Abstract
The delayed adverse neurologic sequelae of radiation therapy, including radiation leukoencephalopathy, hydrocephalus, and cerebral atrophy, represent an increasingly important problem as advances in cancer therapy lead to prolonged patient survival. A variety of pathophysiologic mechanisms for late radiation injury have been proposed, including neurovascular injury, impaired neurogenesis secondary to neural progenitor cell injury, and inflammatory changes. Patient age and comorbidities, as well as radiation dose schedule and the use of concurrent chemotherapy, all appear to be important determinants of long-term outcome. Leukoencephalopathy, or diffuse white matter injury, is associated with progressive and irreversible neurocognitive decline, as well as personality changes, neuropsychiatric abnormalities, Parkinsonism, tremor, and seizures. Hydrocephalus is a common sequela, leading to cognitive impairment, gait instability and urinary incontinence. Radiation therapy is also known to result in progressive brain volume loss, with particular vulnerability in regions associated with higher-order cognitive performance and endogenous neural repair mechanisms. Management strategies are supportive, including CSF diversion in cases of hydrocephalus and neurostimulants and acetylcholinesterase inhibitors in radiation-associated dementia. More work on strategies aimed at neuroprotection and brain repair is needed to serve this growing patient population.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Late radiation injury
- Radiation neurotoxicity
- Leukoencephalopathy
- Hydrocephalus
- Radiation-associated cerebral atrophy
Learning Objectives
-
To learn about delayed adverse effects of cranial irradiation on the central nervous system.
-
To recognize common clinical syndromes induced by cranial irradiation, such as leukoencephalopathy, brain atrophy, and cognitive impairment.
-
To review common risk factors associated with delayed adverse effects of cranial irradiation.
-
To review the current understanding of potential mechanisms underlying delayed neurotoxic effects of cranial irradiation.
-
To learn about potential management strategies to prevent and treat neurotoxic syndromes following cranial irradiation.
Introduction
As survival of primary and secondary malignancies of the central nervous system improves with the efficacy of anticancer therapies , the delayed adverse effects of radiotherapy have been increasingly recognized as an important clinical problem facing patients and clinicians seeking to maximize both survival and quality of life in the posttreatment period.
Complications of cranial irradiation are conventionally categorized into early manifestations, including acute and early delayed radiation injury , and chronic manifestations, or late radiation injury [1]. Early manifestations include fatigue, alopecia, dermatitis, cerebral edema, and the somnolence syndrome and typically manifest within weeks (acute) to 6 months (early delayed). While debilitating, these changes are typically transient and reversible. Manifestations of late radiation injury , including leukoencephalopathy, hydrocephalus, and cerebral atrophy, typically manifest after 6 months and in some cases years after treatment and can be associated with severe, debilitating, and usually irreversible clinical sequelae. These include progressive cognitive impairment, urinary incontinence, and gait dysfunction [2,3,4,5,6]. The current chapter will focus on the pathophysiology, diagnosis, and management of delayed leukoencephalopathy and cerebral atrophy.
Pathophysiology
A variety of pathophysiologic mechanisms have been implicated in late radiation injury, including neurovascular toxicity, neuroinflammation, and damage to diverse neural cell types.
It is known from preclinical studies that radiation induces vascular endothelial damage and endothelial cell apoptosis [7]. This may lead to fibrinoid necrosis of small vessels, in turn giving rise to capillary leakage, cerebral edema, spongiosis, and rarefaction of the white matter [2, 8]. Cerebrovascular mortality from stroke and intracerebral hemorrhage is increased in patients who have received cranial irradiation [9,10,11]. The adverse vascular effects of delayed radiation injury may be mediated by local tissue ischemia and the release of vascular endothelial growth factor (VEGF) [12].
Indeed, inhibition of VEGF with the monoclonal antibody bevacizumab has been shown to attenuate the radiographic abnormalities associated with delayed radiation necrosis and has been used in patients suffering from this complication [13].
A growing literature from preclinical and clinical studies also implicates damage to neuronal and glial progenitor cells within the dentate gyrus of the hippocampus, subventricular zone, and subcortical white matter as an important cause of delayed radiation injury and cognitive decline [14,15,16,17,18,19,20].
