Abstract
Component separation techniques to address large ventral hernias have dramatically evolved during the past decade. The touted benefits of a posterior component separation with transversus abdominis release (TAR), with its broad adaptability and favorable wound morbidity/recurrence profile, have led to its wide adoption for large, complex, and multiply-recurrent repairs. Concurrent development of the robotic platform and surgeon ingenuity have more recently culminated in the evolution of a minimally invasive TAR technique made practical and adaptable by robotic technology (rTAR). Here, we provide a detailed explanation of the benefits this technique offers in the context of alternative repairs described during the past 30 years. Though the operation is actively evolving, we will also provide a detailed depiction of our approach. While early retrospective reviews tout a shortened length of hospital stay compared to modern open and laparoscopic counterparts in order to justify the increased cost of the robotic platform, improved patient outcomes unique to the approach may ultimately provide enough value to substantiate the vitality of the operative approach.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Historical Context: The Evolution of Component Separation Techniques
For large ventral hernias, primary fascial closure and recreation of the linea alba can be difficult to achieve without undue tension. Component separation techniques involve strategic division of fascial and muscular layers of the abdominal wall that relieve such tension and thereby allow for an increased abdominal domain. In the 1980s, Jean Rives and René Stoppa described division of the posterior rectus sheath in their series of large incisional hernias. This retrorectus dissection provides both medial fascial advancement and allows for placement of a prosthetic reinforcement in the retrorectus space [1]. However, when bilateral release of the posterior rectus sheathes is insufficient to gain adequate medial advancement, further myofascial release is necessary. In 1990, Oscar Ramirez described division of the external oblique fascia from its insertion on the internal oblique aponeurosis in a cadaver study, coining the term “component separation.” Importantly, he first quantified the medial advancement gained by a bilateral posterior rectus sheath release (Rives-Stoppa technique) as 6, 10, and 6 cm in the upper, middle, and lower thirds of the abdominal wall, respectively. Adjunctive bilateral division of the external oblique myofascial layer allowed for additional advancement, crudely measured to be 10, 20, and 6 cm [2]. This approach would become one of the most common ways to achieve sufficient facial medialization for large ventral incisional hernias, and today some still consider the term “component separation” to specifically regard division of the external oblique myofascial layer.
While Ramirez’s technique grew in popularity, limitations were noted. Access to the external oblique aponeuroses’ insertion on the internal oblique typically requires significant undermining of skin and subcutaneous tissue anterior to the rectus fascia. These soft tissue flaps, reliant on blood supply from anterior perforators of the epigastric vessels, can be at risk of devascularization and subsequent wound morbidity has been reported from 26 to 63% [3, 4]. Such wound morbidity could prove to be more significant if a prosthetic enforcement is placed in the onlay position—anterior to the fascia and just beneath the soft tissue flaps—leaving the prosthetic directly exposed to and involved with any superficial surgical site morbidity. In order to minimize soft tissue mobilization and devascularization, modifications to Ramirez’s external oblique release were developed. The periumbilical “perforator sparing” technique preserves some of the anterior epigastric perforating vessels to the skin flaps. Saulis and colleagues retrospectively reported a dramatic reduction in wound morbidity (2%) when compared to the traditional technique (20%) at their institution [5]. Completely obviating the need for soft tissue flaps, Lowe et al. described division of the external oblique muscle through either a paramedian incision or an intramuscular tunnel in the avascular plane between the external and internal oblique muscles utilizing a balloon dissection and laparoscopic equipment [6]. A recent meta-analysis of 3055 patients confirmed a decrease in wound morbidity from 35 to 21% utilizing the endoscopic approach when compared to the traditional open technique [7].
Still, limitations to external oblique component separation and variations persist. There are scenarios when periumbilical perforator sparing techniques may not be possible: (1) large ventral hernias with loss of domain where the skin and soft tissue may tether fascial medialization or (2) previous mesh onlay. Large recurrences after a previous external oblique component separation also proved to be another challenging group of patients. Most notably, regardless of the specific approach, no external oblique division technique has an ideal space for prosthetic reinforcement. As previously mentioned, onlay prosthetics are susceptible to superficial wound morbidity. Perforating sparing techniques are a catch-22 in that they limit the space in which to place the prosthetic while a larger subcutaneous pocket for wider overlap paradoxically potentiates superficial soft tissues devascularization. A mesh underlay leaves the abdominal viscera exposed to a prosthetic akin to laparoscopic repairs. Despite the use of coated or barrier meshes, long-term sequelae of intraperitoneal mesh include longer re-operative times, secondary mesh infection, and increased incidence of an unplanned bowel resection or enterotomy in the 25% of these patients who will require a future abdominal operation [8, 9]. The Rives-Stoppa retrorectus space is limited laterally by the linea semilunaris above the arcuate line. Finally, the absence of an ideal space for wide prosthetic overlap is most vexing when managing subxyphoid, suprapubic, and non-midline defects adjacent to boney prominences. These limitations of external oblique release inspired the conception of other component separation techniques that have gained wide popularity in the last decade.
