Abstract
Nosema ceranae is a relatively new and widespread parasite of the western honeybee Apis mellifera that provokes a new form of nosemosis. In comparison to Nosema apis, which has been infecting the honeybee for much longer, N. ceranae seems to have co-evolved less with this host, causing a more virulent disease. Given that N. apis and N. ceranae are obligate intracellular microsporidian parasites, needing host energy to reproduce, energetic stress may be an important factor contributing to the increased virulence observed. Through feeding experiments on caged bees, we show that both mortality and sugar syrup consumption were higher in N. ceranae-infected bees than in N. apis-infected and control bees. The mortality and sugar syrup consumption are also higher in N. apis-infected bees than in controls, but are less than in N. ceranae-infected bees. With both microsporidia, mortality and sugar syrup consumption increased in function of the increasing spore counts administered for infection. The differences in energetic requirements between both Nosema spp. confirm that their metabolic patterns are not the same, which may depend critically on host–parasite interactions and, ultimately, on host pathology. The repercussions of this increased energetic stress may even explain the changes in host behavior due to starvation, lack of thermoregulatory capacity, or higher rates of trophallaxis, which might enhance transmission and bee death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is obvious that, when a parasite outcompetes its host for the nutrient resources available, the host itself will suffer severe energetic stress. Infected hosts may appear to compensate for such a situation by feeding more, although they also are less efficient in obtaining energy than uninfected hosts. Therefore, not only do parasites take nutrients and energy away from hosts, but they also lower the rate at which energy becomes available for hosts to carry out vital functions (Walkey and Meakins 1970). Changes in feeding behavior following infection are just one example of the many potential alterations that might arise due to the need to assimilate more nutrients (Milinski 1985), and indeed, energetic stress typically underlies many of the physiological and behavioral alterations induced by infection (Milinski 1984).
Microsporidia are spore-forming fungal pathogens that develop as obligate intracellular organisms and infect a wide variety of hosts, ranging from insects to mammals (Adl et al. 2005). As they lack mitochondria and reproduce rapidly within a host cell, taking up ATP from their surroundings, parasitic microsporidia are particularly likely to exert severe energetic stress on their hosts (Williams 2009). Nosema apis is a well-studied microsporidian gut parasite that has co-evolved with the western honeybee Apis mellifera and is known to cause a series of metabolic changes (Bailey 1981). However, the extent of the energetic stress induced on the honeybee through N. apis infection remains unclear. While a decrease in feeding has been observed following the infection of caged bees (Rinderer and Elliot 1977), in another study, food consumption increased with no increase in oxygen consumption, suggesting that the parasite itself is likely to be responsible for producing more hunger rather than the host augmenting its metabolic activity (Moffett and Lawson 1975). By contrast, Nosema ceranae is a relatively new microsporidian parasite of A. mellifera (Higes et al. 2006; Huang et al. 2007) that was found to cause energetic stress in honeybees, diminishing the survival of infected individuals (Mayack and Naug 2009). By comparison to N. apis, N. ceranae appears to be more virulent at both the individual and colony levels (Higes et al. 2008; Paxton et al. 2007). This increased virulence could reflect the fact that the parasite–host relationship of N. ceranae has evolved over a relatively short period and therefore it exerts more energetic stress on its host. The implications of energetic stress on virulence at the colony level must be taken into consideration since foragers carry a disproportionate parasite load following N. ceranae infection (Higes et al. 2008). About 30% of the colonies' energy is spent on foraging, and thus, a decrease in the energy available to individuals in association with the changes in metabolic rate for foraging following infection can strongly affect the energy balance at the colony level and, by consequence, the success of the colony (Harrison and Fewell 2002).
In recent reviews of N. ceranae, the need for more comparative studies of N. apis and N. ceranae was highlighted in order to distinguish the intrinsic mechanisms associated with the increased virulence observed in infected bees (Paxton 2010; Fries 2009). The virulence associated with each parasite–host complex can either drastically increase or decrease over time, and as a result, each must be studied in a case by case manner (May and Anderson 1990). Therefore, feeding experiments on caged bees have been performed to quantify the energetic stress induced by N. apis and N. ceranae infection. In addition, the mortality of the bees associated with either parasite has been monitored as an indicator of virulence.
