Abstract
Low vitamin D levels are associated with cardiovascular disease and its precursors, hypertension, and diabetes mellitus. In this chapter, we review mechanistic studies in animals and tissue culture which suggest the role of vitamin D in controlling the renin-angiotensin-aldosterone system. Multiple observational studies in humans have shown an association between low vitamin D levels and cardiovascular disease and hypertension and diabetes mellitus. Mendelian randomization studies and randomized clinical trials have shown mixed results. Current ongoing clinical trials will clarify whether the vitamin D and cardiovascular disease connection is a causal one.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Vitamin D’s role in bone health and mineral metabolism has been recognized for a long time, but discovery of vitamin D receptors in multiple human tissues, including the heart and blood vessels, generated a surge of research interest and consequently a surge of publications on the vitamin D’s relationship with cardiovascular disease [1]. In the early 1980s, Dr. Scragg provided an alternative explanation for the high cardiovascular morbidity and mortality at high latitudes and in the winter months by correlating it with low ultraviolet radiation resulting in low levels of vitamin D [2]. This was followed by a surge of research interest and publications in the coming decades, which now stands at more than 5000 articles listed in PubMed with the search terms vitamin D and cardiovascular disease. Many animal models, observational studies, and clinical interventional randomized, controlled trials have tried to understand complex relationship between vitamin D and cardiovascular health. In this chapter will review animal models, observational studies, and randomized, controlled trials to summarize our current understanding and offer clinically relevant information about vitamin D and cardiovascular health.
Mechanistic Studies
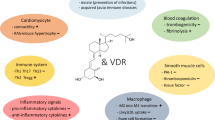
Several animal models suggest an association between vitamin D and cardiovascular health. Mice with knockout vitamin D receptor (VDR) or Cyp27b1 (1-alpha-hydroxylase) genes, mice who effectively have low-activated vitamin D levels, have myocardial hypertrophy, hypertension, increased thrombogenicity, and overexpression of renin-angiotensin-aldosterone system (RAAS) [3].
Myocardium
The VDR is present in cardiac myocyte and fibroblast, which in cardiomyocyte-specific VDR knockout mice results in myocardial hypertrophy [3]. In mice models, the VDR regulates the extracellular matrix by altering the expression of the matrix metalloproteinases [4]. Also, the VDR has an effect on cardiac contractility. Mice with 1-alpha-hydroxylase knockout have reduced systolic function, which is reversed by activated vitamin D treatment [5].
Vasculature
Experimental studies on macrophages from diabetic patients suggest a crucial role of the vitamin D receptor signal pathway in the inhibition of foam cell formation and increased cholesterol efflux in macrophages [6]. Vitamin D and its molecular effects on blood vessels have been extensively reviewed [7]. In vascular smooth muscle cells, 1,25-dihydroxyvitamin D induces production of vascular endothelial growth factor (VEGF) and promotes endothelial repair [7]. Other effects on endothelial cells and macrophages include downregulation of tissue factor (F3) and upregulation of thrombomodulin (THBD) and increased endothelial production of nitric oxide [7]. Endothelial cells in VDR knockout mice show significant alteration in vascular function, showing increased sensitivity to circulating angiotensin-2 when compared to controlled mice [8]. Vitamin D also plays a major role in controlling inflammation and the immune response within physiological limits. Major actions include downregulation of pro-inflammatory cytokines (such as IL-1, IL-2, IL-6, IL-23, interferon-γ, tumor necrosis factor-α) and upregulation of anti-inflammatory cytokines (IL-4 and IL-10) [6]. Vitamin D acts as an immune modulator by suppression of TH1 and TH17 cells and favoring Treg and TH2 cells [9]. These immune functions may play a role in atherosclerosis as administration of oral calcitriol reduced atherosclerosis by inducing tolerant dendritic cells and by inducing regulatory T cells [10].
