Abstract
In an increasingly obese and aging population, metabolic chronic diseases, low bone mass, and osteoporotic fractures are major public health concerns. During the last decades, obesity and osteoporosis have become dramatic global health problems with an increasing prevalence worldwide. Interestingly, the belief that obesity is protective against osteoporosis has come into question, as demonstrated by recent epidemiologic and clinical studies, which have shown that high level of fat mass might be a risk factor for osteoporosis and fragility fractures, both in men and women. Several potential mechanisms have been proposed to explain the complex relationship between adipose tissue and bone. This chapter considers recent data in the literature to further evaluate the relationship between fat and bone tissue in men.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In an increasingly obese and aging population, metabolic chronic diseases, low bone mass, and osteoporotic fractures are major public health concerns. In fact, during the last decades, obesity and osteoporosis have become important global health problems with an increasing prevalence worldwide [1,2,3,4]. Furthermore, the belief that obesity is protective against osteoporosis has come into question as demonstrated by recent epidemiologic and clinical studies, which show that high level of fat mass might be a risk factor for osteoporosis and fragility fractures, both in men and women [5,6,7,8]. In particular, we have demonstrated that TF negatively correlates with BMD independently from vitamin D levels, reduced IGF-1, and increased inflammatory markers [7].
Several potential mechanisms have been proposed to explain the complex relationship between adipose tissue and bone, and understanding how obesity determines low bone mass and modulates fracture risk is important to identify and treat people in order to prevent fractures. Most available evidences indicate that a significant number of fractures occur in obese men. Body mass index (BMI) is positively associated with bone mineral density (BMD), and the mechanisms of this association in vivo might include increased loading and higher aromatase activity [9]. Indeed, fat tissue is one of the major sources of aromatase, an enzyme also expressed in the gonads, which from androgen precursors synthesizes estrogens, steroid hormones which play a pivotal role in the maintenance of skeletal homeostasis and protecting against osteoporosis by reducing bone resorption and stimulating bone formation [9]. However, some fat depots, as visceral fat, might have negative effects on the bone by producing cytokines, molecules able to modulate bone metabolism as pro-resorptive factors [10,11,12]. Adipose tissue, in fact, secretes various inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), resistin, leptin, and adiponectin, which affect human energy and metabolic homeostasis but are also involved in bone metabolism [13,14,15,16]. Moreover, high intramuscular fat content is associated with poorer muscle function, attenuating loading effects and increasing the risk of falls [9]. A recent study has demonstrated that in older men, the condition of sarcopenic obesity is associated with increased fall rates compared with non-sarcopenic obese subjects [10].
On the other hand, since the demonstration that bone cells express several specific hormone receptors [14,15,16,17], and since recent observations have shown that osteocalcin (OCN) and osteopontin (OPN), bone-derived factors, affect body weight control and glucose homeostasis [18,19,20], the bone has come to be considered an endocrine target organ and an endocrine organ itself [21]. These considerations suggest a possible role of bone as a player of a potential feedback mechanism between the skeleton and the other endocrine organs [21]. Thus, the cross talk between fat and bone likely constitutes a homoeostatic feedback system in which adipokines and bone-derived molecules represent the link of an active bone-adipose axis.
Moreover, adipocytes and osteoblasts originate from a common progenitor, a pluripotent mesenchymal stem cell (MSC) [22], which has an equal propensity for differentiation into adipocytes or osteoblasts (or other lines) upon the influence of several cell-derived transcription factors. This process is complex, suggesting significant plasticity and multifaceted mechanism(s) of regulation within different cell lineages, among which are adipocytes and osteoblasts [23, 24].
Finally, obesity is associated with gonadal dysfunction: in women, obesity is associated with androgen excess disorders, mostly the polycystic ovary syndrome, whereas androgen deficiency is frequently present in obese men [25].
2 Fat, Bone, and Fat Bone Marrow Interplay
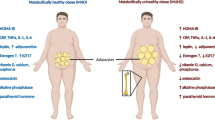
Obesity has always been recognized as a risk factor for cardiovascular and metabolic chronic diseases [2]. Nevertheless, it has been considered a protective factor for bone loss and osteoporosis, which is defined as a bone metabolic disease, characterized by a decrease in bone strength leading to an increased risk of developing spontaneous and traumatic fractures. Even though body fat and lean mass have been positively correlated with BMD, since obesity apparently exerts protection against bone loss, during the last decades, numerous evidences have described an opposite event, suggesting an inverse relationship between obesity and osteoporosis and showing that an increased abdominal fat tissue might be considered a risk factor for osteoporosis and fragility fractures [5, 7, 8] (Fig. 12.1).