Specifically, the hippocampal dentate gyrus is an important region for adult neurogenesis and critical for the maintaining of the cellular hippocampal architecture that underlies learning and memory, spatial processing, and pattern separation [21,22,23,24,25,26,27,28]. Not surprisingly, disruption of adult hippocampal neurogenesis has been identified as a potential cause of radiation-induced cognitive impairment [19]. In addition, the subventricular zone (SVZ) and parenchymal white matter, known to harbor self-renewing glial progenitor cells essential to neuroplasticity, myelination, and endogenous neural repair, have been shown to be sensitive to the effects of radiation, and their depletion may contribute to the delayed cognitive decline, demyelination, and cerebral atrophy that commonly afflict long-term survivors [16, 29,30,31].
In an effort to minimize radiation-induced damage to hippocampal structures relevant to learning and memory, hippocampal-sparing radiotherapy techniques have been developed and appear to improve long-term cognitive outcomes in patients with brain metastases, though this may or may not be an option depending on the extent and distribution of a patient’s disease [32]. A phase III study is ongoing to corroborate the results of this phase II trial.
Lastly, the role of inflammatory changes has been increasingly recognized as a key factor in radiation-induced neurotoxicity. Radiation is known to induce microglial activation and release of neurotoxic cytokines including tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) [14, 33,34,35,36,37]. These inflammatory changes likely contribute to neurovascular damage and direct cytotoxicity, though the precise role of the inflammatory process in delayed radiation injury remains an area of current investigation.
Risk Factors
A variety of risk factors predicting delayed radiation injury have been established. Chief among them are radiation dose, fractionation schedule, the use of concurrent medications, and underlying patient-specific factors. Radiation doses of more than 20 Gy are considered sufficient to cause white matter changes on magnetic resonance imaging (MRI) in patients surviving longer than 1 year (Fig. 39.1). Doses of 72 Gy or higher are associated with increased risk for focal radiation necrosis in at least 5% of patients, though radiation necrosis can also be encountered with lower radiation doses and with some heterogeneity among brain regions in their inherent propensity for tissue damage (Fig. 39.2). There is also evidence that fraction sizes exceeding 2 Gy are more commonly associated with delayed neurotoxicity [38,39,40,41].
MRI scan of a 66-year-old female with metastatic non-small cell lung cancer , treated with whole brain radiation (35 Gy). (a) Axial T2/FLAIR MRI scan prior to radiation therapy without evidence of leukoencephalopathy. (b) Axial T2/FLAIR MRI scan 3–4 years after whole brain radiation therapy showing diffuse subcortical leukoencephalopathy. The patient was symptomatic with cognitive decline and gait difficulties
MRI scan of a 55-year-old male treated with proton radiation for nasopharyngeal carcinoma . Four years later, he developed headaches and altered consciousness. The gadolinium-enhanced MRI of the brain identified a necrotic lesion with abnormal enhancement (a) with associated cerebral edema and focal leukoencephalopathy characterized by T2/FLAIR signal hyperintensity surrounding the enhancing lesion (b), consistent with delayed radiation necrosis
Aside from the absolute radiation dose and fraction size, it is well established that whole brain radiation therapy (WBRT) confers significantly greater risk for delayed toxicities than partial radiation therapy. Regimens that combine WBRT and stereotactic radiosurgery (SRS) appear to induce the greatest declines in performance across an array of neurocognitive domains in surviving patients [42,43,44]. Recognition of these risk factors has led to a shift in practice toward smaller fraction sizes and, when possible, focal rather than whole brain regimens that attempt to spare vulnerable structures [32, 45].
While radiation therapy alone can cause delayed injury, the concurrent use of chemotherapy appears to significantly increase the risk of complications in survivors, perhaps as a result of synergistic injury to the blood-brain barrier, white matter tracts, or brain regions with neurogenic potential [31, 46].
Patient-specific factors also appear to be important predictors of long-term outcomes following radiation therapy. Young patients are particularly vulnerable to late injury, presumably due to disruption of normal neurodevelopmental processes and neural networks that rely on rapid cell division and progenitor cell function [16]. In a recent study in medulloblastoma patients treated with craniospinal irradiation, the cumulative incidence of neurovascular injury was highest in patients treated at age younger than 20 and followed a progressive pattern in the absence of additional treatment [47].