Modulation of Vascular Calcification
Effects of vitamin D on vascular calcium balance are complex, as both excess and deficiency of vitamin D result in vascular calcification [7]. VDR activation increases phosphate levels, which in turn activates fibroblast growth factor-23 (FGF-23), a regulator of phosphate excretion by kidney. VDR activation also enhances expression of Klotho, which has a co-receptor function for FGF-23 and is protective against vascular calcification [7]. This suggests complex interplay between vitamin D, FGF23, and klotho-regulating vascular calcification.
Cardiovascular Risk Factors: Mechanistic Studies
Hypertension : Antihypertensive effects of vitamin D are mediated by RAAS. VDR activation blocks cAMP response in the renin gene promoter and blocks transcription of renin gene [11]. Other studies have suggested that, low calcium and high parathyroid hormone (PTH) levels aside, vitamin D deficiency can directly increase vascular resistance and vasoconstriction and contribute to arterial hypertension [12].
Diabetes mellitus : Antidiabetic effects of vitamin D include increased insulin secretion, insulin sensitivity, and reduced β-cell death and dysfunction induced by cytokines [13].
Lipid Metabolism: Effects of vitamin D on lipid metabolism remains to be determined [14]. High-calcium diet-fed VDR null mice when compared to wild-type mice showed less body fat mass, lower plasma cholesterol, and triglyceride levels even when placed on high-fat diet [15]. In another study done by Wang et al. showed VDR null mice on regular diet had increased cholesterol in both male and female mice [16], while HDL cholesterol and apolipoprotein A-I (APOA1) increased only in male VDR null mice [16]. Effects were reversed by changing to a special diet which normalized mineral metabolism suggesting minimal direct role of VDR in cholesterol regulation [16]. Cell culture studies showed that 1,25 dihydroxyvitamin D decreased both APOA1 and its mRNA in a dose-dependent manner, and effects were reversed by a vitamin D antagonist [17].
PTH regulation : VDR activation has been shown to have suppressive effect on PTH levels. High PTH levels have been linked to chronic kidney disease, obesity, and with increased risk for adverse cardiovascular events [18]. As vitamin D and PTH are intertwined, it is hard to separate out effects of high PTH versus low vitamin D.
Other: VDR activation has been implicated in regulation of inflammation by blocking nuclear factor κB (NFκV) activation. Vitamin D activation is also associated with prevention of kidney diseases through protecting podocytes and other proteinuric effects and associated with regulation of coagulation and oxidative stress [19].
Observational Studies
An association between low concentrations of vitamin D and increased cardiovascular risk has been consistently reported across various observational studies. These observational studies fail to provide a causal relationship due to study design.
A large meta-analysis with prospective studies from across Europe and the United States using individual participant data from the CHANCES Cohort (Consortium on Health and Aging: Network of Cohorts in Europe and the United States), as well as the NHANES III (National Health and Nutrition Examination Survey), found that the lowest 25-hydroxyvitamin D (25(OH)D) quintile was associated with increased all-cause mortality (relative risk (RR), 1.57; 95% confidence intervals (CI), 1.36–1.81) including cardiovascular mortality (RR, 1.41; 95% CI, 1.18–1.68) in subjects with no cardiovascular disease at baseline [20]. Researchers analyzed results for differences in population, sex, age, and seasons; the blood was drawn and found that the results were consistent across all parameters [20]. These results were similar to that reported by Wang et al. in a meta-analysis of 65,994 patients with a RR of 1.03 (95% CI, 1.00–1.06) per 25 nmol/L decrement in 25(OH)D levels for CVD [21]. The pooled RR was 1.52 (95% CI, 1.30–1.77) for total CVD and 1.42 (95% CI, 1.27–2.10) for CVD mortality when comparing lowest to the highest 25(OH)D levels [21]. This association remained significant even on exclusion of studies with baseline CVD and controlling for various confounding factors [21].
