Abstract
Bone disease is one of the most common complications of multiple myeloma. It is the result of increased osteoclast activity which is not compensated by osteoblast activity and leads to osteolytic lesions characterized by bone pain and increased risk for pathological fracture, spinal cord compression, and need for radiotherapy or surgery to the bone. Imaging techniques for the diagnosis of multiple myeloma bone disease include whole-body X-rays, whole-body low-dose CT (WBLDCT), magnetic resonance imaging (MRI), and PET-CT. Bisphosphonates (BPs) are the cornerstone in the treatment of myeloma-related bone disease. Recent studies have revealed novel pathways and molecules that are involved in the biology of myeloma bone disease including the receptor activator of nuclear factor-kappa B ligand/osteoprotegerin pathway, the Wnt signaling inhibitors dickkopf-1 and sclerostin, macrophage inflammatory proteins, activin A, and others. The thorough study of these pathways has provided novel agents that may play a critical role in the management of myeloma-related bone disease in the near future, such as denosumab (anti-RANKL), sotatercept (activin-A antagonist), romosozumab (anti-sclerostin), or BHQ-880 (anti-dickkopf 1). In this chapter, we will focus in the imaging techniques used for the diagnosis of multiple myeloma bone disease, as well as the current and future options for its management.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Multiple myeloma (MM) is a common hematological malignancy characterized by the accumulation of abnormal plasma cells in the bone marrow. Even though survival has been improved after the introduction of novel agents [1, 2], MM remains an incurable plasma-cell malignancy [3, 4]. MM is characterized by osteolytic bone disease due to an elevated function of osteoclasts which is not balanced by a comparable elevation of osteoblast function [5,6,7]. Osteolytic lesions are detected in 70–80% of patients at diagnosis and increase the risk for skeletal-related events (SREs) (pathologic fractures, spinal cord compression (SCC), requirement for surgery or palliative radiotherapy to bone). SREs have a serious impact on the quality of life (QoL) and survival of MM patients and affect both clinical and economic aspects of their life [8,9,10,11,12,13]. The novel International Myeloma Working Group (IMWG) criteria for the diagnosis of symptomatic MM have revealed the value of modern imaging for the management of MM patients, as they include (1) the presence at least one lytic lesion detected not only by conventional radiography but also by computed tomography (CT), whole-body low-dose CT (WBLDCT) or positron emission tomography/CT (PET-CT) and (2) the presence of >1 focal bone marrow lesions on magnetic resonance imaging (MRI) studies [14]. Furthermore, novel imaging techniques, such as MRI and PET-CT, provide prognostic information and have been recently proven of value, for the better definition of response to antimyeloma therapy. Bisphosphonates (BPs) are the cornerstone of therapeutic management of myeloma bone disease, offering considerable benefit in preventing or delaying skeletal-related events and relieving pain [15]. This chapter reviews the latest available details of imaging and treatment of myeloma-related bone disease.
8.2 Pathophysiology of Multiple Myeloma Bone Disease
In the adult skeleton, skeletal integrity is coordinated by the synchronized activity of three cell types. Osteoblasts create new bone matrix, osteoclasts are responsible for bone resorption, and osteocytes regulate bone turnover. In MM patients, bone disease is the result of an uncoupling in bone remodeling. It consists of an increase in the osteoclast-mediated bone resorption, which is combined with suppression in the osteoblast, mediated bone mineralization, and defects on osteocyte functions [16]. Until today, several direct and indirect interactions between myeloma cells and cells of the bone marrow microenvironment have been recognized. The fact that osteolytic lesions occur close to MM cells suggests that factors secreted by tumor cells lead to direct stimulation of osteoclast-mediated bone resorption and inhibition of osteoblast-mediated bone formation [6]. In addition to that, the increased bone resorptive progress leads to the release of growth factors that increase the growth of MM cells, leading to a vicious cycle of tumor expansion and bone destruction. Apart from that, interactions via adhesion between MM cells and bone marrow cells result in the production of factors that promote angiogenesis and make the myeloma cells resistant to chemotherapy [17, 18]. The biologic pathway of the receptor activator of nuclear factor-kappa B (RANK), its ligand (RANKL), and osteoprotegerin (OPG) which is the decoy receptor of RANKL is of major importance for the increased osteoclast activity observed in MM. Myeloma cells disrupt the balance between RANKL and OPG by increasing the expression of RANKL and decreasing the expression of OPG. The resulting increase in RANKL favors the formation and activation of osteoclasts, leading to increased bone resorption [19, 20]. More recently, activin A has been implicated in MM bone disease, through stimulating RANK expression and inducing osteoclastogenesis [21, 22]. In addition to their stimulatory effect on osteoclasts, myeloma cells have been shown to suppress bone formation [23]. The Wingless-type (Wnt) signaling pathway is one pathway that has been shown to play a key role in osteoblast differentiation and has been implicated in osteoblast suppression in myeloma. The Wnt signaling inhibitors dickkopf-1 (Dkk-1) and sclerostin are secreted by myeloma cells and have been found to be increased in the serum of myeloma patients, leading to the block of osteoblast differentiation and activity [24,25,26,27]. Soluble frizzle-related protein-2 (sFRP-2), another inhibitor of Wnt signaling, has also been implicated in suppression of bone formation in myeloma [28]. Although the circulating levels of the above molecules and mainly of sclerostin have not been found to be elevated in myeloma patients in all published studies, the importance of Wnt inhibition in the biology of myeloma-related bone disease is undoubted.