The mechanisms whereby increased central adiposity leads to metabolic alterations, cardiovascular morbidity, and bone loss have been largely based on the demonstration that adipose tissue secretes a number of cytokines and bioactive compounds, named adipokines.
The adipokines, which include a variety of pro-inflammatory peptides, are involved in many physiological or pathological processes, and their dysregulation is a strong determinant of the low-grade inflammatory state of obesity, which promotes a cascade of metabolic alterations leading to cardiovascular complications, insulin resistance (or diabetes mellitus), and bone loss [11, 13].
Leptin, the first identified adipose tissue-derived factor, is an anorexigenic hormone secreted by adipocytes in proportion to body fat content, and its levels are typically elevated in obesity, which is considered a leptin-resistant state [26]. Interestingly, in obese subjects hyperleptinemia has been widely recognized as an independent cardiovascular risk factor associated with hyperinsulinemia and insulin resistance [27], whereas its effect on the bone appears composite, since both negative and positive actions have been reported on BMD, both in men and women [28, 29]. Leptin-deficient ob/ob mice and leptin receptor-deficient db/db mice are extremely obese, with increased vertebral trabecular bone volume due to increased bone formation [30], while intra-cerebroventricular infusion of leptin in both ob/ob and wild-type mice has shown to decrease vertebral trabecular bone mass [30]. In vivo studies indicate that the effect of leptin might depend on its site and mode of action [31], and it has been proposed that peripheral administration of leptin could increase bone mass by inhibiting bone resorption and increasing bone formation, while inhibiting bone formation through a central nervous system effect [28]. In vitro studies also indicate that leptin can act directly on bone marrow-derived mesenchymal stem cells (BMSCs) to enhance their differentiation into osteoblasts and to inhibit their differentiation into adipocytes [32]. Finally, leptin inhibits the expression of neuropeptide Y (NPY), a hypothalamus-derived peptide, essential for the regulation of food consumption, energy homeostasis, and bone remodeling [33]. Specific NPY-knockout mice display a significant decrease in body weight, a significant increase in food intake, and twofold increase in trabecular bone volume compared with wild-type animals [34].
Adiponectin exerts a protective role on cardiovascular system and glucose metabolism, and in contrast with leptin, its serum levels are reduced in obese and diabetic subjects and increase after weight loss [35]. Indeed, low levels of adiponectin are a common feature of obesity and correlate with insulin resistance [36]. Moreover, adiponectin levels are inversely related to the circulating levels of C-reactive protein (CRP), TNF-α and IL-6, powerful inhibitors of adiponectin expression, and secretion in cultured human adipose cells [37]. Interestingly, human osteoblasts express adiponectin and its receptors, and in vivo and in vitro studies show that adiponectin increases bone mass by suppressing osteoclastogenesis and activating osteoblastogenesis [38], likely indicating that a rise in adiponectin, upon fat reduction, could beneficially affect BMD.
Resistin is produced by macrophages and visceral adipocytes. Resistin is elevated in obesity and regulates insulin sensitivity in skeletal muscle and liver, and it is positively associated with insulin resistance and glucose tolerance in both human and animal models [39]. Resistin might also play a role in bone remodeling, increasing osteoblast proliferation, cytokine release, and osteoclast differentiation [40] (Table 12.1).
TNF-α is a pro-inflammatory cytokine which plays important regulatory effects on lipid metabolism, adipocyte function, insulin signaling, and bone remodeling [41]. Its expression correlates with percent body fat, insulin resistance, and osteoclast activity in humans [42, 43]. Osteoclasts are cells tasked with resorbing bone and the identification of three different molecules: the receptor activator of NF-kB ligand (RANKL), an osteoclastogenic cytokine, its receptor (RANK), and its inhibitor osteoprotegerin (OPG) built the bases of the modern bone biology [44]. RANKL is the key osteoclastogenic cytokine effector, inducing osteoclast formation and promoting osteoclast resorptive activity [45]. TNF-α promotes RANKL production by BMSCs and mature osteoblasts, reduces OPG production, and upregulates the receptor RANK on osteoclast precursors, increasing their sensitivity to prevailing RANKL concentrations [46]. Additionally, TNF-α turns out to have another property that is relatively unique among the inflammatory cytokines; it has potent effects on osteoclastogenesis as it not only promotes RANKL production but synergizes with RANKL to amplify osteoclastogenesis and to intensify osteoclastic resorption by directly modulating RANKL-induced signal transduction pathways [47].