Another large meta-analysis of 73 studies involving 849,412 participants found inverse associations of circulating 25(OH)D with risks of deaths due to cardiovascular disease [22]. The data showed pooled relative risks of 1.35 (95% CI, 1.13–1.60) for deaths from cardiovascular disease and 1.35 (95% CI, 1.22–1.49) for all-cause mortality when comparing the bottom versus top thirds of baseline 25(OH)D levels [22]. These results have been consistently reproduced in similar systematic reviews of observational studies with an increased risk of cardiovascular mortality and cardiovascular events associated with 25(OH)D deficiency [20,21,22,23,24,25,26,27,28,29,30].
Although the association of low vitamin D levels and cardiovascular mortality is fairly consistent, less consistent data is available in regard to specific underlying diseases and mechanisms. Several authors have reported that low vitamin D levels are associated with a higher risk of coronary artery disease (CAD) and congestive heart failure [31, 32]. However, other studies failed to find a similar association [33, 34].
Despite some contrary studies, there is much evidence of an association between vitamin D deficiency and cardiovascular disease mortality and events. However, a cause and effect relationship cannot be determined without larger randomized clinical trials.
Cardiovascular Risk Factors: Observational Studies
Hypertension : The relationship between vitamin D and blood pressure was initially described in studies that reported increased risk of arterial hypertension with low UVB exposure and increased prevalence of hypertension at higher latitudes and during the winter season [2, 35,36,37]. Kunutsor et al. in their meta-analysis of 283,537 individuals reported a 12% reduction in the risk of future hypertension for every 10 ng/mL increment in circulating 25(OH)D levels [35]. The relative risk was 0.70 (95% CI, 0.58–0.86) when comparing the incidence of hypertension in extreme tertiles of baseline 25(OH)D levels [35]. Another recent meta-analysis of 53,375 individuals from seven different studies found significant associations between vitamin D deficiency and incident hypertension (hazard ratio (HR), 1.24; 95% CI, 1.08–1.41) [38]. Although accumulating evidence suggests the association between low vitamin D levels and the risk of hypertension, these studies fail to show a causal relationship and are limited by confounding factors.
Diabetes mellitus : Various observational studies have reported vitamin D deficiency as a risk factor for developing type 1 and 2 diabetes mellitus [13, 39,40,41,42]. These include studies using data from NHANES and other large cohorts from the United States and across Europe. A recent meta-analysis by Ye et al. which combined data from 22 longitudinal cohorts reported an increased risk of type 2 diabetes mellitus (RR, 1.21; 95% CI, 1.16–1.27) for 25 nmol/L lower 25(OH)D concentration [43]. Similarly, Song et al. showed an inverse association between 25(OH)D levels and risk of type 2 diabetes mellitus with a 4% lower risk of diabetes (95% CI, 3–6) for each 10 nmol/L increase in 25(OH)D levels [42]. The association between vitamin D and diabetes mellitus is reviewed in Chap. 7 in this publication.
Lipids: The relationship between levels of vitamin D and lipid profile is not well defined. Various cross-sectional studies have linked low 25(OH)D concentrations to elevated LDL cholesterol and triglyceride levels along with low HDL cholesterol levels [14, 44,45,46,47,48,49]. Ponda et al. also reported an increase in mean total cholesterol (TC) and HDL cholesterol levels when 25(OH)D levels increased from <20 to ≥30 ng/mL over time [44]. Nevertheless, no significant change was noted in LDL cholesterol or triglyceride levels [44]. Similar findings were reported by Faridi et al. in a study of 13,039 Atherosclerosis Risk in Communities (ARIC) participants studied over a period of 5 years with cross-sectional and prospective associations between 25(OH)D deficiency and higher LDL cholesterol and non-HDL cholesterol and triglycerides [48]. There was no prospective association seen for LDL cholesterol [48]. This increased risk for incident dyslipidemia in demographic adjusted models (RR, 1.19; 95% CI, 1.02–1.39) was however attenuated on adjusting for other clinical variables [48].
