Abstract
Quantifying plant drought resistance is important for understanding plant species' association to microhabitats with different soil moisture availability and their distribution along rainfall gradients, as well as for understanding the role of underlying morphological and physiological mechanisms. The effect of dry season drought on survival and leaf-area change of first year seedlings of 28 species of co-occurring woody tropical plants was experimentally quantified in the understory of a tropical moist forest. The seedlings were subjected to a drought or an irrigation treatment in the forest for 22 weeks during the dry season. Drought decreased survival and growth (assessed as leaf-area change) in almost all of the species. Both survival and leaf-area change in the dry treatment ranged fairly evenly from 0% to about 100% of that in the irrigated treatment. In 43% of the species the difference between treatments in survival was not significant even after 22 weeks. In contrast, only three species showed no significant effect of drought on leaf-area change. The effects of drought on species' survival and growth were not correlated with each other, reflecting different strategies in response to drought. Seedling size at the onset of the dry season had no significant effect on species' drought response. Our study is the first to comparatively assess seedling drought resistance in the habitat for a large number of tropical species, and underlines the importance of drought for plant population dynamics in tropical forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On a global scale, primary productivity, plant species' distribution and the diversity of plant communities are correlated with rainfall gradients and moisture availability (e.g., Lieth 1975; Boyer 1982; Currie and Paquin 1987; O'Brien 1993). Even within the moist and wet tropics, species' distributions and diversity gradients strongly correlate with annual rainfall (e.g., Gentry 1988; Condit 1998; Swaine 1996; Bongers et al. 1999) and soil moisture availability may be one of the main factors influencing habitat associations of tropical trees, shrubs and herbs (e.g., Whitmore 1984; Richards 1998; Sollins 1998; Webb and Peart 2000). Nevertheless, the responses of tropical rainforest plants to soil water availability and drought have received little attention, perhaps because they were assumed to experience little drought stress.
In tropical forests, annual rainfall varies from about 800 mm to >10,000 mm (Walsh 1998). In most of the tropics, dry season droughts occur regularly once or twice per year (Windsor 1990; Walsh and Newbery 1999) and, even in many aseasonal equatorial areas, dry periods of 15–35 days occur at least every other year (Becker 1992; Burslem et al. 1996; Walsh and Newberry 1999). Especially severe droughts in tropical regions occur in association with El Niño climatic events (e.g., Toma et al. 2000; Nakagawa et al. 2000).
During the dry season, plants in tropical forests can be exposed to considerable drought stress: wilting has been observed, and pre-dawn water potentials of down to −3.5 MPa have been measured (Becker and Wong 1993; Veenendaal et al. 1995; Tobin et al. 1999). Drought has been associated with increased mortality and decreased growth rates in tropical plants (e.g., Turner 1990; Fisher et al. 1991; Veenendaal et al. 1995; Condit et al. 1995). Thus species' differences in drought resistance may be a major factor influencing species distributions in the wet tropics. However, the link between species' drought resistance and plant distribution and diversity remains poorly understood, mainly because comparative quantitative assessments of the effects of drought on plant growth and survival are largely missing.
We use the term drought resistance as "the capacity of a plant to withstand periods of dryness" (Larcher 1980), i.e., the ability to survive drought while minimizing reductions in growth, and ultimately fitness. We experimentally quantified drought resistance for first year seedlings of 28 co-occurring species of woody plants as the survival and growth under drought relative to irrigated conditions in the natural habitat. This method allows us to isolate the effects of drought from other ecological factors that determine survival and growth. We chose young seedlings because, due to their limited root system and competition with adult plants for soil resources (Lewis and Tanner 2000), they are likely to be the life stage most sensitive to drought. By working with a large number of species, we can assess the degree to which species occurring in the same area differ in their responses to drought. This approach may allow us to link variation in species' drought resistance with patterns of distributions and abundance across gradients of soil water availability in tropical rainforests.
Materials and methods
Site and plant material
The experiment was conducted in Panama at the Buena Vista peninsula which is part of the Barro Colorado Nature Monument (BCNM), a protected area of about 5,400 ha administered by the Smithsonian Tropical Research Institute. Average annual rainfall is approximately 2,600 mm with a pronounced 4-month dry season that usually lasts from about mid-December through mid-April (Windsor 1990). Our study site has semi-deciduous, second growth forest about 60 years old. Experimental plots were situated in the understory of the forest in an area of about 25 ha.
We worked with seedlings of 28 woody plant species (Table 1). Species were chosen according to: (1) availability and germination of sufficient seeds, (2) sufficient degree of seedling shade-tolerance for regeneration in the understory, (3) availability of information about habitat affiliation and distribution relative to indicators of soil moisture whenever possible. Seedlings were from 21 different families and 12 orders, and represented different leaf phenologies (evergreen or deciduous, Table 1), different life forms (shrubs and trees) and a range of seed sizes. In the following we will refer to the species by their genus. Seeds were collected in 2000 within the BCNM. For the "seedlings" of Piper trigonum, which frequently propagate vegetatively in nature, we used cuttings. Cuttings included one node and about the proximal fifth of the attached leaf. Propagules of each species were obtained from a minimum of three (mostly many more) mother plants. Within a few days after collection, fleshy seed covers, wings, etc. were removed, and the seeds were set out in seedling trays for germination in the greenhouse under moderately low light conditions (5–10% full sunlight). Seedlings were then transplanted into pots (0.3–1.0 l according to seedling size) and maintained in the greenhouse until transplanting into the field. Seedlings were transplanted to plots in the forest from September through November 2000. For all seedlings, the dry season of 2000/2001 was the first dry season they experienced. According to the time of fruiting and germination, the seedlings were between 2 and 9 months old at the onset of their first dry season and the start of the experiment. Seedling size varied between species, with average seedling heights of 36–308 mm and average leaf areas of 10–263 cm2 (Table 1).
