, respectively. Our understanding of the role of astrocytes, the most numerous type of glial cells, continues to evolve, but they have an established function as part of the blood–brain barrier (BBB), as mechanical support cells within the CNS, in the metabolism of neurotransmitters and ions such as calcium and potassium, and as the main mediators of gliosis, the response to injury in the brain parenchyma. Ependymal cells line the ventricles of the brain as well as the central canal of the spinal cord and produce cerebrospinal fluid (CSF). Microglia, derived from a monocytic (hematopoietic) cell lineage, are the primary phagocytes of the CNS and play a role in acute brain injury (Fig. 3a, b).
Although neurons themselves are likely incapable of mitotic division, the adult human brain has recently been established to have a neuron-generating stem cell compartment, contradicting previously held dogma. Neurogenesis has been constitutively demonstrated within the dentate gyrus of the hippocampus as well as the subventricular zone and olfactory bulb, and a population of pluripotent neural stem cells (capable at least in vitro of dividing into neurons, astrocytes, and oligodendroglia) exists throughout the brain and spinal cord. Regenerative neurogenesis in response to injury has been demonstrated in the corticospinal tract, neocortex, and striatum. These cell populations are the subject of intense research (Emsley et al. 2005).
3 Biology, Physiology, and Pathophysiology
3.1 Radiobiology of the Central Nervous System
The pyramidal cells are the major neurons of the brain. They are considered post-mitotic cells and as such are unable to be replaced. However, there is evidence of a stem cell region in the brain, located in the hippocampus, which may be capable of regenerating neurons (as illustrated in Fig. 4).
Distribution of stem cells in the adult human brain (adapted with permissions from Barani et al. 2007)
Classical radiobiology teaches that CNS tissue is a “late-responding” tissue, characterized by a low α/β ratio, delayed manifestation of radiation injury, and extreme variability in response as the fraction size is altered (Hall 2006a). Recovery of neural tissue from radiation injury was predicted by this model to be extremely limited. This has been contradicted by a landmark study in rhesus monkeys published by Ang et al. (2001). Rhesus monkeys were reirradiated at varying doses (44 Gy followed by either 57.2 or 66 Gy) at varying time intervals (1 or 2 years). The animals were then observed for up to 66 months. Histologic studies of the monkey spinal cords suggested significant repair at one year, with recovery continuing to increase through the third year following reirradiation. A dose-response relationship predictive for myelopathy was also observed. From these data, the authors extrapolated conservative estimates of human spinal cord recovery from myelopathy, following an initial dose of 45 Gy, as 50, 60, and 65–70 % at 1, 2, and 3 years, respectively.
Structural differences between the brain and spinal cord result in a major difference in radiation tolerance between the two tissues. Within the brain, islands of eloquent tissue are intermingled with large areas of brain parenchyma which can be significantly damaged or even removed without compromising essential functions. Consequently, many areas of the brain can tolerate high doses of focal radiation without the development of catastrophic damage. In contrast, the spinal cord is a tightly compacted cable of tissue, nearly all of which is functional. Transection or damage to one segment of the spinal cord results in loss of all downstream function of the cord, which has important implications for radiation dose prescription and treatment planning (Fig. 5).
Physiology: coarse distribution of functions within the brain, a Left lateral view; b Medial view of the right section (with permission from Tillman 2007)
3.2 Pathology and Pathophysiology of Radiation Damage Within the Nervous System
No discrete lesion is pathognomonic for radiation injury to the CNS, which is classically associated with pathologic changes common to most other mechanisms of CNS injury, including demyelination, malacia (decrease in white and gray matter volume), gliosis (scarring), and vascular damage. Foci of liquefactive necrosis associated with significant edema and gliosis may develop in areas receiving high doses of radiation. Figure 6a, b illustrates key features of radiation necrosis. Glial and endothelial cells appear to be the key target cells for radiation damage in the CNS. Because adult neurons are not actively dividing cells, radiation damage to neurons at typical therapeutic doses is therefore unlikely to contribute significantly to CNS toxicity. However, increasing evidence suggests that neural stem cells, an actively dividing cellular compartment, may be subject to radiation damage and play a significant role in late radiation toxicity, particularly with respect to neurocognitive effects.
Clinically, radiation toxicity in the CNS is divided into three phases (acute, early delayed, and late), which correlate with different pathophysiologic mechanisms (Kim et al. 2008). In the acute phase, acute inflammation related to cytokine activity and disruption of the BBB dominates. Acute radiation toxicity within the CNS is characterized by headache, fatigue, and, in severe cases, signs of increased intracranial pressure. Transient demyelination is thought to be responsible for early delayed reactions in the CNS. The primary manifestation of early delayed CNS reaction is the somnolence syndrome, which is seen most frequently in children who receive whole brain radiation and intrathecal methotrexate; it typically occurs approximately 6–12 weeks following the completion of whole brain radiation therapy. The hypersomnolence is typically self-limited and its correlation with the development of late neurotoxicity is controversial (Ch’ien et al. 1980; Berg 1983). The manifestations of late neurotoxicity (developing at least 3 months after radiation exposure) are highly variable, ranging from subtle cognitive deficits to severe encephalopathy associated with diffuse white matter damage. Radiation necrosis, variably associated with cerebral edema and focal neurologic deficits, may develop in areas of the brain receiving high (>60 Gy) radiation doses. Late neurotoxicity is mediated by a combination of vascular lesions, cytokine-induced tissue damage, impaired neurogenesis, and reactive oxygen species.
One of the most consistent features of late radiation damage in the CNS is white matter damage (necrosis and demyelination). Oligodendrocytes are responsible for myelinating neurons and appear to be the most radiosensitive glial cells (Barbarese and Barry 1989; Vrdoljak and Bill 1992). Damage to oligodendroglia was thus hypothesized to be the primary mechanism of radiation-induced demyelination. However, damage to endothelial tissue within the CNS appears to play a key role in post-radiation demyelination. This was elegantly demonstrated by a boron-neutron-capture experiment in which the borated compound (BSH) was unable to cross the BBB. Due to the extremely short range of the alpha particles generated by boron neutron capture therapy, the endothelium was selectively irradiated while brain parenchyma was spared. White matter necrosis and demyelination were nonetheless observed, suggesting that glial cells are not the primary target cells in the development of these lesions (Coderre et al. 2006).
An increase in vascular endothelial growth factor (VEGF) production, triggered by BBB disruption, appears to play a key role in the pathogenesis of white matter lesions. In rodent models, radiation damage to the BBB occurs in two phases. Acute apoptosis of endothelial cells is observed within 24 h of radiation, with regeneration of the endothelium complete approximately 14 days after a single fraction of radiation is administered (Li et al. 2006). The late phase of BBB disruption is associated with increasing vascular permeability beginning approximately 3 months after radiation (in the mouse model) (Yuan et al. 2006). Because VEGF itself causes increased vascular permeability, a positive feedback loop is created which ultimately results in significant local edema, inflammation, and hypoxia. Although VEGF levels eventually rise to a level sufficient to trigger angiogenesis, the structure of the BBB in irradiated areas does not return to normal. The loss of normal endothelium is thought to contribute significantly to the development of late white matter necrosis. Anti-VEGF therapies such as bevacizumab are the subject of active investigation as possible modulators of this late response (Gonzalez et al. 2007).