Mendelian Randomization Studies
Several studies have examined the relationship between single nucleotide polymorphisms (SNPs) in the VDR and other vitamin D-related genes and cardiovascular health, but results have been mixed. In the context of heart failure, low levels of 25(OH)D were independently associated with incident heart failure in white but not in black participants in ARIC and in those genetically predisposed to have high vitamin D-binding protein gene [50]. Genome-wide association studies found four genetic loci [DHCR7 (related to vitamin D synthesis), CYP2R1 (hepatic 25-hydroxylation), DBP (also known as GC; transport), and CYP24A1 (catabolism)] associated with 1–4% variance in 25(OH)D levels [51]. Proteins encoded by these genes are involved in vitamin D production. These SNPs can be used to perform Mendelian randomization studies and evaluate if genetic variations in 25(OH)D levels are associated with CVD risk. These studies might be limited because only <5% of the variation in 25(OH)D levels can be explained by SNPs. Still, Mendelian randomization studies are considered strong causal evidence as genetically determined variations are in general not confounded and indicate a lifelong exposure [52].
Obesity: Recent bidirectional Mendelian analysis of multiple cohorts suggested that high BMI leads to lower 25(OH)D levels, but no association was seen in the opposite direction [53].
Diabetes mellitus Type 2: Studies investigating relationship between type 2 diabetes and low 25(OH)D levels have conflicting results, with one study showing increased risk but others failed to replicate [43, 54].
Lipid profile: The effects of vitamin D on lipid profile have been conflicting, with one Mendelian randomization study showing high 25(OH)D associated with favorable lipid profile [55]. However, another larger study failed to confirm a causal association [56].
Hypertension: Vimaleswaran et al. showed an 8.1% decrease in odds of hypertension with each 10% increase in genetically determined 25(OH)D levels [57]. Another study, which concentrated on vitamin D-binding protein failed to show association with mean arterial blood pressure [58].
Overall, some Mendelian randomization studies failed to show associations between 25(OH)D levels and risk of ischemic heart disease or myocardial infarction and overall mortality [59, 60]. In a large Mendelian randomization study, genetically low 25(OH)D levels were associated with increased all-cause mortality and cancer mortality but not with cardiovascular mortality [61]. The conflicting evidence in Mendelian randomization studies may stem from the low genetically determined variability in 25(OH)D levels, thus requiring a very large sample size to detect associations.
Randomized Clinical Trials
Observational studies have shown association between the vitamin D deficiency and cardiovascular diseases [62,63,64,65,66,67]. Possible mechanisms leading to cardiovascular disease include endothelial dysfunction, over activity of the RAAS, vascular non-compliance, inflammation, effects relating to PTH, direct effects of calcium flux affecting myocardial contractility, and increased risk of diabetes [26, 62, 68,69,70]. However, none of these observational studies prove causality, emphasizing the need for randomized controlled trials. There have been several studies studying the effects of vitamin D supplementation alone or along with calcium to study non-skeletal outcomes. Over the past couple of years, a number of meta-analyses have been published to reanalyze the data and provide better evidence.
A meta-analysis of existing randomized controlled trials to estimate the effects of vitamin D supplements with or without calcium on myocardial infarction or ischemia or cerebrovascular disease, cancer, total fractures, fractures, and mortality was assessed by Bolland et al. [71]. Analyzing data from a total nine trials with 48,647 people for myocardial infarction or ischemic heart disease using a risk reduction threshold of 5% for mortality and 15% for other outcomes, no effect of vitamin D supplementation was seen [71]. When individual outcomes were evaluated separately, the results were similar to the pooled data—myocardial infarction (RR, 1.04; 95% CI, 0.91–1.17), ischemic heart disease or cardiovascular disease (RR, 1.12; 95% CI, 0.78–1.62), stroke (RR, 1.00; 95% CI, 0.88–1.13), and stroke (RR, 1.05; 95% CI, 0.82–1.34) [71].