Experimental set-up
The seedlings were exposed to two treatments, irrigated and dry, for 22 weeks in the dry season (18 December 2000–12 June 2001). Sixty plots (0.8 m×1.0 m) were established in the understory. Plot positions were chosen along the trails with a minimum distance of 10 m between plots, and all plots were situated near large trees to allow for root competition for water. All plots were caged with wire mesh (1.1 cm×1.1 cm mesh width) to exclude vertebrate herbivores, and to minimize damage through leaf-, twig- and branch-fall. Within each plot, we initially marked 45 positions, permitting the inclusion of up to 45 species, and randomly assigned the 28 available species to them. One seedling of each species was transplanted directly into the soil of each plot for a total of 28 plants per plot, and their survival and leaf-area change followed during the course of the experiment. At the beginning and the end of the experiment, light conditions in the plots were assessed with hemispherical photographs (Model Coolpix-950; Nikon, Melville, N.Y.; analyzed with Hemiview 2.1, Delta-T Devices, Cambridge) and the average of the direct site factor (DSF; proportion of direct light reaching the understory in relation to the light reaching the canopy) was determined (cf. Engelbrecht and Herz 2001). To ensure the same range of light conditions in both treatments, the plots were paired by initial light conditions as predicted by the Hemiview analysis, and one plot of each pair was randomly assigned to each treatment.
Dry plots were covered with rain-out shelters (1.2 m×1.4 m) made from transparent plastic sheets to protect them from any dry season rains. During rain events, water collected in the rain-out shelters, and was manually discarded away from the plots. Light intensities (photosynthetic photon flux density) decreased by approximately 20% due to the plastic cover (assessed by LICOR quantum sensor measurements). The resulting range of DSF was 4.5–9.5% in the dry plots, and 5.8–12.6% in the wet plots (means±SD were 6.6%±1.3% and 7.7%±1.5%, t -test: P <0.05). Wet plots were watered regularly with water from Gatun Lake. Initially, 15 mm/m2 water was applied with watering cans 3 times a week, equivalent to 193 mm of monthly rain. Later in the dry season the amount of water applied was increased because competition from neighboring plants decreased the relative soil water content even in the wet plots. The amount of water applied was increased individually for the different plots according to occasional visible wilting of the seedlings.
Soil water content and water potential measurements
Censuses of gravimetric soil water content, seedling survival, plant wilting stage and leaf area were initially conducted monthly, later biweekly throughout the experiment. Gravimetric soil water content of the upper 10 cm of soil was determined. Samples were extracted in each census at a random position in each plot with a soil corer (1 cm diameter). Samples were placed in sealed plastic bags, fresh weight was determined in the laboratory (balance to 0.0001 g). The samples were dried to constant weight at 100°C for at least 48 h, and dry weight was determined. Gravimetric soil water content (G) was calculated as:
After 17 weeks of treatment, when gravimetric soil water content was at its minimum, we measured profiles of soil water potentials in seven dry and two wet plots. Soil samples of about 1 ml were extracted at different depths and immediately placed in field-portable psychrometer chambers (Merrill Specialty Equipment, Logan, Utah). Psychrometers were equilibrated in a water bath for 3.5 h to room temperature (approximately 23°C), and water potentials determined after 15 and 45 s cooling time (CR7; Campbell Scientific Equipment, Logan, Utah).
Assessments of growth, survival and wilting stages
Leaves of all plants were numbered with water-based, water-proof markers, and length and width of each leaf were measured. Expanding and mature leaves were color-coded, with leaf stage based on color and texture (and on comparison with earlier measurements). Regressions between the product of length and width, and leaf area, computed from direct measurements on leaves (and cotelydons, if applicable) of seedlings of equivalent size, were used to calculate leaf areas from these measurements (mean R 2=0.97, range=0.74–0.99). For each leaf we also noted (in 10% intervals) the amount of leaf area dead or missing due to pathogen and/or herbivore damage or to necrosis for other reasons (e.g., drought stress), and subtracted the appropriate area from the area of the intact leaf. In subsequent censuses, we noted: (1) presence/absence for each leaf; (2) changes in the amount of herbivory, pathogen damage and/or necrosis; (3) measured leaves that were not yet fully expanded in the last census; and (4) marked and measured newly developed leaves. With this information, we calculated the leaf area of each seedling at each census. Growth was assessed as (net) leaf-area change relative to the beginning. Leaf-area change can be caused by an increase in leaf area, due to development and expansion of new leaves, and/or a leaf-area loss, e.g., through leaf abscission, or through herbivore or pathogen damage. Dry weights of roots and stems as well as specific leaf areas of seedlings in the understory do not change significantly within the duration of one dry season in this habitat, and are not affected by drought conditions (Baker, Engelbrecht, Tyree, Kursar, unpublished data).
Wilting stage of each leaf was classified at each census according to visual characteristics (Table 2).
Seedling survival was based upon the presence of living above-ground tissues. In many species living stems were green, and living plants could clearly be distinguished even without leaves. For some species, distinguishing dead from alive plants was more difficult because even living stems were grayish. For these, the commencement of disintegration of the stem and increased fracture susceptibility were considered indicators of death. In species for which death was difficult to score, seedlings were followed after rewatering at the end of the experiment, and the scoring confirmed.
Data analysis
We quantified the species' drought resistance in terms of survival ( D S) as the percent survival in the dry treatment relative to the irrigated treatment:
where S W and S D are the number of seedlings after 22 weeks relative to the actual sample size at the beginning in the wet and the dry treatment, respectively (Appendix 1). The drought resistance in terms of leaf-area change ( D LA)—as a measure of plant growth—was quantified in the equivalent way as:
where LAW and LAD are the leaf area after 22 weeks relative to the leaf area at the beginning in the wet and the dry treatment, respectively.
Soil water census data were analyzed by repeated measures ANOVA (STATISTICA; StatSoft, Tulsa, Okla.). An ANCOVA with light conditions as a covariable was used to analyze for overall treatment and species effects on leaf-area change, and for treatment effects on the survival per plot (STATISTICA). Differences within species between the treatments in leaf-area change and survival were analyzed with t -tests and Fisher's exact tests, respectively (InStat; GraphPad Software, San Diego, Calif.). Additionally, the significance levels were adjusted in a sequential Bonferroni test for an experimental error rate α=0.05 (Rice 1989). However, since we were mainly interested in individual species responses, the Results and Discussion are based on unadjusted values (Perneger 1998). The effects of leaf phenology (adult and/or seedling, see Table 1) on average species survival and leaf-area responses to drought were assessed with an ANOVA, and Spearman rank correlations were used to evaluate the effect of initial leaf area and seedling height (STATISTICA).
