Abstract
Climate is known to be a critical factor controlling the broad-scale distribution of plants but often the physiological basis for species distribution limits is not well understood, nor is the extent to which populations within species are locally adapted to climate. Reciprocal transplant experiments designed to test for local adaptation are difficult to conduct and interpret in long-lived species, like oaks. Linking the physiological tolerances of species to their climatic distributions is an alternative approach to understanding adaptation to climate, and is important in predicting future distributions of species under changing climatic conditions. Here we synthesize a series of studies in a single lineage of American oaks that span the temperate tropical divide and encompass a range of precipitation and edaphic regimes, to determine (1) the physiological basis for adaptation to seasonal winter and seasonal drought and (2) the variation among populations that associated with climate variation and can be interpreted as local adaptation . We focus primarily on a series of common gardens that allow us to determine the genetically based differences in functional and physiological traits as well as the genetically based responses to contrasting temperature or precipitation regimes. We show that variation in freezing tolerance among closely related species is greater than variation among populations within species. Nevertheless, freezing tolerance varies predictably with climate of origin and is negatively associated with growth rate. In contrast, drought tolerance mechanisms vary more among populations within a single species, at least for the most widely distributed species, Quercus oleoides, than between species. Within this species, climate of origin predicts a suite of leaf physiological traits, and there is evidence for evolutionary trade-off between desiccation avoidance and desiccation resistance . Combined, these results show evidence for local adaptation to both freezing and drought stress within species, as well as adaptive differentiation between closely related species, despite phylogenetic conservatism in functional traits and highly similar physiognomy across the American live oak clade. The results inform conservation efforts aimed at preventing extinction of tree species in the face of global change.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
4.1 Introduction
A central biological question under rapidly changing global climatic conditions is the extent to which species are locally adapted to climate. Climate is a driving force in evolution (Etterson 2004a; Jump et al. 2006; Ramírez-Valiente et al. 2010; Shaw and Etterson 2012), even though niche conservatism is widespread (Crisp et al. 2009b; Wiens et al. 2010). Physiological traits that are linked to tolerance of seasonal temperature variation and water availability are known to vary considerably among woody taxa and are thought to delimit species distributions across climatic gradients in both temperate and tropical biomes (Larcher 1960, 2000; Sakai and Larcher 1987; Koerner and Larcher 1988; Engelbrecht and Kursar 2003; Tyree et al. 2003; Brodribb and Holbrook 2006; Engelbrecht et al. 2006). Such traits are likely to be under strong selection in relation to climate. We have used a small “model clade” of live oaks ( Quercus section Virentes Nixon) that span a range of climates from the temperate zone to the tropics to examine evidence for adaptive evolution in response to seasonal winter and seasonal drought . The evolutionary history and ecological distribution of the live oaks are well understood, providing a platform for investigation of adaptive change. More broadly, the oaks of the Americas contribute a large fraction of the total forest biomass and woody diversity of North America in both the US and Mexico (Cavender-Bares 2016). Climate change scenarios predict warmer climates in southern and southeastern regions of eastern North America and drier climates in most regions of Mexico Central America by 2100 (IPCC 2007), but the seasonal timing of decreases in rainfall are uncertain (Karmalkar et al. 2008). We used the live oaks as a system to address the question of whether variation between populations and between species in both sensitivity to chilling and freezing stress and resistance to drought corresponds to the climate of origin. We compare our studies of the American live oaks, trees adapted to wet summers and either dry or cold winters, to parallel work on old world Mediterranean oak species, which also experience seasonality in precipitation and temperature but are adapted to cold temperatures that are seasonally offset from low rainfall . These oaks are similar in appearance and many functional attributes but display important physiological and phenological differences as a consequence of the contrasting patterns in seasonality.
Our goal was to examine evidence for local adaptation to climate using a series of common garden experiments, where environmental variation was limited and individual plants were randomized across this variation. Distinguishing between plasticity and genetically-based variation has important implications for understanding range limits and how plants respond to changing environments. As part of these investigations, we tested for environmentally-induced changes in resistance to cold and to drought in plants grown under contrasting and experimentally manipulated climate regimes (temperature or precipitation ). These experiments allowed us to examine plasticity in response to contrasting climatic regimes and to evaluate evidence in support of adaptive plasticity . We examined variation within and among species in response to freezing stress in five of the live oaks but focused on the two most widespread species, Quercus virginiana (temperate biome) and Quercus oleoides (tropical biome). In examining adaptation to drought and seasonality in precipitation , we focused primarily on Quercus oleoides, which spans a range of precipitation regimes, all of which fall within the classification of seasonally-dry tropics. We planted common gardens in the field and also reciprocally transplanted populations to test directly for local adaptation and supplemented water at two times during the year to decipher consequences of water limitation . We review these common garden studies to synthesize what we have learned about genetically based variation within and among closely related species of this lineage in response to the range of climatic variation that it encompasses. We suggest future steps needed to (1) better understand the evolution of climate responses in oak ecosystems and the evolutionary potential of oaks to adapt to increasing drought severity expected in future decades and (2) provide guidance on conservation to prevent extinction of threatened oaks.
4.2 The Live Oaks, Quercus Section Virentes , as a Study System
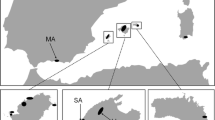
The live oaks consist of seven species of interfertile brevideciduous or semievergreen oaks that span the tropical temperate divide in southern USA, Mexico and Central America and the Caribbean (Muller 1961; Nixon 1985; Nixon and Muller 1997; Cavender-Bares et al. 2015) (Fig. 4.1).
The live oaks, Virentes, are a monophyletic lineage of interfertile American oaks that span the southeastern US, Mexico, Central America and the Caribbean and occur across a range of climates that vary in both minimum temperature and precipitation . The live oaks fall within the white oak group (Nixon and Muller 1997; Cavender-Bares et al. 2015). Shown are the geographic distributions of the seven species in the lineage , their phenotypes, and their climatic distribution in terms of mean annual precipitation (cm) and minimum temperature of the coldest month (°C). Modified from Cavender-Bares et al. (2015)
Q. virginiana and Q. oleoides are the two most broadly distributed members of the live oaks. Q. virginiana extends from the outer banks of southern Virginia and North Carolina in the U.S. into northern Mexico, and Q. oleoides extends from northern Mexico to northwestern Costa Rica. We hypothesized that the wide range of climatic variation encountered by the two species throughout their ranges has led to both interspecific and intraspecific variation as a consequence of adaptation to contrasting climates. The majority of the work we present on population -level differentiation is within and between these two species.
4.3 Adaptation to Winter Stress: Species and Population Responses to Freezing Under Different Climate Regimes
Freezing is considered a major barrier to migration and a strong selective force (Sakai and Weiser 1973; Larcher 2000; Cavender-Bares 2005; Zanne et al. 2014). Freezing temperatures can cause lethal injuries in living plant tissues, and the ability of different species to avoid or tolerate freezing stress through various mechanisms can go a long way in explaining their geographic distributions (Parker 1963; Burke et al. 1976). Live oaks are likely strongly limited by freezing, given that they are not deciduous . Deciduousness is a very common adaptation to freezing winters, and has evolved repeatedly in oaks (Hipp et al. 2017). However, the live oaks are restricted to mild climates and are considered evergreen or brevideciduous , but not deciduous , in taxonomic treatments (Miller and Lamb 1985; Nixon 1985; Nixon and Muller 1997). While they occur in the temperate zone, they are found only in climates where winters are fairly mild but where subzero temperatures are nevertheless frequent. Tree species, such as the live oaks, that remain active during winter are subject to freezing of living and nonliving tissues. Freezing can cause intracellular ice formation, which can kill the cells, or extra-cellular ice formation, which may lead to cellular dehydration and cell membranes damage (Fujikawa and Kuroda 2000).
We tested the hypothesis that populations at different latitudes within species are differentially adapted to cold and freezing stress. However, given the tendency for phylogenetic conservatism in traits (Ackerly and Reich 1999; Wiens et al. 2010), we hypothesized that, alternatively, populations within species could be equally tolerant of cold and freezing, due to ancestral acquisition and conservatism of such traits, even though only some populations currently experience these stresses. The ability to cold acclimate is itself an evolved trait , and the capacity to undergo morphological shifts that protect against freezing damage is characteristic of temperate species. We therefore anticipated that the growth climate would influence the freezing response, and exposure to winter temperatures would lead to cold acclimation in populations adapted to cold such they would show less damage in response to freezing stress.