Ford et al. analyzed 21 studies (n = 13,033) in their systemic review that compared vitamin D to placebo or control and found no significant reduction in the risk event for heart failure (HR, 0.82; 95% CI, 0.58–1.1), myocardial infarction (HR, 0.96; 95% CI, 0.83–1.10), and stroke (HR, 1.07; 95% CI, 0.91–1.29) [72]. Cholecalciferol, calcitriol, ergocalciferol, and vitamin D analogs [doxercalciferol, alfacalcidol, 2-methylene-19-nor-(20S)-1α, 25-dihydroxyvitamin D3, or 1α,25-dihydroxy-2β-(3-hydroxypropyloxy) vitamin D3] were the different forms of vitamin D used in the randomized clinical trials in the meta-analysis [72].
Neither beneficial nor harmful effects are reported in various other meta-analyses comparing interventions with vitamin D and analogs on cardiovascular events including myocardial infarction, heart failure, stroke, or mortality [71,72,73,74,75,76,77]. However, a recent collaborative European study in a paper analyzing various meta-analyses of randomized clinical trials (RCTs) on non-skeletal outcomes of vitamin D could not rule out the association from numerous observational studies linking low vitamin D levels to CVD due to lack of RCTs specifically designed to study supplementation effects on CV health especially in patients with low 25(OH)D levels [78]. Thus, current evidence from meta-analyses of vitamin D supplementation does not suggest an effect; however, most studies were performed for other outcomes, used disparate doses and vitamin compounds, and did not evaluate participants specifically with low 25(OH)D levels. Thus, results of currently ongoing clinical trials are highly anticipated [79].
Cardiovascular Risk Factors: Randomized Clinical Trials
Hypertension : Numerous cross-sectional and observational studies have shown inverse association between levels of 25(OH)D levels and blood pressure. However, as observational studies are marked by various confounding factors and do not infer causality, several RCTS have been designed to study vitamin D’s effect on blood pressure. The results have been inconclusive so far.
A recent meta-analysis by Golzarand et al. of 30 RCTs with 4744 participants found no effect on systolic blood pressure (SBP) (−0.68 mmHg; 95% CI, −2.19–0.84) and diastolic blood pressure (DBP) (−0.57 mmHg; 95% CI, −1.36–0.22) [80]. However, a subgroup analysis showed significant reduction in both SBP and DBP when a daily dose of >800 IU/day of vitamin D3 was given to subjects ≥50 years for <6 months [80]. Vitamin D at dose ≥800 IU/day increases serum 25(OH)D levels more effectively than doses <800 IU/day. Similar results were also reported by Wu et al. who showed significant reduction in SBP with vitamin D supplementation when compared to calcium and placebo (weighted mean difference (WMD), −2.44; 95% CI, −4.86 and −0.02) [81]. No significant effect on DBP was reported (WMD, −0.02; 95% CI, −4.04 and 4.01) [81]. But this study was limited by small number of trials as they included only 4 RCTs involving 429 individuals of oral vitamin D supplementation in normotensive or hypertensive individuals [81].
A small, statistically significant reduction in DBP (−3.1 mmHg; 95% CI, −5.5 to −0.6) was reported in another meta-analysis [82]. Although a nonsignificant reduction in SBP in vitamin D group was noted when compared to placebo (−3.6 mm Hg; 95% CI, −8.0 to 0.7), subgroup analysis suggested a greater drop in SBP when inactivated form of vitamin D was used rather than activated vitamin D (−6.2 mmHg; 95% CI, −12.32 to −0.04 versus +0.7 mmHg; 95% CI, −4.8 to 6.2) [82]. This was similar to the results reported by Lee et al. in a meta-analysis of 15 RCTs for patients with type 2 diabetes mellitus receiving vitamin D supplementation with a combined effect estimate of −0.16 mmHg (95% CI, −0.30 to −0.02; p = 0.02) [83]. A significant reduction in DBP (−1.31; 95% CI, −2.28 to 0.34 mmHg) was reported by Kunutsor et al. in a meta-analysis of 16 trials although the effect was limited to participants with pre-existing cardio-metabolic disease [84]. A nonsignificant reduction in SBP (−0.94; 95% CI, −2.98 to 1.10 mmHg) and DBP (−0.52; 95% CI, −1.18 to 0.14) with heterogeneity and publication bias was noted in pooled meta-analysis [84].