Results
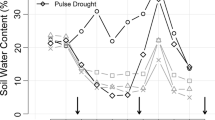
The dry season of 2000/2001 was about average both in terms of amount of rainfall and duration, but it began and ended 3–4 weeks later in the year than normal (Fig. 1A). Soil water content was already significantly lower in the dry than the wet treatment in the first census (after 4 weeks) and stayed significantly lower throughout the experiment (Fig. 1B; repeated ANOVA: P <0.0001 for treatment and time effects, as well as their interaction). The lowest gravimetric soil water contents that were reached at the end of the dry season under the rain-out shelters in the dry treatment were slightly below the values reached in a "normal" dry season in the area (Engelbrecht, Tyree, Kursar, unpublished data), and are probably comparable to those reached in severe El Niño-associated dry seasons. Even in the wet treatment the gravimetric soil water content decreased, due to competition for water from neighboring plants. A rise in soil water content in weeks 20 and 22 coincided with rain events (cf. Fig. 1A, B). The rehydration was probably due to lateral water movement through soil capillaries and roots, i.e., lateral hydraulic "lift" (Caldwell et al. 1998). Soil water potentials and their vertical distribution also differed strongly between treatments (Fig. 1C). In the dry treatment soil water potentials fell to as low as −6 MPa at the soil surface, and there was a strong increase towards lower soil layers. In contrast, soil water potentials measured in the wet treatment were all above −1 MPa, and there was no vertical gradient.
Rainfall and soil moisture conditions in the wet and dry treatments. A Weekly rainfall on Barro Colorado Island (●) during the experiment and 71-year average (–); we calculated the weekly rainfall as the sum of the rainfall for the day plotted plus for the 3 days before and after. The total rainfall during the experiment was 563 mm (➙ indicates 239 mm rainfall in week 2) versus a 70-year average of 473 mm over the same calendar dates. Data from Steve Paton, Smithsonian Environmental Science Program. B Gravimetric soil water content in the upper 10 cm of soil in the experimental plots (○ dry treatment, ● irrigated treatment; n =30). ■ Represents the gravimetric water content at field capacity and □the average gravimetric water content for the wet season of 2001. Data are averages±SE. C Soil water potential profiles measured in week 17 when soil water content was at a minimum (symbols as before; dry treatment n =7, wet treatment n =2)
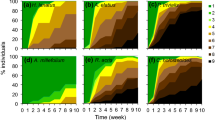
In most species, dry treatment conditions caused wilting, and reduced survival and leaf-area increase. The effects of drought differed strongly among species. As an example, Fig. 2 shows the wilting behavior and mortality of nine species in the dry treatment over time. In some species (e.g., Beilschmiedia, Calophyllum) wilting started very early in the experiment, plants went quickly through all wilting stages, and most individuals died early. At the other extreme (e.g., Ouratea, Garcinia), few plants showed any visible sign of drought stress, and mortality in the dry treatment was extremely low. There was a continuum of reactions (e.g., Tabebuia, Sorocea, Hybanthus, Psychotria) with different combinations of wilting behavior and mortality. Pseudobombax was clearly deciduous with all plants shedding their leaves but having low mortality. Partial recovery from the more advanced wilting stages was evident in weeks 20 and 22 and this correlated with the increased soil water content observed in Fig. 1B.
In 22 of the species, survival over 22 weeks was extremely high in the wet treatment (≥90%, Fig. 3A). In the other six species ( Andira, Beilschmiedia, Dipteryx, Hymenaea, Inga, Pouteria), >10% of the individuals died even in the irrigated treatment. For Beilschmiedia we could not always maintain soil water contents in the irrigated treatment above stress levels (wilting was observed), and mortality even in the wet treatment may have been partly caused by drought. For the other five species, herbivore and pathogen damage were probably the main reasons for mortality in the wet treatment (personal observation).
Survival over 22 weeks in the irrigated and the dry treatment. A Survival in the two treatments relative to the number of individuals at the beginning ( black bars wet treatment, gray bars dry treatment). B Survival in the dry treatment relative to the wet treatment (D S= S D/ S W×100). Species are sorted by increasing drought resistance. Results of Fisher's exact test for treatment effects within each species are given in A [*** P <0.0001, ** P <0.005, * P <0.05, (*) P =0.05, not significant n.s. P>0.05]. The horizontal bar marks those species for which treatment differences were significant after stepwise Bonferroni adjustment. For abbreviations, see Table 1
Seedling survival over 22 weeks was higher in the wet treatment than in the dry treatment in all except one species (Fig. 3A), so that survival in the dry treatment relative to the wet treatment ranged from 0% (Beilschmiedia) to 100% (Dipteryx, Fig. 3B). The watering treatment had a significant effect on seedling survival per plot (ANCOVA: F =50.24, P <0.0001), whereas light as a covariable did not have a significant effect. Within species, 22 weeks of drought caused a significant reduction of survival in 16 of the species. In the 12 other species, the effect was not significant.
Net leaf-area change of the surviving individuals (a measure of growth) varied between treatments and species (Fig. 4A). In most cases, leaf area increased in the irrigated treatment and decreased in the dry treatment. However, in some species leaf area increased and in other species leaf area decreased in both treatments. Overall, there was a significant effect of species (ANCOVA: F =11.71, P <0.0001, see Appendix 1 for n) and treatment ( F =195.52, P <0.0001) on leaf-area change of the surviving individuals and the effect varied among species (species×treatment interaction, F =3.28, P <0.0001). Including light as a covariable did not have a significant effect on leaf-area change. The maximum effect on leaf-area change of the survivors (Fig. 4B) was in Pseudobombax, where the leaf area in the drought treatment was 2% of that in the irrigated treatment. In contrast, in three species there were no significant differences between treatments ( Swartzia, Andira, Piper). Analyzing leaf-area change for survivors only focuses on those individuals contributing to future population dynamics. This approach ignores the fact that all plants that died in the experiment suffered complete leaf loss prior to death, and thus on a population level leaf-area loss was much more pronounced in those species with lower survival. We therefore also calculated leaf-area change for the entire population of each species (Appendix 1).