Elderly patients are also particularly vulnerable to late injury, likely as a result of premorbid age-related brain changes including microvascular disease and cerebral atrophy, which may serve to lessen patient’s neurologic and cognitive reserve [2]. Indeed, patients with pre-existing white matter changes at the time of treatment are more likely to develop progressive white matter changes on MRI at a given radiation dose than patients without white matter changes at the time of treatment [48]. Patients with pre-existing demyelinating diseases such as multiple sclerosis may also be more vulnerable to radiation-induced neurotoxicity [49].
Leukoencephalopathy
Leukoencephalopathy, or diffuse white matter injury , is one of the most commonly encountered delayed complications of radiation therapy. Its incidence is not clearly known and varies widely among published case series, though conservative estimates indicate a range from 5 to 30% in patients undergoing radiation therapy alone, with higher rates in patients receiving concurrent chemotherapy or a combination of WBRT and SRS [2, 50, 51].
Leukoencephalopathy was initially recognized in patients undergoing high-dose radiation therapy for cerebral metastatic disease . For instance, in a series of 12 patients who were radiographically considered cured of CNS malignancy following WBRT, DeAngelis and colleagues found that all patients developed a delayed, insidious, and progressive syndrome of neurologic and cognitive decline [52]. Onset ranged from 5 to 36 months of treatment and was typically characterized by non-specific symptoms including dizziness, fatigue, and headache. Development of an overt neurocognitive syndrome was common, beginning with mild memory impairment and progressing to disorientation, confusion, and ultimately severe dementia. Gait abnormalities were universal, initially manifesting as mild gait instability and progressing to disabling ataxia resulting in frequent falls and traumatic injuries. Urinary urgency, progressing to urinary incontinence, was found in half of the patients. Other symptoms that have been recognized in association with radiation-induced leukoencephalopathy include personality changes, neuropsychiatric abnormalities, Parkinsonism, tremor, and seizures [50, 51, 53,54,55].
An increasing variety of imaging modalities has been used to detect and follow the progression of radiation-induced leukoencephalopathy, though MRI and computed tomography (CT) remain the most commonly employed [5, 56, 57]. MRI is generally preferred for patient evaluation, as it is more sensitive than CT to detect structural changes. Leukoencephalopathy typically appears as increased signal with the periventricular and deep white matter on T2-weighted MRI sequences. Increased diffusivity is seen on diffusion-weighted imaging (DWI), reflecting an increase in the free movement of water as hydrophobic structures degenerate in response to radiation damage. In early stages of this syndrome, T2 hyperintensity may only be seen as capping the frontal and occipital horns of the lateral ventricles. As the disease process ensues, however, those changes typically evolve into confluent and patchy white matter hyperintensity extending from the ventricles to the corticomedullary junction. Foci of contrast enhancement may develop, reflecting disruption of the blood-brain barrier. CT may be used in patients who are unable to undergo MRI due to an implanted device or some other contraindication. The CT signature of leukoencephalopathy is diffuse white matter hypodensity (Fig. 39.3) that typically does not enhance with iodinated contrast.
CT and MRI scan of a 70-year-old female treated with whole-brain radiation therapy for lung cancer . Two to three years after radiation therapy, an axial computed tomography (CT) scan shows ventricular enlargement and periventricular hypodensities (a), consistent with diffuse subcortical leukoencephalopathy, which can be better visualized on a corresponding axial T2/FLAIR MRI scan (b)
The histopathologic hallmarks of leukoencephalopathy include loss of blood-brain barrier integrity and demyelination. Injury to the blood-brain barrier, as discussed above, leads to increase in capillary permeability and chronic vasogenic edema. Injury to the white matter and oligodendroglia that serve to maintain myelin integrity leads to demyelination and replacement of hydrophobic myelin tracts with spongiform vacuoles and gliosis [2, 4, 52]. The combined effect of these changes is an increase in free tissue water, which underlies the T2 hyperintense signal on MRI and the hypodense signal on CT.
In its most severe form, a necrotizing leukoencephalopathy may develop, typically in patients who have received combined radiation and chemotherapy [53]. The latency between treatment and onset of leukoencephalopathy tends to be shorter than for patients treated with radiation therapy alone. Vascular injury is prominent in necrotizing leukoencephalopathy, leading to a higher degree of coagulative necrosis relative to milder forms, and in addition to the MRI features described above, patients may display tumorlike enhancement or disseminated hemorrhagic changes (Fig. 39.4). These findings portend a poor prognosis and are typically irreversible and progressive [4].