In 2008, Carbonell et al. described a novel progression to the Rives-Stoppa retrorectus dissection that allows for wide prosthetic overlap lateral to the semilunar line [10]. After release of the medial posterior rectus sheath and lateral retrorectus dissection, the lateral posterior rectus sheath—consisting solely of fibers from the posterior lamina of the internal oblique—can be divided to expose the underlying transversus abdominis muscle. This allows the plane between the internal oblique and transversus abdominis muscles to be accessed and matured laterally. A subtle but critical anatomical point that allows for this dissection is that the transversus abdominis muscle and its associated aponeurosis inserts onto the posterior rectus sheath more medially than indicated by some anatomical texts. Completely detaching the posterior rectus sheath medially and laterally was termed a “posterior component separation ” (PCS), and Ramirez’s external oblique release somewhat retroactively became known as an “anterior component separation ” (ACS). While Carbonell’s PCS and intramuscular dissection addressed the issue of providing a space for wide lateral prosthetic reinforcement by laterally extending the Rives-Stoppa retromuscular plane, limitations persist. As opposed to an ACS, PCS does not divide any of the lateral abdominal wall muscles opposing medial tension. Also, laterally perforating neurovascular bundles traveling in the intramuscular plane between the internal oblique and transversus abdominis muscles are sacrificed during this lateral dissection. While the clinical significance of subsequent rectus muscle denervation is unknown, division of these nerves and vessels seems to counter one of the theoretical aims of recreating the linea alba—improving core abdominal function by restoring the rectus muscles to the midline and giving lateral abdominal muscles a stable insertion point.
Subsequently in 2009, Novitsky reported a distinct adjunct to the Rives-Stoppa retrorectus dissection , now known as a posterior component separation with transversus abdominis muscle release (TAR). In this technique, the posterior rectus sheath is again divided medially and the retrorectus space is matured laterally in a Rives-Stoppa fashion. At the lateral extent of the retrorectus dissection , just medial to laterally perforating neurovascular bundles, the posterior lamina of the internal oblique is divided to expose the underlying transversus abdominis muscle. This step is similar to the Carbonell’s PCS, with the conscious effort to preserve lateral neurovascular bundles by dividing the posterior rectus sheath medial to these perforators that pierce the posterior lamina of the internal oblique to enter the retrorectus space. Once the transversus abdominis muscle is exposed, it can be separated from the underlying peritoneum and divided to access the retromuscular space between the transversus abdominis muscle and peritoneum. Maturing the retromuscular plane can be done laterally all the way to the psoas muscle. This retromuscular dissection serves two critical purposes. One, it creates a large peritoneal sac contiguous with the posterior rectus sheath that can be used to completely isolate the viscera and allow for “giant prosthetic reinforcement of the visceral sac” originally utilized by Rives and Stoppa in the descriptions of large inguinoscrotal hernia repairs [11]. Specifically, a TAR allows for wide prosthetic reinforcement of the visceral sac above the arcuate line. The second reason to develop this plane is that in our own cadaver studies, retromuscular dissection was the critical step that allowed for anterior facial medialization (akin to Ramirez’s ACS cadaver study) to allow for repair of large (~20 cm) defects [12]. The retromuscular plane also can be matured superiorly to the preperitoneal space beneath the xyphoid and cephalad to the central tendon of the diaphragm. Inferiorly, below the arcuate line, the preperitoneal plane is matured below the pubis into the space of Retzius to expose the Cooper’s ligaments bilaterally. Given the wide retromuscular plane of dissection, subxyphoid, suprapubic, and off-midline hernias can also be addressed. To review, TAR allows for numerous advantages in regard to large ventral incisional hernia repair:
-
Myofascial release—Division and separation of the transversus abdominus muscle allowing for considerable rectus coplex medialization without the need for any soft tissue flaps and the associated wound morbidity encountered during ACS.
-
Division of a muscle—transversus abdominis—whose vector of force directly opposes fascial medialization.
-
Can be utilized when a previous ACS has been done [13].
-
-
A lateral extension of the Rives-Stoppa retrorectus dissection that creates a cephalad extension of the visceral sac above the arcuate line for giant prosthetic reinforcement.
-
Further allows for management of off-midline, subxyphoid, and suprapubic herniations adjacent to boney prominences.
-
The wider retromuscular space allows prosthetic placement in a plane with bilaminar fascial coverage to potentiate ingrowth, while also providing an environment isolated from the viscera and superficial wound morbidity.
-
Knowledge of favorable mesh characteristics in regard to preventing chronic mesh infection when placed in a contaminated scenarios (wound class II–III), coupled with a favorable space for prosthetic placement makes repairs in contaminated fields less of a surgical faux pas [14, 15].
