Abstract
The unique presentation of complex hernias requires a wide range of repair options. Posterior component separation via a transversus abdominis muscle release has increased in popularity owing to the natural anatomic extension from a retromuscular approach. However, endoscopic anterior component separation remains an important and effective technique in selected patients as an adjunct to both open and laparoscopic AWR. Advantages of ECS include the ease of operation with recognizable anatomy, minimal risk of dividing the incorrect layer and destabilizing the lateral abdominal wall, and finally, its effectiveness and low morbidity. Additionally, when paired with minimally invasive AWR techniques (intraperitoneal onlay mesh [IPOM], transabdominal preperitoneal [TAPP], extended-view totally extraperitoneal [eTEP]), endoscopic anterior component separation allows for a fast, efficient, and safe complementary technique to facilitate midline advancement.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Endoscopic component separation
- Ventral hernia
- Incisional hernia
- Subfascial approach
- Subcutaneous approach
- Primary closure
- Abdominal wall reconstruction
32.1 Background/Historical Perspective
Complex ventral hernias remain a challenging surgical problem with successful outcomes requiring a combination of techniques and tools, including tissue-based repairs, prosthetic reinforcement, fascial releases, and myofascial advancement flaps. The goal of abdominal wall reconstruction (AWR) is to provide a durable structural, functional, and cosmetic repair.
More recently, primary closure of defects has been considered an essential aspect of AWR because it attempts to recreate the anatomy and physiology of the abdominal wall while reducing dead space and its consequences [1, 2]. Ventral hernias with defects up to three can be repaired by simple primary closure, whereas for larger defects some type of tension-free reconstruction is advised. This can be accomplished using various muscle relaxation techniques, including surgical, pharmacological, and mechanical methods, with component separation (CS) techniques being the most common. Almost invariably, repair is reinforced with a mesh.
Anterior component separation (ACS) of parts was first introduced by Ramirez in 1990 as a method to reestablish the linea alba with autologous fascia [3]. This technique creates a myofascial advancement flap by partitioning one component of the redundant lateral musculature to enlarge and advance the abdominal wall, assisting in the primary closure of defects without undue tension. The external oblique aponeurosis is released lateral to the semilunar line, and the avascular intermuscular plane between the external and internal obliques is developed. If additional advancement is required, the posterior rectus sheath can be vertically divided and advanced. In general, 8–10 cm of unilateral advancement can be achieved enabling medialization of the rectus abdominis complexes and a tension-free or reduced tension closure. Recurrence rates of 5–30 % have been reported in the absence of mesh reinforcement, a respectable rate for complex abdominal wall hernias.
The major morbidity of open ACS techniques results from the creation of the lipocutaneous flaps. This dissection traditionally sacrifices the anterior perforator complexes, leading to potential flap ischemia/necrosis and the creation of large potential spaces that increase the risk of hematoma, seroma, and infectious complications . Modifications of open component separation result in the preservation of the periumbilical perforating vessels and the creation of smaller spaces thereby reducing wound complication rates [4].
Minimally invasive approaches have been formulated to minimize morbidities resulting from perforator loss and flap creation. Lowe and associates reported an open assisted subcutaneous endoscopic ACS using a balloon in 2000 allowing for incision of the external oblique aponeurosis from the subcutaneous plane [5]. Maas described a laparoscopic balloon-assisted subfascial approach in 2002 consisted of endoscopically performed dissection, with release through a small cutaneous counter incisions [6]. Rosen is credited with popularization of endoscopic ACS in 2007 as an adjunct to AWR in combination with mesh reinforcement [7]. Chen described a modification that simplified the transfascial approach by making the initial incision medial to the anterior superior spine and working cephalad with the help of an additional port, making it more ergonomic and easier to perform. Finally, Daes described in 2010 a totally endoscopic subcutaneous approach in which preoperative skin marking of the semilunar line under ultrasonic guidance precedes creation of a subcutaneous space with a balloon dissector and division and undermining of the external oblique aponeurosis [8]. This modification imitates Ramirez approach and is ergonomic and familiar to surgeons.
32.2 Indications for ECS
-
1.
As part of the totally laparoscopic abdominal wall reconstruction (AWR) together with endoscopic or transfascial closure of defects and the placement of a barrier underlay mesh or an unprotected sublay mesh. This has been the main indication for subcutaneous ECS in our group.