IL-6 is a cytokine which has a wide range of actions; it is secreted by several cell types, including fibroblast, endothelial cells, and adipocytes; and its plasma levels are significantly upregulated in human obesity and insulin resistance [48]. As TNF-α also IL-6 is a well-recognized stimulator of osteoclastogenesis and bone resorption. Several data show that IL-6 mRNA is expressed in preosteoblasts and osteoblasts [49] and that it stimulates osteoblast proliferation and differentiation by controlling the production of local factor [50, 51].
Mature bone cells secrete factors that modulate insulin sensitivity and glucose metabolism, such as OCN, by which the skeleton could function as an endocrine organ itself [50, 52]. OCN is an osteoblast-specific protein and a major non-collagenous protein in the extracellular matrix. Karsenty and colleagues demonstrated that uncarboxylated OCN, acting as a pro-hormone, can increase β-cell proliferation, insulin secretion, insulin sensitivity, and adiponectin expression [53]. Thus, osteoblasts might be able to regulate glucose metabolism by modulating the bioactivity of OCN. In addition, more recent studies showed that OCN bioactivity is modulated by enhanced sympathetic tone driven by leptin, which has been shown to suppress insulin secretion by β-cells [54], and three recent studies have demonstrated an inverse correlation between serum OCN and plasma glucose levels, supporting a role for this pathway in humans [55]. Thus, a novel picture has emerged linking glucose metabolism, adipose stores, and skeletal activity.
OPN is an active player in many physiological and pathological processes, including biomineralization, tissue remodeling, and inflammation. Modulation of immune cell response by OPN has been associated with various inflammatory diseases and might play a pivotal role in the development of adipose tissue inflammation, insulin resistance, and diabetes [56]. OPN expression is drastically upregulated by 40- and 80-fold in adipose tissue from diet-induced and genetically obese mice, respectively [57], and it has been demonstrated that OPN expression in adipose tissue and circulating OPN levels were substantially elevated in obese, diabetic, and insulin-resistant patients compared with lean subjects and conversely that dietary weight loss significantly decreased OPN concentrations [58, 59].
Emerging evidence points to a critical role for the skeleton in several homeostatic processes including energy balance and adipose metabolism, and the connection between fuel utilization and skeletal remodeling seems to begin in the bone marrow with lineage allocation of MSCs into adipocytes or osteoblasts. Adipocytes and osteoblasts, in fact, originate from a common progenitor, a pluripotent mesenchymal stem cell [60], which has an equal propensity for differentiation into adipocytes or osteoblasts or other lines, such as chondrocytes, fibroblast, and endothelial cells, under the influence of several cell-derived transcription factors. This process is complex, suggesting significant plasticity and multifaceted mechanism(s) of regulation within different cell lineages, among which are adipocytes and osteoblasts [22, 61].
Transdifferentiation is the switching of differentiated cells that sometimes occurs during disease [62], and it interests partially differentiated cells (e.g., pre-osteoblasts) that switch to another lineage (e.g., adipocytes) [63]. Fat bone marrow is indicative of aging, and it is frequently observed in the presence of osteoporosis [64]. One possible cause of bone marrow fat deposition is the aberrant commitment of BMMSCs into adipocytes because of their inability to differentiate into other cell lineages, such as osteoblasts. There exists an inverse relationship between bone marrow fat production and bone formation during osteoporosis; in fact an inhibited adipogenesis in subjects with a high bone mass has been observed [65]. Recently, a correlation between the osteo-adipogenic transdifferentiation of bone marrow cells and numerous bone metabolism diseases has been established. Human BMMSC-derived osteoblasts, adipocytes, and chondrocytes had the potential to transdifferentiate to each lineage, and these findings provided new insights on the pathogenesis of skeletal diseases such as osteoporosis in both sexes [66].