In an initial controlled environment experiment with two populations of both Q. virginiana (temperate species) and Q. oleoides (tropical species), Cavender-Bares (2007) found ecotypic differentiation in cold and freezing sensitivity between populations within species and between species across a latitudinal gradient, when grown under either tropical or temperate growth conditions. In response to short term freezing, both the North Carolina and Florida populations from the temperate Q. virginiana showed small losses of photosynthetic function (dark acclimated quantum yield, assessed as variable to maximum chlorophyll fluorescence , F v/F m), 24 h after freezing at −10, −5, −2 °C compared to before freezing. In contrast, Belize and Costa Rica populations from the tropical Q. oleoides showed very large losses in photosynthetic function after freezing at −10 °C. However, the extent of damage to the photosynthetic apparatus was a consequence of growing conditions, and populations within Q. virginiana showed different degrees of damage, depending on climate of origin. Plasticity in the responses of populations to freezing stress is an important adaptation . Plasticity is adaptive if it results in a higher fitness across environments (van Kleunen and Fisher 2005). The case for claiming adaptive plasticity for increased freezing tolerance in response to cold exposure is not disputed. The widely observed changes in cell wall properties and other functions that reduce intracellular freezing and frost damage among most freezing tolerant plants, called “cold acclimation,” increases plant survival (Steponkus 1984; Wisniewski and Ashworth 1985; Huner et al. 1993; Cavender-Bares 2005). Cold acclimation encompasses the range of physiological and morphological changes that occur in response to chilling and prepare a plant to encounter freezing stress. Overall, Q. virginiana plants showed an ability to cold acclimate, while Q. oleoides populations did not. When grown in a tropical treatment exposed to consistently warm growth conditions both Q. virginiana populations showed greater loss of photosynthetic function when exposed to decreasing minimum temperatures, as indicated by a decline in F v/F m, than when acclimated to three months of winter chilling (Fig. 4.2a, b). Across populations and species, the decline in F v/F m under both climate conditions corresponded to climate of origin. Under the tropical treatment (Fig. 4.2a), while both populations in both species suffered declines in F v/F m after freezing at −10 °C, the northern most population from North Carolina showed only a minimal decline in F v/F m and plants in the Florida population of Q. virginiana showed an intermediate response at −10 °C. The ability of Q. virginiana populations grown in the temperate treatment to cold acclimate was apparent from the lack of decline in F v/F m, even at −10 °C. In contrast, the two Q. oleoides populations showing very large declines under both treatments with values of F v/F m that dropped below 0.2 at −10 °C.
Effects of minimum temperature (0, −2, −5, and −10 °C) on recovery of photochemical efficiency in dark leaves a in plants grown under warm conditions all year (tropical treatment), and b in plants acclimated to cold temperatures (temperate treatment). Dark F v/F m was measured on leaves of branches with a stem submersed in water before and 24 h after a dark freezing cycle. Immediately after the freezing cycle, branches were placed in a dark cabinet at room temperature . Plants were measured in February. c Percentage of leaf loss during the interval between November and February in the tropical and temperate growth treatments. Q. virginiana is represented by triangles (open = North Carolina; closed = Florida), and Q. oleoides is represented by circles (open = Belize; closed = Costa Rica). Redrawn from Cavender-Bares (2007)
We also observed a striking difference in leaf abscission in responses to cold exposure both between populations within Q. virginiana and between the two species that corresponded to latitude (Fig. 4.2c), which again demonstrates adaptive plasticity . Abscission in response to cold provides evidence for evolution towards deciduousness. The northern population of Q. virginiana from NC lost nearly 60% of its leaves in response to prolonged cold exposure that reached 4 °C at night, while the Florida population abscised less than 40% compared to only 6% for all populations in the tropical treatment. Meanwhile, the tropical Q. oleoides showed only approximately 10% abscission in response to the same cold exposure, and not significantly different from the abscission rate in the tropical treatment.
In a second study (Cavender-Bares et al. 2011), we again found genetically based differences in freezing sensitivity between Q. virginiana and Q. oleoides; although variation within species was not significant in this case. As before, F v/F m was measured in a replicated controlled environment experiment under tropical and winter -treated temperate conditions, before freezing and 12 h after freezing at −5, −7 and −10 °C. Critical freezing temperatures (the freezing temperature at which F v/F m = 0.4) were more negative for Q. virginiana than Q. oleoides, indicating higher freezing tolerance in the temperate species. However, populations within species did not differ significantly (Fig. 4.3c). In the temperate treatment, where plants were acclimated to cold temperatures for three months prior to freezing, all Q. virginiana populations showed an increase in freezing tolerance relative to when they were grown in consistently warm conditions. Q. oleoides populations showed no ability to increase freezing tolerance when exposed to cold .
Genetically based differences in freezing sensitivity between Q. virginiana and Q. oleoides. The dark-acclimated quantum yield of photosynthesis (F v/F m) was measured in a common garden experiment under a tropical conditions and b winter -treated temperate conditions, before freezing and 12 h after freezing at −5, −7 and −10 °C. Asterisks indicate temperatures and treatments for which the two species were significantly different (α = 0.05). Small symbols to the right in b indicate dark-acclimated F v/F m values of leaves warmed at 22 °C for 48 h. c Climatic distributions showing the percentage of herbarium record occurrences at each temperature for the mean minimum temperature in the coldest month. These occurrence localities were used in the Maxent model to predict climatic distributions for Q. oleoides and Q. virginiana, which showed that minimum temperature was an effective climate variable for predicting species occurrence and was significantly different between the species (P < 0.0001). d A ~3 °C difference in critical freezing temperatures between the two species was apparent. Quadratic curves fitted to F v/F m responses of individuals to three freezing temperatures (not shown) allowed the prediction of critical freezing temperatures (the freezing temperature at which F v/F m = 0.4) for each population . Redrawn from Cavender-Bares et al. (2011)
In the most extensive study of the series examining freezing tolerance , Koehler et al. (2012), tested maternal families from multiple populations within five species of the Virentes for freezing tolerance and response to cold exposure (Fig. 4.4). In this study we used the electrolyte leakage method to examine intracellular cell death of stems in response to freezing, as well as loss of chlorophyll function in leaves, as before. Minimum temperatures in the climate of origin of maternal families across all species strongly predicted freezing tolerance , cold acclimation ability, and growth rates. Maternal families from climates with colder winters had slower growth rates and greater freezing tolerance than those from milder climates. As a consequence, we found evidence for an evolved trade-off between freezing tolerance and growth rate, such that the maternal families from warmer latitudes within and across species showed faster growth rates but lower freezing tolerance than the maternal families from colder latitudes. Live oaks from lower latitudes had much high freezing tolerance and ability to acclimate to freezing, but lower growth rates in the absence of cold stress .
Minimum temperature of the coldest month in the climate of origin predicts leaf a and stem b freezing tolerance in maternal families grown under nonstressed tropical (gray) and after exposure to cold temperatures in temperate conditions (black) based on leaf decline in F v/F m after freezing at −10 °C and stem index of injury after freezing at −15 °C. Minimum temperature of the coldest month further predicts leaf cold acclimation ability ((tropical -temperate )/tropical for decline in F v/F m after freezing at −10 °C) c and stem cold acclimation ability ((tropical -temperate )/tropical for index of injury after freezing at −15 °C) d. e and f show the trade-offs between growth rate (absolute growth rate, AGR) in tropical and temperate conditions and freezing tolerance across maternal families from four live oak species. Leaf freezing tolerance and stem freezing tolerance are both higher in maternal families within and among species with lower growth rates. Species: Q. virginiana, squares; Q. geminata, crosses; Q. fusiformis, circles; Q. oleoides, triangles. Redrawn from Koehler et al. (2012). Regressions are shown as least squares fitted lines
In a study on the same species, populations and maternal families, Ramírez-Valiente et al. (2015) found significant variation among populations and species, as well as increasing anthocyanin content with minimum temperature in the climate of origin. The maternal families with higher freezing tolerance (Fig. 4.4) had lower anthocyanin accumulation (Fig. 4.5). Our interpretation is that maternal families with lower freezing tolerance use anthocyanins as a general protective mechanism in response to cold , by attenuating light and/or neutralizing reactive oxygen species to diminish the risk of photodamage under low temperatures (Pietrini et al. 2002; Gould 2004; Hughes et al. 2012).