Methods
Nosema-free honeybees for experimental infection
Frames of capped brood were obtained from a healthy colony of Apis mellifera iberiensis (Nosema-free confirmed by PCR) located in an experimental apiary 20 km from the “Centro Apícola”, and they were kept in an incubator at 34°C (±1°C) to provide a supply of newly emerged Nosema-free honeybees. The emergent worker bees were carefully removed, and groups of 25 bees were confined to 40 different cages (175 mm long, 45 mm in diameter) that were kept in the incubator for 5 days. The bees were fed ad libitum with a sucrose solution (50% w/w in distilled water) combined with 2% Promotor L (Calier Lab.), a commercial mixture of amino acids and vitamins. Honey and pollen were not used to feed the bees to avoid possible contamination with infective Nosema spp. spores.
Production of viable N. apis and N. ceranae spores
N. apis and N. ceranae spores were obtained from experimentally infected bees as described previously (Higes et al. 2007). Spores were isolated from adult honeybee samples of naturally infected Spanish honeybee colonies sent to our laboratory for pathological studies. The abdomens of bees were macerated in distilled water (PCR grade) using a sterile manual tissue grinder. The ground contents were filtered through a Whatman mesh (no. 4), and the resulting suspension was re-suspended in distilled water (PCR grade), which was then centrifuged. Each time a pellet of mature spores formed at the bottom of the tube, the liquid at the top was discarded. This procedure was repeated three times to remove all the contaminating debris, and the spores were then quantified using a hemocytometer (OIE 2008), while the Nosema species was confirmed by PCR (Martín-Hernández et al. 2007). The spores were divided into batches, stored in distilled water, and they were maintained at a constant temperature until they were used to induce infection.
Experimental design to determine energy demands and survival
Experimental infection was induced in 5-day-old bees as described previously (Higes et al. 2007), and the experimental groups were classified as indicated in Table 1. The concentrations of N. apis or N. ceranae spores administered were 0, 103, 104, 5 × 104 and 105 spores per bee, and each of the ten groups was established with four replicates of 25 bees in a cage.
Before placing the bees in the cages, the bees were starved for 2 h, and then, they were fed individually with 2 μl of 50% sucrose solution containing the appropriate concentration of the inoculum. To achieve the correct dosage, honeybees were anesthetized with CO2 for ease of handling. When each bee woke up, the droplet of 50% sucrose solution mixed with the spores was administered by touching a micropipette to the bee's mouthparts until the entire droplet was consumed. Bees that did not consume the entire droplet were discarded (Malone et al. 1999). Uninfected control bees were fed with 2 μl of the 50% sucrose solution alone.
After inducing infection, the bees in each cage were fed ad libitum with a sugar syrup made up of 50% sucrose solution with 2% Promotor L (Calier Lab.) through an individual feeder attached to the cage. In order to measure the nutritional demand of infected and uninfected bees, the amount consumed was used as a measure of energetic stress, as described previously (Mayack and Naug 2009). On days 1 (D1), 2 (D2), and 6 (D6) post-infection (p.i.), the amount of syrup consumed by each cage of bees was recorded by weighing the feeder, and the mean amount consumed per bee at each time point was then calculated (on the 6th day p.i., the total ingested food was calculated as the daily average consumed food). Two different incubators (Memmert® Mod. IPP500, ±0.1°C) were maintained at 33°C; one contained the N. ceranae-infected bees, and the other, N. apis-infected bees, in order to avoid cross-contamination. In addition, a group of uninfected bees were kept in each of the two incubators with the infected bees. The cages were observed daily for bee mortality, and all dead bees were removed when detected.
On the seventh day post-infection, all the remaining living bees were sacrificed by freezing, and the entire abdomen was removed to confirm the Nosema species present using the multiplex PCR method described previously (Martín-Hernández et al. 2007). Bees of both control groups served as negative controls and confirmed the absence of infection.