Estrogens can regulate several molecular signals within bone metabolism and play a pivotal role in the development of bone marrow fat [67,68,69]. Recent studies have shown that estrogens suppress osteo-adipogenic transdifferentiation via canonical Wnt signaling, which regulates bone development, adipogenic differentiation, and gene expression in the whole process of bone metabolism [65, 70]. Specifically, canonical Wnt/beta-catenin signaling is highly expressed in mesenchymal precursor cells and pluripotent cells, especially toward the osteoblast lineage, while inhibiting adipogenic differentiation [71]. Canonical Wnt signaling stabilizes and promotes cellular and nuclear beta-catenin levels, which inhibit adipogenesis [72], and the suppression of Wnt signaling is crucial for PPAR gamma induction and preadipocyte differentiation [73].
PPARγ plays a central role in initiating adipogenesis, and mutations of the PPARγ gene are associated with an altered balance between bone and fat formation in the bone marrow [61]. PPARγ insufficiency led to increased osteoblastogenesis in vitro and higher trabecular bone volume in vivo, confirming the key role of mesenchymal stem cell lineage allocation in the skeleton [60]. Interestingly, aged mice exhibit fat infiltration into the bone marrow, and enhanced expression of PPARγ-2, along with reduced mRNA expression of bone differentiation factors [73], and mice with premature aging (the SAM-P/6 model) show nearly identical patterns of adipocyte infiltration, with impaired osteoblastogenesis [74], indicating that aging, or events that accelerate aging, results in significant bone marrow adiposity and in defect in osteoblastogenesis in mice [75].
Estrogens and androgens can both modulate several molecular signals within bone metabolism and play a role in the development of bone marrow fat. Moreover, BMMSCs express androgen receptor (AR), and a recent study shows that androgens, independently of their aromatization, are able to prevent rosiglitazone-induced adipogenesis in human mesenchymal stem cells [76].
3 Obesity, Androgen Deficiency, and Bone Metabolism
Estrogens and androgens modulate bone remodeling by regulating the activity of the abovementioned molecules, thus protecting against bone loss by regulating the activity of genes responsible for osteoclastogenesis and mesenchymal cell replication, exerting pro-apoptotic effects on osteoclasts and anti-apoptotic effects on osteoblasts and osteocytes. Conversely, hypogonadism leads to increased bone resorption, both in men and women [77].
Testosterone deficiency syndrome is becoming recognized as an increasingly frequent problem in the aging male population [78], and low serum testosterone is more common in men with type 2 diabetes mellitus, metabolic syndrome, cardiovascular disease, and obesity than in the general population [79,80,81]. Interestingly, it is known that obesity in men is associated with low testosterone and reduced sex hormone-binding globulin (SHBG) levels. An increased BMI is associated with a low measured, or calculated, free and bioavailable testosterone. Specific pathogenetic mechanisms involved in this phenomenon are complex and not completely understood, but evidence indicates that testosterone deficiency induces increased adiposity, while increased adiposity induces hypogonadism [82].
The prevalence of secondary hypogonadism in adult male subjects affected by type 2 diabetes has been estimated to be 29% (range 25–40%), with a higher prevalence of 50% when obesity and type 2 diabetes coexist. Indeed, several studies indicate that men who are obese at baseline and at follow-up, either if fat tissue excess is measured by BMI or by central obesity prevalence (waist/hip ratio or waist circumference), exhibit a greater decline of total and free testosterone compared to men who were never classified as obese [83], mainly due to higher amounts of visceral fat [84]. Visceral adiposity is associated with elevated concentrations of insulin, C-peptide, and glucose intolerance, which are negatively correlated to total and free testosterone levels [85, 86]. The link between obesity and (decreased) SHBG is mainly explained by the effects of obesity-induced insulin resistance, resulting in higher insulin levels that subsequently suppress hepatic production of SHBG that would then result in reduced delivery of testosterone to the peripheral tissues and increased availability of free testosterone as a substrate for aromatase to convert into estradiol [87, 88].
Male obesity is associated with increased aromatase activity within adipocytes [89], and estradiol in turn exerts a negative feedback effect on LH secretion from the pituitary [90]. This may worsen obesity and promote increased fat mass that represents a vicious circle perpetuating the hypogonadal state, thus resulting in a reduction in muscle mass and an increase in the volume of visceral fat [91]. Another mechanism that mediates obesity-related effects on the male hypothalamic-pituitary-testicular axis is mediated by increased plasma leptin levels that exert a direct negative action on LH-/hCG-stimulated testicular androgen production and decrease Leydig cell responsiveness to gonadotropin stimulation [92]. Finally, inflammatory mediators, such as C-reactive protein, have been demonstrated to contribute to the suppression of the hypothalamic-pituitary-testicular axis function and to the development of male secondary hypogonadism [93].
