Abstract
Nosemosis, a disease caused by a microsporidian infection, is one of the most frequently observed parasitic pathologies affecting adult honeybees. Presently, Nosema ceranae seems to be the main microsporidian infection in Apis mellifera. The antibiotic fumagillin is the only compound available to treat Nosema diseases; however, it is no longer licensed in most EU member states; therefore, the need to identify new molecules/substances prevails. The intent of this paper is to test bacterial metabolites by Bacillus and Enterococcus strains, isolated from bee midgut and honey. The toxicity on bees and the antiparasitic activity on N. ceranae were assessed under laboratory conditions. Results did not yield toxicity for the administered surfactin or bacteriocin concentrations. Spores exposed to direct contact with a particular surfactin revealed a significant infectivity reduction when inoculated on bees. This surfactin, administered ad libitum from the individuals’ emergence, led to a significant reduction in parasitosis development when bees were infected with untreated spores 7 days postemergence. Based on the results obtained, one of the surfactins is herein postulated as a molecule capable of reducing N. ceranae development, acting either by direct exposure to purified spores or incorporated into the digestive tract of the bee.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxonomically, microsporidia are defined as highly specialized fungi (Sina et al. 2005), parasitizing a wide range of hosts. When it comes to honeybees, the microsporidian Nosema ceranae is one of the etiologic agents of nosemosis, a current worldwide disease (Klee et al. 2007; Giersch et al. 2009). This intracellular parasite was first described for the Asian honey bee, Apis cerana (Fries et al. 1996), and afterward for the European bee, Apis mellifera (Higes et al. 2006). The host cell penetration occurs through the mechanical injection of the polar filament, which protrudes from the spore, forced by water influx (Delbac and Polonais 2008) and takes the infectious sporoplasm to the host cell cytoplasm, where the organism replicates.
The antibiotic fumagillin (dicyclohexylammonium), obtained from Aspergillus fumigatus, is the most widely antimicrobial agent available nowadays to treat Nosema diseases. Its efficacy has been proven by temporally reducing the parasitosis caused by N. ceranae in A. mellifera (Williams et al. 2008). However, this drug is no longer licensed in most EU member states. Efforts have been done in finding alternatives to control this disease, among them experimenting with different compounds, such as thimerosal (Gontarski and Wagner 1954; Blatz 1955; Schlüter 1957; Furgala and Bosch 1970; Liu 1988), itraconazole (Liu and Myrick 1988), thymol (Yücel and Doğaroğlu 2005; Maistrello et al. 2008), Sinefungin (Sichtova et al. 1993), organic acids (Forsgren and Fries 2005; Underwood and Currie 2009), herbal hydroalcoholic extracts (Pohorecka 2004), essential oils, lisozima, and phytoalexin resveratrol (Maistrello et al. 2008), among others.
Many bacteria strains are commonly isolated from the hive environment (Gilliam 1979; Gilliam and Prest 1978; Audisio et al. 2005; Sabaté et al. 2009; Alippi and Reynaldi 2006) and bees’ digestive tract (Gilliam 1978; Gilliam and Valentine 1976; Audisio et al. 2010). Most bacteria synthesize a wide range of molecules with potential inhibitory effects on bacteria and other organisms (Jack et al. 1995; Katz and Demain 1977; Nielsen and Sorensen 1997). These molecules include toxins, metabolic by-products such as organic acids, hydrogen peroxide, bacteriolytic enzymes, bacteriocin, or surfactin-type molecules, etc.
Surfactant molecules are cyclic lipopeptides with powerful antimicrobial properties including antifungal, antibacterial, antiviral, and antimycoplasma activity (Pooja and Swaranjit 2004). Bacteriocins, in turn, have the ability to interfere with the growth of bacteria phylogenetically related to the producing strain (Jack et al. 1995). Even though great progress has been achieved and extensive efforts are being made in the study of these molecules properties, no reports have yet been made on their anti-microsporidial activity.
The aim of the present study was to evaluate the toxicity of bacterial metabolites produced by bacteria isolated from bee’s midgut on A. mellifera individuals and to determine their antiparasitic properties against N. ceranae.