a Means for populations (within species) under temperate (black) and tropical (gray) treatments for anthocyanin concentration measured in the reddest leaf of the plants. Bars indicate standard errors. Different letters indicate significant differentiation (P < 0.05) within the temperate and tropical treatments. Populations: Q. fusiformis (circles), TX_FUS—Texas; Q. geminata (diamonds), FLN_GE—Northern Florida; NC_GE—North Carolina; Q. oleoides (triangles), CR_OL—Costa Rica; BZ_OL—Belize; MX_OL—Mexico; Q. virginiana (squares), FLS_VIR—Southern Florida; FLN_VI—northern Florida; LA_VIR—Louisiana; TX_VIR—Texas; NC_VIR—North Carolina. b Relationship between anthocyanins and minimum temperatures of the coldest month in the source of origin of the maternal families under temperate (black) and tropical (gray) treatments. Q. fusiformis (circles), Q. geminata (diamonds), Q. oleoides (triangles) and Q. virginiana (squares). Points represent mean maternal-family values
All of these studies provide clear evidence for adaptive divergence in freezing tolerance and the ability to acclimate to cold winters among species with contrasting climates of origin. Within Q. virginiana, we also found adaptive differentiation among populations in cold acclimation ability and freezing tolerance . In other words, families and populations within the species have different levels of adaptive plasticity in response to cold , depending on climate of origin. The ability to increase freezing tolerance after cold exposure is entirely absent in the tropical species, Q. oleoides, and remains untested in Q. brandegeei and Q. sagraena in Cuba. Lacking freezing and cold tolerance , which is hypothesized to be maintained at significant metabolic cost (Burke et al. 1976; Guy 2003; Savage and Cavender-Bares 2013). This lack of cold acclimation ability and freezing tolerance , more generally, helps explain why Q. oleoides appears to use anthocyanins as a general mechanisms to reduce photoprotective stress under cold conditions, particularly in young leaves (Ramírez-Valiente et al. 2015). Our current working hypothesis is that the live oaks lost freezing tolerance after radiation into Mexico and Central America , given that the oaks colonized the temperate zone first (Hipp et al. 2017). The alternative possibility is that the live oaks originated in tropical climates and gained freezing tolerance . Regardless, these studies demonstrate population level local adaptation in freezing tolerance in the widely distributed temperate Q. virginiana, and either adaptive loss of freezing tolerance and cold acclimation ability in the tropical Q. oleoides, or adaptive acquisition of these attributes in Q. virginiana. Molecular evidence from candidate genes lends support to the hypothesis that the live oaks lost freezing tolerance , given strong conservatism and purifying selection in a core gene responsible for cold acclimation ability (Meireles et al. 2017). In these same populations of Q. virginiana and Q. oleoides, we studied two cold response candidate genes ICE1, a key gene in the cold acclimation pathway, and HOS1, which modulates cold response by negatively regulating ICE1. Meireles et al. (2017) found that that HOS1 experienced recent balancing selection. This finding indicates that evolution has favored diversity in cold tolerance modulation through balancing selection in HOS1, perhaps due to the range of climatic environments the species experience across their ranges. At a deeper evolutionary scale, a codon based model of evolution revealed the signature of negative (or purifying) selection in ICE1. In the same analysis, three positively selected codons were identified in HOS1, possibly a signature of the diversification of Virentes into warmer climates from a freezing adapted lineage of oaks. It thus appears that, while evolution has favored diversity in cold tolerance modulation through balancing selection in HOS1, it has maintained core cold acclimation ability, given evidence for purifying selection in ICE1.
4.4 Species and Population Responses to Water Availability Under Different Climate Regimes
Water limitation is a second major barrier in the ability of plants to occupy a given biome and accounts for major shifts in the Earth’s species composition (Pennington 2006; Crisp et al. 2009a; Anderegg et al. 2016). Within the same climatic zone, water limitation can be caused by topographic variation and soil type. In these cases, water availability and soil fertility often covary (Cavender-Bares et al. 2004). Quercus virgininiana and Quercus geminata, two co-occurring temperate live oak species, known to be sister species, occur in contrasting soils and hydraulic regimes in the southeastern US (Cavender-Bares and Pahlich 2009). They provide a good test of sympatric divergence in function in relation to microhabitat water availability. Quercus virginiana occurs on moister and richer soils than Q. geminata based on ecological studies in Florida (Myers 1990; Cavender-Bares et al. 2004) as well as taxonomic treatments of the species (Kurz and Godfrey 1962; Nixon and Muller 1997). Cavender-Bares et al. (2004) found significant niche differentiation across soil types between the two species based on soil moisture and soil fertility (pH, calcium content, exchangeable NH4 and NO3, and exchangeable P). Q. virginiana occurrs on moister, more nutrient rich, and higher pH sites than Q. geminata. Quercus virginiana also has a broader distribution across the range of variation in all of the edaphic factors relative to Q. geminata (Cavender-Bares and Pahlich 2009). When sympatric Florida populations of each species that occur in different microhabitats are grown in a common environment, Q. virginiana has faster growth and higher photosynthetic rates per gram of leaf tissue than Q. geminata, corresponding to its more resource rich native habitat. The resource allocation patterns of Q. virginiana support its faster growth strategy; it has thinner leaves (higher specific leaf area , SLA ) and allocates more to leaf area relative to total plant biomass , thus maximizing light capture and total plant photosynthetic capacity. Quercus virginiana also allocates less to root mass than shoot mass. In contrast, the slower growth strategy of Q. geminata is accompanied by a greater investment in roots relative to shoots and lower allocation to leaf area per unit biomass . Lower evaporative surface area and greater proportional belowground biomass permits Q. geminata to conserve water. This conservative strategy matches the lower water availability in their native habitat. Based on measurements from stems naturally grown in the field, Q. virginiana has higher stem specific hydraulic conductance than Q. geminata and lower Huber values than Q. geminata (Cavender-Bares and Holbrook 2001). The significant functional differentiation between the species observed both in common gardens and in naturally occurring populations corresponds to habitat differentiation and provides evidence for adaptive divergence between these sympatric sister species. Adaptive differentiation must have either occurred in sympatry, a possibility given contrasting phenology and flowering times (Cavender-Bares and Pahlich 2009) or in allopatry prior to secondary contact. In a subsequent series of studies, we found similar kinds of adaptive differentiation within a single species that spans a range of climates and soils as we explain in Sect. 4.5.
4.5 Intraspecific Variation in Seasonally-Dry Tropical Climates in the Widely Distributed Tropical Live Oak , Quercus Oleoides
Across latitudes, variation in the timing and amount of precipitation establishes contrasting selection pressures that may be anticipated to lead to adaptive differentiation in populations and local adaptation . Yet it is not well understood the extent to which local adaptation occurs in long-lived tree species. Maintenance of high genetic variation and plastic responses to the environment are other important means of persisting in variable environments, particularly when generation times are long and an individual tree may experience a range of environments throughout the course of its lifespan (Shaw and Etterson 2012; Meireles et al. 2017). Quercus oleoides is a long-lived species widely distributed in seasonally dry tropical forests (SDTFs) of Central America . This species usually forms mono-dominant stands and influences local hydrologic budgets and soil conservation (Boucher 1981; Klemens et al. 2011). It is considered to have a evergreen or brevi-deciduous leaf habit depending on the population of origin (Muller 1942). This species is a useful study system to explore evolution of drought resistance strategies in SDTFs because it spans a large gradient of dry-season aridity and wet season rainfall in the region. Here we review and synthesize the main findings from recent studies relative to the drought response exhibited by Q. oleoides to seasonal water variation.
Vast areas in tropical latitudes are characterized by seasonally dry climates, which are particularly abundant in Mesoamerica. Seasonally dry tropical forests (SDTFs) in this region exhibit nearly constant temperatures throughout the year but have marked variation in precipitation . Usually, rainfall exhibits a bimodal distribution with maximums in June and October and minimums between March and April. The length of the dry season might vary between two and seven months, and its severity is variable across the region. Xeric environments can also experience drought events during the wet season (Ananthakrishnan and Soman 1989; Nicholls and Wong 1990).