Emerging data suggest that bone mass, energy metabolism, and reproductive function might be coordinately regulated. The main mediator of this axis is undercarboxylated osteocalcin (uOCN), a bone-derived hormone, which has recognized effects as the improvement of insulin secretion from the pancreas; the amelioration of systemic insulin sensitivity, in particular in skeletal muscle; and the stimulation of the global endocrine activity of the Leydig cell, including vitamin D 25-hydroxylation and testosterone production [94]. A rising interest toward the non-classical effects of 25-hydroxycholecalciferol 25(OH)D (vitamin D) exists, based on the presence of its receptors in tissues other than the bone, gut, and kidneys [95]. Several studies have suggested the involvement of vitamin D in the pathogenesis of CVD, cancer, and metabolic syndrome [96,97,98]. The association of low vitamin D levels and metabolic syndrome is more pronounced in overweight and obese than in normal-weight individuals [99]. A recent study confirmed the lowest vitamin D concentrations and the highest prevalence of vitamin D deficiency in type 2 diabetes patients with hypogonadism, particularly in those with secondary hypogonadism [100]. Several mechanisms have been proposed to explain the role of vitamin D in the pathogenesis of insulin resistance, and adiponectin has been proposed as a major player with its strong association with impaired glucose tolerance, independent from adiposity [101]. Adiponectin and glucose homeostasis are both correlated to OCN levels, an osteoblast hormone linked to vitamin D metabolism, as mentioned above [94, 102]. Interestingly, animal studies suggest that bone might be a positive regulator of male fertility and that this action might be mediated through OCN, via binding to a specific receptor present on Leydig cells that favors testosterone biosynthesis. OCN-deficient mice show a decrease in testicular, epididymal, and seminal vesicle weights and sperm count, and Leydig cell maturation appears to be halted in the absence of OCN [103]. Androgens favor periosteal bone formation in men and maintain trabecular bone mass and integrity by inhibiting IL-6 production [104]. Also, androgens stimulate the proliferation of osteoblast progenitors and the differentiation of mature osteoblasts by decreasing osteoclast formation and bone resorption, via increased production of OPG by osteoblasts [77]. The net result of these functions leads to an accrual in bone formation [105]. Finally, our group has recently demonstrated an association between visceral fat mass, altered insulin sensitivity, OCN, and testosterone levels in aging obese male subjects that are significantly correlated with skeletal health [106]. In this view, OCN might be considered a new important marker of metabolic and gonadic function in obese men, other than the well-established function as a marker of bone remodeling.
4 Conclusions
Body fat and bone interplay through several adipokines and bone-derived molecules, such as OCN, which modulate bone remodeling, adipogenesis, body weight control, and glucose homeostasis. Thus, the existence of a cross talk between fat and bone tissue suggests a homoeostatic feedback system in which adipokines and bone-derived molecules form part of an active bone-adipose axis.
In conditions such as aging, hypogonadism, obesity, or metabolic alterations, an osteo-adipogenic transdifferentiation and an aberrant commitment of BMMSCs into adipocytes might occur. In particular, since BMMSCs express androgen receptor, androgens can modulate several molecular signals within bone metabolism and might play a role in the development of bone marrow fat, which might explain several mechanisms linking obesity to an increase of male skeletal alterations as compared to subjects with normal body weight.
Finally, obesity is associated with gonadal dysfunction, leading to androgen deficiency. Since androgens promote bone formation, and bone tissue might be a positive regulator of male fertility, through OCN, and since an association between visceral fat mass, insulin sensitivity, OCN, and testosterone levels in obese men has been observed, OCN might be considered a new important marker of metabolic and gonadic functions in adult obese men, other than the well-established function as a marker of bone remodeling.
References
Kado DM, Huang MH, Karlamangla AS, Barrett-Connor E, Greendale GA (2004) Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc 52:1662–1667
Rossner S (2002) Obesity: the disease of the twenty-first century. Int J Obes Relat Metab Disord 26(Suppl 4):S2–S4
Hu FB (2003) Overweight and obesity in women: health risks and consequences. J Womens Health (Larchmt) 12(2):163–172
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Tech Rep Ser. 2000;894:1–253.