Statistical analysis
A generalized linear model (GLM) was used to study the dependent variables (syrup consumption at three different time points, D1, D2, and D6) and the effects of two fixed factors. These factors included infection at three levels (no infection, infection with N. apis, infection with N. ceranae), the spore dose administered, and the model used (intercept, infection, and nested spore dose in infection). The differences were calculated to a 95% confidence level with a Wald chi-square test, and the same test was used to estimate the parameters selected to compare the effect between each dose and to calculate the differences between them.
The data were represented in bar graphs. The differences in syrup consumption on the 3 days post-infection were averaged according to the spore doses administered, and then, the differences between the spore doses administered averaged over the 3 days were compared using repeated measures ANOVA with a post hoc Bonferroni analysis.
Results
N. ceranae-infected bees consumed significantly more syrup than N. apis-infected or uninfected bees throughout the experiment. In infected bees, the amount of syrup consumed increased with the dose of the N. ceranae spore inoculum at all the time points analyzed.
Nutritional demands
The overall amount of syrup consumed increased significantly up to day 2 p.i., but it decreased significantly from days 2 to 6 p.i. On days 3, 4, and 5 p.i., the amount of syrup fed to the bees was not recorded as the quantity consumed was negligible. On day 1 p.i. (D1 time point), the fixed factors of the GLM showed an effect of infection on syrup consumption in the bees (Wald chi-square = 480.2; p < 0.0001), and on the 2nd day p.i. (D2 time point), the results were similar to those observed on D1. On the 6th day p.i. (D6 time point), only N. ceranae-infected bees consumed more syrup than N. apis and uninfected bees (Wald chi-square = 45.1, p < 0.0001; Fig. 1).
The amount of syrup consumed on the 3 days post-infection (days 1, 2, and 6 p.i.) by N. apis (N = 16; 4 replicates × 4 spore doses for each day; striped bars) and N. ceranae (N = 16; 4 replicates × 4 spore doses for each day; solid bars) compared to their respective control group (N = 4; 4 replicates for day and Nosema species; clear bars). For clarity, the infected data are pooled across the 1,000–100,000 spore parasite loads administered at the beginning of the experiment. The data represent the mean values for each group, and their standard deviation bars and the multiple asterisks indicate highly significant differences between means tested at the 0.05 alpha level
Parasite load
As the spore dose increased, there was a significant increase in the amount of syrup consumed by infected bees (Wald chi-square = 152.3; p < 0.0001). At all spore doses, N. ceranae-infected bees consumed significantly more syrup than N. apis-infected or uninfected bees, and N. apis-infected bees consumed more syrup than uninfected ones. Considering the combined effects in N. ceranae-infected bees (Nosema infection and spore dose), the highest amount of syrup consumption was recorded in bees infected with the largest dose of spores, and a dose of 105 spores per bee was the only dose that produced significantly higher consumption than the rest of the spore doses assayed (Wald chi-square = 104.7, p < 0.0001). The same was true for N. apis infection except that the highest amount of spores administered was not significantly different from the rest (Wald chi-square = 22.6, p < 0.0001; Fig. 2).
The amount of syrup consumed by bees infected with N. apis (N = 12; 4 replicates × 3 days for spore dose; striped bars), N. ceranae (N = 12; 4 replicates × 3 days for spore dose; solid bars), and their respective controls (N = 12; 4 replicates × 3 days; clear bars), plotted as a function of the 1,000–100,000 parasite load administered at the beginning of the experiment. For clarity, the infected data are pooled across the three measurements post-infection. The data represent the mean values for each group with the standard deviation bars and letters indicating significant differences between means at the 0.05 alpha level
Bee mortality
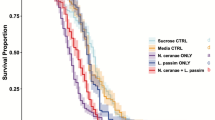
Honeybee mortality was assessed at different time points during the experiment (Table 2), and no mortality was observed until day 6 p.i. in some of the infected groups. The mortality rates of infected bees showed a similar pattern to their overall energetic demands and the corresponding Nosema spore doses. Not only did bees infected with the higher doses of N. apis spores suffer higher mortality, but even greater mortality was seen in those infected with N. ceranae spores. As such, the highest mortality was evident in the bee group infected with the largest dose of N. ceranae spores (105 spores/bee), which produced 60.7% of death among the bees 6 days p.i. Indeed, mortality reached 93.1% after 7 days p.i. in this group of bees, at which time the mortality was higher in all N. ceranae-infected groups than in the control groups. In the case of N. apis infection, only the higher doses of spores (105 and 5 × 104 spores/bee) produced more mortality than in the control group of bees.