In an initial study of local adaptation to contrasting precipitation regimes using a reciprocal transplant experiment within Costa Rica, Deacon and Cavender-Bares (2015) found that upland and lowland populations of Q. oleoides both had higher fitness , in terms of both growth and survival , in upland environments, where precipitation was higher and water limitation less severe during the dry season. The results clarified that water was more limiting to fitness in the lowland environment than in the upland. A later field common garden study in the same lowland region again showed that water limitation during the dry season reduced seedling fitness from both the upland and lowland populations by decreasing survival . Furthermore, water supplementation at the low elevation site during the dry season resulted in an increase in emergence of seedlings and subsequent fitness from seeds produced late in the season (Center et al. 2016). The upland and lowland Costa Rican populations originate from environments that span the full range of precipitation variation across the entire species range. However, in two separate transplant experiments, we found no evidence of local adaptation of these two populations within Costa Rica through reciprocal transplanting , despite barriers to gene flow that could have permitted it (Center 2015; Deacon and Cavender-Bares 2015). In the latter study Center (2015), reciprocal transplanting included populations and sites in the upland and lowland regions in Costa Rica as well as in a very xeric region in southern Honduras. However, even including this broader span of populations we found no evidence for local adaptation , although biotic factors may have interfered (Center 2015). Detecting local adaptation under complicated field conditions in long-lived species is difficult, however, and sometimes better evidence can be obtained for adaptive differentiation in physiological function in controlled environments.
Making inferences about local adaptation based on functional differentiation in traits requires a clear understanding of the expectations for how traits should vary with climatic and soil conditions. Seasonally dry tropical forests in Central MesoAmerica are typically dominated by trees with very different leaf life spans, including drought deciduous and evergreen species (Borchert et al. 2002; Givnish 2002; Bowman and Prior 2005; Klemens et al. 2011; Vico et al. 2015). Deciduous species usually have thin leaves with high specific leaf area , short life spans and high investment in photosynthetic tissues per leaf mass. For this reason, they are thought to sustain high photosynthetic rates in the wet season under high soil water potentials but to abscise their leaves in the drought season to reduce water loss via transpiration (Reich and Borchert 1984; Eamus and Prichard 1998). Theoretical models predict that this acquisitive resource-use strategy is beneficial for carbon, nutrients and water balances in SDTFs when the dry season is longer or more severe because it maximizes carbon uptake and nutrient use when water is not limiting and minimizes water loss during the long dry season (Cornelissen et al. 1996; Givnish 2002; Bowman and Prior 2005; Poorter and Markesteijn 2008). In contrast, as the dry season becomes shorter or less severe, a conservative resource use strategy with increased drought tolerance is hypothesized to be beneficial for species that inhabit SDTFs because it allows carbon assimilation throughout the entire year including during the dry season (Oertli et al. 1990; Niinemets 2001; Read and Sanson 2003; Wright et al. 2005; Bowman and Prior 2005; Poorter et al. 2009; Markesteijn et al. 2011). In general, a conservative resource use strategy is associated with leaves with greater investment in structural components with low SLA , high leaf thickness and high lignin concentration (Reich 2014). These leaves have lower photosynthetic rates but can maintain function much longer (Parkhurst and Loucks 1972; Fetcher 1981; Niinemets 2001; Read and Sanson 2003; Markesteijn et al. 2011). Species that use resources conservatively also tend to possess adaptations traits that allow them to be functionally active at low soil water potentials such as adaptations that reduce xylem cavitation (e.g., narrow vessels with resistant pit membranes, high stem wood density ) and traits that maintain leaf turgor (Brodribb et al. 2003). Photoprotection is also expected to vary with leaf lifespan and exposure to dry season drought (Demmig-Adams and Adams 2006; Savage et al. 2009). To the extent that leaves remain functional during the dry season, they would be expected to increase xanthophyll pigments that aid in energy dissipation when water becomes limiting, stomatal conductance declines and photosynthesis quenches less of the incoming absorbed solar radiation.
In several common garden studies testing for ecophysiological differentiation among populations, Ramírez-Valiente et al. (2015), Ramírez-Valiente and Cavender-Bares (2017) and Ramírez-Valiente et al. (2017) demonstrated evolutionary divergence in leaf functional traits among populations of Q. oleoides from contrasting precipitation regimes that vary in dry season length and severity. They found, somewhat counterintuitively, that more mesic populations tend to face greater water stress because they maintain functional leaves for longer during the dry season. Ramírez-Valiente et al. (2015) observed that in response to drought , Q. oleoides populations originating from mesic areas increased the de-epoxidation rates of the xanthophyll cycle more than xeric populations (Fig. 4.6). They showed that differences in physiological mechanisms, particularly the activation of the xanthophyll cycle, were much higher among populations within species than among different species. The nature of variation within and among species thus contrasts that observed for freezing tolerance , which showed greater differentiation between species than within them. Differences in SLA among Q. oleoides populations were also higher than differences observed among live oak species. This study showed for the first time that populations from more mesic areas tended to have more sclerophyllous leaves with higher capacity for photoprotection in this tropical oak (Ramírez-Valiente et al. 2015). The interpretation is that the mesic populations maintain leaves for longer during the dry season and need to continue to maintain function with increasing water stress.
Relationship between the de-epoxidation state of the xanthophyll cycle measured in Quercus oleoides seedlings established in a greenhouse experiment and the index of moisture (precipitation —potential evapotranspiration) of families in their climate of origin. Red: dry treatment, blue: well-watered treatment. Redrawn from Ramírez-Valiente et al. (2015). Circles represent family means
Consistent patterns of variation in leaf thickness and specific leaf area with the index of moisture in the location of the source populations were found in multiple common garden studies in both the greenhouse and in the field (Ramírez-Valiente and Cavender-Bares 2017; Ramírez-Valiente et al. 2017). Regardless of the location of the experiment, watering treatment and sampled populations, a consistently negative association between specific leaf area and the index of moisture of the source has been observed (Fig. 4.7). Differences in SLA among populations were mainly due to leaf thickness and to a lesser extent to leaf density . In fact, a positive association between leaf thickness and the index of moisture of the source were also observed across studies (Ramírez-Valiente and Cavender-Bares 2017; Ramírez-Valiente et al. 2017).
Relationship between specific leaf area and the index of moisture (Precipitation − potential evapotranspiration) in Quercus oleoides seedlings established in three common garden experiments: a Greenhouse experiment (red: dry treatment, blue: well-watered treatment) (Ramírez-Valiente and Cavender-Bares 2017), b Field experiment a, established in Honduras in 2011 with eight populations (red: dry season, blue: wet season) (Ramírez-Valiente et al. 2017) and c Field experiment b, established in Honduras in 2012 with fourteen populations (measured only in the dry season) (Ramírez-Valiente and Cavender-Bares unpublished). Circles represent population means. Bars indicate standard errors. Index of moisture values are population means in the climate of origin
These studies further revealed that Q. oleoides populations had similar values of stomatal conductance and water use efficiency (WUE) (Ramírez-Valiente and Cavender-Bares 2017; Ramírez-Valiente et al. 2017) but significantly differed in water potential at the turgor loss point and leaf abscission in response to drought . Differences in these two traits were again associated with the climate of origin, consistent with variation in leaf morphology . Overall, our findings reveal that populations from more mesic sites have smaller sclerophyllous leaves (lower SLA and higher thickness) and greater drought tolerance (lower πtlp) (Fig. 4.8a) than populations from more xeric sites, which have larger mesophyllous leaves (higher SLA and lower thickness) and increase leaf abscission in response to drought (Fig. 4.8b). Since populations had similar stomatal conductance and WUE , leaf senescence in response to drought may have been favored in populations from more xeric soil conditions as a means of reducing water loss (Jonasson et al. 1997; Condit et al. 2000; Franklin 2005; Stevens et al. 2016). The increased drought tolerance and more durable leaves observed in the most mesic areas within the distribution range of Q. oleoides would allow maintaining photosynthetic activity under lower water potentials. Thus, the association between drought resistance strategies, leaf morphology and climate of populations for Q. oleoides agrees with the postulates by the resource-use hypothesis in SDTFs (Borchert 1994; Medina 1995; Condit 1998; Givnish 2002; Bowman and Prior 2005; Choat et al. 2007; Tomlinson et al. 2013; Vico et al. 2015). Our findings for Q. oleoides are consistent with temporal studies, which show that decreasing rainfall in the dry season enhances the relative abundance of deciduous species in tropical dry forests over time (Enquist and Enquist 2011) and by spatial analyses at small scales, which show that dry deciduous species preferentially occupy drier microhabitats than evergreen species (Comita and Engelbrecht 2009). In sum, these findings suggest that water availability is a key factor driving the spatial and temporal dynamics of functional strategies in the tropics and provide experimental evidence that selection has favored an increase in deciduousness with increasing dry season severity in Quercus oleoides.