Reactive oxygen species are responsible for approximately 2/3 of X-ray induced DNA damage. Although radiation-generated ROS are themselves short-lived, radiation damage is associated with a prolonged ROS cascade in the damaged normal tissue and chronic oxidative stress. A variety of mechanisms contribute to chronic oxidative stress in irradiated areas. In areas of the CNS which have been damaged by radiation, the BBB is disrupted and the production of pro-inflammatory cytokines (e.g., TNF-α, INF-γ, ICAM-1) is upregulated (Belka et al. 2001). Activated leukocytes as well as CNS microglia are recruited to the area and release large quantities of ROS as they participate in the local inflammatory reaction. Neuronal excitotoxicity is also associated with the release of ROS, as is chronic hypoxia resulting from damage to small blood vessels.
As noted above, certain areas of the brain (primarily the hippocampal dentate gyrus and the subventricular zone) retain constitutive neurogenic stem cell activity throughout life. Memory and learning abilities appear to be correlated with stem cell activity in these regions, at least in available rodent models, and damage to NSC’s in irradiated adults is likely partly responsible for post-radiation neurocognitive deficits (Barani et al. 2007). Neurogenic stem cells appear to be significantly radiosensitive, (Peissner et al. 1999) with rapid and prolonged loss of cell population in the stem cell compartment following radiation (Tada et al. 2000). Juvenile rats have a higher density of active NSC’s and thus appear to be at higher risk for neurocognitive sequelae of brain radiation (Fukuda et al. 2005). This correlates well with the inverse relationship between age at irradiation and the severity of cognitive deficits observed clinically in humans.
4 Clinical Syndromes
4.1 Clinical Syndromes
Exposure of the CNS to radiation results in a variety of clinical manifestations. In an attempt to standardize the evaluation and reporting of neurotoxicity, formal scoring tables for the evaluation and description of acute and late neurotoxicity have been developed. Table 1 summarizes the Late Effects Normal Tissue Task Force–Subjective, Objective, Management, and Analytic (LENT–SOMA) and the National Cancer Institute CTC (V.4) Common Terminology Criteria for Adverse Events (CTCAE) grading systems for neurotoxicity. The clinical expression depends on a host of factors, including total dose, fraction size, treated volume, treatment time, and age of the patient. Other factors contribute to CNS toxicity in many patients undergoing radiotherapy to the brain and spinal cord. These include surgery, medications (e.g., steroids, opioids, benzodiazepines, anticonvulsants), chemotherapy, and pre-existing medical comorbidity). The importance of recurrent or persistent malignancy as a contributor to neurological and neurocognitive sequelae in patients undergoing radiation therapy for brain tumors also should not be underestimated.
The clinical endpoints are summarized in Table 2 as Focal and Global events. Late CNS toxicity may be broadly, and somewhat arbitrarily, segregated into categories as shown.
4.1.1 Cerebrovascular Syndrome
Acute exposure to high total body doses (≥20–30 Gy) causes the cerebrovascular syndrome. Fatal within 24–48 h, this syndrome is associated with systemic loss of vascular permeability and the rapid onset of cerebral edema and multiorgan failure. The few reported human cases have been associated with prodromal symptoms including fever, confusion, and weakness. These are followed by a brief (5–6 h) latent period where recovery of mental status and blood pressure may occur. This latent phase rapidly progresses to the final stage of the cerebrovascular syndrome, associated with fever, diarrhea, refractory hypotension, and progressive cerebral edema causing worsening mental status and death (state when death usually occurs) (Hall 2006b; Waselenko et al. 2004).
4.1.2 RT-Induced Neurocognitive Deficits in the Adult Population
Brain radiation induces late cognitive changes in the adult brain as well. The precise evaluation of these changes is complicated by a number of factors. Many patients with disorders (brain metastases, malignant glioma) requiring brain RT have a limited lifespan, and do not survive long enough to develop late neurocognitive changes, which can develop years after cranial RT. Surgery, chemotherapy, medications, and disease recurrence also cause neurotoxicity, further complicating the precise evaluation of radiation’s contribution to cognitive deficits (see above). Finally, accurate assessment of neurocognitive deficits requires serial neurocognitive testing for years following radiation, which frequently is not feasible (Crossen 1994). The RTOG has investigated a battery of previously validated neurocognitive tests which can be administered in 45 min, shown in Table 3 (Regine et al. 2004).
Radiation-induced dementia associated with diffuse leukoencephalopathy is characterized by depression, emotional liability, and deficits in memory and attention which progress to gait disturbance and incontinence in approximately 80% of patients, as shown in Fig. 7. An important differential diagnosis is normal-pressure hydrocephalus. Spontaneous improvement is rare. The only available therapy is supportive care, and the time to death after developing symptoms of radiation-induced dementia ranges from 1 month to 2 years (Keime-Guibert et al. 1998). The use of concurrent chemotherapy increases the incidence of radiation-induced dementia (Frytak et al. 1989).
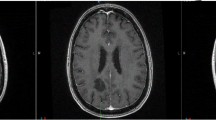
Radiation-Induced Diffuse White Matter Abnormality: a 73 year-old man presented with a single brain metastasis from colon cancer. He received 37.5 Gy in 2.5 Gy fractions of whole brain radiation followed by radiosurgery boost. He recurred with multiple new enhancing lesions (all subcentimeter) suspicious for metastasis. He received 21.6 Gy in 1.8 Gy fractions of repeat whole brain radiation. Six months later, his MRI revealed diffuse white matter changes on both FLAIR (a) and T2 (b) sequences
Typically, however, neurocognitive deficits in adults are subtle, and outcomes are generally favorable with modern fractionation schemes (Brown et al. 2003). Armstrong et al. reported on a series of young patients with supratentorial, favorable-histology brain tumors who received partial brain RT with doses ranging from 46 to 63 Gy (Armstrong et al. 1995). Serial neurocognitive testing (at baseline and at regular intervals up to 3 years after completing RT) was performed; the RT patients were also compared with a group of age-matched controls. Patients experienced “subtle early-delayed memory changes [that were] followed by a rebound of ability” by 1 year after completing RT. Disease control (Regine et al. 2001) and pre-treatment cognitive function (Brown et al. 2001) also appear to be important predictors of post-RT cognitive status. Temporal lobe radionecrosis has also been correlated with neurocognitive deficits (Cheung et al. 2000).
Of particular interest is a study published by Klein et al. (2002) in which the authors attempted to differentiate effects of tumor, radiotherapy, anticonvulsants, and surgical intervention in a group of 195 patients with low-grade glioma, 104 of whom had undergone radiotherapy. The group was compared to 100 patients with low-grade hematologic malignancies non-Hodgkin lymphoma and chronic lymphocytic leukemia (NHL/CLL) as well as 195 healthy controls. The use of anticonvulsants also had a significant negative impact on cognitive function. Glioma patients as a group had poorer cognitive functioning than both the NHL/CLL patients and the healthy controls. The use of radiation was correlated with cognitive deficits when irradiated glioma patients were compared with glioma patients who did not receive radiation. This effect was strongly dose- and fraction size-dependent. Patients who received >2 Gy/day of radiation accounted for nearly all cases of cognitive disability in this series. The authors concluded that tumor effects were responsible for the majority of cognitive deficits in low-grade glioma patients, although the delivery of radiation doses >2 Gy/day also had a significant impact on cognitive function (Klein et al. 2002).