Discussion
These results presented here clearly show that N. ceranae imposes greater energetic stress on infected bees, suggestive of a stronger virulence in comparison to N. apis. Energetic stress is related to the increasing doses of spores for infection, and it is highest in N. ceranae-infected bees, which also suffer the worst survival. Furthermore, these data are consistent with previous studies that demonstrate the detrimental effects of N. ceranae on individual bees and on colony survival as a whole (Higes et al. 2008; Mayack and Naug 2009; Naug and Gibbs 2009; Alaux et al. 2010). Energetic stress imposed by N. ceranae is much more substantial than previously thought, and it persists for much longer, lasting for up to 6 days post-infection.
The severe energetic stress observed in Nosema-infected bees is probably due to the parasite itself competing directly with its host for key nutrients and energy resources, like most microsporidian species. N. ceranae and N. apis only develop when in direct contact with the host cell cytoplasm (Fries et al. 1996; De Graaf et al. 1994; Weidner et al. 1999; Higes et al. 2007; Chen et al. 2009a), indicating that the parasite may require some external energy supply to reproduce (Weidner et al. 1999). Microsporidia lack mitochondria, and they have long been suspected to either take up ATP from the host cell environment or gain ATP by metabolizing host cell carbohydrates through the glycolytic pathway (Weidner et al. 1999). Consistent with this notion of ATP uptake, microsporidia have frequently been seen to be surrounded by host mitochondria, which probably facilitate the uptake of ATP from host cells (Dufort et al. 1987; Sokolova et al. 1988; Williams 2009).
In these experiments, the parasite dependence on host energy is manifested by the increase in syrup consumption by infected bees, as seen in earlier studies (Mayack and Naug 2009; Alaux et al. 2010). However, it is interesting to note that we found significantly higher feeding in N. ceranae-infected bees than in N. apis-infected bees. Some years ago, it was found that heavily infected cells of the gut lining may either be dead or dying, provoking poor nutrient absorption in the midgut of the bee and eventually leading to the early death of bees due to starvation (Liu 1984). Intriguingly, there was no difference in virulence between N. apis- and N. ceranae-infected bees fed ad libitum when administered the same doses of spores as in this experiment (Forsgren and Fries 2010) probably due to differences in the method of infection in the laboratory (e.g., house adult worker bees obtained from combs versus newborn bees obtained in an incubator, etc.). Therefore, the stronger virulence (mortality) of N. ceranae-infected bees at the same parasite load as N. apis is probably due to the severe energetic stress caused by the increased lack of nutrient absorption, which is likely to be due to more aggressive destruction of the gut lining. As a matter of fact, the degeneration of epithelial ventricular cells of the gut lining is more severe in N. ceranae-infected bees than in N. apis-infected bees, diminishing nutrient absorption (Higes et al. 2007) The more aggressive damage to the gut lining in N. ceranae-infected caged worker bees has also been detected in naturally infected worker and queen bees (Higes et al. 2008; 2009a, b).
Malnutrition that results in a higher within-host parasite load does not necessarily suppress immunocompetence that leads to a higher number of infected individuals in the population, as seen with microsporidia infecting vertebrate hosts (reviewed by Wakelin 1989 and Lloyd 1995). Invertebrate generation times are much shorter than those of vertebrates, and infection of invertebrates is usually chronic. Invertebrate parasites depend strongly on host resources (Pulkkinen and Ebert 2004), and thus, in some instances, severe starvation limits the growth of the parasite within invertebrate hosts. Indeed, severe energetic stress was shown to impede parasite spread and the decrease of the number of infected individuals at a population level when Daphnia was infected with the microsporidian Glugoides intestinales (Pulkkinen and Ebert 2004). However, this does not appear to be the case in this study, where consuming significantly more syrup over the period tested probably yields enough additional energy to support the reproduction and larger parasite load of N. ceranae-infected bees (Chen et al. 2009b).