a Relationship between the index of moisture (Precipitation − potential evapotranspiration) and water potential at the turgor loss point (πtlp) in one-year old Quercus oleoides seedlings established in a greenhouse experiment (red: dry treatment, blue: wall-watered treatment) (Ramírez-Valiente and Cavender-Bares 2017). Circles represent population means. Bars indicate standard errors. b Relationship between the index of moisture (Precipitation − potential evapotranspiration) and leaf abscission in one-year old Quercus oleoides seedlings established in a greenhouse experiment (red: dry treatment, blue: wall-watered treatment). Redrawn from Ramírez-Valiente and Cavender-Bares (2017). Circles represent population means. Bars indicate standard errors
4.6 Comparison of the Seasonally Dry Tropical Q. Oleoides to Mediterranean Oaks
It is interesting to note that the trends observed in Q. oleoides are opposite to those reported by Barlett et al. (2012) at global scale, who found a positive relationship between water availability and πtlp in a metanalysis. This inconsistency across studies probably reflects contrasting patterns in the evolution of drought tolerance among- and within-biomes. Differences in πtlp among functional types within biomes are broadly documented (Bartlett et al. 2012). In fact, species adapted to avoid or escape from dry seasons usually exhibit lower drought tolerance (increased osmotic potentials and turgor loss points) in dry tropical and temperate ecosystems (Medina 1995).
Our results also contrast with those found in intraspecific studies on evergreen oak species from seasonally-dry temperate zones (i.e. Mediterranean-type ecosystems), which show that populations from xeric climates with long dry seasons have a conservative resource-use strategy (Gratani et al. 2003; Ramírez-Valiente et al. 2010, 2014; Niinemets 2015). We speculate that the differences in the patterns of variation of functional traits between tropical and Mediterranean oaks are probably related to temperatures in the wet season. In Mediterranean-type ecosystems carbon assimilation is limited by both water deficit in summer and low temperatures in winter (Larcher 2000; Nardini et al. 2000; Cavender-Bares 2005; Flexas et al. 2014; Granda et al. 2014; Niinemets 2016). In contrast to Mediterranean ecosystems, which have cold winters, in seasonally-dry tropical ecosystems, temperature is not a limiting factor for photosynthesis in the wet season. Mediterranean species face a range of severity in drought stress in summer and mild to more significant freezing stress in winter . Across species of Mediterranean oaks, generally, those with longer leaf lifespans, including the evergreen Q. ilex (holm oak ), which maintains its leaves for well over two years, and Q. suber (cork oak ), which maintains leaves for well over one full year, have much lower SLA than species with shorter leaf lifespans, including the deciduous species such as Q. afares and Q. faginea, from northern Africa , (Fig. 4.9a). Species with lower SLA also have lower leaf nitrogen concentration and are much more resistant to freezing (Cavender-Bares et al. 2005). This direction of variation in these traits is consistent with leaf economic spectrum. Within the two evergreen species, Q. ilex and Q. suber, the nature and severity of seasonal stress drives leaf variation either in the same direction as the LES or in the opposite direction, similar to Q. oleoides. We hypothesize that a conservative resource-use strategy with long leaf life span , thick leaves, high density tissues and high water use efficiency is beneficial in terms of carbon, nutrient and water balance for species inhabiting areas with long dry seasons and cold winters. Thick leaves with higher investment in structural tissues are more resistant to water stress but also to freezing temperatures (Cavender-Bares 2005; Granda et al. 2014). They have higher construction costs but might be offset by a longer payback interval (Williams et al. 1989; Eamus and Prichard 1998).
a Specific leaf area (SLA , cm2 g−1) in relation to leaf lifespan for four Mediterranean species from northern Africa and southern Europe , grown and measured in a common garden in France, showing species means and standard errors from sampled individuals. Modified from Cavender-Bares et al. (2005). b Relationship between summer precipitation and SLA in seven-year-old saplings of Quercus suber established in a field common garden experiment (Ramírez-Valiente et al. 2010). Circles represent population means. Bars indicate standard errors. c Relationship between the index of moisture and specific leaf area (SLA) in seedlings of five populations of Quercus ilex from contrasting climates (data from García-Nogales et al. 2016). Circles represent population means. Bars indicate standard errors
The patterns of response to cold and drought found in oak species that inhabit seasonally-dry areas as well as those that span the temperate -tropical divide provide key results to understand patterns of resource-use strategies in oaks. For example, in the Mediterranean evergreen oak species, Q. suber, one of the studied populations exhibited higher SLA than expectations based on its low precipitation in summer . That “outlier” population was located in the southernmost area of their distribution ranges in the Iberian Peninsula (Cadiz province, Spain), characterized by mild temperatures in winter . Q. suber from this location was also found to be highly sensitive to low temperatures in winter (Aranda et al. 2005). Similar results have been reported for the evergreen oaks Q. ilex . In a recent study with five holm oak populations, García-Nogales et al. (2016) found that populations from the Iberian Peninsula characterized by cold winters tended to have a positive association between SLA and precipitation , indicating that mesic populations had higher SLA as observed in Q. suber and Q. faginea. In contrast, southern populations from North Africa with significantly lower index of moisture had higher SLA than Iberian populations, following the pattern shown by Q. oleoides in the seasonally dry tropical forest, where winter is absent.
4.7 Population Differentiation in Growth and Photosynthesis
Despite the strong population patterns observed for leaf morphology and drought resistance strategies, our results for growth and photosynthetic rates were not consistent across studies. In analyses performed with data from four common garden trials established in Honduras, we found population -level differentiation in height growth (Fig. 4.10). Populations from areas with longer or more severe dry seasons had higher growth rates in height than mesic populations, which agrees with the resource-use hypothesis for SDTFs . However, the reverse pattern of variation was also observed. Specifically, the results derived from a greenhouse experiment with five Q. oleoides populations revealed a positive association between growth rates and the index of moisture (Ramírez-Valiente and Cavender-Bares 2017), contrary to expectations based on the resource-use hypothesis. One possibility for this unexpected positive association between the index of moisture and growth could be the influence of the Rincón population , a mesic isolated population from Costa Rica. This population showed an outstanding growth rate in this experiment. In fact, once it was removed from the analysis, the relationship between the index of moisture and growth rate became no longer significant (R = 0.330, P = 0.001 including Rincón vs. R = −0.066, P = 0.578 excluding Rincón). Rincón de la Vieja is a high elevation population from Costa Rica that exhibits a marked neutral genetic differentiation relative to lowland populations (Deacon and Cavender-Bares 2015). This isolation could have leaded the evolution of particular traits conferring high relative growth rates in this population , even though we detected no fitness advantage in this population .
Relationship between growth in height (cm) and index of moisture (Precipitation —potential evapotranspiration) measured in four common garden experiments established in Honduras with natural and manipulated precipitation regimes (blue: water supply in the dry season, red: no water supply in the dry season). Redrawn from Ramírez-Valiente et al. (2017)
Patterns of population -level variation for photosynthetic rates were not consistent with the resource-use hypothesis. Populations from xeric areas did not show higher photosynthetic rates as expected. Furthermore, SLA was negatively associated with A mass or relative growth rate (RGR) under well-watered conditions in different studies (Ramírez-Valiente et al. 2017). Xeric populations undergo a short period of water deficit (July–August) within the wet season during which, precipitation is lower than potential evapotranspiration. This “little dry season” has an impact on physiology of species similar to the actual dry season. It is possible that this ‘little dry season’ may have constrained the evolution of increased A mass and RGR under favorable conditions of water and have promoted some leaf drought resistance during the wet season in xeric populations of Q. oleoides that experience this unpredictable water shortage (Choat et al. 2007). Consistent with this idea, we found a positive relationship between turgor loss point (πtlp) and index of moisture when Q. oleoides grew under well-watered conditions and the lack of plasticity in πtlp in response to water availability for xeric populations (see also next section).