-
-
Preservation of laterally perforating neurovascular bundles that supply the rectus muscles. To support the importance of preserving this innervation, we have demonstrated that restoration of the linea alba improves rectus abdominis function after TAR [16].
-
Restoration of the midline via TAR also allows for reversal of atrophy and compensatory hypertrophy of the external and especially synergistic internal oblique muscles demonstrated on CT imaging [17].
-
As major proponents of this technique, we also understand the importance of introspection and critical review. Some skeptics highlight the importance of the transversus abdominis muscle as an internal girdle whose circumferential tension stabilizes the lumbosacrum. Potential associations between transversus abdominis dysfunction and low back pain as well as spinal instability are theoretical causes for concern given complete transection during a TAR [18]. To date, no such deleterious effects have been reported, and subsequent reversal of atrophy of the external and internal oblique muscles may provide a mechanism of compensation.
Complimentary Limitations of Modern Techniques Inspire Ingenuity
While no technique is ideal for all scenarios, the TAR appears to be an incredibly useful operation for the armamentarium of the general surgeon, as attributed by its growing popularity during the past decade. Still, our largest series of 428 TARs repaired with synthetic mesh generated a wound morbidity rate of 18.7%, including a 9.1% rate of surgical site infection. The large operations generated a median hospital stay of 6 days, with associated morbidity including a 6.8% rate of urinary tract infections and 6.3% rate of venous thromboembolic events [19]. So while the TAR operation is versatile and effective—offering a recurrence rate of 3.7%—it relegates the patient to the consequences of a large laparotomy. Adaptation of a less invasive approach, offering the same benefits of open repair, would seem to be the next logical step.
Meanwhile, undergoing its own evolution in parallel since 1993, laparoscopic ventral hernia repair (LVHR) has been adopted by general surgeons to address 20–27% of ventral hernias [20, 21]. However, unlike open retromuscular repairs, these techniques have traditionally culminated in the placement of an intraperitoneal prosthetic directly exposed to the underlying viscera at the expense of the aforementioned sequelae. Defects bridged by a prosthetic in the absence of fascial approximation leave a dead space for seroma formation, fail to recreate the anatomy of a functional abdominal wall, and are subject to mesh eventration or “pseudo-recurrence” [22, 23]. Conversely when primary fascial closure precedes intraperitoneal onlay mesh, it is done so in the absence of any fascial release to mitigate tension. Finally, despite demonstrating improvements in length of hospital stay, time to recovery, wound morbidity, and recurrence, LVHR is notoriously painful, suppressing some of the benefits anticipated with a less invasive approach [24,25,26,27].
Given the outlined benefits of an open TAR technique for large ventral hernias at the expense of a large laparotomy, and the inverse technical sacrifices made during LVHR to reap the benefits of a minimally invasive approach, one can conceptually appreciate everything a minimally invasive TAR would accomplish. Conveniently, as advanced minimally invasive techniques to address ventral hernias were being conceptualized, so too was robotic technology. The da Vinci robot (Intuitive Surgical, Sunnyvale, CA, USA) touts several advantages over traditional laparoscopy including six degrees of motion, three-dimensional images, superior ergonomics, and tremor-less precision during intracorporeal suturing [28]. Approved by the Food and Drug Administration in 2000, it was first used for ventral hernia repair in 2002 by Ballantyne [29]. The robot was initially utilized to mimic traditional laparoscopic repairs with intraperitoneal mesh placement or preperitoneal mesh placement in the absence of any myofascial release [30, 31]. Not until 2012 did Abdallah et al. describe a robotic retrorectus dissection akin to a Rives-Stoppa technique in series of small herniations associated with rectus diastasis [32]. While a review article in 2015 and two recently published hernia textbooks offer early descriptions of the evolving robotic TAR (rTAR) technique [21, 33, 34], a manuscript offering outcomes of the robotic retromuscular dissection was only recently published by Warren et al. less than a year from the time this chapter is being written [35]. As experience and technical considerations for rTAR are evolving, herein we will aim to describe our approach to this fairly challenging robotic repair.
Patient Selection
Early considerations of attempting a rTAR were obviously met with skepticism. Because of the complexity of recurrent ventral incisional herniations addressed with an open TAR, minimally invasive attempts were understandably difficult for most surgeons to envision. Patient selection is obviously going to be critical. As permutations of robotic hernia repairs are evolving, so are the inclusion criteria. Conservatively, to optimize the technical feasibility and safety of the technique, rTAR candidates ideally have:
-
Midline defects of 8–15 cm without loss of domain.
-
Smaller defects may be amendable to intraperitoneal, preperitoneal, or an isolated retrorectus repair done either open, laparoscopically, or robotically.
-
Larger defects may create too much tension at the time of fascial closure, depending on abdominal wall compliance.