Materials and methods
Bees collection and assays conditions
Experiments were conducted in the Arthropods Laboratory of the UNMdP, Mar del Plata, Argentina, between May and August 2008. Newly emerged A. mellifera bees were obtained from a healthy colony located in the experimental apiary J. J. Nagera of the Arthropods Laboratory (Lat.: 38°10′06″ S; Long.: 57°38′10″ W) and kept under incubator conditions during the experiment (33°C ± 0.79; 60% ± 3.3 HR).
N. ceranae characterization
The Nosema spores used for inoculation were purified from a laboratory strain developed in confined workers. Newly emerged bees were inoculated, supplied with sugar cane syrup 66%, and sacrificed to retrieve fresh spores at the time of assays 2 and 3. Spores were molecularly characterized following Martin-Hernández et al. (2007). The PCR fragments were purified and sequenced in the Pro-papa Laboratory EEA, INTA, Balcarce, Argentina. Afterward, they were sequenced and matched with respect to those published in the GenBank (NCBI).
Individual infection was achieved using a technique similar to that proposed by Rinderer and Thomas (1976). This methodology enables a uniform handling of bees and obviates trofalaxia among individuals. By means of this technique, consumption is induced by previous starvation, thereby preventing oral structures manipulation to force intake and so minimizing the stress resulting from prolonged handling.
Bacterial metabolites
Surfactins
Two cyclic lipopeptides synthesized by Bacillus subtilis, were employed S1 (Mori2) and S2 (C4) (Sabaté et al. 2009). They were recovered as precipitates of different cell-free supernatants (CFS), resulting from the acidification with 55 μL of concentrated HCl. Then, lipopeptides were extracted with methanol; the solvent was evaporated, and the precipitate was dissolved in sterile distilled water at pH 8 at a final concentration of 10 mg/mL. These solutions were used as the surfactin samples 1 and 2, respectively.
Bacteriocins
Two different samples were employed: bacteriocin B1, synthesized by Enterococcus avium DSMZ17511 (Audisio et al. 2005) and bacteriocin B2, produced by Enterococcus faecium CRL1385 (Audisio et al. 1999, 2001). Both samples were produced by each bacterium in BHI broth (Britania, Argentina) and used as they were present in the CFS applying no further purification steps. The CFS was assayed at the final pH after cell growth (4.5–5.0–5.5) and at pH 6.5–7.0, adjusted with NaOH 2 N.
Metabolite concentrations were determined in duplicate by the critical dilution method (Daba et al. 1991), using Listeria monocytogenes 01/155 as the indicator culture. The resulting titers were expressed in arbitrary units per milliliter (AU ml-1) and calculated as follows: (1000)/(V s × D), where V s is the bacteriocin volume tested (microliter) and D is the highest dilution still inhibiting cell growth. Also, the lactic acid present in the cell-free supernatant of enterococci cultures was quantified by HPLC.
Assay 1—toxicity of bacteriocin and surfactin on A. mellifera
Metabolites were administered ad libitum either through a sucrose syrup (SSY)–metabolite solution (50:50) or pure solution, providing candy (powdered sugar and glucose) as carbon source. Individuals were subjected to diets from the first hour of imago, replacing solutions on a daily basis and providing fresh beebread. Diets consisted of surfactin S2 (C4) and bacteriocins B1 (pH 5.5, 7) and B2 (pH 4.5, 5.5, 7). Distilled water (surfactin was resuspended in this substance) and sterile culture medium (BHI broth, Britania, Argentina) were used as control treatments, since it was in the cultured medium where enterococci produced enterocins. The syrup was prepared at 66% w/v of sugar concentration.
Three replicates per treatment were produced, 15 bees in each one. The average solution consumption and mortality were recorded on a daily basis, quantifying the volume loss caused by evaporation.
Since infective spores were detected in pollen samples (Higes et al. 2008), a contamination control was implemented. It was made up of 15 newly emerged individuals, fed on pollen and sucrose syrup (66% w/v). Dead and alive individuals were analyzed under microscope at the end of the experiment, and spore presence was determined in the entire abdomen.