4.1.3 Neurocognitive Decline in Patients with CNS Metastasis
Historically, brain radiation has been frequently cited as the major cause of neurocognitive decline in patients treated for metastases. One of the most misinterpreted studies on this subject is the Memorial Sloan-Kettering experience from DeAngelis et al. who reported an 11 % risk of radiation-induced dementia in patients undergoing WBRT for brain metastasis (DeAngelis et al. 1989a). Of the 47 patients who survived 1 year after WBRT, 5 patients (11 %) developed severe dementia. When these 5 patients are examined, all were treated in a fashion that would significantly increase the risk of late radiation toxicity (i.e., large daily fractions and concurrent radiosensitizer). Three patients received 5 and 6 Gy daily fractions, while a fourth patient received 6 Gy fractions with concurrent adriamycin. Only one patient received what is considered a standard radiation fractionation scheme (i.e., 30 Gy in 10 fractions), but this patient received a concurrent radiosensitizer (lonidamine). No patient who received the standard 30 Gy in 10 fractions WBRT alone experienced dementia. Even though the study included 232 patients in the initial analysis, it only examined the 47 patients who survived at least 1 year. The principles of conditional probability dictate that the 11 % risk is accurate only if a patient survives 1 year, which is significantly longer than most reported series. Therefore, a radiation-induced dementia risk of ≈2 % (5/232) might be a better estimate of the true probability ab initio for patients with brain metastasis treated with the various radiation doses and drugs used in that study.
Recent studies that have used sophisticated neurocognitive testing are clearly demonstrating that the brain tumor itself (presence, recurrence, and progression) has the greatest effect on neurocognitive decline. In the large phase III motexafin gadolinium study, the neurocognitive battery examined memory recall, memory recognition, delayed recall, verbal fluency, pegboard hand coordination, and executive function (Meyers et al. 2004). This study demonstrated that 21–65.1 % of patients had impaired functioning at baseline before treatment with WBRT (30 Gy in 10 daily fractions). Furthermore, patients who progressed in the brain after treatment experienced significantly worse scores in all of these individual tests.
Patients frequently present to the radiation oncologist already started on prophylactic anticonvulsants. This represents one of the most preventable causes of neurocognitive decline in brain tumor patients. Anticonvulsants are clearly known to adversely affect quality of life and neurocognition. In a study of 156 patients with low-grade glioma (85 % experiencing a seizure), Klein and colleagues correlated seizure-burden with quality of life and neurocognitive function (Klein et al. 2003). This study convincingly demonstrates the significant correlation between the increase in the number of anticonvulsants (even with lack of seizures) and the decrease in quality of life and neurocognitive function. Based on four negative randomized trials, the American Academy of Neurology recommends that prophylactic anticonvulsants not be initiated in newly diagnosed brain tumor patients who have not experienced a seizure (Glantz et al. 2000).
4.1.4 Radiation Necrosis
As described above, CNS radionecrosis is a focal, well-circumscribed lesion that develops in regions of the brain near the primary tumor that have received high doses of radiation. Necrosis is typically associated with focal neurologic deficits that correspond to the lesion’s location. Distinguishing these lesions from tumor recurrence can be problematic, but specialized imaging techniques may aid in diagnosis (Fig. 8). The lesion is frequently associated with significant cerebral edema, which precedes the development of radiation necrosis (Delattre et al. 1988). Edema and accompanying breakdown of the BBB increase parenchymal susceptibility to radiation necrosis by facilitating a cascade of local inflammatory mediators. This can be effectively inhibited with steroid therapy. Early (i.e., when focal edema is present but the area treated has not yet frankly necrosed) treatment with dexamethasone appears to improve outcomes significantly (Lee et al. 2002). If steroid therapy is delayed until the development of a cystic lesion, improvement of symptoms with medications alone is unlikely, and surgical excision of the mass may be indicated (Gutin 1991).
Resolving Radiation Necrosis: the above images illustrate a case of proven radiation necrosis. The patient was a 62 years old female with 1.5 cm metastatic solitary brain metastasis from non-small cell lung cancer. She received 37.5 Gy in 2.5 Gy fractions of whole brain radiation, followed by radiosurgery boost to 24 Gy. Two years after radiosurgery boost, she developed radiation necrosis. Patient was almost asymptomatic; therefore, she was managed conservatively with no medical intervention. Over a course of 12 months, MRI slowly normalized. Images in the first row show T1 post-contrast axial images of a heterogeneously enhancing lesion with mass effect with subsequent resolution. (a 2-year, b 3-year) Lower images demonstrate FLAIR abnormalities indicating significant edema (c 2-year, d 3-year)
Necrosis develops months to years after RT (Sloan and Arnold 2003). Total dose and fraction size clearly predict for the development of radionecrosis (DeAngelis et al. 1989b; Sheline et al. 1980). Data from a large series (n = 1,032) of patients treated with RT for nasopharyngeal cancer show an increased risk of necrosis with fraction sizes ≥2 Gy/day (median total dose 62.5 Gy), twice-daily RT, shorter treatment times, and an increased value of the product of total dose and fraction size (Lee et al. 2002). The recent QUANTEC analysis summarized the incidence of necrosis following fractionated partial brain irradiation for patients receiving variable doses per fraction (Fig. 9) (Lawrence et al. 2010).
Relationship between biologically effective dose (BED) and radiation necrosis after fractionated radiotherapy. Fit was done using nonlinear least-squares algorithm using Matlab software (The MathWorks, Natick, A). Nonlinear function chosen was probit model (similar functional form to Lyman model). Dotted lines represent 95 % confidence levels; each dot represents data from specific study, n = patient numbers as shown. a Fraction size <2.5 Gy, b fraction size ≥2.5 Gy (data too scattered to allow plotting of ‘‘best-fit’’ line), and c twice-daily radiotherapy (with permissions from Lawrence et al. 2010)
Among patients undergoing stereotactic radiosurgery [mean dose of 28.6 Gy (range 18.2–53.3)], increased tumor volume, treated volume, V10 (volume of tissue receiving >10 Gy), low conformality index, and repeated treatments to the same lesion have been found to be correlated with an increased incidence of radiation necrosis (Chin et al. 2001; Valéry et al. 2003). The summary data from QUANTEC is reproduced in Fig. 10. Note the steep increase in incidence of necrosis with dose.
Relationship between volume receiving high-dose irradiation and incidence of radiation necrosis in single-fraction stereotactic radiosurgery. Studies differed in their completeness of follow-up, definition of volume, and definition of radiation necrosis. Volume plotted as a point, representing mid-point of volume range. V10 = volume receiving 10 Gy, V12 = volume receiving 12 Gy, RxV = treatment volume. Flickinger data is shown for patients with either radiologic or symptomatic evidence of necrosis (marked as “All”), or only those with symptomatic necrosis (Symp). The other authors’ data refers to symptomatic necrosis (with permissions from Lawrence et al. 2010)
4.2 Detection and Diagnosis: Imaging characteristics of radiation-induced CNS lesions
Radiologic findings consistent with demyelination, white matter necrosis, and parenchymal volume loss mirror the pathologic changes induced by brain and spinal cord radiation. Magnetic resonance imaging (MRI) is a sensitive imaging modality for white matter lesions (Tsuruda et al. 1987). Figure 7 depicts the typical MRI appearance of radiation-induced white matter changes. Both the incidence and severity of white matter lesions in the brain are directly related to increasing RT dose and worsen with time. Data from RTOG 83-02, which was a prospective dose-escalation study in malignant gliomas (see Table 4), shows a clear dose-response relationship for radiation necrosis (Corn et al. 1994) A more recent publication has reported on a group of 24 patients who underwent serial MRI following whole brain radiation (Fujii et al. 2006). Low-grade white matter changes were noted as early as 2 months after WBRT, with a median time to onset of 5.5 months, and continued to evolve for as long as 2 years. No patient developed grade 3 or greater changes before 6 months post-RT, and the median time to onset of these more severe changes was 12.5 months. Radiation myelopathy has a variable appearance on MRI, and may appear as cord edema, increased T2 white matter signal, and gadolinium-enhancing T1 white matter lesions. Changes may progress to cord atrophy or radiation necrosis (Maranzano et al. 2001; Alfonso et al. 1997).