8.3 Imaging for the Diagnosis of Multiple Myeloma Bone Disease
The imaging techniques used for the diagnosis of multiple myeloma bone disease are:
-
Whole-body X-rays (WBXR)
-
Whole-body CT (WBCT)
-
Magnetic resonance imaging (MRI)
-
PET-CT
8.4 Whole-Body X-rays (WBXR)
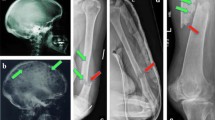
Conventional radiography has been widely used for the identification of osteolytic lesions both at diagnosis and during the course of the disease. The “skeletal survey” (whole-body X-rays (WBXR)) at diagnosis should include plain radiographs of the whole skeleton (anteroposterior and lateral views of the skull posteroanterior view of the chest; anteroposterior and lateral views of the thoracic lumbar and cervical spine (including an open mouth view), humeri, and femora; and anteroposterior view of the pelvis) [29]. In addition, symptomatic areas should also be specifically visualized. Osteolytic lesions have the typical appearance of “punched-out” lesions with absence of reactive sclerosis and are more common in the vertebrae, ribs, skull, and pelvis [30]. Although the WBXR was the standard of care for many years, it has several limitations: (1) for a lytic lesion to become apparent, >30% loss of trabecular bone must occur; (2) difficulty of assessment of certain areas, such as the pelvis and the spine; (3) limitations in the detection of lytic lesion response to antimyeloma therapy because of delayed evidence of healing; (4) reduced specificity for the differential diagnosis of myeloma-related versus benign fracture (very important, particularly in cases of new vertebral compression fractures in the absence of other criteria of relapse); (5) observer dependency (there is very low reproducibility among centers; higher number of osteolytic lesions detected in academic vs. nonacademic centers); and (6) prolonged study length, often not tolerable from patients in severe pain [29, 30]. Thus, the development of novel imaging methods has led to the replacement of WBXR by more advanced techniques, such as the WBLDCT in many European centers or by PET-CT in the USA.
8.5 Whole-Body Low-Dose CT (WBLDCT)
WBLDCT was introduced to allow the detection of osteolytic lesions in the whole skeleton with high accuracy, no need for contrast agents and low radiation dose compared to standard CT (two- to threefold lower radiation dose vs. conventional CT) [31, 32]. In several studies, WBLDCT was found to be superior to WBXR for the detection of osteolytic lesions [31, 33,34,35,36,37]. In one of the largest studies staging myeloma patients, 61% of patients with normal WBXR had more than one osteolytic lesions on WBLDCT [36]. According to the latest criteria for symptomatic myeloma, these patients should receive therapy. In the same study, the total number of lesions detected by WBLDCT was 968 vs. 248 for WBXR (p < 0.001). The only limitation of this study was its retrospective origin [36]. In a more recent prospective study, which included 52 myeloma patients at diagnosis, WBLDCT revealed osteolyses in 12 patients (23%) with negative WBXR and proved to be more sensitive than WBXR mainly in the axial skeleton (p < 0.001). WBLDCT was superior in the detection of lesions in patients with osteopenia and osteoporosis [37].