-
2.
To facilitate an open AWR , especially for central defects not amenable to closure by tension-free primary repair. However, when performing a Rives procedure requiring mesh coverage of an area wider than the retrorectus space; posterior component separation-transversus abdominus release (PCS-TAR) is a better option.
-
3.
When planning tension-free primary closure of ventral hernias during colostomy reversal, colon resection, or in other contaminated or infected fields, often without the use of mesh.
-
4.
Endoscopic component separation (ECS) can be performed in the presence of a stoma without para-stomal hernias. In this case, ECS is performed lateral to the ostomy site without the need to relocate the stoma.
-
5.
Finally, ECS can be performed to assist in the management of abdominal compartment syndrome.
32.3 Contraindications for ECS
-
1.
Severe skin dystrophy requiring extensive resection or the creation of extensive flaps.
-
2.
Defects that can be closed primarily without undue tension.
-
3.
Defects disproportionally wider than longer.
-
4.
Patients with noncompliant abdominal walls from multiple previous repairs/meshes. In these cases, an onlay or PCS-TAR may be more appropriate.
-
5.
Patients who have undergone previous bilateral PCS-TAR. However, it is possible to use a PCS-TAR approach on one side (for stoma reversal) and an anterior CS on the other side.
32.4 Operative Steps
32.4.1 Preoperative Preparation
Skin preparation extends from the nipples to the upper thighs and should be laterally extended to beyond the posterior axillary lines. For clean operations, a single dose of a first-generation cephalosporin is administered during anesthetic induction. Urinary catheters are used in complex cases or when pelvic dissection is anticipated. Pneumatic compression devices are used in all patients. During clean contaminated or contaminated cases, ECS should be performed first.
32.4.2 Techniques of ECS
The three approaches to anterior ECS create exactly the same compound myofascial advancement flap. The subfascial approach has been used most extensively, followed by the modified subfascial approach and the more recently described subcutaneous ECS approach. The latter two approaches are considered more ergonomic and easier to perform because they imitate the traditional open technique. Moreover, they avoid the difficulty of dissecting in the costal area, they avoid operating in parallax, and they require only one additional trocar.
32.4.3 Operative Technique
32.4.3.1 Transfascial Approach
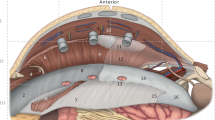
In this technique, the patient is placed in the supine position with both arms abducted. A 12-mm incision is made just below the tip of the eleventh rib using a S retractor. The subcutaneous tissues are bluntly divided, exposing the external oblique aponeurosis. The external oblique is sharply incised, exposing the internal oblique muscle . The potential space between the external and internal oblique aponeuroses is developed lateral to the semilunar line using a bilateral balloon dissector . A structural 12-mm balloon port is then placed and the space maintained with a CO2 insufflation pressure of 12 mmHg. The areolar attachments are bluntly dissected under direct vision using a 10-mm 30° laparoscope. Two additional 5-mm ports are created, one at the level of the umbilicus on the posterior axillary line and another just above the inguinal ligament lateral to the rectus. This entire plane between the external and internal oblique muscles is dissected, extending from just above the costal margin to the inguinal ligament and from the semilunar line medially to the posterior axillary line laterally, where the oblique muscles meet the latissimus dorsi. Coagulating scissors are used for component separation, with the division of the external oblique aponeurosis released from the costal margin to the inguinal ligament. The external oblique muscle will be at the top of the screen, the internal oblique muscle at the bottom, and the semilunar line present medially. This process is repeated on the opposite side. Each of the lateral compartments is drained with a closed suction drain. A video of the technique can be found at https://www.youtube.com/watch?v=lKtKXDKIiRM.