Focal areas of radiation necrosis are often indistinguishable from tumor recurrence, even with high-resolution contrast enhanced MRI (Wen et al. 2010). A typical lesion of radiation necrosis is shown in Fig. 8. T1-weighted sequences show a ring-enhancing lesion with a necrotic center. On FLAIR (or T2-weighted) sequences, significant surrounding cerebral edema can be seen. Spontaneous regression of this lesion after 1 year of observation was noted (Brandsma et al. 2008). New imaging modalities, such as FDG–PET and magnetic resonance spectroscopy, may be helpful in the evaluation of radiation necrosis. The use of FDG–PET scanning (which utilizes a radiolabeled glucose molecule) for the evaluation of intracranial radiation necrosis is complicated by the brain’s high intrinsic rate of glucose metabolism. Radiolabeled amino acids and radiolabeled thymidine are more specific markers for metabolic activity within the brain and have the potential to differentiate areas of active tumor from areas of radiation necrosis (Herholz et al. 2007). Magnetic resonance spectroscopy is able to differentiate between the molecular resonance frequencies of different constituents of the CNS, although its spatial resolution is poor. CNS lesions have distinctive magnetic resonance “fingerprints” based on their relative concentrations of choline (associated with the cell wall), creatine (normalization element), N-acetyl aspartate (NAA associated with mature neurons), lactate, and lipid (both associated with necrotic tissue). Specifically, radiation necrosis is characterized by: low choline signal relative to both NAA and creatinine, high NAA signal relative to creatinine and choline, and high lactate/lipid signal relative to choline (Rock et al. 2002). The addition of diffusion sequences to MRS may further aid in differentiating areas of tumor from areas of necrosis (Rock et al. 2004).
MRI is the ideal modality to detect the gradual whitening of the cerebral cortex indicating progressive leukoencephalopathy. Radionecrosis is best visualized with a contrast enhanced MRI, although CT may image the necrotic area in some cases. To establish a distinction between tumor necrosis and radiation induced necrosis. MRS may be useful in some cases.
5 Radiation Tolerance: Current Recommendations for Normal Tissue Dose Limits in the Nervous System
In 1991, Emami et al. published an exhaustive summary of data then available regarding radiation toxicity in normal tissue (Emami et al. 1991). Clinical outcomes, quoted as 5 and 50 % risk of complication within 5 years (TD5/5 and TD 50/5) were correlated with volume of normal tissue radiated. At the time, three-dimensional treatment planning was not widely used, and radiation dose estimates for normal tissue were inaccurate. These data have since been updated in a second review published in 2007 (Milano et al. 2007).
Rubin’s original estimate for fractionated partial brain RT (5 % risk at 5 years for one-third brain, 60 Gy) appears to be somewhat conservative. The QUANTEC review concluded that the 5 % risk at 5 years of the partial brain for normally fractionated RT is 72 Gy (range, 60–84). For standard fractionation, a 5 and 10 % risk of symptomatic radiation necrosis is predicted to occur at a BED of 120 Gy (range, 100–140) and 150 Gy (range, 140–170), respectively [corresponding to 72 Gy (range, 60–84) and 90 Gy (range, 84–102) in 2 Gy fractions]. The brain is especially sensitive to fraction sizes >2 Gy (Mayo et al. 2010). Thus, partial-brain fractionated RT to 54–60 Gy in 1.8–2 Gy daily fractions, a very common regiment for many brain lesions, is also well tolerated, with a low incidence of late neurocognitive effects or radio-necrosis. When choosing a fractionation scheme for whole brain radiation, the patient’s life expectancy must be considered carefully. Late radiation side effects are clearly correlated with daily fraction sizes that exceed 3 Gy and total dose, but this toxicity is less important in patients with a limited life expectancy, who will benefit from a shorter RT schedule (e.g., due to convenience) and the higher probability of tumor control afforded by a treatment scheme such as 30 Gy in 10 fractions. For patients with a longer (>6 months) life expectancy, more prolonged treatment schemes (40 Gy in 2 Gy/day fractions or 37.5 Gy in 2.5 Gy/day) are typically recommended, although confirmatory evidence is modest. Table 5 summarizes widely applied dose limitations for brain, spinal cord, and other critical structures in the CNS for conventional fractionation.
For radiosurgery, the risk of complications increases with the size of the target volume. Toxicity increases rapidly once the volume of the brain exposed to >12 Gy is >5–10 cm3. Eloquent areas of the brain (brain stem, corpus callosum) require more stringent limits (Mayo et al. 2010). For lesions involving the brainstem parenchyma, dose for single fraction radiosurgery is often limited to 15 Gy or lower. Nevertheless, small portions of the brainstem can tolerate higher doses as there is a strong volume effect. For example, stereotactic radiosurgery doses of 15–18 Gy (10–20 % line of prescribed 75–90 % to Dmax) are routinely administered to small areas of the brainstem surface for the treatment of trigeminal neuralgia.
6 Chemotherapy
There is strong evidence that chemotherapy contributes significantly to neurocognitive outcomes in cancer patients (Brezden et al. 2000; Tchen et al. 2003). Double-blinded randomized trials of healthy volunteers have demonstrated that corticosteroids can have significant impact on recall testing, attention, EEG testing, and hippocampal volume and activity (Newcomer et al. 1994; Schmidt et al. 1999; Brown et al. 2004). Studies of healthy volunteers have further demonstrated that medications commonly used in cancer patients, such as benzodiazepines and opioids, can cause profound neurocognitive deficits (Zacny and Gutierrez 2003).
A number of chemotherapeutic agents have been historically associated with acute and late toxicities (Table 6) (Anderson et al. 1997; Watterson et al. 1994; Madhu et al. 1993). Entities such as acute and chronic encephalopathy, necrotizing leukoencephalopathy, acute cerebellar syndromes, and peripheral neuropathies, reflect both drug and radiation toxicities. This is of particular concern in children (Allen and Siffert 1996; Prassopoulos et al. 1997).
7 Special Topics
7.1 Cranial Nerves
Optic Nerves
Radiation-induced pathology of the optic nerve is associated with two discrete clinical syndromes. Anterior ischemic optic neuropathy is believed to be secondary to vascular injury of the distal portion of the optic nerve. Patients present with gradual, painless visual loss, with median time to onset 2–4 years after completing RT. Funduscopic examination reveals edema of the optic disk which progresses to atrophy over the course of months to years. Retrobulbar optic neuropathy results from damage to proximal segments of the optic nerve. Visual field deficits and rapidly progressive vision loss, sometimes associated with ocular, periorbital, or retrobulbar pain, are characteristic of retrobulbar optic neuropathy, and disk abnormalities are infrequently observed. Both forms of optic neuropathy are refractory to treatment with steroids and hyperbaric oxygen. Both types of injury are correlated with increasing patient age, total RT doses >59 Gy, and daily fraction size >2 Gy (Parsons et al. 1994).