Leaf-area change over 22 weeks of the surviving individuals in the irrigated and dry treatment. A Leaf-area change relative to the leaf area at the beginning ( black bars wet treatment, gray bars dry treatment). Values >1 and <1 are net leaf-area increases and losses, respectively. The values are averages±SE, n are given in Appendix 1. B Leaf-area change in the dry treatment relative to the wet treatment ( D LA=LAD/LAW×100). Results of t -tests for treatment effects in each species are given in A (*** P <0.0001, ** P <0.005, * P< 0.05, n.s. P> 0.05). The t -test for unequal variances was used where necessary. Horizontal bars mark those species for which treatment differences were significant after stepwise Bonferroni adjustment. For abbreviations, see Table 1
Species' survival and leaf-area change of the survivors in dry relative to wet conditions ( D S and D LA) did not correlate with each other (Fig. 5). Points were scattered, forming a triangular shape with three "extremes": both high relative survival and leaf-area change (e.g., Swartzia) (group a), high relative survival combined with a strong decrease in leaf area (e.g., Dipteryx, Pseudobombax) (group b), and a low relative survival combined with an intermediate effect on leaf-area change (Virola, Calophyllum, Piper) (group c). However, when species with facultatively deciduous seedlings (see Fig. 5) were excluded from the analysis, there was a significant relationship between D s and D LA ( r 2=0.29, P <0.05). An analysis of leaf-area change in the whole population of all species (Appendix 1) showed a significant correlation between survival and leaf-area change in dry relative to irrigated conditions (r 2=0.36, P <0.01).
Relation between D S and D LA of survivors in the dry relative to the wet treatment. The three species that overlap at the right of center are HYM, XYO and TRC. * Species a priori known to be deciduous as adults (Croat 1978), ** additional species whose seedlings were found to be facultatively deciduous in this study. For abbreviations, see Table 1
Deciduous species (as reported by Croat 1978 for adults, see Table 1) showed a significantly stronger effect of drought on leaf-area change than evergreen species (ANOVA: F =18.38, P <0.0005), but survival did not differ between deciduous and evergreen species (ANOVA: P >>0.05). The same was true when we based our definition of deciduousness on our direct observations of the seedlings in the experiment and added Hybanthus, Inga , and Thevetia, species that are not deciduous as adults (see Fig. 5). Initial seedling leaf area or seedling height did not have any effect on relative survival or relative leaf-area change ( P>>0.05 in Spearman rank correlations).
Discussion
Survival and growth reduction due to drought
A trend towards decreased seedling survival was observed in all except one of the 28 species (Fig. 3A). The effect of drought on survival varied considerably and continuously among species (Fig. 3B). Few studies have conclusively shown drought-induced death of tropical seedlings (Turner 1990; Fisher et al. 1991; Veenendaal et al. 1995), although many present circumstantial evidence (e.g., Garwood 1982; Schupp 1988; Brown and Whitmore 1992; Gilbert et al. 2001). That survival of 43% of the species in this study was not significantly affected by the severe drought conditions in the experiment, shows that many species in the BCNM forest are well adapted to drought conditions, and may survive severe dry seasons and/or in dry microsites. The high number of drought-resistant species may seem surprising, given that tropical rainforest plants have been viewed as extremely drought sensitive. However, drought conditions do occur even in wet tropical forests (see Introduction). Adaptations to drought, allowing high survival rates under drought conditions, therefore may be more advantageous than had been recognized previously, especially for plants in areas with a pronounced dry season. These findings are concurrent with results from a recent study where seedlings of three common shade-tolerant tree species in the Barro Colorado Nature Monument did not show increased mortality in a severe El Niño dry season compared to normal years (Engelbrecht et al. 2002).
Our measure of growth (leaf-area change) was significantly lower in the dry than the irrigated treatment in most species, and the effect varied widely and significantly (Fig. 4). Seedlings in the dry treatment showed reduced development and expansion of new leaves, and/or increased leaf loss compared to seedlings under irrigated conditions. Growth reduction poses a cost on plants. It is therefore likely that individuals that suffer little growth reduction will have less drought-related fitness losses than those with strong decreases in growth rates. Reduced growth and leaf shedding as a result of drought stress are well known phenomena (e.g., Jarvis and Jarvis 1963; Kozlowski and Pallardy 1997 and citations therein). However, few studies have directly compared the extent of growth reduction due to drought between species (e.g., Jarvis and Jarvis 1963; Evans and Etherington 1991; Espigares and Peco 1995; Fernandez and Reynolds 2000). Of the relevant studies of drought effects on growth of woody rainforest plants, most have shown that droughted seedlings, as well as saplings and mature trees, have reduced growth compared to plants under wet conditions (e.g., Unwin and Kriedemann 1990; Fisher et al. 1991; Veenendaal et al. 1995; Poorter and Hayashida-Oliver 2000; Ito et al. 2000; Chandrashekar et al. 1998). Methodological differences and the very limited number of species investigated do not allow comparisons based on the available data.
Species' drought responses both in terms of survival and growth varied continuously. Any separation into "drought-resistant" versus "drought-sensitive" species must therefore remain arbitrary. This result indicates the difficulty of defining drought-related functional groups in tropical forests in order to reduce ecological complexity for modeling of forest dynamics, consequences of disturbance or global climate change (cf. Swaine and Whitmore 1988; Gitay and Noble 1997).
Strategies of drought resistance
The reductions in survival and in growth (leaf-area change) of the surviving plants due to drought were not correlated. The combinations of responses indicate three extreme strategies of coping with drought (see Fig. 5):
-
1.
Little effect on both growth and survival: drought of the same length and intensity as in the present experiment, comparable to a severe drought at the study site, does not stress these species. We conclude that these species are well adapted to drought. The adaptations may include deep and extended root systems (especially in relation to their leaf area), low drought-induced xylem dysfunction, and osmotic adjustment, so that transpiration, photosynthesis and cell expansion continue at similar rates under wet and dry conditions.