32.4.3.2 Modified Subfascial Approach
An analogous transfascial endoscopic anterior component separation technique has been employed, using this same operative plane with equivalent release and a simplified operative approach. The external oblique aponeurosis is accessed 2 cm medially to the anterior superior iliac spine. In this location, the anatomy is easily recognized as the external oblique is less muscular and almost entirely aponeurotic. After making a 1-cm incision in the aponeurosis, a bilateral laparoscopic inguinal hernia balloon dissector is used to develop the plane in a similar fashion (Fig. 32.1). A structural 10-mm balloon port is placed, and CO2 insufflation is initiated, to a pressure of 12 mmHg (Fig. 32.2). A single 5-mm port is inserted at the level of the umbilicus on the posterior axillary line. The areolar attachments between these muscle layers are dissected in similar fashion. Component separation is performed by incising the external oblique aponeurosis 2 cm lateral to the semilunar line . This release is continued well above the costal margin to the insertion of the external oblique on the ninth and tenth ribs . The inferior release from the port site to the inguinal ligament can be easily performed in an open fashion, using shears to divide the aponeurosis 2–3 cm to the inguinal ligament under direct visualization (Fig. 32.3). A closed suction drain is passed through the lateral 5-mm port and inserted into the intermuscular space. A video of the technique can be found at: https://www.dropbox.com/sh/6kri5u3ew2qoijg/AAAEHORMtofcfYgiw9OP_dMxa?dl=0&preview=Component+Separation+Project+Right+side.wmv.
32.4.3.3 Endoscopic Subcutaneous CS Approach
The patient is placed in a supine position with both arms tucked and padded at their sides. Under ultrasound guidance, the semilunar lines lateral to the rectus abdominis muscle are identified and marked on the skin bilaterally. Marking can be performed by the surgeon using portable ultrasound equipment immediately before skin preparation or can be performed by a radiologist in advance using indelible ink. A 12-mm incision is made in the lower lateral quadrant of the abdomen, lateral to the previously marked semilunar line. A balloon dissector is introduced and advanced over the anterior aponeurosis until the tip reaches the costal margin. The balloon is inflated at two levels using eight to ten pumps (Fig. 32.4). Occasionally, in obese or post-bariatric patients or in patients who have undergone previous abdominoplasty, a blunt rod (trocar interchanger) is used to create a subcutaneous tunnel over the fascia before introducing the balloon dissector . The balloon is then replaced by a simple 10–12-mm trocar. The space is maintained with CO2 insufflation at a pressure of 10 mmHg. An additional 5-mm port is introduced at a position lateral and slightly superior to the camera port (Fig. 32.5). The external oblique aponeurosis is incised laterally to the left semilunar line, using the marking on the skin as a guide. Exposure of the fatty tissues without visualization of muscle ensures entry into the correct plane (Fig. 32.6). If muscle can be visualized at this level, either the rectus sheath medially or the muscular part of the external oblique laterally has been divided.
The external oblique aponeurosis is incised from this level to 4–6 cm above the costal margin. Above the costal margin, the aponeurosis changes to muscle and division should be performed carefully to avoid bleeding. An ultrasonic device may be useful for this purpose. Scissors and judiciously used cautery can be used to dissect under the external oblique muscle laterally in an avascular plane to provide maximum advancement (Fig. 32.7). With the camera turned downward, the incision in the external oblique muscle is continued below the camera port to include the inguinal ligament. Drains are not used routinely during this technique. The subcutaneous space is re-insufflated at the end of AWR to verify hemostasis. A video of the technique can be found at https://www.youtube.com/edit?video_id=4SpWz7U5uZ0&video_referrer=watch.
32.4.4 Pearls and Pitfalls
-
1.
ECS is performed first when used as an adjunct to open AWR in clean contaminated and contaminated cases. It can also be undertaken first in totally laparoscopic AWR , when clinical examination and CT scanning provide thorough information; otherwise, laparoscopic exploration should be performed first.
-
2.
Many times there is no need to perform a bilateral ECS . We have been able to laparoscopically close most defects 6–15 cm in width with a unilateral subcutaneous CS without dehiscence or abdominal wall asymmetry.
-
3.
ECS can be used to repair any suitable lateral defect, not just central defects.
-
4.
When defects are close to the semilunar line, ECS can be performed on the same side by dividing the external oblique muscle more laterally, thus avoiding the division of the semilunar line.
-
5.
A vertical posterior rectus fascia release may be added to an ECS to assist in relieving tension on the closure.
-
6.
To facilitate endoscopic closure of defects during laparoscopic AWR , a provisional single transfascial suture can be placed at the midline of the defect to elongate the components and approximate the borders of the defect.
-
7.