Assay 2—effect of bacteriocin and surfactin on N. ceranae spores viability after direct exposure
To analyze metabolites activity on N. ceranae spores after direct exposure, fresh spores were obtained (1.54 × 107 spores/mL) from individuals infected with the characterized strain. Spores were stored for 2 h at 4°C in distilled water. One milliliter (for each treatment) of the homogenized initial solution was centrifuged to precipitate the spores, discarding the supernatant. The volume was completed with the pure solutions of bacteriocin B1 (pH 5.5 and 7.0), bacteriocin B2 (4.5, 5.5, and 7.0), or surfactin S2, and the spores were resuspended in these solutions. They were placed in darkness at room temperature (25–27°C) and shaken periodically for 40 h. Then, they were centrifuged once again to discard metabolites, completing the volume with distilled water and centrifuging again to wash the remaining substances. The spores were resuspended/homogenized in sucrose solution, and individual inoculation was performed. Ten microliters of solution were administered to each individual (1.54 × 105 spores) on day 7 postemergence. In bacteriocins B1 and B2 (at both pH) assays, BHI broth was used as control solution. In the surfactin assay, in turn, distilled water was employed.
During the experiments, individuals were supplied with sugar cane syrup and fresh pollen. To quantify infection development, the surviving individuals were sacrificed 10 days post-infection (p.i.). The digestive tract was removed by pinching the last abdominal segments and cutting the midgut afterward. Spores number (parasitosis intensity) was individually quantified, counting the number of spores in suspension samples with a hemacytometer and light microscopy (Cantwell 1970). A contamination control treatment similar to the one described in assay 1 was also adopted.
Assay 3—effect of prolonged consumption of bacteriocins or surfactins on the development of N. ceranae
To analyze the effect of a prolonged consumption of metabolites on infection development, metabolites were administered ad libitum, including surfactin S1 in the treatments (not used in previous assays).
Solutions were administered upon individuals’ emergence, replacing the volumes on a daily basis until the experiment conclusion. Metabolites were supplied in cane sugar syrup so as to force bees to incorporate them in each feeding. Fresh pollen was also administered ad libitum. The treatments are specified in Table 1. On day 7 (after emergence), bees were individually infected with 10 μL of inoculum. A low spore inoculum was preferred so as to prevent the rapid mortality registered in infected bees subjected to a pollen diet (Rinderer and Thomas 1976; Porrini 2008) and, by so doing, prolonging metabolites consumption. Solutions were replaced daily. Ninety individuals per treatment, confined to experimental wooden boxes with a plastic mesh (11 × 9 × 6 cm3) participated in this assay.
On day 20 p.i., the surviving bees were sacrificed in order to individually quantify the number of spores in the midgut just as described in assay 2.
Solution’s evaporation was controlled to correct the consumed volume, and a pollen contamination control was adopted, as described in assay 1.
Statistical analysis
The statistical analysis of the results obtained was conducted applying α = 0.05. The spore loads recorded upon the experiment conclusion were compared to those of the control group. A t test or a Mann–Whitney test was performed if homoscedasticity had not been achieved. ANOVA and Tukey’s test were applied to compare the average consumed volumes and the accumulated mortality.
Results
N. ceranae characterization
After PCR multiplex, the fragments obtained from the spores samples only exhibited a band corresponding to 218 bp, in agreement with Martín-Hernández et al. (2007). Sequencing results were entered in the GenBank BLASTn, which yielded 98% homology with N. ceranae (accession N° FJ425736).
Bacterial metabolites
Two different sample of bacteriocins and two of surfactins were assayed. Arbitrary units (AU) were used to estimate the substances titers, quantifying the inhibitory effect against L. monocytogenes. It was 102,400 AU/mL for CFS B1, 25,600 AU/mL for CFS B2, 2,000 AU/mL for surfactin S1 (Mori2), and 5,000 AU/mL for surfactin S2 (C4). The lactic acid concentration produced by Enterococcus and determined by HPLC analyses was close to 32 mM.
Toxicity analyses of bacteriocin or surfactin on A. mellifera
No significant mortality was recorded in treated individuals after 30 days of confinement with syrup administration (ANOVA, p = 0.60) as well as in metabolites administered with no syrup (ANOVA, p = 0.57). The average consumption of sucrose solution–metabolite (50:50) was 23.05 μL/bee/day and 15.3 μl/bee/day for the administration of the pure metabolites and candy. Both values did not diverge from the quantities consumed by the control group (ANOVA, p = 0.58 and p = 0.47, respectively).