-
-
Limited redundant soft tissue and no chronic skin infections/ulcerations that would typically be removed during open repairs.
-
No large amounts of previous mesh or concern for chronic mesh infection that would also typically be excised during an open repair.
-
Ability for safe laparoscopic access, port placement, and subsequent lysis of adhesions to free the viscera from the anterior abdominal wall.
-
No or limited history of obstructive symptoms that would compel the surgeon to lyse inter-loop adhesions. This is a relative contraindication.
As comfort with the robotic technique evolves, inclusion and exclusion criteria will as well. Optimal patients should be identified for early attempts, and candid conversations should be had regarding the risk of technical unfeasibility. If laparoscopic access cannot be achieved, the patient and surgeon should agree preoperatively on whether to abort the procedure or convert to an open repair, and the informed consent form should reflect this. Not only should the surgeon be well trained in the robotics platform, but he/she should be comfortable with the open technique, if necessary. Furthermore, a thorough understanding of abdominal wall anatomy and subtle points appreciated during the open TAR technique aid in the robotic dissection.
While a complete discussion of our patient-driven medical optimization goals for complex ventral hernia patients are beyond the scope of this chapter, some details are worth mentioning. We expect that patients will take an active and conscientious role in losing weight before surgery and we often refer patients for medically monitored weight loss through a protein sparing modified fast regimen for extreme cases refractory to traditional weight loss attempts. Diabetics are expected to optimize their hemoglobin A1c to below 7.5, and preoperative levels >9 will prompt endocrinology consultation and case cancellation . Smokers are expected to quit for a minimum of 4 weeks before their operation and appropriate preoperative blood testing can be done to confirm patient sincerity when indicated. Our center for perioperative medicine coordinates universal decolonization of methicillin-resistant staph aureus (MRSA) before surgery and MRSA-positive patients receive perioperative antibiotic prophylaxis that includes coverage of MRSA (typically vancomycin). Finally, preoperative nutritional optimization with arginine and omega-3 fatty acid supplements is provided and encouraged for all patients starting 5 days before surgery. These have traditionally been our expectations before an open TAR. If the surgeon decides to proceed with robotic repair in an un-optimized patient, these factors should play a role in the decision to convert to open if a minimally invasive approach is not technically feasible . For example, the patient may be counseled that if laparoscopic peritoneal access cannot be gained, that an open repair will be deferred until the patient loses more weight.
rTAR Operative Details
-
Patients are placed in a supine position and arms are tucked so that the arm boards are not an obstacle during movement of the robot patient-side cart (Fig. 10.1 ).
-
We utilize a double-dock technique when performing a rTAR, meaning that the retromuscular dissection on each side of the abdominal wall is achieved with the robot docked on the contralateral side. The da Vinci Xi has the ability to rotate its boom 180° so that bilateral docking can be achieved without moving the patient or the patient-side cart. Earlier models of the da Vinci (ex. Si) require movement of the robot to the other side of the patient, or rotating the patient 180° for the contralateral dissection depending on the setup of the operating room. When necessary, this transition should be discussed and negotiated with the anesthesia team and operating room staff before the operation. At our institution, we rotate the foot of the operating table away from where the patient-side cart will approach the bed, and the da Vinci Xi boom obviates the need to move the bed or side cart when docking on the contralateral side.
-
The abdomen should be widely prepped and draped in the event that open conversion is necessary.
-
We prefer to gain intra-abdominal access using a 5 mm optical trocar and 0° laparoscope away from previous incisions. Typically, this is done just beneath the costal margin just lateral to the mid-clavicular line. Either side is feasible but we prefer the left when possible.
-
Pneumoperitoneum to 15 mmHg of carbon dioxide is achieved.
-
The next 8 mm trocar is then placed 1–2 finger breadths medial and cephalad to the anterior superior iliac spine. The long bariatric trocars are helpful here to minimize collisions with hips and thighs during upper abdominal dissection.
-
The subcostal port is upsized to the 8-m robotic trocar and the 3rd port is placed in between the first 2 at approximately anterior axillary line (Fig. 10.2 ).
-
At this point, initial adhesiolysis can be done using traditional laparoscopic equipment and may have already been necessary to make room for lateral port placement. During adhesiolysis, a conscious effort should be made to preserve the peritoneum that will eventually provide a barrier to the retromuscular prosthetic. Alternatively, docking of the robot could be done to aid adhesiolysis, understanding that the benefits of improved visualization and ergonomics are at the cost of losing haptic feedback. Loss of haptic feedback and a contained visual field are important considerations and require utmost care to minimize risks of visceral injuries. There should be a low threshold to perform a standard laparoscopic lysis of adhesions until an adequate working space for the robot has been achieved. Finally, if the adhesions are considered treacherous or one encounters a “frozen” abdomen where preservation of the visceral sac seems unlikely, a minimally invasive approach should be abandoned.