The QUANTEC review concludes, “The Emami estimate of 5 % probability of blindness within 5 years of treatment for a dose of 50 Gy appears inaccurate. From the present data review, 50 Gy is closer to a “near zero” incidence. The incidence of RION was unusual for a Dmax <55 Gy, particularly for fraction sizes <2 Gy. The risk increases (3–7 %) in the region of 55–60 Gy and becomes more substantial (>7–20 %) for doses >60 Gy when fractionations of 1.8–2.0 Gy are used. The patients with RION treated in the 55–60 Gy range were typically treated to doses in the very high end of that range (i.e., 59 Gy). For particles, most investigators found that the incidence of RION was low for a Dmax < 54 CGE. One exception to this range was for pituitary tumors, in which investigators used a constrained Dmax of <46 Gy for 1.8 Gy/fraction. For single-fraction SRS, the studies have indicated the incidence of RION is rare for a Dmax < 8 Gy, increases in the range of 8–12 Gy, and becomes >10 % in the range of 12–15 Gy.”
The recent QUANTEC review generated a nice dose–response curve for optic nerve injury following conventional fractionation (Fig. 11) (Mayo et al. 2010). The tolerance (<1 % risk) of the optic nerve at single fraction radiosurgery doses appears to be 8–10 Gy provided that the patient does not have a history of external radiation (Tishler et al. 1993; Stafford et al. 2003).
Comparison of incidence of radiation-induced optic neuropathy (RION) versus maximum dose (Dmax) to optic nerves (from Mayo et al. 2010). Selected studies generally used fraction sizes with range of 1.8–2.0 Gy, assessed the dose to the nerve directly from their best estimate of dose distribution in the structure (i.e., not as a partial volume average), did not include pituitary lesions (lower tolerance), and selected patient age <70 years (if segregated). Bars illustrate range of doses for groups characterized by incidence values. Points offset from 0 to ≤1 % were shifted to clearly show range bars. For points displayed at 0 %, available range information was outside 50–70 Gy. Threshold for RION appears to be 55–60 Gy. However, range bars illustrate treatment in 60–65 Gy range for some studies without RION. Data estimated from tables, figures, and text reported in the studies, because exact incidence data were not always provided (with permission from Mayo et al. 2010)
7.1.1 Other Cranial Nerves
The olfactory optic nerves are similar to CNS brain tissue in radiation sensitivity. The data presented above regarding the tolerance of the optic nerve cannot be extrapolated to the remainder of the cranial nerves. The majority of cranial nerves are similar to peripheral nerves as to their radiosensitivity. Cranial nerves within the cavernous sinus appear to be radioresistant and have been reported to tolerate single-fraction doses of up to 40 Gy (Tishler et al. 1993). The trigeminal nerve at the root entry zone is routinely irradiated to 90 Gy (Dmax) for trigeminal neuralgia and cumulative doses of 160 Gy or higher have been reported with minimal risk for subsequent sensory abnormalities (Pollock 2005). The vestibulocochlear nerve (CN VIII) appears to be somewhat less radioresistant, with hearing loss reported at doses above 54 Gy in conventional fractionation (Johannesen et al. 2002). This may be related to radiation-induced bone sclerosis within the middle ear.
7.2 Fetal Effects
Exposure of the fetal nervous system to ionizing radiation between the gestational ages of 8 and 25 weeks is associated with microcephaly and mental retardation. This was most clearly demonstrated in the Japanese populations exposed to radiation from the atomic bombs of Hiroshima and Nagasaki. The highest risk period for the development of radiation-associated mental retardation appears to be between the gestational ages of 8 and 16 weeks, which coincides with neuronal proliferation, differentiation, and migration into the developing cerebral cortex. Although the dosimetry associated with the atomic bomb exposures is somewhat uncertain, the incidence of mental retardation appears to be linearly associated with dose, with a possible threshold dose of 0.12–0.2 Gy. An exposure of 1 Gy at the most sensitive gestational age of 8–15 weeks is associated with an estimated 43 % risk of mental retardation. Radiation exposure between 16 and 25 weeks is associated with increased risk of mental retardation as well, but the incidence is lower than at the 8–16 week period. Less pronounced cognitive impairment (measured in the atomic-bomb survivors by IQ testing and school performance) is also evident among individuals exposed to lower doses of radiation between 8 and 26 weeks gestational age, with a linear relationship between decreased IQ/school performance and increased radiation doses. No effect on IQ nor school performance was observed among children who were exposed to radiation before 8 weeks nor after 26 weeks (Hall 2006c; National Academy of Sciences/National Research Council 1990).
7.3 Effects in Childhood
Neurocognitive and neuropsychologic consequences of brain radiation in children may occur in up to 50 % of children who undergo brain radiation (Walter et al. 1999; Packer et al. 1987). The clinical manifestations are variable, ranging from overt mental retardation among children receiving high doses of radiation at a young age to subtler cognitive and behavioral deficits. In infants and toddlers, mental retardation, growth delay, and leukoencephalopathy are sufficiently profound that cranial radiotherapy is preferentially delayed until after age 3, except in extreme circumstances (Duffner et al. 1993). Serial IQ testing is commonly used to quantify the extent of neurocognitive impairment among children who have received brain radiation. Declines in IQ have been observed in multiple trials to be age- and dose-dependent (Silber et al. 1992; Merchant et al. 2005; Mulhern et al. 1998). Among survivors of childhood cancer who received radiation, deficits in attention and concentration appear to be particularly significant contributors to cognitive decline (Langer et al. 2002; Briere 2008). It is important to note, however, that many children with brain tumors have cognitive deficits at baseline (i.e., before radiation therapy begins), likely secondary to tumor effect as well as pre-radiotherapy interventions such as surgery (Merchant et al. 2002; Sonderkaer et al. 2003). It is unlikely that cranial radiotherapy will be completely eliminated as part of the treatment paradigm for pediatric malignancies such as medulloblastoma, high-risk acute lymphoblastic leukemia (ALL), and ependymoma. Continuing clinical investigation is focusing on minimizing the cognitive impact of cranial RT in children by attempting to lower total RT dose and volume as well as by utilizing technologies such as proton-beam RT (Merchant et al. 2009).
8 Management of Nervous System Toxicity
Corticosteroids are the mainstay of treatment for many radiation-induced CNS toxicities, including radiation necrosis, cranial nerve palsy, and peripheral neuropathy. Treatment with corticosteroids often affords significant symptomatic benefit. It may also alter the pathophysiology of radionecrosis by reducing local edema and interrupting local inflammatory cascades. However, the side effects of steroids are manifold, including worsening diabetic hyperglycemia, immunosuppression, loss of muscle mass, osteoporosis, peripheral edema, weight gain, skin changes, and psychosis. Therefore, if steroid therapy is initiated, it should only be continued if effective, and efforts should be made to taper steroids quickly as symptoms begin to resolve.