In total WBLDCT advantages over WBXR include (1) superior diagnostic sensitivity for depiction of osteolytic lesions, especially in areas where the WBXR detection rate is low, i.e., pelvis and spine; (2) superiority in estimating fracture risk and bone instability; (3) duration of the examination, which is ≤5 min, an important issue for patients in extreme pain; (4) production of higher-quality 3D high-resolution images for planning biopsies and therapeutic interventions; and finally (5) demonstration of unsuspected manifestations of myeloma or other disease, especially in the lungs and kidneys (33% in the study by Wolf et al.; 37, 31–37). Major disadvantages of WBLDCT include increased length of time required for radiologists to report their findings, lack of availability in several centers [14, 31], and lack of specificity for the differential diagnosis between malignant and osteoporotic fractures, despite improvements during the last years [38]. Furthermore, although exposure to radiation is much lower compared to standard CT, it continues to be higher than WBXR: mean dose of WBLDCT is approximately 3.6 and 2.8 mSv for females and males, respectively, versus 1.2 mSv for WBXR [39]. Nevertheless, the higher diagnostic accuracy of the WBLDCT and patient comfort is particularly important for the elderly, and often suffering group renders the dose/quality ratio favorable for WBLDCT. For these reasons, the European Myeloma Network has suggested that WBLDCT should replace conventional radiography as the standard imaging technique for evaluation of bone disease in MM, where available [40].
8.6 Magnetic Resonance Imaging
Techniques. Several MRI techniques have been developed for the assessment of the bone marrow involvement in MM. Conventional MRI protocols include T1-weighted, T2-weighted with fat suppression, short time inversion recovery (STIR), and gadolinium T1-weighted with fat suppression [41]. Myeloma lesions show typically a low signal intensity on T1-weighted images, a high signal intensity on T2-weighted and STIR images, and often enhancement on gadolinium-enhanced images [42, 43].
Limitations of MRI are the prolonged acquisition time, availability issues the high cost, the exclusion of patients with metal devices in their body, the difficulties in cases of claustrophobic patients, and the limited field of view. To override these restrictions, a Whole body MRI (WB-MRI) methodology, which does not usually require contrast infusion, was developed. The time of WB-MRI is approximately 45 min. Although of interest, this newer technique is not yet widely employed.
All above MRI methods use MRI exquisite contrast and spatial resolution for the depiction of the WB anatomy and specific tissue composition in details.
Novel MRI techniques include diffusion-weighted imaging, dynamic contrast-enhanced MRI, and PET-MRI.
A novel and promising MRI sequence is the diffusion-weighted imaging (DWI-MRI) which derives its contrast mainly from differences in the diffusivity of water molecules in the tissue environment. This functional technique demonstrates alterations in intra- and extracellular water content from disruption of the transmembrane water flux that are visible before identified changes on the morphologic routine sequences [44,45,46]. DWI-MRI uses the calculation of apparent diffusion coefficient (ADC) values to better evaluate myeloma burden and MRI infiltration patterns [47, 48]. DWI can be used to detect regions with bone marrow infiltration for both diagnosis and monitoring treatment response [49], because ADC values are higher in MM patients at diagnosis, compared with patients in remission 20 weeks after initiation of treatment [50]. In MM patients, the ADC was reproducible [51] and correlated with bone marrow cellularity and microvessel density (MVD) [52]. One disadvantage of DWI is that the ADC is not exclusively influenced by diffusion but also by perfusion. However, improved sequences are under development to differentiate both influences [53]. DWI-MRI was found superior to WBXR for the detection of bone involvement in 20 patients with relapsed/refractory MM in all areas of the skeleton except of the skull, where both examinations had equal sensitivity [54]. In another small study with 24 myeloma patients (both treated and untreated), DWI-MRI was found more sensitive than F18-fluorodeoxyglucose (FDG)-PET in the detection of myeloma lesions [55]. In a recent study, 17 patients were evaluated with DWI-MRI and FDG-PET-CT, and the findings were compared with bone marrow biopsy data. In all studied regions, WB-DWI scores were higher compared to FDG-PET-CT. DWI-MRI was of particular accurance in diagnosing diffuse disease (diffuse disease was observed in 37% of regions imaged on WB-DWI scans versus only 7% on FDG-PET-CT); both techniques were equally sensitive in the detection of focal lesions. [56] Preliminary reports suggest that DWI-MRI may be used for the better definition of response to therapy, but this has to be confirmed in larger studies and in comparison with PET-CT results [48, 57].