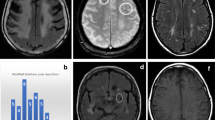
Axial MRI scan of a 42-year-old male with a left parieto-occipital IDH-mutant glioblastoma , treated with involved-field radiation therapy and concurrent chemotherapy with temozolomide. Approximately 3–5 years later, he developed progressive T2/FLAIR signal abnormalities within both parieto-occipital lobes (a), associated with interval hemorrhages best seen on gradient echo (GRE) sequences (b) and abnormal enhancement on T1 post-contrast MRI (c). Under the initial assumption of recurrent disease, he underwent repeat surgery. Pathology revealed hemorrhagic tissue necrosis in the absence of solid tumor, consistent with delayed treatment effects
Leukoencephalopathy is frequently associated with worsening communicating hydrocephalus, which can be seen on MRI or CT as an enlargement of the ventricles out of proportion to cerebral atrophy, with evidence of increased periventricular edema, also known as transependymal flow , which results from a pressure gradient driving fluid from the ventricular space into the periventricular tissue [54]. Hydrocephalus is thought to result from leptomeningeal fibrosis and disruption of CSF resorption by the arachnoid villi, leading to communicating hydrocephalus. It is therefore perhaps not surprising that there is such overlap between the clinical manifestations of radiation leukoencephalopathy and those of normal pressure hydrocephalus, in which the hallmark features include dementia, ataxia, and urinary incontinence. Relief of intracranial pressure by means of ventriculoperitoneal shunting (VPS) is a possible intervention for hydrocephalus associated with radiation leukoencephalopathy and has been shown to improve symptoms and quality of life in a subset of patients [50, 58]. In one early series, Thiessen and DeAngelis demonstrated a 6-month duration in symptomatic benefit (primarily in ataxia and urinary incontinence, with minimal effect on cognitive outcomes), though without a demonstrable prolongation of survival [52, 54].
Cerebral Atrophy and Neurocognitive Decline
Cerebral atrophy is a concerning and frequent manifestation of delayed radiation injury [59,60,61,62,63] (Fig. 39.5). In a landmark early study, Constine and colleagues found evidence on MRI and CT of ventricular expansion and enlarged sulci in approximately half of patients [2]. Cerebral atrophy has long been considered a late change, typically observed in advanced stages of radiation-induced neurotoxic injury [1]. More recent studies, however, suggest that atrophy may already begin within weeks of treatment onset, continuing to progress for months after completion of treatment. The ventricles may expand to as much as double their pretreatment volume in some cases [30].
MRI scan of a 72-year-old female with neuroendocrine carcinoid lung cancer treated with prophylactic whole brain radiation . She developed cognitive decline and gait difficulties 3–4 years after cranial irradiation. (a) Contrast-enhanced axial T1-weighted MRI scan at time of whole brain radiation therapy. (b) Contrast-enhanced axial T1-weighted MRI scan 4 years after radiation therapy, showing significant global brain atrophy and enlargement of ventricular spaces
Loss of both white and gray matter contributes to the decrease in total brain volume [62]. Within the white matter, radiation-induced demyelination and spongiform vacuolization, coupled with injury to neural progenitor cell-mediated endogenous neural repair, lead to diffuse gliosis and contraction of the white matter space. While the mechanism of cortical atrophy is less well understood, it is thought that vascular injury to distal perforating arteries plays a central role to its pathophysiology [4].
The precise relationship between cerebral atrophy and neurocognitive performance remains under current investigation, though more recent studies suggest preferential vulnerability of hippocampal structures [64] and of cortical areas relevant for higher cognitive function to radiation treatment [65].
It has been established that higher degrees of cortical atrophy are associated with poorer cognitive outcomes and overall performance status [66, 67]. Outcomes are worse after WBRT compared to partial radiation therapy and appear to be particularly unfavorable for patients undergoing regimens that combine WBRT and SRS [42, 55]. In one recent series, Brown and colleagues found that 92% of patients undergoing WBRT and SRS experienced cognitive decline compared to 64% of patients receiving SRS alone. Early recall, delayed recall, and verbal fluency were particularly affected [43].