The benefit of hyperbaric oxygen (HBO) therapy for radiation-induced CNS toxicity has been investigated in a number of small trials (Hulshof et al. 2002; Ohguri et al. 2007; Chuba et al. 1997). No consistent benefit has been reported and no large randomized trial has been conducted. At this point, there is insufficient evidence to recommend for or against HBO in patients with CNS toxicity secondary to radiotherapy. Pentoxifylline, which may also improve tissue oxygenation and down-regulate inflammatory cytokines, has also been suggested as a treatment for radionecrosis and RT-induced cranial and peripheral nerve injury. However, no well-designed, prospective trials have been performed to demonstrate its efficacy. While there is increased tissue oxygen delivery and down-regulation of inflammatory cytokines with pentoxifylline, there is also evidence that tumor oxygenation and increased tumor growth can occur (Vernimmen et al. 1994; Grzela et al. 2003). Similarly, HBO increases tumor oxygenation which most likely explains the multiple reports of explosive tumor recurrences and accelerated disease progression following HBO therapy (Wang 1999; Bradfield 1996). While erythropoietin has neuroprotective effects after injury, this has not been studied in radiation patients; moreover, there have been multiple randomized trials recently reported that demonstrate its negative effect on overall survival in cancer patients (van der Kooij et al. 2008; Leyland-Jones et al. 2005; Smith et al. 2008). Therefore, these agents should be routinely avoided in patients with active tumor or in those who are still in the window in which the risk of disease recurrence is high.
A variety of psychoactive drugs have been utilized to treat post-RT cognitive dysfunction. Methylphenidate, a stimulant used to treat attention deficit disorder, may improve psychomotor slowing and arousal in patients with lassitude or lethargy following radiation (Meyers et al. 1998). Donepezil is an acetylcholinesterase inhibitor used to improve cognitive function in patients with dementia. Donepezil significantly improved mood, cognitive function, and health-related quality of life in a phase II trial of irradiated brain tumor patients (Shaw et al. 2006). A recently opened Phase III trial (RTOG 0614) is investigating another anti-dementia agent, memantine, in patients undergoing whole brain radiation for brain metastases. Memantine is an N-methyl-D-aspartate receptor antagonist which slows the progression of and improves symptoms related to vascular dementia. Because vascular injury plays a key role in the pathogenesis of radiation injury to the brain, memantine is an attractive therapeutic agent. Bevacizumab, as described above, has the potential to modulate post-RT vascular pathology as well and is under active investigation.
9 Future Research: Stem Cell Transplant
The transfusion of pluripotent stem cells to regenerate parenchymal and endothelial cells is no longer the impossible dream. The new exciting advances in the bioengineering of adult skin cells by insertion of three genes into histocompatible stem cells opens the door for an improved therapeutic ratio, that is, the stabilization and reversal of the radiation Biocontinuum (Gutin 1991).
An apocryphal study utilized rat embryonic grafts, implanted as a core of tissue in irradiated adult rat brain; appeared to reverse most the morphologic and functional aspects of neuronal damage (Fig. 12a, b) (Pearlman et al. 1990).
Coronal (30 μm) section through the anterior hippocampus, stained with cresyl violet. In comparison to normal controls (a), the 30 Gy irradiated animals (b) showed marked disruption of the cytoarchitecture of the hippocampal formation, large holes, and almost complete degeneration of the fimbria (FI) (with permission from CURED LENT II 2008)
10 Review of Literature/Historical Highlights
1937 O’Connell and Brunschwig: After a thorough analysis of the literature and cases, concluded that the brain and its blood vessels are injured. Suggested that 15,000 R not be exceeded and that the optimal dose is 4,500 R.
1943 Smithers, Clarkson and Strong: Reported a case of Brown-Sequard syndrome one year and three months after irradiation of the esophagus (5,800 R in 39 days).
1948 Pennybacker and Russell: Presented a clinical and pathologic review of five cases of brain necrosis following therapy for brain tumor (except one case of rodent ulcer). The damage was due to thrombosis of small vessels.
1951 and onward: Lars Leksell pioneers the concept of stereotactic radiosurgery.
1954 Arnold, Bailey and Laughlin: Conducted an experimental study of a wide range of single doses to the brain and primates, concluding that the brain is more radioresponsive than generally conceded.
1954 Arnold, Bailey and Harvey: In experimental studies, suggested that the brain stem and hypothalamus are more sensitive to irradiation than the cerebrum.
1958 Berg and Lindgren: Conducted an excellent experimental study of time-dose relationship and morphology of delayed radiation lesions of the brain in rabbits.
1963 Berg and Lindgren: Presented data relating tolerance of the brain to field size, using an experimental situation in which select fields were used.
1964 Haley and Snider: Conducted a multidisciplinary symposium on the response of the nervous system to ionizing irradiation with emphasis on cytologic, histologic, anatomic, functional, biochemical and behavioral aspects.
1965 Vaeth: Offered a time-dose plot for radiation myelitis.
1966 Bouchard: Presented the most recently published treatise on the radiation therapy of brain tumors and the tolerance of the brain to irradiation.
1968 Rubin and Cassarett: Presented the bio-continuum paradigm to chart clinical pathophysiologic events in an early/late timeline.
1988: Kjellberg and Abe suggest a series of ‘iso effect’ curves for various doses/volumes for single fraction radiosurgery, based on combined animal and human data (Kjellberg and Abe 1988). The 1 % iso-effect line is latter suggested by Marks and Spenser (based on a literature review) to closer to a 3–8 % risk, (Marks and Spencer 1991) and by Flickinger, Schell, Larson (based on clinical data) to be closer to a 3 % risk (Flickinger et al. 1990).
1990: Pearlman, Rubin, White, et al. Fetal hypothalamic transplants into irradiated brains of rats: restore the histopathology to normal (Pearlman et al. 1990).
1991: Gutin, Leibel and Sheline publish “Radiation Injury to the Nervious System”.
1993 Tishler, Loeffler, Lunsford, et al. (Tishler et al. 1993) demonstrate a steep dose response for optic nerve injury following radiosurgery.
1993 Flickinger, Lunsford, Kondziolka, et al. illustrate the higher rate of imaging-defined brain injury (vs. symptom-defined injury) following radiosurgery, and the importance of ‘location’ within the brain in estimating the risk of injury with radiosurgery (Flickinger et al. 1992).
1996 and onwand: Shaw and other investigators at the RTOG define volume dependent tolerance doses for brain radiosurgery (Shaw et al. 1996).
2001: Ang et al. report marked recovery of “tolerance” in primate spinal cord. (Ang et al. 2001).