-
2.
Little effect of drought on survival, but a strong effect on growth: the seedlings of these species shed their leaves under dry conditions and kept (most of) them in the wet treatment. They are facultatively deciduous. Leaf shedding may be crucial for maintaining above-lethal water potentials in meristems and roots of seedlings. Although leaf shedding does have a pronounced negative impact on growth, this strategy may allow survival through strong drought conditions, and successful colonization of relatively dry habitats.
-
3.
A strong effect of drought on survival with the surviving individuals showing an intermediate growth reduction: these species may be taking a gamble on maintaining high photosynthesis, transpiration and growth rates while rapidly approaching lethal desiccation levels, possibly combined with shallow roots. This strategy may be successful in habitats where droughts are usually mild or short but not in habitats with extended or severe droughts. The absence of species with both low survival and growth under drought may reflect the climatic conditions in our study area, where soil moisture declines regularly in the dry season. Such highly drought-sensitive species may be more common in continuously wet habitats.
The scatter of points when relating drought effects on survival and growth was continuous, despite the three extremes identified above (Fig. 5). This suggests that plant strategies to cope with drought cannot be clearly separated from each other, but rather represent a continuum and are the outcome of different combinations of traits. Accordingly, dry season measurements of midday leaf water potentials on saplings of four of our species showed strong species differences (averages of −1.8 to −4.5 MPa, Tobin et al. 1999), but no relation to drought resistance in terms of survival or growth in our study. That different strategies to cope with drought can lead to similar survival rates is supported by our unexpected result that species with deciduous seedlings did not have higher relative survival than non-deciduous species. Our data also suggest that strategies to cope with drought may change throughout the life cycle of plants: the seedlings of three of our species ( Inga, Hybanthus and Thevetia) were facultatively drought deciduous in the experiment, whereas adults are not known to be deciduous (Croat 1978).
Even though seedling size varied tenfold, we found no relation between seedling size at the beginning of the experiment and the magnitude of the drought effect on either leaf-area increase or survival across species. This is contrary to expectations that seedling size at the onset of a drought might be a good predictor of drought resistance, reasoning that larger plants have deeper roots and thus can better exploit the higher water potentials in deeper soil layers. Lower susceptibility to drought in larger plants has been shown in some tropical plants (Cao 2000; Poorter and Hayashida-Oliver 2000). The lack of a trend towards a smaller drought effect in species with larger seedlings may have two possible reasons: (1) species' leaf area and seedling height may poorly reflect rooting depth; (2) other mechanisms of desiccation-avoidance and desiccation-tolerance may have a larger effect on species' survival and growth under drought conditions, e.g., drought deciduousness may be a determining factor in some of our species.
Our data set showing large differences among species' drought resistance may permit us to provide a better understanding of the relative importance of the underlying morphological and physiological mechanisms across species. We have developed methods to assess the desiccation-tolerance of individual species (Tyree et al. 2002), and studies to link mechanisms of desiccation-tolerance and -avoidance to species' performance in the habitat are currently being conducted.
Quantifying drought responses
Survival and growth of plants under drought conditions in the field are not only influenced by drought effects, but also by other abiotic and biotic factors, namely light intensity, herbivore and pathogen pressure (e.g., Howe 1990). To distinguish between drought effects and other factors and to assess the relative importance of drought effects requires direct comparison between performance under dry and irrigated conditions. In such an experiment all differences between treatments are caused by drought effects—both direct and through interactions between drought and various non-drought related factors. For example drought may lead to reduced allocation to plant defenses, and make plants more susceptible to herbivore or pathogen attack (Schoeneweiss 1986). Because non-drought related factors affecting plant performance vary spatially and temporally (e.g., Wright 1996; Wolda 1988), approaches comparing wet and dry sites, years, or seasons do not distinguish between drought- and non-drought-related effects. The value of a direct comparison between dry and irrigated conditions is exemplified by our result that several of the species showed pronounced mortality even under irrigated conditions, mainly due to herbivores or pathogens. An experimental analysis without an irrigation treatment would have underestimated the drought resistance of these species. To our knowledge, only three studies have been conducted where growth and/or survival of tropical plants under dry season conditions were directly compared to irrigated conditions, each working on only one to five species (Fisher et al. 1991; Mulkey and Wright 1996; Poorter and Hayashida-Oliver 2000).
The temporal and spatial dynamics of soil water availability are important for the effectiveness of mechanisms of desiccation-tolerance and desiccation-avoidance (especially rooting depth), but are difficult to mimic in potted plants under greenhouse conditions. Conducting the experiments in the natural habitat allowed for natural soil moisture depletion rates as well as natural profiles of soil water potentials (Fig. 1C) by taking advantage of root competition from neighboring plants and evaporation from the soil surface.
Ecological implications
The moist tropical forest in the Barro Colorado Nature Monument (BCNM) is subjected to annual dry seasons of varying intensity that result in pronounced decreases in soil moisture (Windsor 1990). The significant negative drought effect on seedling survival and growth of most of the species we studied (Figs. 3, 4) suggests that drought is a strong selective agent in the moist tropical forest in the BCNM, with an especially important role in severe El Niño dry seasons. Decreases in available soil moisture affected the seedlings of our study species to very different extents (Figs. 3, 4), which likely results in differential influences on species' recruitment patterns. Recruitment of those species identified as most drought resistant will not be affected by the average dry season drought in the area, whereas recruitment of the most drought-sensitive species will be affected, restricting successful recruitment of these species to wet microhabitats or to exceptionally wet years. Decreased seedling survival in the dry season in the BCNM has been shown for drought-sensitive species ( Virola and Ocotea, Fisher et al. 1991; Howe 1990; Gilbert et al. 2001), but for an intermediately drought-resistant species there was no drought effect even in a severe El Niño dry season ( Trichilia, Engelbrecht et al. 2002). Differential drought effects on species' recruitment success may explain why seedlings of many of our study species that are common in the BCNM forest were rather drought resistant, whereas only a few species were very drought sensitive (Figs. 3, 4).