Treatment of late cognitive decline is generally supportive and relies on a combination of pharmacotherapy and neurocognitive rehabilitation. There are mixed data to support the use of neurostimulants. For instance, low-dose methylphenidate appears to improve attention and social cognition in patients with known neurocognitive impairment following WBRT [68,69,70]. Acetylcholinesterase inhibitors , including donepezil, have also been shown to yield significant improvements in attention, concentration, memory, and mood, with the greatest benefit conferred to those with low baseline cognitive scores [71]. While the data for such treatment strategies is equivocal, these medications are generally well tolerated and may be reasonable to consider in selected patients for whom non-pharmacologic treatments have failed.
The use of memantine , an inhibitor of the glutamatergic NMDA receptor, to prevent radiation-induced cognitive impairment following whole-brain radiation therapy has recently been studied in a randomized and prospective clinical trial and was associated with improved cognitive function over time and a reduced rate of decline in executive function, memory, and processing speed [72]. A phase III study is ongoing to corroborate these results.
Future strategies to prevent or minimize the neurotoxic effects of radiation on the brain are likely to include radiation dose alterations, modifications of the radiation field, delay of radiation therapy, and combined use of pharmacological and non-pharmacological interventions aiming at neuroprotection and enhanced brain repair.
Conclusions and Key Points
Radiation therapy remains an essential treatment modality and means of prolonging survival in patients with CNS malignancy. It is, however, associated with an array of early and delayed complications. As more patients achieve longer survival periods with the increasing efficacy of anticancer therapies, the delayed neurotoxicities of cranial irradiation represent an important challenge in an effort to not only maximizing survival but also quality of life.
-
Early complications of CNS radiation occur within weeks to 6 months of treatment onset, including side effects such as cerebral edema, and are typically transient and reversible. Late complications typically occur after 6 months and include leukoencephalopathy, cerebral atrophy, and progressive neurocognitive decline.
-
Radiation causes injury to the CNS through a variety of pathophysiologic mechanisms, including (a) damage to large and small cerebral blood vessels; (b) depletion of neural progenitor cell populations leading to impaired neurogenesis, gliogenesis, and endogenous cellular repair; and (c) inflammatory changes including cytokine release and microglial activation.
-
An individual patient’s risk for developing delayed central nervous system toxicity depends on the total radiation dose administered, the fraction size, the extent of radiation (whole brain versus partial), the concurrent use of chemotherapy, and independent patient specific factors including age and premorbid functional status.
-
Leukoencephalopathy is characterized clinically by progressive neurocognitive decline and may also result in urinary incontinence and gait instability. On a cellular level, chronic vasogenic edema and loss of white matter are seen, resulting in gliosis and vacuolization that appear on MRI as confluent patches of T2 white matter hyperintensity. It is often complicated by hydrocephalus, for which ventriculoperitoneal shunting may be considered in selected patients.
-
Cerebral atrophy results from white matter destruction, as above, and loss of gray matter, possibly due to neural progenitor cell damage and chronic ischemia secondary to radiation-associated vasculopathy. While patients may respond to neurostimulants, acetylcholinesterase inhibitors, and cognitive rehabilitation, the neurocognitive impairment that accompanies these delayed complications can be profound and is commonly irreversible.
Self-Assessment Questions
-
1.
Which of the following is NOT a common feature of delayed radiation injury?
-
A.
Hydrocephalus.
-
B.
Cerebral atrophy.
-
C.
Cerebral edema.
-
D.
Leukoencephalopathy.
-
A.
-
2.
Radiation-induced leukoencephalopathy is characterized on neuroimaging by all of the following features EXCEPT:
-
A.
Diffuse white matter hypodensity on CT.
-
B.
Increased T2 white matter intensity on MRI.
-
C.
Multifocal subcortical infarcts.
-
D.
Patchy foci of contrast enhancement.
-
A.
-
3.
Ventriculoperitoneal shunting in radiation-induced leukoencephalopathy has been shown to:
-
A.
Prolong patient survival by an average of 6 months.
-
B.
Improve cognitive outcomes.
-
C.
Improve quality of life without prolonging survival.
-
D.
Increase the rate of fatal CNS infection.