The behavior of the treated individuals was normal, being active during inspections throughout the experiment, and with no excessive dejections during confinement.
It is worth noting that no pollen contamination with spores was reported, as no spores were found in contamination control bees at the end of the experiment.
Bacteriocin and surfactin effect on N. ceranae spore viability after direct exposure
Sample 2 of surfactin, synthesized by B. subtilis C4, was the only treatment that significantly reduced spores viability after a 40-h exposure, decreasing parasite intensity nearly 50% after 10 days of having infected the individual with the treated inoculum.
No significant bee mortality was accounted during the 17-day trial. Figure 1 depicts the intensity values of spores recorded for each individual, grouped according to an arbitrary scale of intensity assessment (number of spores in midgut): low intensity (0–1 × 106), medium intensity (1–10 × 106), and high intensity (>10 × 106). The general average intensity and intensities for each scale range and both deviations are listed in Table 2.
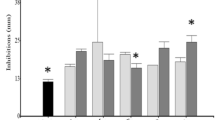
Assay 2 (n = 90 bees/treatment). Percentage of individuals at each level of infection on the arbitrary scale for each treatment (day 10 p.i.). %LOW, %MEDIUM, %HIGH: percentage of individuals with low (0–1 × 106), medium (1–10 × 106), or high (>10 × 106) intensity of spores in midgut. Bars indicate percentages above 3%. S2 surfactin 2 produced by C4, B1 bacteriocin from E. avium DSMZ17511 (pH 5.5, 7), B2 bacteriocin from E. faecium CRL1385 (pH 4.5, 5.5, 7), CTRL control groups of CFS and S2. Asterisk indicates significant differences between spore intensities when compared to the control group (Mann–Whitney p < 0.001)
As it was determined before, no spores of Nosema were detected in the control group.
Effect of prolonged consumption of bacteriocin and surfactin on N. ceranae development
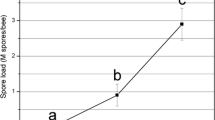
The antiparasitic effect recorded in assay 2 was also obtained when the diluted solution of surfactin S2 was administered ad libitum for 24 days. Figure 2 illustrates the intensity values registered for each individual, in agreement with the scale described above. Table 2 provides the average intensities for each scale range, the general average, and deviations; also the daily average of consumption and the cumulative mortality at 10 and 20 days p.i. (Table 3). In the contamination control, no spores were detected in individuals.
Assay 3 (n = 90 bees/treatment). Percentage of individuals at each level of infection on the arbitrary scale for each treatment (day 20 p.i.).%LOW, %MEDIUM, %HIGH: percentage of individuals with low (0–1 × 106), medium (1–10 × 106), or high (>10 × 106) intensity of spores in midgut. Bars indicate percentages above 3%. S1 surfactin 1 produced by Mori2, S2 surfactin 2 produced by C4, B1 bacteriocin of E. avium DSMZ17511 (pH 4.5, 6.5), B2 bacteriocin of E. faecium CRL1385 (pH 4.5, 6.5), CTRL control groups of CFS (B1 and B2) and surfactins (S). Asterisk indicates significant differences between spore intensities when compared to control group (Mann–Whitney p = 0.038)
Discussion
The present study constitutes the first record of administration of "bacteriocin or surfactin" metabolites to A. mellifera individuals. It is also the first attempt to test the antiparasitic action of these compounds on Microsporidia in an invertebrate host.
The administered metabolites revealed no toxic effect on worker bees after 30 days of consumption, thereby demonstrating that the surfactins and bacteriocins interaction with the luminal environment and the microbiota incorporated with pollen had no negative effect on bees. Particularly worth of mention is the fact that the compounds safety was obtained, either by administering pure metabolites (resuspended surfactin precipitate), with no prior purification (bacteriocins and lactic acid), or by administering sugar syrup to both solutions. Metabolites administration did not play a key role in bees behavior nor in the solutions’ consumed volumes. Surfactin S2, the cyclic lipopeptide produced by B. subtilis C4, was the only compound administered that decreased parasitosis intensity after 40 h of metabolite–spore contact. This antiparasitic effect, obtained with 10 mg/ml of surfactin concentration, was observed once again when a metabolite concentration of 5 mg/ml was supplied ad libitum before and after bees infection. In this last experiment, the suppression in the parasitic load was significant, even when individuals were inoculated with a spore dose six times greater than that administered in bacteriocins treatments. As stated by Mayack and Naug (2008), who demonstrated an increased hunger level in N. ceranae infected bees, S2 was the only solution less consumed than the control solution during the post infection period.