The severe energetic stress caused by N. ceranae when compared with N. apis may be related to the immune suppression seen in infected bees. Indeed, there is a clear trade-off between energy acquisition and activation of the insect's immune system to fight infections (Schmid-Hempel 2005). Short-term food deprivation in insects leads to a downregulation of the immune system, resulting in less resistance when challenged with infection. When fed under ideal conditions, the immune system is restored, demonstrating the energetic cost associated with maintaining an effective immune system (Siva-Jothy and Thompson 2002; Feder et al. 1997). In contrast to the activation of the immune system by N. apis, N. ceranae causes immune suppression (Antúnez et al. 2009), much like the Varroa mite that induces energetic stress and facilitates multiple co-infections in honeybees (Gregory et al. 2005; Yang and Cox-Foster 2005).
It is important to note that energetic stress was observed in infected bees maintained under ideal laboratory conditions, while under natural conditions, a combination of other factors may represent additional negative influences on honeybees. For example, at sub-lethal levels, pesticides like neonicotinoids have been shown to cause a further increase in energetic stress in N. ceranae-infected honeybees (Alaux et al. 2010). Energetic stress has important implications for the success of bee foraging since carbohydrates are their main source of fuel for flight, and foraging is a metabolically expensive activity (Rothe and Nachtigall 1989). Moreover, energetic stress is suspected to be the cause of the poor thermoregulation in infected foragers when they are chilled, and infected bees seek warmer locations within the hive to compensate when they feel cold. This deficient thermoregulation increases the probability of foragers suffering hypothermia, leading to their incapacity to sustain flight and provoking forager starvation outside of the colony (Campbell et al. 2010). Indeed, free-flying foragers infected with N. ceranae have lower hemolymph trehalose levels due to energetic stress, and based on the differences in sugar levels, it would be predicted that infected foragers could only fly two thirds the distance of an uninfected forager (Mayack and Naug 2010). The fact that the pollinators' habitat is declining adds yet further stress on honeybee foragers, causing an increase in the distance a honeybee forager has to fly to collect nectar and pollen for the colony (Naug 2009).
Not only would N. ceranae infection have some individual behavioral effects on foragers, but the colony could also be affected. Increased hunger due to N. ceranae infection leads to differences in trophallaxis rates within the colony, which may increase the rate of transmission and the spread of the disease (Feigenbaum and Naug 2010; Naug and Gibbs 2009). An increase in an individual's hunger through infection may increase the overall rate of foraging in the colony, as well as the colony's energetic demand regulating foraging. Indeed, hunger at the level of individual foragers within the colony increases foraging rates (Howard and Tschinkel 1980; Toth et al. 2005). Moreover, it is probable that vitellogenin levels can be indirectly modulated by nutritional stress, thereby inducing infected bees to start foraging earlier (Amdam and Omholt 2003; Nelson et al. 2007). The probability of infected foragers starving to death would also increase as they might be unable to fly back to the hive due to energetic stress (Mayack and Naug 2009; Naug 2009). As such, starvation may contribute to colony depopulation, highly infected foragers having been found dead far from their hives (Higes et al. 2008).
Conclusion
This study contributes to the growing body of literature demonstrating that N. ceranae is more virulent than N. apis (reviewed in Higes et al. 2010). The increased virulence associated with the energetic stress observed could be due to the shorter co-evolution of the N. ceranae parasite–host complex, given that this is a relatively new parasite of the western honeybee when compared to N. apis (Higes et al. 2006; Klee et al. 2007). As described here and elsewhere, N. ceranae has a relatively important influence in terms of the fitness of its host, both alone or in combination with other agents. N. ceranae is probably less efficient in terms of the physiological integration of the host–parasite complex, and it must draw more food from its host due to less efficient energy conversion. Therefore, it is plausible that the stronger virulence observed following N. ceranae infection is due to additional energetic stress imposed by this relatively new parasite, over and above that of N. apis. Individual energetic stress from infection may have more far-reaching effects in honeybees as they are social insects, affecting the regulation of foraging or immune function, which may potentially affect the survival of infected bees.