Differences in drought resistance among species, combined with spatial and temporal differences in soil moisture availability are expected to have profound effects on species' distribution patterns both on a small and large scale, i.e., drought-sensitive species occur in wetter sites and are excluded from drier areas, and species with high drought resistance occur in drier sites than drought-sensitive species.
Variation in soil moisture at the microhabitat scale is due to differences in topography, drainage, soil type and texture, and root density. Much evidence indicates that trees, palms and herbs of the wet tropics have habitat associations with variables that are indicative of rhizosphere water availability (reviews by Whitmore 1984; Richards 1998; Sollins 1998; Webb and Peart 2000). However, we are unaware of studies that have determined whether the relationship between soil water availability and species drought resistance explains microhabitat distribution. In a 50-ha forest dynamics plot on Barro Colorado Island (BCI), which is about 4 km south-west of our study site, 50% of the tree and shrub species showed a positive association with topographic habitat classifications (plateaus, slopes or streams; Harms 2001). Slopes are wetter than ridge or plateau sites on BCI (Becker et al. 1988). Interestingly, the most drought-sensitive species in our study, Calophyllum , Virola , Xylopia and Beilschmiedia, showed positive habitat associations with (wet) slopes, whereas Garcinia and Ouratea, which were highly drought resistant in our study, were among the relatively few species positively associated with the (dry) plateau (Harms 2001). These preliminary data suggest that further analysis of the relation between species' drought resistances and their habitat associations will provide new insights into the underlying processes of the observed patterns.
On a larger scale, plant abundance, distribution and diversity in tropical forests vary along rainfall gradients (Condit 1998; Veenendaal and Swaine 1998; Bongers et al. 1999). The mechanisms leading to these changes of tropical forest communities remain open (Givnish 1999; Hubbell 2001). Especially debated are the processes that allow for species coexistence in highly diverse tropical forest communities, and that lead to gradients of diversity (e.g., Hubbell 2001; Wright 2002). Variation in forest composition along rainfall gradients suggests that changes in rainfall may have profound impacts on tropical forest communities. Shifts in rainfall patterns, together with an increase of the frequency of El Niño events, are the main effects of global climate change expected for the tropics (Hulme and Viner 1998; Timmermann et al. 1999). Drying trends could cause the local extinction of drought-sensitive species, and lead to the invasion of more drought-resistant species (Condit 1998). Quantitative assessments of differential drought resistance provide a baseline for linking species' ecological characteristics to distribution patterns at different spatial scales, and therefore contribute to the understanding of gradients of species diversity in the tropics, and to predicting climate-induced changes in tropical forest communities.
References
APG (1998) An ordinal classification for the families of flowering plants. Ann Miss Bot Gard 85:531–553
Becker P (1992) Seasonality of rainfall and drought in Brunei Darussalam. Brunei Mus J 7:99–109
Becker P, Wong M (1993) Drought-induced mortality in tropical heath forest. J Trop Sci 5:416–417
Becker P, Rabenold PE, Idol JR, Smith AP (1988) Water potential gradients for gaps and slopes in a Panamanian tropical moist forest's dry season. J Trop Ecol 4:173-184
Bongers F, Poorter L, Van Rompaey RSAR, Parren MPE (1999) Distribution of twelve moist forest canopy tree species in Liberia and Cote d'Ivoire: response curves to a climatic gradient. J Veg Sci 10:371–382
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Brown ND, Whitmore TC (1992) Do dipterocarp seedlings really partition tropical rain forest gaps? Phil Trans R Soc Lond B 335:369–378
Burslem DFRP, Grubb PJ, Turner IM (1996) Responses to simulated drought and elevated nutrient supply among shade-tolerant tree seedlings of lowland tropical forest in Singapore. Biotropica 28:636–646
Caldwell MM, Dawson TE, Richards JH (1998) Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113:151–161
Cao K-F (2000) Water relations and gas exchange of tropical saplings during a prolonged drought in a Bornean heath forest, with reference to root architecture. J Trop Ecol 16:101–116
Chandrashekar TR, Nazeer MA, Marattukalam JG, Prakash P, Annamalainathan K, Thomas J (1998) An analysis of growth and drought tolerance in rubber during the immature phase in a dry subhumid climate. Exp Agric 34:287–300
Condit R (1998) Ecological implications of changes in drought patterns: shift in forest composition in Panama. Clim Change 39:413–427
Condit R, Hubbell SP, Foster RB (1995) Mortality rates of 205 neotropical tree and shrub species and the impact of severe drought. Ecol Monogr 65:419–439
Croat TB (1978) The flora of Barro Colorado Island. Stanford University Press, Stanford
Currie DJ, Paquin V (1987) Largescale biogeographical patterns of species richness of trees. Nature 329:326–327
Engelbrecht BMJ, Herz H (2001) Evaluation of different methods to estimate understory light conditions in tropical forest. J Trop Ecol 17:207–224
Engelbrecht BMJ, Wright SJ, DeSteven D (2002) Effects of El Niño drought on survival and water relations of seedlings of three neotropical rainforest species in Panama. J Trop Ecol 18:569–579
Espigares T, Peco B (1995) Mediterranean annual pasture dynamics: impact of autumn drought. J Ecol 83:135–142
Evans CE, Etherington JR (1991) The effect of soil water potential on seedling growth of some British plants. New Phytol 118:571–579
Fernandez RJ, Reynolds JF (2000) Potential growth and drought tolerance of eight desert grasses: lack of a trade-off? Oecologia 123:90–98
Fisher BL, Howe HF, Wright SJ (1991) Survival and growth of Virola surinamensis yearlings: Water augmentation in gap and understory. Oecologia 86:292 –297
Garwood NC (1982) Seasonal rhythm of seed germination in a semideciduous tropical forest. In: Leigh EG Jr, Rand AS, Windsor DM (eds) The ecology of a tropical forest: seasonal rhythms and long-term changes. Smithsonian Institution Press, Washington, D.C., pp 173–199
Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Miss Bot Gard 75:1–34
Gilbert GS, Harms KE, Hamill DN, Hubbell SP (2001) Effects of seedling size, El Niño drought, seedling density and distance to nearest conspecific adult on 6-year survival of Ocotea whitei seedlings in Panama. Oecologia 127:509–516
Gitay H, Noble IR (1997) What are functional types and how should we seek them? In: Smith TM, Shugart HH, Woodward FI (eds) Plant functional types. Their relevance to ecosystem properties and global change. (International Geosphere-Biosphere Programme book series) Cambridge University Press, Cambridge, pp 3–19
Givnish TJ (1999) On the causes of gradients in tropical tree diversity. J Ecol 87:193–210
Harms KE, Condit R, Hubbell SP, Foster RB (2001) Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J Ecol 89:947–959
Howe HF (1990) Survival and growth of juvenile Virola surinamensis in Panama: effects of herbivory and canopy closure. J Trop Ecol 6:259–280
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press. Princeton, N.J.