Greco EA, Fornari R, Rossi F, Santiemma V, Prossomariti G, Annoscia C, Aversa A, Brama M, Marini M, Donini LM, Spera G, Lenzi A, Lubrano C, Migliaccio S (2010) Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract 64(6):817–820
Kim KC, Shin DH, Lee SY, Im JA, Lee DC (2010) Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J 51(6):857–863
Greco EA, Francomano D, Fornari R, Marocco C, Lubrano C, Papa V, Wannenes F, Di Luigi L, Donini LM, Lenzi A, Aversa A, Migliaccio S (2013) Negative association between trunk fat, insulin resistance and skeleton in obese women. World J Diabetes 4(2):31–39
Compston JE, FlahiveJ HDV, Watts NB, Siris ES, Silverman S, Saag KG, Roux C, Rossini M, Pfeilschiffer J, Nieves JW, Netelenbos JC, March L, AZ LC, Hooven FH, Greenspan SL, Gehlbach SH, Diez-Perez A, Cooper C, Chapurlat RD, Boonen S, Anderson FA Jr, Adami S, Adachi JD, GLOW Investigators (2014) Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J Bone Miner Res 29(2):487–493
Walsh JS, Vilaca T (2017) Obesity, Type 2 diabetes and bone in adults. Calcif Tissue Int 100(5):528–535
Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM, Hirani V (2017 Mar) Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: The Concord Health and Ageing in Men Project. J Bone Miner Res 32(3):575–583
Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92:73–78
Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, Simon I, Soler J, Richart C (2004) Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes Res 12:962–971
Magni P, Dozio E, Galliera E, Ruscica M, Corsi MM (2010) Molecular aspects of adipokine-bone interactions. Curr Mol Med 10(6):522–532
Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL (1988) Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 241(4861):84–86
Kim HJ (2010) New understanding of glucocorticoid action in bone cells. BMB Rep 43(8):524–529
Komm BS, Terpening CM, Benz DJ, Graeme KA, Gallegos A, Korc M, Greene GL, O’Malley BW, Haussler MR (1988) Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science 241(4861):81–84
Migliaccio S, Davis VL, Gibson MK, Gray TK, Korach KS (1992) Estrogens modulate the responsiveness of osteoblast-like cells (ROS 17/2.8) stably transfected with estrogen receptor. Endocrinology 130(5):2617–2624
Gomez-Ambrosi J, Rodrıguez A, Catalan V, Fruhbeck G (2008) The bone-adipose axis in obesity and weight loss. Obes Surg 18:1134–1143
Takeda S (2008) Effect of obesity on bone metabolism. Clin Calcium 18:632–637
Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME (2006) Playing with bone and fat. J Cell Biochem 98:251–266
Fukumoto S, Martrin TJ (2009) Bone as an endocrine organ. Trends Endocrinol Metab 20(5):230–236
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H (2004) PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855
Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC (1996) Peroxisome proliferator activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol 50:1087–1094
Rodriguez JP, Montecinos L, Rios S, Reyes P, Martinez J (2000) Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem 79:557–565
Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella-Carretero JI (2017) Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 23(4):390–408
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF (1996) Serum immunoreactive leptin concentrations in normal-weight and obese humans. N Engl J Med 34:292–295
Martin SS, Qasim A, Reilly MP (2008) Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol 52:1201–1210
Kontogianni MD, Dafni UG, Routsias JG, Skopouli FN (2004) Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res 19:546–555
Goulding A, Taylor RW (1998) Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 63:456–458
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 100:197–207
Thomas T (2004) The complex effects of leptin on bone metabolism through multiple pathways. Curr Opin Pharmacol 4:295–300
Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638
Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H (2002) Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109:915–921
Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hokfelt T, Herzog H (2002) Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA. 99:8938–8943
Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE (2003) Structure-function studies of the adipocytes-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278:9073–9085
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudio K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with body lipoatrophy and obesity. Nat Med 7:941–946
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R (2002) Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 290:1084–1089
Jurimae J, Rembel K, Jurimae T, Rehand M (2005) Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res 37:297–302
Ukkola O (2002) Resistin—a mediator of obesity-associated insulin resistance or an innocent bystander? Eur J Endocrinol 147:571–574