Mesh should be used to cover the ECS site, at least while surgeons are learning the procedure and when in doubt.
32.4.5 Evaluation of Results
A review of the literature involving endoscopic ACS found only 13 studies eligible for inclusion [9]. The authors concluded that, in general, these studies lacked selection criteria, long-term clinical follow-up, and a clear description of outcomes, and very few described follow-up imaging protocols. All were retrospective reviews. We recently submitted the first prospective evaluation of endoscopic subcutaneous ACS, with long-term clinical and imaging follow-up [10]. Twenty consecutive patients between 2012 and 2015 were evaluated. These patients had defects 6–15 cm in width, with length greater than size, and without skin dystrophy, loss of domain, or active infection. None of these patients had undergone multiple previous repairs/meshes and there was no reasonable suspicion of severe adhesions. Most ECSs were performed unilaterally as adjuncts to totally laparoscopic AWR (IPOM plus) and were followed clinically and by CT imaging for up to 38 months (mean, 21 months). In 19 of these patients, the repair remained sound clinically and by CT imaging, whereas one patient had a small limited disruption well protected by the underlying mesh. In eight patients, in which the area was not covered by mesh, there was no defect at the CS site. Morbidity was low, with no patient experiencing surgical site infection (SSI) or mesh-related complications . Cosmetic results were excellent; in particular, despite almost all ECSs being unilateral, we did not observe abdominal wall asymmetry and the degree of patient satisfaction was high.
32.5 Conclusion
The unique presentation of complex hernias requires a wide range of repair options. Posterior component separation via a transversus abdominis muscle release has increased in popularity owing to the natural anatomic extension from a retromuscular approach. However, endoscopic anterior component separation remains an important and effective technique in selected patients as an adjunct to both open and laparoscopic AWR . Advantages of ECS include the ease of operation with recognizable anatomy, minimal risk of dividing the incorrect layer and destabilizing the lateral abdominal wall, and finally, its effectiveness and low morbidity. Additionally, when paired with minimally invasive AWR techniques (intraperitoneal onlay mesh [IPOM], trans-abdominal preperitoneal [TAPP], extended-view totally extraperitoneal [eTEP]), endoscopic anterior component separation allows for a fast, efficient, and safe complementary technique to facilitate midline advancement.
References
Nguyen DH, Nguyen MT, Ashkenazy EP, Kao LS, Liang MK. Primary fascial closure with laparoscopic ventral hernia repair: systematic review. World J Surg. 2014;38:3097–104.
Clapp ML, Hicks SC, Awad SS, Liang MK. Trans-cutaneous closure of central defects (TCCD) in laparoscopic ventral hernia repairs (LVHR). World J Surg. 2013;37:42.e51.
Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519.e526.
Saulis AS, Dumanian GA. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg. 2002;109:2275–80.
Lowe JB, Garza JR, Bowman JL, Rohrich RJ, Strodel WE. Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg. 2000;105:70–729.
Maas SM, de Vries RS, van Goor H, de Jong D, Bleichrodt RP. Endoscopically assisted “components separation technique” for the repair of complicated ventral hernias. J Am Coll Surg. 2002;194:388–90.
Rosen M, et al. Laparoscopic component separation in the single-stage treatment of infected abdominal wall prosthetic removal. Hernia. 2007;11:435–40.
Daes J. Endoscopic subcutaneous approach to component separation. J Am Coll Surg. 2014;218:e1–4.
Ferretis M, Orchard P. Minimally invasive component separation techniques in complex ventral abdominal hernia repair: a systematic review of the literature. Surg Laparosc Endosc Percutan Tech. 2015;25:100–5.
Daes J, Dennis RJ. Endoscopic subcutaneous component separation as an adjunct to abdominal wall reconstruction. Surg. Endosc. 2016;22:1–5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Daes, J., Chen, D.C. (2017). Endoscopic Component Separation Techniques. In: Hope, W., Cobb, W., Adrales, G. (eds) Textbook of Hernia. Springer, Cham. https://doi.org/10.1007/978-3-319-43045-4_32
Download citation
DOI: https://doi.org/10.1007/978-3-319-43045-4_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43043-0
Online ISBN: 978-3-319-43045-4
eBook Packages: MedicineMedicine (R0)