Hulme M, Viner D (1998) A climate change scenario for the tropics. Clim Change 39:145–176
Ito S, Nishiyama Y, Kustiawan W (2000) Responses of Dipterocarp seedlings to drought stress. In: Guhardja E, Fatawi M, Sutisna M, Mori T, Ohta S (eds.) Rainforest ecosystems of East Kalimantan. El Niño, drought, fire and human impacts. Springer, Berlin Heidelberg New York, pp 143–150
Jarvis PG, Jarvis MS (1963) The water relations of tree seedlings. 1. Growth and water use in relation to soil water potential. Physiol Plant 16:215–235
Kozlowsky TT, Pallardy SG (1997) Growth control of woody plants. Academic Press, San Diego, Calif.
Larcher W (1980) Physiological plant ecology, 2nd edn. Springer, Berlin Heidelberg New York
Lewis SL, Tanner EVJ (2000) Effects of aboveground and belowground competition on growth and survival of rain forest tree seedlings. Ecology 81:2525–2538
Lieth H (1975) Primary production of the major vegetation units of the world. In: Lieth H, Whittaker RH (eds) Primary productivity of the biosphere. Springer, Berlin Heidelberg New York, pp 203–231
Mulkey SS, Wright SJ (1996) Influence of seasonal drought on the carbon balance of tropical forest plants. In: Smith AP, Mulkey SS, Chazdon RL (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 187–216
Nakagawa M, Tanaka K, Nakashizuka T, Ohkubo T, Kato T, Maeda T, Sato K, Miguchi H, Nagamasu H, Ogino K, Teo S, Hamid AA, Seng LH (2000) Impact of severe drought associated with the 1997–1998 El Niño in a tropical forest in Sarawak. J Trop Ecol 16:355–367
O'Brien EM (1993) Climatic gradients in woody plant species richness: towards an explanation based on an analysis of southern Africa's woody flora. J Biogeogr 20:181–198
Perneger TV (1998) What's wrong with Bonferroni adjustments? Br Med J 316:1236–1238
Poorter L, Hayashida-Oliver Y (2000) Effects of seasonal drought on gap and understory seedlings in a Bolivian moist forest. J Trop Ecol 16:481–498
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Richards PW (ed) (1998) The tropical rain forest: an ecological study, 2nd edn. Cambridge University Press, Cambridge
Schoeneweiss D (1986) Water stress disposition to disease—an overview. Water, fungi and plants. In: Ayres P (ed) Cambridge University Press, New York
Schupp EW (1988) Factors affecting post-dispersal seed survival in a tropical forest. Oecologia 76:525–530
Sollins P (1998) Factors influencing species composition in tropical lowland rainforest: Does soil matter? Ecology 79:23–30
Swaine MD (1996) Rainfall and soil fertility as factors limiting forest species distributions. J Ecol 84:419–428
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical rain forests. Vegetatio 75:81–86
Timmermann A, Oberhuber J, Bacher A, Esch M, Latif M, Roeckner E (1999) Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature 398:694–697
Tobin MF, Lopez OR, Kursar TA (1999) Responses of tropical understory plants to a severe drought: tolerance and avoidance of water stress. Biotropica 31:570–578
Toma T, Marjenah, Hastaniah (2000) Climate in Bukit Soeharto, East Kalimantan. In: Guhardja E, Fatawi M, Sutisna M, Mori T, Ohta S (eds) Rainforest ecosystems of East Kalimantan. El Niño, drought, fire and human impacts. Springer, Berlin Heidelberg New York, pp 107–117
Turner IM (1990) The seedling survivorship and growth of three Shorea species in a Malaysian tropical rain forest. J Trop Ecol 6:469–478
Tyree MT, Vargas G, Engelbrecht BMJ, Kursar TA (2002) Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest seedling-tree, Licania platypus (Hemsl.) Fritsch. J Exp Bot 53:2239–2247
Unwin GL, Kriedemann PE (1990) Drought tolerance and rainforest tree growth on a North Queensland rainfall gradient. For Ecol Manage 30:113–123
VAST nomenclatural database (2002) Missouri Botanical Garden, St. Louis, Miss. http://mbot.org/W3T/search/vast.html 20
Veenendaal EM, Swaine MD (1998) Limits to tree species distribution in lowland tropical rainforests. In: Newbery DM, Prins HHT, Brown N (eds) Dynamics of tropical forest communities. Thirty-seventh Symposium of the British Ecological Society. Blackwell, Oxford, pp 163–191
Veenendaal EM, Swaine MD, Agyeman VK, Blay D, Abebrese IK, Mullins CE (1995) Differences in plant and soil water relations in and around a forest gap in West Africa during the dry season may influence seedling establishment and survival. J Ecol 83:83–90
Walsh RPD (1998) Climate. In: Richards PW (ed) The tropical rain forest: an ecological study, 2nd edn. Cambridge University Press, Cambridge, pp 159–202
Walsh RPD, Newbery DM (1999) The ecoclimatology of Danum, Sabah, in the context of the world's rainforest regions, with particular reference to dry periods and their impact. Phil Trans R Soc Lond B 354:1391–1405
Webb CO, Peart DR (2000) Habitat associations of trees and seedlings in a Bornean rain forest. J Ecol 88:464–478
Whitmore TC (1984) Tropical rainforests of the Far East, 2nd edn. Oxford University Press, Oxford
Windsor DM (1990) Climate and moisture availability in a tropical forest. Long-term records from Barro Colorado Island, Panama. Smithsonian Institution Press, Washington, D.C.