Thommesen L, Stunes AK, Monjo M, Grosvik K, Tamburstuen MV, Kjobli E, Lyngstadaas SP, Reseland JE, Syversen U (2006) Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem 99(3):824–834
Fasshauer M, Klein J, Krahlisch S, Lossner U, Klier M, Bluher M, Paschke R (2003) GH is a positive regulator of tumor necrosis factor-alpha-induced adipose related protein in 3T3-L1 adipocytes. J Endocrinol 178:523–531
Hotamisligil G, Arner P, Caro J, Atkinson R, Spiegelman B (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409–2415
Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV (1991) Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A 88(12):5134–5138
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennet L, Boone T, Shimamoto G, DeRose M, Elliot R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone Density. Cell 89(2):309–319
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390(6656):175–179
Wei S, Kitaura H, Zhou P, Ross P, Teitelbaum SL (2005 Feb) IL-1 mediates TNF-induce osteoclastogenesis. J Clin Invest 115(2):282–290
Cenci S, Weitzmann MN, Roggia C, Namba N, NovackD WJ, Pacifici R (2000) Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-𝛼. J Clin Invest 106(10):1229–1237
Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE (2001) Circulating IL-6 in relation to adiposity, insulin action and insulin secretion. Obes Res 9:414–417
Dodds A, Merry K, Littlewood A, Gowen M (1994) Expression of mRNA for IL1 beta, IL6 and TGF beta 1 in developing human bone and cartilage. J Histochem Cytochem 42:733–744
Taguchi Y, Yamamoto M, Yamate T, Lin SC, Mocharla H, DeTogni P, Nakayama N, Boyce BF, Abe E, Manolagas SC (1998) Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc Assoc Am Physicians 110:559–574
Sims NA, Jenkins BJ, Quinn JM, Nakamura A, Glatt M, Gillespie MT, Ernst M, Martin TJ (2004) Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest 113:379–389
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A 105:5266–5270
Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG Jr, Chua SC Jr, Kim JK, Kaestner KH, Karsenty G (2008) The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol 183:1235–1242
Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ (2006) The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 4:291–302
Scatena M, Liaw L, Giachelli CM (2007) Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 27:2302e2309
Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, Aszmann O, Ludvik B, Silberhumer GR, Prager G, Stulnig TM (2008) Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 149:1350e1357
Sarac F, Basoglu OK, Gunduz C, Bayrak H, Biray Avci C, Akcicek F (2011) Association of osteopontin and tumor necrosis factor-alpha levels with insulin resistance in obese patients with obstructive sleep apnea syndrome. J Clin Invest 34:528–533
You JS, Ji HI, Chang KJ, Yoo MC, Yang HI, Jeong IK, Kim KS (2013) Serum osteopontin concentration is decreased by exercise-induced fat loss but is not correlated with body fat percentage in obese humans. Mol Med Rep 8:579–584
Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ (2004) Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res 19:256–264
Martin RB, Zissimos SL (1991) Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 12:123–131
Burke ZD, Tosh D (2005) Therapeutic potential of transdifferentiated cells. Clin Sci (Lond) 108:309–321, 30
Schilling T, Kuffner R, Klein-Hitpass L, Zimmer R, Jakob F, Schutze N (2008) Microarray analyses of transdifferentiated mesenchymal stem cells. J Cell Biochem 103:413–433
Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT (2010) Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res 25:757–768
Gao B, Huang Q, Lin Y-S, Wei B-Y, Guo Y-S, Sun Z, Wang L, Fan J, Zhang H-Y, Han Y-H, Li X-J, Shi J, Liu J, Yang L, Luo Z-J (2014) Dose-dependent effect of estrogen suppresses the osteo-adipogenic transdifferentiation of osteoblasts via canonical Wnt signaling pathway. PLoS One 9(6):e-99137
Song L, Tuan RS (2004) Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 18:980–982
Abdallah BM, Ditzel N, Mahmood A, Isa A, Traustadottir GA, Schilling AF, Ruiz-Hidalgo MJ, Laborda J, Amling M, Kassem M (2011) DLK1 is a novel regulator of bone mass that mediates estrogen deficiency induced bone loss in mice. J Bone Miner Res 26:1457–1471
Kamiya Y, Chen J, Xu M, Utreja A, Choi T, Drissi H, Wadhwa S (2013) Increased mandibular condylar growth in mice with estrogen receptor beta deficiency. J Bone Miner Res 28:1127–1134
Song L, Zhao J, Zhang X, Li H, Zhou Y (2013) Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor mediated ERK and JNK signal activation. Eur J Pharmacol 714:15–22
Colaianni G, Brunetti G, Faienza MF, Colucci S, Grano M (2014) Osteoporosis and obesity: Role of Wnt pathway in human and murine models. World J Orthop 5(3):242–246
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209
Qiu W, Chen L, Kassem M (2011) Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochem Biophys Res Commun 413:98–104