No beneficial effects were reported by Beveridge et al., Pittas et al., and Elamin et al. in their meta-analyses [73, 85, 86]. On the contrary, Manousopoulou et al. reported a significant increase in SBP after vitamin D supplementation (fixed-effects model, standardized mean difference, 0.24; 0.11–0.37; random-effects model, standardized mean difference, 0.24; 0.09–0.39; I 2 = 23.0) [87]. Golzarand et al. had also reported an increase in SBP and DBP in subgroup analysis of their meta-analysis, although it was only seen in patients with vitamin D in combination with calcium supplementation and vitamin D supplementation alone in overweight and obese subjects [80]. Thus, the evidence currently is mixed about vitamin D blood pressure-lowering effects.
Diabetes mellitus : An association between low 25(OH)D and impaired glucose tolerance, hemoglobin A1c levels, and diabetes status has been shown in various observational studies. Various RCTs and meta-analyses have been designed to evaluate causality.
A recent meta-analysis by George et al. showed a significant improvement in fasting glucose levels (−0.32 mmol/L; 95% CI, −0.57 to −0.07) and a small improvement in insulin resistance (standard mean difference, −0.25; 95% CI, −0.48 to −0.03) [88]. However, the study was restricted to patients with diabetes or impaired glucose tolerance [88]. In another meta-analysis including 12 RCTs and 1181 overweight and obese individuals, no effect on glucose concentration, insulin levels, and HOMA-IR values was seen [89]. A subgroup analysis taking supplement dose, time of supplementation, and baseline of 25(OH)D concentration also did not show an effect [89]. Similar results were also reported by another meta-analyses by Haroon et al. with no significant effect on hemoglobin A1c, beta-cell function, and insulin resistance in long-term studies (follow-up >3 months) [90]. However, most of these trials were small, and not all the patients included reported vitamin D deficiency [90].
Conclusion
In this review, we have described animal and human observational studies and randomized clinical trials of vitamin D and cardiovascular disease. Animal and tissue culture studies suggest that vitamin D deficiency may cause cardiovascular disease including its precursors of hypertension and diabetes mellitus. Interestingly, very high vitamin D levels are also associated with vascular calcification in animal models. Thus, there may be a target level that is not too high or too low. Observational studies fairly consistently show an association between low vitamin D levels and cardiovascular disease. However, randomized clinical trials have not conclusively shown a decrease in cardiovascular disease with vitamin D supplementation. Randomized trials have used different doses and formulations of vitamin D, different length of treatment, and different 25(OH)D level entry criteria and often had endpoints that were not primarily cardiovascular disease. Thus, there continues to be a need for well-designed, large randomized clinical trials evaluating the effects of an adequate dose of vitamin D on cardiovascular disease.
References
Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13:719–64.
Scragg R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int J Epidemiol. 1981;10:337–41.
Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–47.
Weber KT, Weglicki WB, Simpson RU. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res. 2009;81:500–8.
Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–9.
Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98.
Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–93.
Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014;64:1290–8.
Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80.
Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, Ishida T, Hirata K. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30:2495–503.
Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282:29821–30.
Chen S, Sun Y, Agrawal DK. Vitamin D deficiency and essential hypertension. J Am Soc Hypertens. 2015;9:885–901.
Pilz S, Kienreich K, Rutters F, de Jongh R, van Ballegooijen AJ, Grubler M, Tomaschitz A, Dekker JM. Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr Diab Rep. 2013;13:261–70.
Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50:303–12.