References
Adl SM, Simpson AGB, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MFJR (2005) The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol 52:399–451
Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces LP, Le Conte Y (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12:774–782
Amdam GV, Omholt SW (2003) The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J Theor Biol 223:451–464
Antunez K, Martin-Hernandez R, Prieto L, Meana A, Zunino P, Higes M (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290
Bailey L (1981) Honey bee pathology. Academic Press, London
Campbell J, Kessler B, Mayack C, Naug D (2010) Behavioral fever in infected honeybees: parasitic manipulation or coincidental benefit? Parasitology 137:1487–1491
Chen YP, Evans JD, Murphy C, Gutell R, Zuker M, Gundensen-Rindal D, Pettis JS (2009a) Morphological, molecular and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee Apis mellifera. J Eukaryot Microbiol 56:142–147
Chen Y, Evans JD, Zhou L, Boncristiani H, Kimura K, Xiao T, Litkowski AM, Pettis JS (2009b) Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J Invertebr Pathol 101:204–209
De Graaf DC, Raes H, Sabbe G, De Rycke PH, Jacobs FJ (1994) Early development of Nosema apis (Microspora: Nosematidae) in the midgut epithelium of the honeybee (Apis mellifera). J Invertebr Pathol 63:74–81
Dufort M, Valero Y, Poguet M (1987) Particular distribución de las mitocondrias de Mytilicola intestinalis en células parasitadas por Unikaryon mytilicolae. Rev Iber Parasitol Vol Ext:1–11
Feder D, Mello CB, Garcia ES, Azambuja P (1997) Immune responses in Rhodnius prolixus: influence of nutrition and ecdysone. J Insect Physiol 43:513–519
Feigenbaum C, Naug D (2010) The influence of social hunger on food distribution and its implications for disease transmission in a honeybee colony. Insectes Soc 57:217–222
Fries I, Feng F, da Silva A, Slemenda SB, Pieniazek NJ (1996) Nosema ceranae sp (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur J Protistol 32:356–365
Fries I (2009) Nosema ceranae in European honey bees (Apis mellifera). J Invertebr Pathol 103:S73–S79
Forsgren E, Fries I (2010) Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet Parasitol 170:212–217
Gregory PG, Evans JD, Rinderer T, de Guzman L (2005) Conditional immune-gene suppression of honeybees parasitized by Varroa mites. J Insect Sci: 5:7
Harrison JF, Fewell JH (2002) Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp Biochem Physiol A Mol Integr Physiol 133:323–333
Higes M, Martín R, Meana A (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol 92:93–95
Higes M, García-Palencia P, Martín-Hernández R, Meana A (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol 94:211–217
Higes M, Martín-Hernández R, Botías C, Bailón EG, González-Porto AV, Barrios L, Nozal MJd, Bernal JL, Jiménez JJ, Palencia PG, Meana A (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10:2659–2669
Higes M, Martín-Hernández R, Garrido-Bailón E, González-Porto AV, García-Palencia P, Meana A, Del Nozal MJ, Mayo R, Bernal JL (2009a) Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ Microbiol Reports 1:110–113
Higes M, Martín-Hernández R, García-Palencia P, Marín P, Meana A (2009b) Horizontal transmission of Nosema ceranae (Microsporidia) from worker honey bees to queens (Apis mellifera). Environ Microbiol Reports 1:495–498
Higes M, Martín-Hernández R, Meana A (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41:375–392
Huang WF, Jiang JH, Chen YW, Wang CH (2007) A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 38:30–37
Howard DF, Tschinkel WR (1980) The effects of colony size and starvation on food flow in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Behav Ecol Sociobiol 7:293–300
Klee J, Besana AM, Genersch E, Gisder S, Nanetti A, Tam DQ, Chinh TX, Puerta F, Ruz JM, Kryger P, Message D, Hatjina F, Korpela S, Fries I, Paxton RJ (2007) Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J Invertebr Pathol 96:1–10
Liu TP (1984) Ultrastructure of the midgut of the worker honey bee Apis mellifera heavily infected with Nosema apis. J Invertebr Pathol 44:282–291