4.8 Plasticity in Response to Drought
In long-lived species, plasticity is a critical means of surviving spatial and temporal environmental variation and may be more important than local adaptation in tolerating seasonal stress. Studies on Q. oleoides have showed a high phenotypic plasticity to water availability in growth rates, gas exchange , leaf morphology and photochemistry (Ramírez-Valiente et al. 2015, 2017; Ramírez-Valiente and Cavender-Bares 2017). Unlike in the case of cold acclimation discussed earlier, whether the plasticity we observed in this suite of traits is adaptive and therefore able to evolve in response to natural selection does not have an easy answer. Several studies on annual or short-lived species have shown an association between plasticity in response to water availability (or other resource) and fitness (Sultan 1995; Dudley 1996; Sultan 1996; Donohue et al. 2000). Plasticity is also considered adaptive if genotypes of a given species differ in phenotypic plasticity and the direction of the response is consistent with expectations based on the environment. Studies on Q. oleoides found population -level differentiation in plasticity of three functional traits: photoprotective pigments, water potential at the turgor loss point and leaf abscission that was associated with the index of moisture of the populations. Specifically, we found that populations originating from mesic areas tended to have higher plasticity in the de-epoxidation rates of the xanthophyll cycle (Fig. 4.11a) and higher capacity of osmoregulation (i.e. high plasticity in the water potential at the turgor loss point ) (Fig. 4.11b) whereas populations from more xeric populations had higher plasticity in leaf abscission (which is probably associated with leaf life span ) (Fig. 4.11c). These observations together with the fact that the direction of the phenotypic change is consistent with expectations based on the response to drought suggest that phenotypic plasticity is adaptive for these traits and could be subjected to natural selection. A trade off between plasticity in drought avoidance via leaf abscission with plasticity in drought tolerance via osmotic adjustment , suggests that plants have evolved flexibility in one kind of response or the other but not both (Fig. 4.11d). It is important to point out, however, that the variation in the response patterns within each of the populations is quite high, reinforcing other work in this system showing high diversity within populations (Center 2015; Deacon and Cavender-Bares 2015; Cavender-Bares et al. 2011).
a Plasticity (Δ) in the de-epoxidation state of the xanthophyll cycle (Δ = dry treatment − well-watered treatment) in relation to the index of moisture . Points represent family means means. Redrawn from Ramírez-Valiente et al. (2015). Circles represent family means. Plasticity (Δ) in turgor loss point b and leaf abscission c (Δ = dry treatment − well-watered treatment) in relation to the index of moisture of the population . Points represent population means. Bars indicate standard errors. d Relationship between plasticity in water potential at turgor loss point (πtlp) and plasticity in leaf abscission (Δ = dry treatment − well-watered treatment). Points represent population means. Bars indicate standard errors. b–d are redrawn from Ramírez-Valiente and Cavender-Bares (2017)
4.9 Response of Oaks to Past Climate Change Provides Lessons for the Future
Understanding the physiological limits of species, and the nature of variation within and among species, is critical to understanding species responses to climate change and in providing guidelines for conservation. To the extent to which species are composed of locally adapted populations with narrow climatic tolerances, on one extreme, or of populations that have little variation among them but broad climatic tolerances, at the other extreme, different conservation strategies are required. There are real limits to adaptive potential in long-lived organisms relative to the rate of climate change . Even during past climatic changes that occurred during the expansion of the desert and Mediterranean biomes some 5-million years ago, evidence is mounting that a live oak species, Quercus brandegeei (Fig. 4.12c, d), currently found only in the Cape region of southern Baja California, underwent severe range retraction in response to increased drought (Cavender-Bares et al. 2015). Coalescence models using molecular data indicate that the species once occupied a much more extensive range and had a population size >100-fold larger than its current population size. The species underwent range contraction as the Earth continued to cool and dry forming both Mediterranean and desert ecosystems globally. Rather than adapting to the novel climatic regime, now inhabited largely by desert and chaparral species, Q. brandegeei became a relictual population restricted to the edges of the ephemeral river beds in the Cape of Baja California. It is now an IUCN red-listed species.
4.10 Evolutionary Potential of Oak Populations
Future work in this system is aimed at addressing the adaptive potential of the live oaks to respond through genetic change to future climate change , a topic of increasingly highlighted importance (Etterson 2004a, b, 2008; Davis et al. 2005; Shaw and Etterson 2012). The genetic architecture of traits determines the potential rate of adaptive evolution in response to a changing environment. Response to natural selection requires genetic variation for traits (Falconer and Mackay 1996), although genetic correlations of various kinds can enhance or impede the rate of evolutionary change (Etterson and Shaw 2001). In particular, volutionary change can be enhanced if the sign of a genetic correlation follows the direction of selection but impeded if these are antagonistic. Facilitating or antagonistic relationships between the genetic architecture of traits and the direction of selection can occur between different life history stages (Schluter et al. 1991), between pairs of traits in a single life history stage (Conner and Via 1992; Caruso 2004) and between trait expression in different environments (Dickerson 1955; Via 1993; Etterson 2004b). Future work elucidating the underlying genetic architecture of physiological traits at different life history stages and as expressed in different environments will help predict the potential for evolutionary responses of these important long-lived species to future climates. Parallel to these efforts, advances in deciphering the genes and gene expression patterns associated with adaptations to climate is critical; important progress in oak systems has been made recently (Gugger et al. 2016, 2017). Understanding the nature and distribution of physiological and genome-wide variation within species facilitates conservation efforts. To that end, we are working with botanical gardens to link the knowledge we have gained to help them with in situ and ex-situ conservation of threatened oak species.
4.11 Conclusions
We synthesized evidence from a series of common garden studies for adaptive differentiation in physiological function both within and among closely related species in response to low temperature and drought . While direct experimental evidence for local adaptation to climatic variation associated with precipitation is lacking, the pattern of trait variation as well as the direction of plasticity are consistent with local adaptation to climate and adaptive plasticity . That nature of the variation in function that exists within and among populations has conservation implications. From these studies we have learned that the variation represented in the species cannot be captured within a single location. At the same time, variation in freezing tolerance is greatest between species, despite important evidence for population differentiation within Q. virginiana. In contrast, variation in drought tolerance , in some cases, is greater among populations within a species than between species of live oaks. The patterns may depend on the degree and nature of climatic variation that the populations within a species encounter. And despite clear evidence for evolution in response to climate, the case of Q. brandegeei demonstrates that even after 5 million years of exposure to drought conditions, the species maintains its conserved niche in well-drained soils with seasonal water availability, unable to migrate into the surrounding desert. As a consequence, it is a relictual species that will very likely perish without human intervention. The work synthesized here is fundamental to understanding and protecting the oaks, a critical group of species that contributes much to human well-being.