-
The robot is docked by bringing the patient-side cart toward the operating room table at 90° to the torso with the center column aligned with the patient’s hip. Arms 1/2/3 or 2/3/4 can be docked to the ports, as only 3 of 4 are typically utilized.
-
For right-handed surgeons, a dV Fenestrated bipolar (or Prograsp) is placed in the left-handed port and the dV monopolar scissors is placed in the right-handed port. A standard angled camera is placed through the middle port.
-
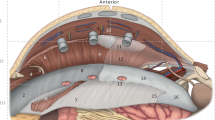
Once the visceral adhesions are cleared from the hernia sac and the anterior abdominal wall, the posterior sheath is incised with the monopolar scissors just lateral to the edge of the hernia sac to expose the rectus muscle (Fig. 10.3 ). This posterior rectus sheath division can be extended superiorly, following the belly of the rectus muscle.
-
The avascular retrorectus plane is then matured laterally to the linea semilunaris and superiorly/inferiorly at least 5–8 cm beyond the defect. The pneumoperitoneum allows for uniform retraction to aid this dissection. Although for smaller hernias, this retrorectus only Rives-Stoppa dissection may be sufficient for closure of the anterior fascia, excessive tension on the posterior closure and limited space for mesh placement limit utilization of this approach in our practice.
-
The lateral extent of the retrorectus dissection reveals the perforating neurovascular bundles that are identified and preserved, similarly to the open technique. Identification and preservation of those bundles is not only important to maintaining innervation of the rectus muscles, but also serves to identify the semilunar line.
-
In the upper third of the abdomen, where the belly of the transversus abdominis muscle is most prominent medially to the semilunar line, the lateral posterior rectus sheath (consisting solely of fibers from the posterior lamina of the internal oblique aponeurosis) is incised just medial to the neurovascular perforators to expose the underlying transversus abdominis muscle (Fig. 10.4 ).
-
Using the neurovascular perforators as a landmark will typically prevent intramuscular dissection or potentially catastrophic division of the semilunar line.
-
-
The transversus abdominis muscle can then be separated from the underlying transversalis fascia and maturation of the pretransversalis plane laterally as far as the psoas muscle allows from wide release of the posterior and anterior components as they become more dissociated. An ideal superior retromuscular dissection leaves the transversus abdominis naked, with an intact visceral sac consisting of transversalis fascia and peritoneum. While dissection in the preperitoneal plane is also possible, we avoid it due to significant risks of tearing thin peritoneum, especially in the subcostal areas.
-
The lateral division of the posterior rectus sheath and transversus abdominis release can be initiated inferiorly, but the medial transversus abdominis becomes aponeurotic at the mid-abdomen. Starting the development of the pretransversalis plane superiorly will aid the inferior retromuscular dissection in our opinion.
-
Eventually, the inferior TAR dissection will culminate in division of the arcuate line just medial to its junction with the semilunar line and the posterior rectus sheath with its contiguous peritoneum/transversalis fascia is completely disconnected from the anterior fascia and muscles of the lateral abdominal wall. The initiated superior and lateral retromuscular dissections will become contiguous with the inferior preperitoneal plane (space of Retzius) utilized for laparoscopic inguinal hernias where Cooper’s ligaments can be visualized. The inferior transversalis fascia fibers below the arcuate line are swept up to the abdominal wall so as not to injure the inferior epigastric vessels.
-
Overall, our preferred plane of the retromuscular dissection is pretransversalis in the upper abdomen and preperitoneal in the lower abdomen with the transition between the two layers at approximately the level of the umbilicus.
-
-
Once the unilateral TAR dissection is complete, any defects in the posterior layers need to be closed. We utilize either interrupted figure of 8’s 2-0 Vicryl sutures or running 3-0 barbed absorbable sutures.
-
Next, three robotic ports are placed on the contralateral side to perform a mirror-image dissection . These ports will enter the retromuscular space directly without piercing the underlying peritoneum. Conversely, when the contralateral TAR is complete, port site defects in the posterior sheath will need to be closed, along with any other posterior sheath tears. Once again, we typically use interrupted 2-0 Vicryl or running 3-0 barbed absorbable sutures.
-
Superiorly, if extension into the subcostal and/or subxyphoid space is necessary for prosthetic overlap, there are a few anatomical considerations of which to be aware. A superior and lateral dissection in the preperitoneal space below the costal margin, exposing the muscle fibers of the diaphragm, confirms development of the correct retromuscular plane after a TAR.
When completed, bilateral posterior component separations with a TAR should allow for primary fascial closure with acceptable tension, as well as a sufficient retromuscular space to accommodate large prosthetic overlap in all directions.
-
First, the medialized posterior rectus sheathes are closed using a running 2-0 V-loc suture with the dV SutureCut needle driver in the dominant hand (Fig. 10.5 ). If too much tension is encountered, this could be a sign of incomplete retroperitoneal dissection.