Surfactin possesses antimicrobial and antifungal properties (Katz and Demain 1977; Joshi et al. 2008; Sabaté et al. 2009), one of the main effects being the detergent action on the lipid bilayer (Peypoux et al. 1999; Pooja and Swaranjit 2004). Therefore, one possible mechanism of antiparasitic action can be through a disruption in the integrity of the spore’s coat. If proteinaceous exospore and chitinous endospore are permeable to metabolites, the plasmatic membrane integrity and germination or replication mechanisms are likely to have been affected. In our experiments, these surfactant properties could have affected not only the spores exposed to metabolite solutions before being inoculated on bees (assay 2) but also the untreated spores inoculated in assay 3 and then exposed to metabolite contact in the digestive tract. Also the spores generated in the midgut after parasite replication could become affected in this last experiment.
In vitro studies have proven the powerful cytotoxicity effect of surfactin on Vero cells (kidney epithelial monkey cells) after 1-h exposure (From et al. 2007), as well as the disruption of the viral lipid membrane and partially of the capsid observed by electron microscopy after a few minutes (Vollenbroich et al. 1997). Indeed, further in vitro and in vivo experiments should be conducted so as to elucidate the optimum exposure time affecting spores viability and to determine the way in which surfactin interacts with the vegetative phase of the parasite’s cycle. The possible enterotoxicity exerted on the bee midgut cells should also be addressed.
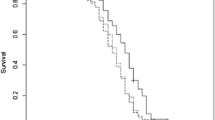
When the activity from two surfactins produced by two different Bacillus strains was compared, surfactin S2 was the only one exhibiting statistically significant antiparasitic activity, yet both metabolites (S1 and S2) extended the infected bees survival. This could be ascribed to a major reduction of the parasitic load, although other mechanisms able to prolong survival should not be discarded.
The antibacterial activity against L. monocytogenes provided by S2 was lower than that obtained with surfactin S1, the lipopeptide produced by B. subtilis Mori 2. Hence, in this particular case, the greater antibacterial effect could be correlated with the antiparasitic effect. Conversely, even when the bacteriocins tested yielded the highest AU values of bactericidal effect, no antiparasitic action was detected on N. ceranae. Despite the lack of anti-microsporidial activity, the results obtained are attractive, as it is the first time that bacteriocins potential has been tested on a target other than bacteria, microorganisms generally affected by such compounds (Jack et al. 1995).
Syrup acidification has been proposed as an effective practice against nosemosis development (Mottoul 1996); however, field and laboratory studies have demonstrated that Nosema prevalence or development is not affected by acidified feed (Vaillant 1989; Forsgren and Fries 2005). This work shows that bacteriocins administrated on acidified medium have no effect on spore viability after contact with metabolites as well as on Nosema development after ad libitum consumption. The lactic acid concentration derived from the strains employed (32 mM) is not very high compared to that of other members of the family, such as Lactobacillus genera, which may produce concentrations close to 175 or 220 mM (Audisio et al. 2010). Therefore, further experiments using higher lactic acid concentrations, or administrating lactic acid exclusively, could offer different results.
Spores refrigerated at 8°C loose nearly 50% viability (Fries and Forsgren 2009) and degrade more quickly when stored at 4°C (Fenoy et al. 2009). Then it is reasonable that spores in assay 3, stored for 24 h at 4°C (treatments with bacteriocin produced by E. avium) yield less infectivity than those stored for 2 h.
This research shows that an alternative treatment for the nosemosis caused by N. ceranae, based on bacterial metabolite activity is feasible. Surfactin postulates as a molecule capable of reducing parasitosis development, acting either by direct exposure to spores or incorporated to the luminal medium of bee midgut. This characteristic, added to the non toxic effect on bees, renders its inclusion as an antiparasitic drug viable. During the experiment, two concentrations of surfactin were used: 50:50 in syrup 66% w/v (assays 1 and 3) or at maximum concentration (assays 1 and 2). Further analyses should be conducted so that minimum inhibitory concentrations are also addressed. Likewise, it would prove interesting to thoroughly analyze the effects of metabolites on bee physiology, such as the mechanisms of antiparasitic action against N. ceranae and other microsporidian parasites.