References
Ackerly DD, Reich PB (1999) Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. Am J Botany 86:1272–1281
Ananthakrishnan R, Soman MK (1989) Statistical distribution of daily rainfall and its association with the coefficient of variation of rainfall series. Int J Climatol 9:485–500
Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, Jansen S (2016) Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc Nat Acad Sci 113:5024–5029
Aranda I, Castro L, Alia R, Pardos JA, Gil L (2005) Low temperature during winter elicits differential responses among populations of the Mediterranean evergreen cork oak (Quercus suber). Tree Physiol 25:1085–1090
Bartlett MK, Scoffoni C, Sack L (2012) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett 5:393–405
Borchert R (1994) Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75:1437–1449
Boucher DH (1981) Seed predation by mammals and forest dominance by Quercus oleoides, a tropical lowland oak. Oecologia 49:409–414
Bowman D, Prior L (2005) Why do evergreen trees dominate the Australian seasonal tropics? Austr J Bot 53:379–399
Brodribb TJ, Holbrook NM (2006) Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant Cell Environ 29:2205–2215
Brodribb TJ, Holbrook NM, Edwards EJ, Gutierrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26:443–450
Burke MJ, Gusta LV, Quamme HA, Weiser CJ, Li PH (1976) Freezing injury in plants. Ann Rev of Plant Physiol 27:507–528
Caruso CM (2004) The quantitative genetics of floral trait variation in Lobelia: potential constraints on adaptive evolution. Evolution 58:732–740
Cavender-Bares J (2005) Impacts of freezing on long-distance transport in woody plants. In: Holbrook MN, Zwieniecki M (eds) Vascular transport in plants. Elsevier Inc., Oxford, pp 401–424
Cavender-Bares J (2007) Chilling and freezing stress in live oaks (Quercus section Virentes): intra- and interspecific variation in PS II sensitivity corresponds to latitude of origin. Photonsynth Res 94:437–453
Cavender-Bares J (2016) Diversity, distribution and ecosystem services of the North American Oaks. Int Oaks 27:37–48
Cavender-Bares J, Holbrook NM (2001) Hydraulic properties and freezing-induced cavitation in sympatric evergreen and deciduous oaks with, contrasting habitats. Plant Cell Environ 24:1243–1256
Cavender-Bares J, Pahlich A (2009) Molecular, morphological, and ecological niche differentiation of sympatric sister oak species, Quercus virginiana and Q. geminata (Fagaceae). Am J Bot 96:1690–1702
Cavender-Bares J, Kitajima K, Bazzaz F (2004) Multiple trait associations in relation to habitat differentiation among 17 Floridian oak species. Ecol Monogr 74:635–662
Cavender-Bares J, Cortes P, Rambal S, Joffre R, Miles B, Rocheteau A (2005) Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: a comparison of cooccurring Mediterranean oaks that differ in leaf lifespan. New Phytol 168:597–612
Cavender-Bares J, Gonzalez-Rodriguez A, Pahlich A, Koehler K, Deacon N (2011) Phylogeography and climatic niche evolution in live oaks (Quercus series Virentes) from the tropics to the temperate zone. J Biogeogr 38:962–981
Cavender-Bares J, Gonzalez-Rodriguez A, Eaton DAR, Hipp AAL, Beulke A, Manos PS (2015) Phylogeny and biogeography of the American live oaks (Quercus subsection Virentes): a genomic and population genetics approach. Mol Ecol 24:3668–3687
Center A (2015) Physiological and fitness consequences of seasonal rainfall variation in neotropical live oak seedlings (Quercus oleoides): implications for global change. University of Minnesota, Saint Paul
Center A, Etterson JR, Deacon NJ, Cavender-Bares J (2016) Seed production timing influences seedling fitness in the tropical live oak Quercus oleoides of Costa Rican dry forests. A J Bot 103:1407–1419
Choat B, Sack L, Holbrook N (2007) Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytol 175:686–698
Comita L, Engelbrecht B (2009) Seasonal and spatial variation in water availability drive habitat associations in a tropical forest. Ecology 90:2755–2765
Condit R (1998) Ecological implications of changes in drought patterns: shifts in forest composition in Panama. Clim Change 39:413–427
Condit R, Watts K, Bohlman S, Pérez R, Foster R, Hubbell S (2000) Quantifying the deciduousness of tropical forest canopies under varying climates. J Veg Sci 11:649–658
Conner J, Via S (1992) Natural selection on body size in Tribolium: possible genetic constraints on adaptive evolution. Heredity 69:73–83
Cornelissen JHC, Diez PC, Hunt R (1996) Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J Ecol 84:755–765
Crisp M, Arroyo M, Cook L, Gandolfo M, Jordan G (2009a) Phylogenetic biome conservatism on a global scale. Nature 458:754–756
Crisp MD, Arroyo MTK, Cook LG, Gandolfo MA, Jordan GJ (2009b) Phylogenetic biome conservatism on a global scale. Nature 458:754–756
Davis MB, Shaw RG, Etterson JR (2005) Evolutionary responses to changing climate. Ecology 86:1704–1714
Deacon NJ, Cavender-Bares J (2015) Limited pollen dispersal contributes to population genetic structure but not local adaptation in Quercus oleoides forests of Costa Rica. PLoS ONE 10:e0138783
Demmig-Adams B, Adams WW (2006) Tansley review: photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
Dickerson G (1955) Genetic slippage in response to selection for multiple objectives. Cold Spr Harb Symp Quant Biol 20:213–224
Donohue K, Messiqua D, Pyle EH, Heschel MS, Schmitt J (2000) Evidence of adaptive divergence in plasticity: density- and site-dependent selection on shade-avoidance responses in Impatiens capensis. Evolution 54:1956–1968
Dudley SA (1996) The response to differing selection on plant physiological traits: evidence for local adaptation. Evolution 50:103–110
Eamus D, Prichard H (1998) A cost-benefit analysis of leaves of four Australian savanna species. Tree Physiol 18:537–545
Engelbrecht BMJ, Kursar TA (2003) Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136:383–393
Engelbrecht BMJ, Dalling JW, Pearson TRH, Wolf RL, Galvez DA, Koehler T, Tyree MT, Kursar TA (2006) Short dry spells in the wet season increase mortality of tropical pioneer seedlings. Oecologia 148:258–269
Enquist B, Enquist C (2011) Long-term change within a Neotropical forest: assessing differential functional and floristic responses to disturbance and drought. Glob Chang Biol 17:1408–1424
Etterson JR (2004a) Evolutionary potential of Chamaecrista fasciculata in relation to climate change I. Clinal patterns of selection along an environmental gradient in the Great Plains. Evolution 58:1446–1458
Etterson JR (2004b) Evolutionary potential of Chamaecrista fasciculata in relation to climate change: II. Genetic architecture of three populations reciprocally planted along an environmental gradient in the Great Plains. Evolution 58:1459–1471
Etterson JR (2008) Evolution in response to climate change. In: Carroll S, Fox C (eds) Conservation biology: evolution in action. Oxford University Press, Oxford, p 145
Etterson J, Shaw R (2001) Constraint to adaptive evolution in response to global warming. Science 294:151–154
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics. Prentice Hall, New York
Fetcher N (1981) Leaf Size and Leaf Temperature in Tropical Vines. Am Nat 117:1011–1014
Flexas J, Diaz-Espejo A, Gago J, Gallé A, Galmés J, Gulías J, Medrano H (2014) Photosynthetic limitations in Mediterranean plants: a review. Environl Exp Bot 103:12–23
Franklin D (2005) Vegetative phenology and growth of a facultatively deciduous bamboo in a monsoonal climate. Biotropica 37:343–350
Fujikawa S, Kuroda K (2000) Cryo-scanning electron microscopic study on freezing behavior of xylem ray parenchyma cells in hardwood species. Micron 31:669–686
Givnish TJ (2002) Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fenn 36:703–743
Gould K (2004) Nature’s Swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 2004:314–320
Granda E, Scoffoni C, Rubio-Casal A, Sack L, Valladares F (2014) Leaf and stem physiological responses to summer and winter extremes of woody species across temperate ecosystems. Oikos 123:1281–1290
Gratani L, Meneghini M, Pesoli P, Crescente M (2003) Structural and functional plasticity of Quercus ilex seedlings of different provenances in Italy. Trees 17:515–521
Gugger PF, Cokus SJ, Sork VL (2016) Association of transcriptome-wide sequence variation with climate gradients in valley oak (Quercus lobata). Tree Genet Genom 12:15
Gugger PF, Peñaloza-Ramírez JM, Wright JW, Sork VL (2017) Whole-transcriptome response to water stress in a California endemic oak, Quercus lobata. Tree Physiol 37:632–644
Guy CL (2003) Freezing tolerance of plants: current understanding and selected emerging concepts. Can J Bot 81:1216–1223
Hipp AL, Manos PS, González-Rodríguez A, Hahn M, Kaproth M, McVay JD, Avalos SV, Cavender-Bares J (2017) Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytol. doi:10.1111/nph.14773
Hughes NM, Burkey KO, Cavender-Bares J, Smith WK (2012) Xanthophyll cycle pigment and antioxicant profiles of winter-red (anthocyanic) and winter-green (acyanic) angiosperm evergreen species. J Exp Bot 63:1895–1905
Huner NPA, Oquist G, Hurry VM, Krol M, Falk S, Griffith M (1993) Photosynthesis, photoinhibition and low-temperature acclimation in cold tolerant plants. Photosynth Res 37:19–39
IPCC (2007) Climate Change 2007: the physical science basis. contribution of working group i to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York
Jonasson S, Medrano H, Flexas J (1997) Variation in leaf longevity of Pistacia lentiscus and its relationship to sex and drought stress inferred from leaf δ13C. Funct Ecol 11:282–289
Jump A, Hunt J, Peñuelas J (2006) Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob Change Biol 12:2163–2174