Wolda H (1988) Insect seasonality: why? Annu Rev Ecol Syst 19:1–18
Wright SJ (1996) Phenological responses to seasonality in tropical forest plants. In: Smith AP, Mulkey SS, Chazdon RL (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 440–460
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14
Acknowledgements
Maria del Carmen Ruiz, David Galvez and Didimo Moran provided essential assistance in all stages of the experiment. Additional assistance was provided by Robert Wolf, Beatriz Baker, Kelly Anderson, Eli Robbins, Ana Matilde Ruiz, Teresa Ruiz and Sebastian Brulez. We thank Mel Tyree for his suggestions throughout the study and for comments on the manuscript. Osvaldo de Leon, Andres Hernandez, Rolando Perez, Salomon Aguilar, Rafael Aizprua, Nayda Flores and Blanca Arauz provided expertise in seed and plant identification. This project was funded by the Andrew W. Mellon Foundation, and the University of Utah.
Author information
Authors and Affiliations
Corresponding author
Appendix 1.
Appendix 1.
Sample sizes at the beginning of the experiment for each treatment, number of survivors, and relative leaf-area changes (DLA) for all individuals (alive and dead)
The data are medians (minimum; maximum in parentheses). For abbreviations, see Table 1.
Species code | N | Alive | Leaf-area change | Mann-Whitney | |||
|---|---|---|---|---|---|---|---|
Wet | Dry | Wet | Dry | Wet | Dry | ||
AND | 30 | 29 | 18 | 12 | 0.000 | 0.000 | n.s. |
(0.000; 1.572) | (0.000; 1.235) | ||||||
BEI | 30 | 30 | 16 | 0 | 0.964 | 0.000 | - |
(0.000; 1.393) | (0.000; 0.000) | ||||||
CAL | 30 | 30 | 30 | 6 | 1.000 | 0.000 | *** |
(0.000; 1.533) | (0.000; 1.180) | ||||||
COR | 28 | 26 | 27 | 19 | 0.965 | 0.347 | *** |
(0.000; 2.315) | (0.000; 0.925) | ||||||
CUP | 30 | 31 | 29 | 26 | 1.079 | 0.957 | ** |
(0.000; 1.951) | (0.000; 1.377) | ||||||
DIP | 30 | 30 | 22 | 22 | 0.764 | 0.000 | ** |
(0.000; 4.789) | (0.000; 0.967) | ||||||
GAR | 30 | 30 | 30 | 29 | 1.000 | 1.000 | ** |
(0.949; 2.120) | (0.000; 1.000) | ||||||
HOR | 30 | 29 | 29 | 27 | 1.076 | 0.861 | *** |
(0.000; 1.803) | (0.000; 1.036) | ||||||
HYB | 30 | 31 | 30 | 26 | 1.228 | 0.368 | *** |
(0.107; 2.480) | (0.000; 1.306) | ||||||
HYM | 30 | 30 | 23 | 18 | 0.801 | 0.246 | * |
(0.000; 1.615) | (0.000; 1.155) | ||||||
ING | 30 | 30 | 25 | 23 | 1.039 | 0.235 | ** |
(0.000; 2.082) | (0.000; 1.190) | ||||||
LAC | 30 | 30 | 30 | 26 | 1.292 | 1.000 | ** |
(0.000; 1.821) | (0.000; 1.392) | ||||||
LAI | 30 | 30 | 30 | 16 | 1.412 | 0.805 | *** |
(0.000; 5.201) | (0.000; 1.222) | ||||||
LIC | 30 | 30 | 27 | 18 | 1.000 | 0.697 | ** |
(0.000; 1.410) | (0.000; 1.116) | ||||||
OUR | 30 | 30 | 30 | 29 | 1.372 | 1.013 | *** |
(0.293; 2.149) | (0.000; 1.310) | ||||||
PIC | 30 | 30 | 28 | 20 | 1.315 | 0.985 | ** |
(0.000; 2.300) | (0.000; 1.512) | ||||||
POU | 30 | 30 | 26 | 17 | 1.012 | 0.415 | ** |
(0.000; 2.533) | (0.000; 1.468) | ||||||
PSE | 30 | 30 | 27 | 24 | 0.471 | 0.000 | *** |
(0.000; 3.624) | (0.000; 0.079) | ||||||
PTE | 26 | 24 | 26 | 17 | 1.000 | 0.790 | ** |
(0.745; 3.056) | (0.000; 1.075) | ||||||
PTRI | 30 | 30 | 28 | 5 | 1.000 | 0.000 | *** |
(0.000; 1.528) | (0.000; 1.031) | ||||||
SOR | 30 | 30 | 30 | 16 | 1.000 | 0.081 | *** |
(0.553; 2.276) | (0.000; 1.296) | ||||||
SWA | 30 | 30 | 30 | 26 | 1.000 | 0.992 | n.s. |
(0.000; 1.634) | (0.000; 1.706) | ||||||
TAB | 30 | 30 | 27 | 12 | 0.651 | 0.000 | *** |
(0.000; 2.929) | (0.000; 0.526) | ||||||
THE | 30 | 29 | 27 | 20 | 1.163 | 0.081 | *** |
(0.000; 1.744) | (0.000; 1.682) | ||||||
TRC | 30 | 29 | 30 | 22 | 1.036 | 1.000 | *** |
(0.949; 2.081) | (0.000; 1.194) | ||||||
VIR | 30 | 30 | 29 | 6 | 1.000 | 0.000 | *** |
(0.000; 2.003) | (0.000; 1.000) | ||||||
XYL | 24 | 25 | 24 | 9 | 1.476 | 0.000 | *** |
(0.988; 2.729) | (0.000; 1.178) | ||||||
XYO | 27 | 25 | 27 | 20 | 1.000 | 0.705 | ** |
(0.182; 1.920) | (0.000; 1.000) | ||||||
Rights and permissions
About this article
Cite this article
Engelbrecht, B.M.J., Kursar, T.A. Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136, 383–393 (2003). https://doi.org/10.1007/s00442-003-1290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1290-8