-
Similarly, closure of the anterior fascial sheath is accomplished using several running nonabsorbable #1 V-loc suture (Fig. 10.6 ). Every 3–4 throws, a bite of the soft tissue or hernia sac is incorporated to minimize the dead space for seroma formation. The sutures are preplaced at a regular pneumoperitoneum, but tightened when the pressure is decreased to 4–5 mmHg.
-
Next, the retromuscular pocket is measured with a ruler to size the prosthetic to subsequently achieve a giant prosthetic reinforcement of the visceral sac.
-
The robot is undocked and a piece of appropriately sized midweight uncoated macroporous polypropylene (SoftMesh, Bard, Murray Hill, NJ, USA) is placed on top of the visceral sac. While fibrin glue, absorbable tacks or transfascial suture fixation have all been tried, and we typically add no additional mesh fixation (Fig. 10.7 ).
-
No port sites need to be closed since the mesh underlays them.
Postoperative Care
We tend to use a multimodal pain control regimen outlined in our previous descriptions of our enhanced recovery pathway [36, 37]. Diet advancement and transition to enteral medications is typically accelerated in comparison to open repairs, and is mostly dictated by the extent of adhesiolysis and duration of the operation. Over the course of 12 months our operative times decreased from greater than 6 h to 2.5–4 h, much like the early experience reported by Carbonell [21]. As our operative times have improved, patients with a minimal adhesiolysis are given clear liquids on the day of surgery and are advanced as tolerated to a regular diet the next day. If they are tolerating a regular diet, ambulating in the halls, and their pain is controlled on enteral pain medication , they are typically ready for discharge the next day.
Outcomes
Presented at the SAGES 2016 annual meeting , Warren et al. reported a retrospective comparison of 103 LVHRs and 53 robotic ventral repairs (rVHR) at their institution between 2013 and 2015 [35]. Techniques were not standardized, and rVHR included preperitoneal (26%), intraperitoneal (4%), retrorectus (27%), and rTAR (43%) mesh placement. The benefits of abdominal wall reconstruction by fascial closure (rVHR 96.2 vs. LVHR 50.5%; p < 0.001) and extraperitoneal mesh placement (96.2 vs. 9.7%; p < 0.001) were achieved in almost all robotic approaches. Longer operative times (245 vs.122 min, p < 0.001) and more frequent postoperative seroma formation (47.2 vs. 16.5%, p < 0.001) for rVHR are countered with equivalent rates of surgical site infection (3.8 vs. 1%, p = 0.592) and a shorter median length of stay (1 vs. 2 days, p = 0.004). While the improvement in length of stay is consistent with our anecdotal experience, no difference was shown in narcotic requirement to suggest the robotic approach was less painful. Notably, LVHR patients in this study were also statistically older (60.2 vs. 52.9 years; p = 0.001) and selection bias no doubt favored the rVHR group. The anomalous 9% rate of bowel injury in the LVHR group and four open conversions speaks to the complexity of the LVHR group and also likely played a role in prolonging that cohort’s median length of stay. While one could attribute the lower incidence of bowel injury and open conversion to improved visualization and ergonomics offered by the robotic technique, this may be a dangerous assumption and can more likely be explained by a less complex robotic cohort. Loss of haptic feedback, fixed camera angles, and instrument exchanges by novice assistants can be dangerous properties of the robotic approach early in the learning curve, especially during visceral adhesiolysis.
The largest retrospective review of robotic ventral hernia repair to date was recently published by Carbonell et al. using data extracted from the Americas Hernia Society Quality Collaborative (AHSQC) database [38]. Their aim was to compare hospital length of stay for 111 robotic retromuscular repairs (rRMR)—including 85% rTAR and 15% robotic retrorectus dissections—with a propensity score matched group of patients who underwent open retromuscular repair (83% TAR, 17% retrorectus) using logistical regression to match patient variables, medical comorbidities, and hernia characteristics. They found a median length of stay of 2 days for rRMR compared to 3 days for open equivalents (p < 0.001) with no difference in readmission rates or surgical site infection. Increased seroma formation was again associated with the robotic approach (25% vs. 4%) and could be related to less frequent use of drains placed after robotic dissections (21% vs. 70%). While the propensity score matching algorithm appears to account for most variables impacting hospital length of stay, the authors admit they were not able to account for surgeon or institutional characteristics. Surgeons pioneering the robotic approach may be more likely to employ enhanced recovery pathways and feel comfortable with accelerated discharge.