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- BBB:
-
Blood–brain barrier
- COWAT:
-
Controlled oral word association test
- CNS:
-
Central nervous system
- CTCAE:
-
Common terminology criteria for adverse events
- HVLT:
-
Hopkins verbal learning test
- HBO:
-
Hyperbaric oxygen
- LENT–SOMA:
-
Late effects normal tissue task force–subjective, objective, management, and analytic
- MRI:
-
Magnetic resonance imaging
- MMSE:
-
Mini-mental status exam
- NHL/CLL:
-
Non-Hodgkin lymphoma and chronic lymphocytic leukemia
- PNS:
-
Peripheral nervous system
- POMS:
-
Profile of mood states short form
- TMT:
-
Trail making test
- WM:
-
White matter
- VEGF:
-
Vascular endothelial growth factor
References
Alfonso E, DeGregorio M, Mateo P et al (1997) Radiation myelopathy in over-irradiated patients: MR imaging findings. Eur Radiol 7(3):400–404
Allen JC, Siffert J (1996) Contemporary chemotherapy issues for children with brainstem gliomas. Pediatr Neurosurg 24:98–102
Anderson V, Godber D, Smibert D et al (1997) Neurobehavioural sequelae following cranial irradiation and chemotherapy n children: an analysis of risk factors. Pediatr Rehabil 1:63–76
Ang K, Jiang G, Feng Y et al (2001) Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys 50(4):1013–1020
Armstrong C, Ruffer J, Corn B et al (1995) Biphasic patterns of memory deficits following moderate-dose partial brain irradiation: Neuropsychologic outcome and proposed mechanism. J Clin Onc 13(9):2263–2271
Barani I, Benedict S, Lin P (2007) Neural stem cells: implications for the conventional radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys 68(2):324–333
Barbarese E, Barry C (1989) Radiation sensitivity of glial cells in primary culture. J Neurol Sci 91(1–2):97–107
Belka C, Budach W, Kortmann R et al (2001) Radiation induced CNS toxicity – molecular and cellular mechanisms. Br J Cancer 85(9):1233–1239
Berg R, Ch’ien L, Lancaster W (1983) Neuropsychological sequelae of post radiation somnolence syndrome. J Dev Behav Pediatr 4(2):103–107
Bradfield JJ, Kinsella JB, Mader JT et al (1996) Rapid progression of head and neck squamous carcinoma after hyperbaric oxygenation. Otolaryngol Head Neck Surg 114(6):793–797
Brandsma D, Stalpers L, Taal W et al (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Brezden CB, Phillips KA, Abdolell M et al (2000) Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695–2701
Briere M, Scott J, McNall-Knapp R et al (2008) Cognitive outcome in pediatric brain tumor survivors: delayed attention deficit at long-term follow-up. Pediatr Blood Cancer 50(2):337–340
Brown P, Buckner J, Brown C et al (2001) The effects of radiation on cognitive function in patients with low-grade glioma. Presented at the American society for therapeutic radiology and oncology 41st annual meeting, San Francisco, 4 Nov 2001
Brown P, Buckner J, Uhm J et al (2003) The neurocognitive effects of radiation in adult low-grade glioma patients. Neuro-Oncol 5(3):161–167
Brown E, Woolston D, Frol A et al (2004) Hippocampal volumes, spectroscopy, cognition and mood in patients receiving corticosteroid therapy. Biol Psychiatry 55:538–545
Ch’ien L, Aur R, Stagner S (1980) Long-term neurological implications of somnolence syndrome in children with acute lymphocytic leukemia. Ann Neurol 8(3):273–277
Cheung M, Chan A, Law S et al (2000) Cognitive function of patients with nasopharyngeal carcinoma with and without temporal lobe radionecrosis. Arch Neurol 57(9):1347–1352
Chin L, Ma L, Dibiase S (2001) Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow-up. J Neurosurg 94(6):899–904
Chuba P, Aronin P, Bhambani K et al (1997) Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer 80(10):2005–2012
Coderre J, Morris G, Micca P et al (2006) Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res 166(3):495–503
Corn B, Yousem D, Scott C et al (1994) White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (radiation therapy oncology group 83–02). Cancer 74(10):2828–2835
Crossen J, Garwood D, Glatstein E et al (1994) Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol 12(3):627–642
DeAngelis LM, Mandell LR, Thaler HT et al (1989a) The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery 24:798–805
DeAngelis L, Delattre J, Posner J (1989b) Radiation-induced dementia in patients cured of brain metastases. Neurology 39:789–796
Delattre J, Rosenblum M, Thaler H et al (1988) A model of radiation myelopathy in the rat: pathology, regional capillary permeability changes, and treatment with dexamethasone. Brain 111(6):1319–1336
Duffner P, Horowitz M, Krischner J et al (1993) Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 328(24):1725–1731
Emami B, Lyman J, Brown A et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21(1):109–122
Emsley J, Mitchell B, Kempermann G et al (2005) Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol 75(5):321–341
Flickinger JC, Schell MC, Larson DA (1990) Estimation of complications for linear accelerator radiosurgery with the integrated logistic formula. Int J Radiat Oncol Biol Phys 19:143–148
Flickinger JC, Lunsford LD, Kondziolka D et al (1992) Radiosurgery and brain tolerance: an analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys 23:19–26
Frytak S, Shaw J, O’Neill B et al (1989) Leukoencephalopathy in small cell lung cancer patients receiving prophylactic cranial radiation. Am J Clin Oncol 12(1):27–33
Fujii O, Tsujino K, Soejima T et al (2006) White matter changers on magnetic resonance imaging following whole brain radiotherapy for brain metastases. Radiat Med 24(5):345–350
Fukuda A, Fukuda H, Swanpalmer J et al (2005) Age-dependent sensitivity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. J Neurochem 92(3):569–584
Glantz MJ, Cole BF, Forsyth PA et al (2000) Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors: report of the quality standards subcommittee of the American academy of neurology. Neurology 54:1886–1893
Gonzalez J, Kumar A, Conrad C et al (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67(2):323–326
Grzela T, Lazarczyk M, Niderla J et al (2003) Pentoxifylline promotes development of murine colon adenocarcinoma-derived metastatic tumors in liver. Oncol Rep 10(6):1805–1809
Gutin P (1991) Treatment of radation necrosis of the brain. In: Gutin P, Leibel S, Sheline G (eds) Radiation injury to the nervous system. Raven Press, New York, pp 271–282
Hall E (2006a) Giaccia A: dose-response relationships for model normal tissues. In: Radiobiology for the radiologist. Lippincott Williams and Wilkins, Baltimore, pp 320–321
Hall E (2006b) Giaccia A: acute effects of total body radiation. In: Radiobiology for the radiologist. Lippincott Williams and Wilkins, Baltimore, pp 118–119
Hall E (2006c) Giaccia A: effects of radiation on the embryo and fetus. In: Radiobiology for the Radiologist. Lippincott Williams and Wilkins, Baltimore, pp 173–175
Herholz K, Coope D, Jackson A (2007) Metabolic and molecular imaging in neuro-oncology. Lancet Neurol 6(8):711–724
Hulshof M, Stark N, van der Kleij A et al (2002) Hyperbaric oxygen therapy for cognitive disorders after irradiation of the brain. Strahlenther Onkol 178(4):192–198
Johannesen T, Rusmussen K, Winther F et al (2002) Late radiation effects on hearing, vestibular function, and taste in brain tumor patients. Int J Radiat Oncol Biol Phys 53(1):86–90
Kagan AR (1993) Nervous system toxicity. In: Madhu JJ, Flam MS, Legha SS et al (eds) Chemoradiation: an integrated approach to cancer treatment. Lea & Febiger, Philadelphia, pp 582–590
Keime-Guibert F, Napolitano M, Delattre J (1998) Neurological complications of radiotherapy and chemotherapy. J Neurol 245(11):695–708
Kim J, Brown S, Jenrow K (2008) Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol 87:279–286
Kjellberg RN, Abe M (1988) Stereotactic bragg peak proton beam therapy. In: Lunsford LD (ed) Modern stereotactic neurosurgery. Martinus Nijhoff, Boston, pp 463–470
Klein M, Heimans J, Aaronson N et al (2002) Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 360(9343):1361–1368
Klein M, Engelberts NH, van der Ploeg HM et al (2003) Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol 54:514–520
Langer T, Martus P, Ottensmeier H et al (2002) CNS late effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol 38(5):320–328