-
A.
-
4.
The following medications have a role in supportive treatment for neurocognitive decline after radiation therapy EXCEPT:
-
A.
Acetylcholinesterase inhibitors.
-
B.
NMDA receptor antagonists.
-
C.
Atypical antipsychotics.
-
D.
Psychostimulants.
-
A.
-
5.
Which of the following is NOT an established risk factor for delayed radiation injury?
-
A.
Patient age.
-
B.
Radiation dose and fractionation schedule.
-
C.
Patient gender.
-
D.
Concurrent chemotherapy.
-
A.
Answers
-
1.
C
-
2.
C
-
3.
C
-
4.
C
-
5.
C
References
Belka C, Budach W, Kortmann RD, et al. Radiation induced CNS toxicity–molecular and cellular mechanisms. Br J Cancer. 2001;85(9):1233–9.
Constine LS, Konski A, Ekholm S, et al. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys. 1988;15(2):319–30.
Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12(3):627–42.
Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991;12(1):45–62.
Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–8.
Arrillaga-Romany IC and Dietrich J. Imaging finings in cancer therapy-associated neurotoxicity. Seminars in Neurology. 2012; 32(3):476–86.
Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004;4(5):273–84.
Murphy ES, Xie H, Merchant TE, et al. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015;122(3):421–9.
Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43(11):3035–40.
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the childhood cancer survivor study. Int J Radiat Oncol. 2013;86(4):649–55.
Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol. 2013;86(4):643–8.
Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–53.
Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94(1):63–8.
Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–62.
Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–30.
Dietrich J, Monje M, Wefel J, et al. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13(12):1285–95.
Hernández-Rabaza V, Llorens-Martín M, Velázquez-Sánchez C, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159(1):59–68.
Hellström NAK, Björk-Eriksson T, Blomgren K, et al. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27(3):634–41.
Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227(2):376–9.
Boström M, Kalm M, Karlsson N, et al. Irradiation to the young mouse brain caused long-term, progressive depletion of neurogenesis but did not disrupt the neurovascular niche. J Cereb Blood Flow Metab. 2013;33(6):935–43.
Squire LR, Ojemann JG, Miezin FM, et al. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci U S A. 1992;89(5):1837–41.
Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–41.
Ekstrom AD, Kahana MJ, Caplan JB, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425(6954):184–8.
Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31(4):163–9.
Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–3.
Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–50.
Knoth R, Singec I, Ditter M, et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. Callaerts P, editor. PLoS One. 2010;5(1):e8809.
Richard GR, Titiz A, Tyler A, et al. Speed modulation of hippocampal theta frequency correlates with spatial memory performance. Hippocampus. 2013;23(12):1269–79.
Peissner W, Kocher M, Treuer H, et al. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71(1):61–8.
Prust MJ, Jafari-Khouzani K, Kalpathy-Cramer J, et al. Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology. 2015;85(8):683–91.
Correa DD, Shi W, Abrey LE, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14(1):101–8.
Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–6.
Mizumatsu S, Monje ML, Morhardt DR, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–7.
Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5.
Han W, Umekawa T, Zhou K, et al. Cranial irradiation induces transient microglia accumulation, followed by long-lasting inflammation and loss of microglia. Oncotarget. 2015;7(50):82305–23.
Morganti JM, Jopson TD, Liu S, et al. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci. 2015;35(2):748–60.
Moravan MJ, Olschowka JA, Williams JP, et al. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 2011;176(4):459–73.
Tomio L, Romano M, Zanchin G, et al. Ultrarapid high-dose course of prophylactic cranial irradiation in small-cell lung cancer: evaluation of late neurologic morbidity in 16 long-term survivors. Am J Clin Oncol. 1998;21(1):84–90.
Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31(5):702–13.
Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–7.
Dietrich J, Gondi V, Mehta M. Delayed complications of cranial irradiation. In: UpToDate, Waltham, MA, 05(2), 2018.
Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–44.
Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA. 2016;316(4):401.
Brown PD, Buckner JC, O’Fallon JR, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J Clin Oncol. 2003;21(13):2519–24.
Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non–small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29(3):279–86.
Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32(6):535–41.
Roongpiboonsopit D, Kuijf HJ, Charidimou A, et al. Evolution of cerebral microbleeds after cranial irradiation in medulloblastoma patients. Neurology. 2017;88(8):789–96.