Limitations and Vitality of the Robotic Technique
Conservatively, rVHR is feasible for a select group of patients . Whether or not the technique has value in terms of reduced cost or improved patient outcomes will be the subject of intense scrutiny in our value-conscious healthcare landscape. The upfront cost a hospital system invests on the purchase of a robot as well as its maintenance, and the disposable cost of multiuse instruments immediately puts the platform at a disadvantage, not to mention the added expense of barbed suture and increased operative times for rVHR. Not surprisingly, the focus of early reports on rVHR reduction in hospital stay can be a major step in justifying the sincere debt. Furthermore, operative times have dropped dramatically as experience accrues, and extraperitoneal placement of uncoated polypropylene prosthetic reinforcement obviates the need for their more expensive barrier mesh counterparts used in most laparoscopic repairs. Future analysis of resources utilized for pain control and time to return to work could also favor the robotic approach.
Most intriguingly, robotic retrorectus and rTAR are unique to general surgery in that they cannot be routinely reproduced in a minimally invasive approach without use of the robot. Most comparisons in the colorectal, foregut, and thoracic literature compare a laparoscopic technique to a robotic replication of that technique. While safety and feasibility can typically be demonstrated, a clinical benefit has not been demonstrated for most procedures, making it difficult to justify the increased cost of the robotic platform. A notable exception has been the evolution of robotic prostatectomy that touts equivalent cancer outcomes to open repair through a minimally invasive technique that is easy to learn and teach [39]. Comparatively, laparoscopic prostatectomy is notoriously challenging and difficult to learn. Retrospective analyses comparing open and robotic prostatectomy confirm a shorter hospital stay and return to normal activity with the robotic approach to justify the increased cost. Interestingly, robotic prostatectomy has been widely accepted into clinical practice despite the absence of any randomized controlled trials.
In this chapter, we have outlined in detail the benefits of an open retrorectus dissection supplemented with the TAR release in comparison to alternative open and laparoscopic techniques. For the right patient, a rTAR can achieve the benefits of the open TAR technique through a minimally invasive approach, simultaneously avoiding limitations of each traditional operation. Importantly, the cost of the robotic approach appears to be offset by savings in decreased hospital stay. The quality of the operation, as substantiated by patient outcomes, could further legitimize its value and vitality. Robotic prostatectomy has already set a precedent for wide adoption of a robotic technique driven by improved patient outcomes despite increased cost. As we move forward, we need to be critical of how we define and study outcomes in our hernia patients, being cognizant that both patients and surgeons can be susceptible to marketing bias.
References
Stoppa RE. The treatment of complicated groin and incisional hernias. World J Surg. 1989;13(5):545–54.
Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86(3):519–26.
Girotto JA, Chiaramonte M, Menon NG, Singh N, Silverman R, Tufaro AP, et al. Recalcitrant abdominal wall hernias: long-term superiority of autologous tissue repair. Plast Reconstr Surg. 2003;112(1):106–14.
Gonzalez R, Rehnke RD, Ramaswamy A, Smith CD, Clarke JM, Ramshaw BJ. Components separation technique and laparoscopic approach: a review of two evolving strategies for ventral hernia repair. Am Surg. 2005 Jul;71(7):598–605.
Saulis AS, Dumanian GA. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg. 2002 Jun;109(7):2275–80.
Lowe JB, Garza JR, Bowman JL, Rohrich RJ, Strodel WE. Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg. 2000;105(2):720–9.
Switzer NJ, Dykstra MA, Gill RS, Lim S, Lester E, de Gara C, et al. Endoscopic versus open component separation: systematic review and meta-analysis. Surg Endosc. 2015;29(4):787–95.
Snyder CW, Graham LA, Gray SH, Vick CC, Hawn MT. Effect of mesh type and position on subsequent abdominal operations after incisional hernia repair. J Am Coll Surg. 2011;212(4):496–502.
Patel PP, Love MW, Ewing JA, Warren JA, Cobb WS, Carbonell AM. Risks of subsequent abdominal operations after laparoscopic ventral hernia repair. Surg Endosc. 2017;31(2):823–8.
Carbonell AM, Cobb WS, Chen SM. Posterior components separation during retromuscular hernia repair. Hernia. 2008;12(4):359–62.
Stoppa RE, Rives JL, Warlaumont CR, Palot JP, Verhaeghe PJ, Delattre JF. The use of Dacron in the repair of hernias of the groin. Surg Clin North Am. 1984;64(2):269–85.
Majumder A, Miller HJ, Sandoval V, Fayezizadeh M, Wen Y, Novitsky YW. Objective assessment of myofascial medialization after posterior component separation via transversus abdominis muscle Releas. J Am Coll Surg. 2016;223(4):S57.
Pauli EM, Wang J, Petro CC, Juza RM, Novitsky YW, Rosen MJ. Posterior component separation with transversus abdominis release successfully addresses recurrent ventral hernias following anterior component separation. Hernia. 2015 Apr;19(2):285–91.
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ. In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg. 2012;16(11):2139–44.
Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg. 2013;217(6):991–8.