Wong KE, Szeto FL, Zhang W, Ye H, Kong J, Zhang Z, Sun XJ, Li YC. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296:E820–8.
Wang JH, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol. 2009;113:222–6.
Wehmeier K, Beers A, Haas MJ, Wong NC, Steinmeyer A, Zugel U, Mooradian AD. Inhibition of apolipoprotein AI gene expression by 1, 25-dihydroxyvitamin D3. Biochim Biophys Acta. 2005;1737:16–26.
van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: a systematic review and meta-analysis of prospective studies. Am Heart J. 2013;165:655–64, 664.e651–5.
Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13:404–17.
Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, Streppel M, Gardiner J, Ordonez-Mena JM, Perna L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656.
Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–29.
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903.
Brondum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32:2794–802.
Fan H, Yu W, Cao H, Li J, Liu B, Wang J, Shao Y, Fan Y, Yang J, Zhang Q, Hu X. Meta-analysis of circulating 25-hydroxyvitamin D levels and risk of cardiovascular and all-cause mortality in elderly population. Int J Cardiol. 2014;176:1025–9.
Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–33.
Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14.
Sokol SI, Tsang P, Aggarwal V, Melamed ML, Srinivas VS. Vitamin D status and risk of cardiovascular events: lessons learned via systematic review and meta-analysis. Cardiol Rev. 2011;19:192–201.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Tomson J, Emberson J, Hill M, Gordon A, Armitage J, Shipley M, Collins R, Clarke R. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12,000 deaths. Eur Heart J. 2013;34:1365–74.
Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100.
Joergensen C, Reinhard H, Schmedes A, Hansen PR, Wiinberg N, Petersen CL, Winther K, Parving HH, Jacobsen PK, Rossing P. Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care. 2012;35:168–72.
Lim S, Shin H, Kim MJ, Ahn HY, Kang SM, Yoon JW, Choi SH, Kim KW, Song JH, Choi SI, et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. J Clin Endocrinol Metab. 2012;97:169–78.
Karohl C, Vaccarino V, Veledar E, Goldberg J, Tangpricha V, Bellasi A, Raggi P. Vitamin D status and coronary flow reserve measured by positron emission tomography: a co-twin control study. J Clin Endocrinol Metab. 2013;98:389–97.
Degerud E, Loland KH, Nygard O, Midttun O, Ueland PM, Seifert R, Strand E, Bleie O, Dierkes J. Vitamin D status was not associated with ‘one-year’ progression of coronary artery disease, assessed by coronary angiography in statin-treated patients. Eur J Prev Cardiol. 2015;22:594–602.
Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–21.
Ullah MI, Koch CA, Tamanna S, Rouf S, Shamsuddin L. Vitamin D deficiency and the risk of preeclampsia and eclampsia in Bangladesh. Horm Metab Res. 2013;45:682–7.
Ullah MI, Uwaifo GI, Nicholas WC, Koch CA. Does vitamin d deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int J Endocrinol. 2010;2010:579640.
Qi D, Nie XL, Wu S, Cai J. Vitamin D and hypertension: prospective study and meta-analysis. PLoS One. 2017;12:e0174298.
Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. 2013;5:3551–62.
Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–8.
Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–8.
Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F, InterAct C, Langenberg C, Wareham NJ, Forouhi NG. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3:35–42.
Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. 2012;126:270–7.
Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr. 2010;64:1457–64.
Kelishadi R, Farajzadegan Z, Bahreynian M. Association between vitamin D status and lipid profile in children and adolescents: a systematic review and meta-analysis. Int J Food Sci Nutr. 2014;65:404–10.
Lupton JR, Faridi KF, Martin SS, Sharma S, Kulkarni K, Jones SR, Michos ED. Deficient serum 25-hydroxyvitamin D is associated with an atherogenic lipid profile: the Very Large Database of Lipids (VLDL-3) study. J Clin Lipidol. 2016;10:72–81.e71.