Sabsevitz DS, Bovi JA, Leo PD, et al. The role of pre-treatment white matter abnormalities in developing white matter changes following whole brain radiation: a volumetric study. J Neurooncol. 2013;114(3):291–7.
Miller RC, Lachance DH, Lucchinetti CF, et al. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: the Mayo clinic experience. Int J Radiat Oncol. 2006;66(4):1178–86.
Perrini P, Scollato A, Cioffi F, et al. Radiation leukoencephalopathy associated with moderate hydrocephalus: intracranial pressure monitoring and results of ventriculoperitoneal shunting. Neurol Sci. 2002;23(5):237–41.
Lai R, Abrey LE, Rosenblum MK, et al. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology. 2004;62(3):451–6.
DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–96.
Cummings M, Dougherty DW, Mohile NA, et al. Severe radiation-induced leukoencephalopathy: case report and literature review. Adv Radiat Oncol. 2016;1(1):17–20.
Thiessen B, DeAngelis LM. Hydrocephalus in radiation leukoencephalopathy: results of ventriculoperitoneal shunting. Arch Neurol. 1998;55(5):705–10.
Monaco EA, Faraji AH, Berkowitz O, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119(1):226–32.
Dietrich J, Klein JP. Imaging of cancer therapy–induced central nervous system toxicity. Neurol Clin. 2014;32(1):147–57.
Mamlouk MD, Handwerker J, Ospina J, et al. Neuroimaging findings of the post-treatment effects of radiation and chemotherapy of malignant primary glial neoplasms. Neuroradiol J. 2013;26(4):396–412.
Fischer CM, Neidert MC, Péus D, et al. Hydrocephalus after resection and adjuvant radiochemotherapy in patients with glioblastoma. Clin Neurol Neurosurg. 2014;120:27–31.
Swennen MHJ, Bromberg JEC, Witkamp TD, et al. Delayed radiation toxicity after focal or whole brain radiotherapy for low-grade glioma. J Neurooncol. 2004;66(3):333–9.
Omuro AMP, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62(10):1595–600.
Shibamoto Y, Baba F, Oda K, et al. Incidence of brain atrophy and decline in mini-mental state examination score after whole-brain radiotherapy in patients with brain metastases: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72(4):1168–73.
Karunamuni R, Bartsch H, White NS, et al. Dose-dependent cortical thinning after partial brain irradiation in high-grade glioma. Int J Radiat Oncol. 2016;94(2):297–304.
Ailion AS, King TZ, Wang L, et al. Cerebellar atrophy in adult survivors of childhood cerebellar tumor. J Int Neuropsychol Soc. 2016;22(5):501–11.
Seibert TM, Karunamuni R, Bartsch H, et al. Radiation dose–dependent hippocampal atrophy detected with longitudinal volumetric magnetic resonance imaging. Int J Radiat Oncol. 2017;97(2):263–9.
Seibert TM, Karunamuni R, Kaifi S, et al. Cerebral cortex regions selectively vulnerable to radiation dose-dependent atrophy. Int J Radiat Oncol. 2017;97(5):910–8.
Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of radiation therapy oncology group randomized trials 0212 and 0214. Int J Radiat Oncol. 2013;86(4):656–64.
Kiehna EN, Mulhern RK, Li C, et al. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. J Clin Oncol. 2006;24(33):5283–90.
Mulhern RK, Khan RB, Kaplan S, et al. Short-term efficacy of methylphenidate: a randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. J Clin Oncol. 2004;22(23):4795–803.
Meyers CA, Weitzner MA, Valentine AD, et al. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998;16(7):2522–7.
Page BR, Shaw EG, Lu L, et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro Oncol. 2015;17(10):1393–401.
Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–9.
Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–37.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Prust, M., Dietrich, J. (2018). Cerebral Atrophy and Leukoencephalopathy Following Cranial Irradiation. In: Chang, E., Brown, P., Lo, S., Sahgal, A., Suh, J. (eds) Adult CNS Radiation Oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-42878-9_39
Download citation
DOI: https://doi.org/10.1007/978-3-319-42878-9_39
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42877-2
Online ISBN: 978-3-319-42878-9
eBook Packages: MedicineMedicine (R0)