Criss CN, Petro CC, Krpata DM, Seafler CM, Lai N, Fiutem J, et al. Functional abdominal wall reconstruction improves core physiology and quality-of-life. Surgery. 2014;156(1):176–82.
De Silva GS, Krpata DM, Hicks CW, Criss CN, Gao Y, Rosen MJ, et al. Comparative radiographic analysis of changes in the abdominal wall musculature morphology after open posterior component separation or bridging laparoscopic ventral hernia repair. J Am Coll Surg. 2014;218(3):353–7.
Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221(6):507–36.
Novitsky YW, Fayezizadeh M, Majumder A, Neupane R, Elliott HL, Orenstein SB. Outcomes of posterior component separation with transversus abdominis muscle release and synthetic mesh sublay reinforcement. Ann Surg. 2016;264(2):226–32.
LeBlanc KA, Booth WV. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg Laparosc Endosc. 1993;3(1):39–41.
Vorst AL, Kaoutzanis C, Carbonell AM, Franz MG. Evolution and advances in laparoscopic ventral and incisional hernia repair. World J Gastrointest Surg. 2015;7(11):293–305.
Carter SA, Hicks SC, Brahmbhatt R, Liang MK. Recurrence and pseudorecurrence after laparoscopic ventral hernia repair: predictors and patient-focused outcomes. Am Surg. 2014 Feb;80(2):138–48.
Orenstein SB, Dumeer JL, Monteagudo J, Poi MJ, Novitsky YW. Outcomes of laparoscopic ventral hernia repair with routine defect closure using “shoelacing” technique. Surg Endosc. 2011;25(5):1452–7.
Heniford BT, Park A, Ramshaw BJ, Voeller G. Laparoscopic ventral and incisional hernia repair in 407 patients. J Am Coll Surg. 2000;190(6):645–50.
Carbonell AM, Harold KL, Mahmutovic AJ, Hassan R, Matthews BD, Kercher KW, et al. Local injection for the treatment of suture site pain after laparoscopic ventral hernia repair. Am Surg. 2003;69(8):688–91.
Ramshaw BJ, Esartia P, Schwab J, Mason EM, Wilson RA, Duncan TD, et al. Comparison of laparoscopic and open ventral herniorrhaphy. Am Surg. 1999;65(9):827–31.
Itani KM, Hur K, Kim LT, Anthony T, Berger DH, Reda D, et al. Comparison of laparoscopic and open repair with mesh for the treatment of ventral incisional hernia: a randomized trial. Arch Surg. 2010;145(4):322–8.
Schluender S, Conrad J, Divino CM, Gurland B. Robot-assisted laparoscopic repair of ventral hernia with intracorporeal suturing. Surg Endosc. 2003;17(9):1391–5.
Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc. 2002;16(10):1389–402.
Allison N, Tieu K, Snyder B, Pigazzi A, Wilson E. Technical feasibility of robot-assisted ventral hernia repair. World J Surg. 2012;36(2):447–52.
Sugiyama G, Chivukula S, Chung PJ, Alfonso A. Robot-assisted transabdominal preperitoneal ventral hernia repair. JSLS. 2015;19(4):1–3.
Abdalla RZ, Garcia RB, RIDD C, CRPD L, Abdalla BMZ. Procedimento de Rives/Stoppa modificado robô-assistido para correção de hérnias ventrais da linha média. ABCD arq bras cir dig. 2012;25(2):129–32.
Ballacer C, Parra E. Robotic ventral hernia repair. In: Novitsky YW, editor. Hernia surgery: current principles. Cham: Springer; 2016.
Hope WW, Cobb WS, Adrales GL, editors. Textbook of hernia. Cham: Springer; 2017.
Warren JA, Cobb WS, Ewing JA, Carbonell AM. Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc. 2017;31(1):324–32.
Belyansky I, Zahiri HR, Park A. Laparoscopic transversus abdominis release, a novel minimally invasive approach to complex abdominal wall reconstruction. Surg Innov. 2016;23(2):134–41.
Majumder A, Fayezizadeh M, Neupane R, Elliott HL, Novitsky YW. Benefits of multimodal enhanced recovery pathway in patients undergoing open ventral hernia repair. J Am Coll Surg. 2016;222(6):1106–15.
Carbonell AM, Warren JA, Prabhu AS, Ballecer CD, Janczyk RJ, Herrera J. et al. Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society Quality Collaborative: Ann Surg; 2017. p. 27.
Estey EP. Robotic prostatectomy: the new standard of care or a marketing success? Can Urol Assoc J. 2009;3(6):488–90.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Petro, C.C., Novitsky, Y.W. (2018). Robotic Component Separation. In: LeBlanc, K. (eds) Laparoscopic and Robotic Incisional Hernia Repair. Springer, Cham. https://doi.org/10.1007/978-3-319-90737-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-90737-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90736-9
Online ISBN: 978-3-319-90737-6
eBook Packages: MedicineMedicine (R0)