Karmalkar AV, Bradley RS, Diaz HF (2008) Climate change scenario for Costa Rican montane forests. Geophys Res Lett 35. doi:10.1029/2008GL033940
Klemens JA, Deacon NJ, Cavender-Bares J (2011) Pasture recolonization by a tropical oak and the regeneration ecology of seasonally dry tropical forests. In: Dirzo R, Young HS, Mooney HA, Ceballos G (ed) Seasonally Dry Tropical Forests. Island Press/Center for Resource Economics, pp 221–237
Koehler K, Center A, Cavender-Bares J (2012) Evidence for a freezing tolerance—growth rate trade-off in the live oaks (Quercus series Virentes) across the tropical-temperate divide. New Phytol 193:730–744
Koerner C, Larcher W (1988) Plant life in cold climates. In: Long SP, Wodward FI (eds) Plants and temperature. Society of Experimental Biology, Cambridge, pp 25–57
Kurz H, Godfrey RK (1962) Trees of Northern Florida. University of Florida, Gainesville
Larcher W (1960) Transpiration and photosynthesis of detached leaves and shoots of Quercus pubescens and Q. ilex during desiccation under standard conditions. Bull Res Counc Isr 8:213–224
Larcher W (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst 134:279–295
Markesteijn L, Poorter L, Paz H, Sack L, Bongers F (2011) Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell Environ 34:137–148
Medina E (1995) Diversity of life forms of higher plants in neotropical dry forests. In: Bullock S, Mooney H, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 221–242
Meireles JE, Beulke A, Borkowski D, Romero-Severson J, Cavender-Bares J (2017) Balancing selection maintains diversity in a cold tolerance gene in broadly distributed live oaks. Genome in press
Miller HA, Lamb SH (1985) Oaks of North America. Naturegraph Publishers Inc, Happy Camp, California
Muller CH (ed) (1942) The central American species of Quercus. United States Department of Agriculture, Washington, DC
Muller SC (1961) The origin of Quercus fusiformis small. J Linn Soc 58:186–192
Myers RL (1990) Scrub and High Pine. In: Myers RL, Ewel JJ (eds) Ecosystems of Florida. University of Central Florida Press, Orlando, pp 150–193
Nardini A, Salleo S, Gullo MAL, Pitt F (2000) Different responses to drought and freeze stress of Quercus ilex L. growing along a latitudinal gradient. Plant Ecol 148:139–147
Nicholls N, Wong KK (1990) Dependence of rainfall variability on mean rainfall, latitude, and the Southern Oscillation. J Clim 3:163–170
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Niinemets Ü (2015) Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol 205:79–96
Niinemets Ü (2016) Does the touch of cold make evergreen leaves tougher? Tree Physiol 36:267–272
Nixon KC (1985) A Biosystematic Study of Quercus Series Virentes (the live oaks) with Phylogenetic Analyses of Fagales, Fagaceae and Quercus, Ph.D. Thesis. University of Texas, Austin
Nixon KC, Muller CH (1997) Quercus Linnaeus sect. Quercus White oaks. In: Flora of North America Committee (ed) Flora of North America, North of Mexico. Oxford University Press, New York, pp 436–506
Oertli JJ, Lips SH, Agami M (1990) The strength of sclerophyllous cells to resist collapse due to negative turgor pressure. Acta Oecologica 11:281–289
Parkhurst DF, Loucks OL (1972) Optimal leaf size in relation to environment. J Ecol 60:505–537
Parker J (1963) Cold resistance in woody plants. Bot Rev 29:123–201
Pennington RT (2006) Neotropical Savannas and seasonally dry forests: plant diversity, biogeography, and conservation. CRC Press, Taylor & Francis Group, New York
Pietrini F, Iannelli M, Massacci A (2002) Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant, Cell Environ 25:1251–1259
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta‐analysis. New Phytol 182:565–588
Poorter L, Markesteijn L (2008) Seedling traits determine drought tolerance of tropical tree species. Biotropica 40:321–331
Ramírez-Valiente JA Cavender-Bares J (2017) Evolutionary trade-offs between drought resistance mechanisms across a precipitation gradient in a seasonally dry tropical oak (Quercus oleoides). Tree Physiol 1–13. doi:10.1093/treephys/tpx1040
Ramírez-Valiente JA, Sánchez-Gómez D, Aranda I, Valladares F (2010) Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol 30:618–627
Ramírez-Valiente J, Valladares F, Sánchez-Gómez D, Delgado A, Aranda I (2014) Population variation and natural selection on leaf traits in cork oak throughout its distribution range. Acta Oecol 58:49–56
Ramírez-Valiente JA, Koehler K, Cavender-Bares J (2015) Climatic origins predict variation in photoprotective leaf pigments in response to drought and low temperatures in live oaks (Quercus series Virentes). Tree Physiol 35:521–534
Ramírez-Valiente JA, Center A, Sparks SP, Sparks KL, Etterson JR, Longwell T, Pilz G, Cavender-Bares J (2017) Population-level differentiation in growth rates and leaf traits in seedlings of the neotropical live oak Quercus oleoides grown under natural and manipulated precipitation regimes. Front Plant Sci 8:585
Read J, Sanson GD (2003) Characterizing sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytol 160:81–99
Reich PB (2014) The world‐wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301
Reich PB, Borchert R (1984) Water stress and tree phenology in a tropical dry forest in the lowlands of Costa Rica. J Ecol 61–74
Sakai A, Larcher W (1987) Frost survival of plants: responses and adaptations to freezing stress. Springer-Verlag, Berlin
Sakai A, Weiser CJ (1973) Freezing resistance of trees in North America with reference to tree regions. Ecology 54:118–126
Savage JA, Cavender-Bares J (2013) Phenological cues drive an apparent trade-off between freezing tolerance and growth in the family Salicaceae. Ecology 94:1708–1717
Savage J, Cavender-Bares J, Verhoeven A (2009) Habitat generalists and wetland specialists in the genus Salix vary in their photoprotective responses to drought. Funct Plant Biol 36:300–309
Schluter D, Price TD, Rowe L (1991) Conflicting selection pressures and life history trade-offs. Proc Roy Soc B 246:11–17
Shaw RG, Etterson JR (2012) Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol 195:752–765
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35:543–584
Stevens N, Archibald S, Nickless A, Swemmer A, Scholes R (2016) Evidence for facultative deciduousness in Colophospermum mopane in semi-arid African savannas. Austr Ecol 41:87–96
Sultan SE (1995) Phenotypic plasticity and plant adaptation. Acta Bot Neerl 44:363–383
Sultan SE (1996) Phenotypic plasticity for offspring traits in Polygonum persicaria. Ecology 77:1791–1807
Tomlinson K, Poorter L, Sterck F, Borghetti F, Ward D, Bie S, Langevelde F (2013) Leaf adaptations of evergreen and deciduous trees of semi-arid and humid savannas on three continents. J Ecol 101:430–440
Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA (2003) Desiccation tolerance of five tropical seedlings in Panama. Relationship to a field assessment of drought performance. Plant Physiol 132:1439–1447
Van Kleunen M, Fischer M (2005) Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol 166:49–60
Via S (1993) Adaptive phenotypic plasticity: target of by-product of selection in a variable environment? Am Nat 142:352–365
Vico G, Thompson S, Manzoni S, Molini A, Albertson J, Almeida-Cortez J, Fay P, Feng X, Guswa A, Liu H, Wilson T, Porporato A (2015) Climatic, ecophysiological, and phenological controls on plant ecohydrological strategies in seasonally dry ecosystems. Ecohydrology 8:660–681
Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Davies TJ, Grytnes JA, Harrison SP, Hawkins BA, Holt RD, McCain CM, Stephens PR (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324
Williams K, Field CB, Mooney HA (1989) Relationships among leaf construction cost, leaf longevity, and light environment in rain-forest plants of the genus Piper. Am Nat 133:198–211
Wisniewski ME, Ashworth EN (1985) Changes in the ultrastructure of xylem parenchyma cells of peach (Prunus persica) and red oak (Quercus rubra) in response to a freezing stress. Am J Bot 72:1364–1376
Wright IJ, Reich PB, Cornelissen JH, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets U, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M (2005) Modulation of leaf economic traits and trait relationships by climate. Glob Ecol Biogeogr 14:411–421
Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, FitzJohn RG, McGlinn DJ, O’Meara BC, Moles AT, Reich PB, Royer DL, Soltis DE, Stevens PF, Westoby M, Wright IJ, Aarssen L, Bertin RI, Calaminus A, Govaerts R, Hemmings F, Leishman MR, Oleksyn J, Soltis PS, Swenson NG, Warman L, Beaulieu JM (2014) Three keys to the radiation of angiosperms into freezing environments. Nature 506:89–92
Acknowledgements
The studies represented here were funded by grants from the University of Minnesota and the National Science Foundation (IOS: 0843665) to J.C-B. and a fellowship from the Severo Ochoa excellence program to J.A.R-V .
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Cavender-Bares, J., Ramírez-Valiente, J.A. (2017). Physiological Evidence from Common Garden Experiments for Local Adaptation and Adaptive Plasticity to Climate in American Live Oaks (Quercus Section Virentes): Implications for Conservation Under Global Change. In: Gil-Pelegrín, E., Peguero-Pina, J., Sancho-Knapik, D. (eds) Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.. Tree Physiology, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-319-69099-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-69099-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69098-8
Online ISBN: 978-3-319-69099-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)