Lloyd S (1995) Environmental influences on host immunity. In: Grenfell T, Dobson AP (eds) Ecology of infectious diseases in natural populations. Cambridge University Press, UK
Malone LA, Giacon HA, Newton MR (1999) Comparison of the responses of some New Zealand and Australian honey bees (Apis mellifera L) to Nosema apis Z. Apidologie 26:495–502
Martín-Hernández R, Meana A, Prieto L, Martínez-Salvador A, Garrido-Bailon E, Higes M (2007) Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol 73:6331–6338
May RM, Anderson RM (1990) Parasite–host coevolution. Parasitology 100:S89–S101
Mayack C, Naug D (2009) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J Invertebr Pathol 100:185–188
Mayack C, Naug D (2010) Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J Insect Physiol 56:1572–1575
Milinski M (1984) Parasites determine a predator's optimal feeding strategy. Behav Ecol Sociobiol 15:35–37
Milinski M (1985) Risk of predation of parasitized sticklebacks (Gasterosteus aculeatus L) under competition for food. Behaviour 93:203–215
Moffet JO, Lawson FA (1975) Effect of Nosema-infection on O2 consumption by honey bees. J Econ Entomol 68:627–629
Naug D (2009) Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol Conserv 142:2369–2372
Naug D, Gibbs A (2009) Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40:595–599
Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV (2007) The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol 5:673–677
Office International des Epizooties (OIE) (2008) Manual of standards for diagnostic test and vaccines [online]. http://www.oie.int/eng/normes/mmanual/2008. Accessed 20 June 2010
Paxton RJ (2010) Does infection by Nosema ceranae cause "Colony Collapse Disorder" in honey bees (Apis mellifera)? J Apic Res 49:80–84
Paxton RJ, Klee J, Korpela S, Fries I (2007) Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38:558–565
Pulkkinen K, Ebert D (2004) Host starvation decreases parasite load and mean host size in experimental populations. Ecology 85:823–833
Rinderer TE, Elliot K (1977) Influence of nosematosis on the hoarding behavior of the honeybee. J Invertebr Pathol 30:110–111
Rothe U, Nachtigall W (1989) Flight of the honey bee. J Comp Physiol B Biochem Syst Environ Physiol 158:739–749
Schmid-Hempel P (2005) Evolutionary ecology of insect immune defenses. Annu Rev Entomol 50:529–551
Siva-Jothy MT, Thompson JJW (2002) Short-term nutrient deprivation affects immune function. Physiol Entomol 27:206–212
Sokolova YY, Timoshenko SA, Issi VI (1988) Morphogenesis and ultrastructure of life cycle stages of Nosema mesnili (Microsporidia, Nosematidae). Citologiya 30:26–33
Toth AL, Kantarovich S, Meisel AF, Robinson GE (2005) Nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol 208:4641–4649
Yang XL, Cox-Foster DL (2005) Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci USA 102:7470–7475
Wakelin D (1989) Nature and nurture: overcoming constraints on immunity. Parasitology 99:S21–S35
Walkey M, Meakins RH (1970) An attempt to balance energy budget of a host-parasite system. J Fish Biol 2:361–372
Weidner E, Findley AM, Dolgidh V, Sokolova J (1999) Microsporidian biochemistry and physiology. In: Wittner M, Weiss LM (eds) The microsporidia and microsporidiosis. ASM Press, Washington, DC, pp 172–195
Williams BAP (2009) Unique physiology of host-parasite interactions in microsporidia infections. Cell Microbiol 11:1551–1560
Acknowledgments
Author contributions: MH, RM-H designed the research; MH, RM-H and CB carried out the assay and collected the data; LB and AM-S performed statistic studies; and MH, AM, C.M. and R.M-H. wrote the paper. RTA2009-00105-C02-01 national research project and MARM-FEAGA founds (Programa Nacional Apícola 2011-2013) provided research facilities and monetary support. We would like to thank to Almudena Cepero, Virginia Albendea, Carmen Abascal, Carmen Rogerio and Teresa Corrales for their technical support. We thank Dr. Naug for revision of the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martín-Hernández, R., Botías, C., Barrios, L. et al. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol Res 109, 605–612 (2011). https://doi.org/10.1007/s00436-011-2292-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2292-9