Faridi KF, Zhao D, Martin SS, Lupton JR, Jones SR, Guallar E, Ballantyne CM, Lutsey PL, Michos ED. Serum vitamin D and change in lipid levels over 5 y: the Atherosclerosis Risk in Communities study. Nutrition. 2017;38:85–93.
Challoumas D. Vitamin D supplementation and lipid profile: what does the best available evidence show? Atherosclerosis. 2014;235:130–9.
Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, Reis JP, Gross M, Eckfeldt JH, Folsom AR. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: the ARIC (Atherosclerosis Risk in Communities) study. JACC Heart Fail. 2015;3:347–56.
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8.
Verduijn M, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant. 2010;25:1394–8.
Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383.
Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:298–306.
Skaaby T, Husemoen LL, Martinussen T, Thyssen JP, Melgaard M, Thuesen BH, Pisinger C, Jorgensen T, Johansen JD, Menne T, et al. Vitamin D status, filaggrin genotype, and cardiovascular risk factors: a Mendelian randomization approach. PLoS One. 2013;8:e57647.
Ooi EM, Afzal S, Nordestgaard BG. Elevated remnant cholesterol in 25-hydroxyvitamin D deficiency in the general population: Mendelian randomization study. Circ Cardiovasc Genet. 2014;7:650–8.
Vimaleswaran KS, Cavadino A, Berry DJ, LifeLines Cohort Study Investigators, Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:719–29.
Leong A, Rehman W, Dastani Z, Greenwood C, Timpson N, Langsetmo L, Berger C, Metastroke, Fu L, Wong BY, et al. The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: a Mendelian randomization study. PLoS Med. 2014;11:e1001751.
Brondum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol. 2015;44:651–61.
Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W, Pieber TR, Obermayer-Pietsch B, Renner W. Vitamin D and mortality: a Mendelian randomization study. Clin Chem. 2013;59:793–7.
Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330.
Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int. 2013;24:2167–80.
Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH. Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol. 2012;27:581–91.
Fleck A. Latitude and ischaemic heart disease. Lancet. 1989;1:613.
Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–63.
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11.
Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60.
Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9.
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38.
Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11:7–12.
Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:307–20.
Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M, RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–55.
Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42.
Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:824–34.
Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ, Korean Meta-Analysis Study Group. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10.
Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, Spuls P, Harmonising Outcome Measures for Atopic Dermatitis Initiative. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. 2013;132:1337–47.
Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–23.
Rejnmark L, Bislev LS, Cashman KD, Eiriksdottir G, Gaksch M, Grubler M, Grimnes G, Gudnason V, Lips P, Pilz S, et al. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One. 2017;12:e0180512.
Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71.
Golzarand M, Shab-Bidar S, Koochakpoor G, Speakman JR, Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: an updated meta-analysis. Nutr Metab Cardiovasc Dis. 2016;26:663–73.
Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. 2010;103:729–37.
Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–54.
Lee KJ, Lee YJ. Effects of vitamin D on blood pressure in patients with type 2 diabetes mellitus. Int J Clin Pharmacol Ther. 2016;54:233–42.
Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. 2014;29:1–14.
Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745–54.
Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29.
Manousopoulou A, Al-Daghri NM, Garbis SD, Chrousos GP. Vitamin D and cardiovascular risk among adults with obesity: a systematic review and meta-analysis. Eur J Clin Invest. 2015;45:1113–26.
George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29:e142–50.
Jamka M, Wozniewicz M, Jeszka J, Mardas M, Bogdanski P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep. 2015;5:16142.
Nigil Haroon N, Anton A, John J, Mittal M. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: a systematic review of interventional studies. J Diabetes Metab Disord. 2015;14:3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Sondarwa, K., Buttar, R.S., Hensley, V., Melamed, M.L. (2018). Vitamin D and Cardiovascular Disease. In: Liao, E. (eds) Extraskeletal Effects of Vitamin D. Contemporary Endocrinology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-73742-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-73742-3_8
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-73741-6
Online ISBN: 978-3-319-73742-3
eBook Packages: MedicineMedicine (R0)