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3:379–389
Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC, Lipschitz DA (1997) Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6: a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res 12:1772–1779
Duque G, Macoritto M, Kremer R (2004) Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity. Biogerontology 5:421–429
Benvenuti S, Cellai I, Luciani P, Deledda C, Saccardi R, Mazzanti B, Dal Pozzo S, Serio M, Peri A (2012) Androgens and estrogens prevent rosiglitazone-induced adipogenesis in human mesenchymal stem cells. J Endocrinol Invest 35(4):365–371
Manolagas SC, Kousteni S, Jilka RL (2002) Sex steroids and bone. Recent Prog Horm Res 57:385–409
Wu FW, Tajar A, Beynon JM, et al.; for the EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–136
Dhindsa S, Prabhakar S, Sethi M et al (2004) Frequent occurrence of hypogonadotrophic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 89:5462–5468
Kapoor D, Aldred H, Clark S et al (2007) Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes. Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30:911–917
Gray A, Feldman HA, Mckinlay JB et al (1991) Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 73:1016–1025
Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, Facchiano E, Sforza A, Forti G, Mannucci E, Maggi M (2013) Body weightlossrevertsobesity-associatedhypogonadotropichypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 168(6):829–843
Derby CA, Zilber S, Brambilla D et al (2006) Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 65:125–131
Svartberg J, Von Muhlen D, Sundsfjord J et al (2004) Waist circumference and testosterone levels in community dwelling men. Tromsø study. Eur J Epidemiol 19:657–663
Seidell JC, Bjorntorp P, Sjostrom L (1990) Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 39:897–901
Pasquali R, Casimirri F, Cantobelli S et al (1991) Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 40:101–104
Hautanen A (2000) Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord 24(2):S64–S70
Kalme T, Koistinen H, Loukovaara M et al (2003) Comparative studies on the regulation of insulin-like growth factor-binding protein-1 (IGFBP-1) and sex hormone-binding globulin (SHBG) production by insulin and insulin like growth factors in human hepatoma cells. J Steroid Biochem Mol Biol 86:197–200
Dandona P, Dhindsa S (2011) Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab 96:2643–2651
Pitteloud N, Dwyer AA, DeCruz S et al (2008) The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrin Metab 93:2686–2692
Caprio M, Fabbrini E, Isidori AM et al (2001) Leptin in reproduction. Trends Endocrinol Metab 12:65–72
Isidori AM, Caprio M, Strollo F et al (1999) Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 84:3673–3680
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25:4–7
De Toni L, Di Nisio A, Rocca MS, De Rocco PM, Ferlin A, Foresta C (2017) Osteocalcin, a bone-derived hormone with important andrological implications. Andrology 5(4):664–670. https://doi.org/10.1111/andr.12359
Bikle D (2009) Nonclassic actions of vitamin D (review). J Clin Endocrinol Metab 94:26–34
Wang TJ, Pencina MJ, Booth SL et al (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511
Lappe JM, Travers-Gustafson D, Davies KM et al (2007) Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85:1586–1591
Martini LA, Wood RJ (2006) Vitamin D status and the metabolic syndrome. Nutr Rev 64:479–486
Lu L, Yu Z, Pan A et al (2009) Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 32:1278–1283
Bellastella G, Maiorino MI, Olita L et al (2014) Vitamin D deficiency in Type 2 diabetic patients with hypogonadism. J Sex Med 11:536–542
Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W et al (2009) The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine 36:205–210
Lee NK, Sowa H, Hinoi E et al (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Oury F, Sumara G, Sumara O et al (2001) Endocrine regulation of male fertility by the skeleton. Cell 144(5):796–809
Jilka RL, Hangoc G, Girasole G et al (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91
Michael H, Harkonen PL, Vaananen HK et al (2005) Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res 20:2224–2232
Migliaccio S, Francomano D, Bruzziches R et al (2013) Trunk fat negatively influences skeletal and testicular functions in obese men: clinical implications for the aging male. Int J Endocrinol 2013:182753. https://doi.org/10.1155/2013/182753
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Greco, E.A., Mocini, E., Marocco, C., Lenzi, A., Migliaccio, S. (2020). Obesity and Male Osteoporosis: Protective Factor?. In: Ferlin, A., Migliaccio, S. (eds) Male Osteoporosis. Trends in Andrology and Sexual Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-96376-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-96376-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96375-4
Online ISBN: 978-3-319-96376-1
eBook Packages: MedicineMedicine (R0)