References
Audisio MC, Oliver G, Apella MC (1999) Antagonistic effect of Ent. faecium J96 against human and poultry pathogenic salmonellae species. J Food Protect 62:751–755
Audisio MC, Oliver G, Apella MC (2001) Effect of different complex carbon sources on growth and bacteriocin synthesis of Enterococcus faecium. Intl J Food Microbiol 63:235–241
Audisio MC, Terzolo HR, Apella MC (2005) Bacteriocin from honeybee beebread Enterococcus avium, active against Listeria monocytogenes. Appl Environ Microb 71(6):3373–3375
Audisio MC, Torres MJ, Sabaté DC, Ibarguren C, Apella MC (2010) Properties of different lactic acid bacteria isolated from Apis mellifera L. bee-gut. Microb Res. doi:10.1016/j.micres-2010-01-003
Alippi AM, Reynaldi FJ (2006) Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources. J Invertebr Pathol 91(3):141–146
Blatz W (1955) Nosemack Südwestdtsch Imker 7(12):358
Cantwell GE (1970) Standard methods for counting Nosema spores. Amer. Bee j 110: 220-223
Daba H, Pandian S, Gosselin JF, Simard RE, Huang J, Lacroix C (1991) Detection and activity of a bacteriocin produced by Leuconostoc mesenteroides. Appl Environ Microb 57:3450–3455
Delbac F, Polonais V (2008) The microsporidian polar tube and its role in invasion. Subcell Biochem 47:208–220
Fenoy S, Rueda C, Higes M, Martín-Hernández R, Aguila C (2009) High resistance of Nosema ceranae, a parasite of honeybee, to temperature and desiccation. Appl Environ Microbiol 75(21):6886–6889. doi:10.1128/AEM.01025-09
Forsgren E, Fries I (2005) Acidic-benzoic feed and nosema disease. J Apia Sci 49(2):81–88
Fries I, Forsgren E (2009) Nosema ceranae fungerar inte som Nosema apis. Nosema ceranae does not function as Nosema apis. Bitidningen 107:20–21
Fries I, Feng F, Da Silva A, Slemenda SB, Pieniazek NJ (1996) Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee (Apis cerana) (Hymenotpera, Apidae). Eur J Protistol 32:356–365
From C, Hormazabal V, Hardy SP, Granum PE (2007) Cytotoxicity in Bacillus mojavensis is abolished following loss of surfactin synthesis: implications for assessment of toxicity and food poisoning potential. Int J Food Microbiol 117:43–49
Furgala B, Bosch R (1970) The effect of Fumidil B, Nosemack and Humatin on Nosema apis. J Apic Res 9(2):79–85
Giersch T, Berg T, Galea F, Hornitzky M (2009) Nosema ceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidol 40:117–123
Gilliam M (1978) Bacteria belonging to the genus Bacillus isolated from selected organs of queen honeybees, A. mellifera. J Invertebr Pathol 31:389–391
Gilliam M (1979) Microbiology of pollen and bee bread: the genus Bacillus. Apidol 10:269–274
Gilliam M, Prest DB (1978) Microbiology of feces of the larval honeybee, Apis mellifera. J Invertebr Pathol 31:389–391
Gilliam M, Valentine DK (1976) Bacteria isolated from the intestinal contents of foraging workers honeybees, Apis mellifera: the genus Bacillus. J Invertebr Pathol 28:275–276
Gontarski G, Wagner O (1954) Quantitative Versuche zur chemotherapeutischen Bekämpfung von Nosema apis Zander bei der Honigbiene, Arzneim. Forsch 4:161–168
Higes M, Martín R, Meana A (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol 92:81–83
Higes M, Martín-Hernández R, Garrido-Bailón E, García-Palencia P, Meana A (2008) Detection of infective osema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J Invertebr Pathol 97:76–78
Jack RW, Tagg JR, Bibek R (1995) Bacteriocins from Gram-positive bacteria. Microbiol Rev 59:171–200
Joshi S, Bharucha Ch, Desai AJ (2008) Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour Technol 99:4603–4608
Katz E, Demain AL (1977) The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev 41:449–474