Lawrence YR, Li XA, El Naqa I et al (2010) Radiation dose–volume effects in the brain. Int J Radiat Oncol Biol Phys 76:S20–S27
Lee A, Kwong D, Leung S et al (2002) Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys 53(1):75–85
Leyland-Jones B, Semiglazov V, Pawlicki M et al (2005) Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 23(25):5960–5972 (Epub 9 Aug 2005)
Li Y, Chen P, Haimovitz-Friedman A et al (2006) Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res 63(18):5950–5956
Maranzano E, Bellavita R, Floridi P et al (2001) Radiation-induced myelopathy in long-term surviving metastatic spinal cord compression patients after hypofractionated radiotherapy: a clincial and magnetic resonance imaging analysis. Radiother Oncol 60(3):281–288
Marks LB, Spencer DP (1991) The influence of volume on the tolerance of the brain to radiosurgery. J Neurosurg 75:177–180
Mayo C, Martel MK, Marks LB et al (2010) Radiation dose–volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 76:S28–S35
Merchant T, Kiehna E, Miles M et al (2002) Acute effects of irradiation on cognition: changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. Int J Radiat Oncol Biol Phys 53(5):1271–1278
Merchant T, Kiehna E, Li C et al (2005) Radiation dosimetry predicts IQ after conformal radiotherapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys 63(5):1546–1554
Merchant TE, Conklin HM, Wu S et al (2009) Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 27:3691–3697
Meyers C, Weitzner M, Valentine A (1998) Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol 16(7):2522–2527
Meyers CA, Smith JA, Bezjak A et al (2004) Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 22:157–165
Milano M, Constine L, Okunieff P et al (2007) Normal tissue tolerance dose metrics for radiation therapy of major organs. Sem Radiat Oncol 17(2):131–140
Mulhern R, Kepner J, Thomas P et al (1998) Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a pediatric oncology group study. J Clin Oncol 16(5):1723–1728
National Academy of Sciences/National Research Council, Committee on the biological effects of ionizing radiations: health effects of exposure to low levels of ionizing radiations. Washington DC, 1990
Newcomer J, Craft S, Hershey T et al (1994) Glucocorticoid-inducd impairment in declarative memory performance in adult humans. J Neurosci 14(4):2047–2053
Ohguri T, Imada H, Kohshi K et al (2007) Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 67(1):248–255
Packer R, Sposto R, Atkins T et al (1987) Quality of life in children with primitive neuroectodermal tumors (medulloblastoma) of the posterior fossa. Pediatric Neurosci 13(4):169–175
Parsons J, Bova F, Fitzgerald C et al (1994) Radiation optic neuropathy after megavoltage external-eam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys 30(4):755–763
Pearlman SH, Rubin P, White HC et al (1990) Fetal hypothalamic transplants into brain irradiated rats: graft morphometry and host behavioral responses. Int J Radiat Oncol Biol Phys 19:293–300
Peissner W, Kocher M, Treuer H et al (1999) Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res 71(1):61–68
Pollock BE, Foote RL, Link MJ et al (2005) Repeat radiosurgery for idiopathic trigeminal neuralgia. Int J Radiat Oncol Biol Phys 61(1):192–195
Prassopoulos P, Cavouras D, Evlogias N et al (1997) Brain atrophy in children undergoing systemic chemotherapy for extracranial solid tumors. Med Pediatr Oncol 28:228–233
Regine W, Scott C, Murray K et al (2001) Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from radiation therapy oncology group study 91–04. Int J Radiat Oncol Biol Phys 51(3):711–717
Regine WF, Schmitt F, Scott C et al (2004) Feasibility of neurocognitive outcome evaluations in patients with brain metastases in a multi-institutional cooperative group setting: results of radiation therapy oncology group BR-0018. Int J Radiat Oncol Biol Phys 58(5):1346–1352
Rock J, Hearshen D, Scarpace L et al (2002) Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurg 51(4):912–919
Rock J, Scarpace L, Hearshen D et al (2004) Association among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurg 54(5):1111–1117
Rubin P, Casarett GW (1968) Alimentary tract: esophagus and stomach. In: Rubin P, Casarett GW (eds) Clinical radiation pathology, vol 1, 1 edn. W. B. Saunders Company, Philadelphia p 517
Schmidt L, Fox N, Goldberg M et al (1999) Effects of acute prednisone administration on memory, attention, and emotion in healthy human adults. Psychoneuroendocrinology 24:461–483
Shaw E, Scott C, Souhami L et al (1996) Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: Initial report of radiation therapy oncology group protocol 90–05. Int J Radiat Oncol Biol Phys 34(3):647–654
Shaw E, Rosdhal R, D’Agostino R et al (2006) Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. J Clin Oncol 24(9):1415–1420
Sheline G, Wars W, Smith V (1980) Therapeutic irradiation and brain injury. Int J Radat Oncol Biol Phys 6(9):1215–1228
Silber J, Radcliffe J, Peckham V et al (1992) Whole brain radiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol 10(9):1390–1396
Sloan A, Arnold S, St. Clair W et al (2003) Brain injury: current management and investigations. Sem Radiat Oncol 13(3):309–321
Smith RE Jr, Aapro MS, Ludwig H et al (2008) Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 26(7):1040–1050 (Epub 8 Jan 2008)
Sonderkaer S, Schmiegelow M, Carstensen H et al (2003) Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol 21(7):1347–1351
Stafford S, Pollock B, Leavitt J et al (2003) A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 55(5):1177–1181
Tada E, Parent J, Lowenstein D et al (2000) X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 99(1):33–41
Tchen N, Juffs H, Downie F et al (2003) Congitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol 21(22):4175–4183
Tillman BN, Elbermani W (2007) (eds) Atlas of human anatomy, clinical edition, 1st edn. Mud Puddle Books Inc, New York, pp. 60
Tishler R, Loeffler J, Lunsford L et al (1993) Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys 27(2):215–221
Tsuruda J, Kortman K, Bradley W et al (1987) Radiation effects on cerebral white matter: MR evaluation. Am J Roentgenol 149:165–171
Valéry CA, Cornu P, Noël G (2003) Predictive factors of radiation necrosis after radiosurgery for cerebral metastasis. Stereotact Funct Neurosurg 81(1–4):115–119
van der Kooij MA, Groenendaal F, Kavelaars A et al (2008) Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev 59(1):22–23, Nov
Vernimmen F, Verheye-Dua F, du Toit H et al (1994) Effect of pentoxifylline on radiatiion damage and tumor growth. Strahlenther Onkol 170(10):595–601
Vrdoljak E, Bill C, va der Kogel A et al (1992) Radiation-induced apoptosis of oligodendrocytes in vitro. Int J Radiat Biol 62(4):475–480
Walter A, Mulhern R, Gajjar A et al (1999) Neurodevelopmental outcome of young children with medulloblastoma at St. Jude’s children’s research hospital. J Clin Oncol 17(12):3720–3728
Wang PH, Yuan CC, Lai CR et al (1999) Rapid progression of squamous cell carcinoma of the cervix after hyperbaric oxygenation. Eur J Obstet Gynecol Reprod Biol 82(1):89–91
Waselenko J, MacVittie T, Blakely W et al (2004) Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann Int Med 140(12):1037–1051
Watterson J, Toogood I, Nieder M et al (1994) Excessive spinal cord toxicity from intensive central nervous system-directed therapies. Cancer 74:3034–3041
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Yuan H, Gaber M, Boyd K et al (2006) Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys 66(3):860–866
Zacny JP, Gutierrez S (2003) Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology (Berlin) 170:242–254. (Epub 29 Aug 2003)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Yovino, S., Kwok, Y., Regine, W.F. (2014). Brain and Cranial Nerves. In: Rubin, P., Constine, L., Marks, L. (eds) ALERT • Adverse Late Effects of Cancer Treatment. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75863-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-540-75863-1_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-75862-4
Online ISBN: 978-3-540-75863-1
eBook Packages: MedicineMedicine (R0)