Klee J, Besana A, Genersch E, Gisder S, Nanetti A, Tam DQ, Chinh TX, Puerta F, Kryger P, Message D, Hatjina F, Korpela S, Fries I, Paxton R (2007) Widespread dispersal of the microsporidium Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J Invertebr Pathol 96:1–10
Liu TP (1988) Ultrastructural changes in Nosema apis in the midgut of the honeybee treated with thimerosal in vitro. Parasitol Res 74:492–494
Liu TPP, Myrick GR (1988) Deformities in the spore of Nosema apis as induced by itraconazole. Parasitol Res 75:498–502. doi:10.1007/BF00930980
Maistrello L, Lodesani M et al (2008) Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 39:436–445
Martín-Hernández R, Meana A, Prieto L, Martínez Salvador A, Garrido-Bailón E, Higes M (2007) Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol 73(20):6331–6338
Mayack C, Naug D (2008) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J Invertebr Pathol. doi:10.1016/j.jip-2008-12-001
Mottoul J (1996) Etude de l´acidification des nourritures contre Nosema apis Zander. La Belgique Apicole 2:39–43
Nielsen P, Sorensen J (1997) Multi-target and médium independent fungal antagonisms by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol Ecol 22:183–192
Peypoux F, Bonmatin JM, Wallach J (1999) Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51:553–563
Pohorecka K (2004) Laboratory studies on the effect of standardized Artemisia absinthium L. extract on Nosema apis infection in the worker Apis mellifera. J Apicul Sci 48 (2) 131–136
Pooja S, Swaranjit SC (2004) Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol 22(3):142–146
Porrini MP (2008) Desarrollo de la parasitosis causada por Nosema ceranae (Microspora: Nosematidae) en individuos de Apis mellifera (Hymenoptera: Apidae) sometidos a diferentes dietas e inóculos de esporas” (Grade Thesis - Universidad Nacional de Mar del Plata)
Rinderer, Thomas E (1976) Honey bees: individual feeding of large numbers of adult workers. J Econ Entomol 69(4):489–491
Sabaté DC, Carrillo L, Audisio MC (2009) Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160:163–169
Schlüter H (1957) Erfahrungen mit Nosemack Reizfütterung im Freien. Dtsch Bienenw 8(4):71–72
Sichtova M, Haque MA, Vavra J, Canning EU, Robert-Gero M (1993) Evaluation of the antibiotic sinefungin as an antimicrosporidial drug. Fol Parasitol 40:85–91
Sina M, Alastair G, Farmer M et al (2005) The new higher level classification of Eukaryotes with emphasis on the taxonomy of Protists. J Eukar Microbiol 52(5):399–451
Underwood RM, Currie RW (2009) Indoor winter fumigation with formic acid for control of Acarapis woodi (Acari: Tarsonemidae) and Nosema Disease, Nosema sp. J Econ Entomol 102(5):1729–1736. doi:10.1603/029-102-0501
Vaillant J (1989) Nourrissement au sirop de sucre acidifié. La santé de l´abeille 110:55–60
Vollenbroich D, Özel M, Vater J, Kamp RM, Pauli G (1997) Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from bacillus subtilis. Biologicals 25:289–297
Williams GR, Sampson MA, Shutler D, Rogers REL (2008) Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J Invertebr Pathol 99:342–344
Yücel B, Doğaroğlu M (2005) The impact of Nosema apis Z. infestation of honey bee (Apis mellifera L.) colonies after using different treatment methods and their effects on the population levels of workers and honey production on consecutive years. Pak J Biol Sci 8(8):1142–1145
Acknowledgments
This research was supported by FONCyT, PICTR 890/2006, to M.E. We thank the support provided by the Animal Production Department, EEA INTA, Balcarce, and the Fares Taie Laboratories Food Division.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porrini, M.P., Audisio, M.C., Sabaté, D.C. et al. Effect of bacterial metabolites on microsporidian Nosema ceranae and on its host Apis mellifera . Parasitol Res 107, 381–388 (2010). https://doi.org/10.1007/s00436-010-1875-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1875-1