The dynamic contrast-enhanced MRI (DCE-MRI) is another MRI technique which evaluates the distribution of a contrast agent inside and outside the blood vessels. Information is assessed by computer-based analysis of repeated images over time. The analysis provides data for blood volume and vessel permeability for the assessment of microcirculation of a specific area [58, 59]. More importantly in MM patients, DCE-MRI-derived parameters correlated with marrow angiogenesis, microvessel density (MVD) [60], as well as in angiogenic response to therapy [61]. Regarding DCE-MRI sampling rate and model, there are two pharmacokinetic models (proposed by Brix and Tofts) that have been applied in the literature. However, a comparison of these models demonstrated that the Brix model is a little bit more robust [62]. Since DCE-MRI has not been established in clinical routine, no definite sequence can be recommended.
Positron emission tomography in combination with MRI (PET-MRI) represents a novel imaging modality in which the PET part detects active focal lesions, while the MRI part shows the location of the lesions and gives information on myeloma cell infiltration of the bone marrow. Especially in patients who reach a complete remission (CR), this technique might be able to localize residual sites of disease activity and therefore may help to guide treatment in the future [63]. In MM, there is only one prospective study, which compared PET-MRI with PET-CT in 30 myeloma patients with both techniques performed sequentially. There was high correlation between the two techniques, regarding number of active lesions and average SUV [64]. Further studies with PET-MRI will reveal if there is any value of this technique for MM patients.
MRI Patterns of Marrow Involvement. Five MRI patterns of bone marrow infiltration in myeloma have been reported: (1) normal appearance of bone marrow, (2) focal involvement (positive focal lesion is considered the lesion of a diameter of at least 5 mm), (3) homogeneous diffuse infiltration, (4) combined diffuse and focal infiltration, and (5) variegated or “salt-and-pepper” pattern with inhomogeneous bone marrow with interposition of fat islands [65, 66]. Low tumor burden is usually associated with a normal MRI pattern, but a high tumor burden is usually suspected when there is diffuse hypointense change on T1-weighted images, diffuse hyperintensity on T2-weighted images, and enhancement with gadolinium injection [67]. In several studies, the percentage of symptomatic patients with each of the abnormal MRI bone marrow patterns ranges from 18 to 50% for focal pattern, 25 to 43% for diffuse pattern, and 1 to 5% for variegated pattern [59]. The Durie-Salmon PLUS system uses the number of focal lesions (from focal or combined focal/diffuse patterns) for the staging of a myeloma patient and not the diffuse or “salt-and-pepper” patterns [68].
MRI Versus Conventional Radiography and Other Imaging Techniques for the Detection of Bone Involvement in Symptomatic Myeloma. MRI is more sensitive compared to WBXR for the detection of bone involvement in MM. In the largest series of patients published to date, MRI was compared to WBXR in 611 patients who received tandem autologous transplantation (ASCT). MRI and WBXR detected focal and osteolytic lesions in 74% and 56% of the imaged anatomic sites, respectively. Furthermore, 52% of 267 patients with normal WBXR had focal lesions on MRI. More precisely, MRI detected more focal lesions compared to lytic lesions in WBXR in the spine (78% vs. 16%; p < 0.001), the pelvis (64% vs. 28%; p < 0.001), and the sternum (24% vs. 3%; p < 0.001). WBXR had better performance than MRI in the ribs (10% vs. 43%; p < 0.001) and the long bones (37% vs. 48%; p = 0.006) and equal results in the skull and the shoulders [69]. Similar results had been previously reported in smaller studies, where MRI was superior to WBXR for the detection of focal vs. osteolytic lesions in the pelvis (75% vs. 46% of patients) and the spine (76% vs. 42%), especially in the lumbar spine [70,71,72,73,74]. A recent meta-analysis confirmed the superiority of MRI over WBXR regarding the detection of focal lesions and showed that MRI especially outscores WBXR in the axial skeleton but not in the ribs [75].
Although it is clear that MRI can detect bone marrow focal lesions long before the development of osteolytic lesions in the WBXR, other imaging techniques such as PET combined with computed tomography (PET-CT), CT, or WBCT detect more osteolytic lesions compared to WBXR [75]. Is there any evidence that MRI is superior to the other techniques in depicting bone involvement in myeloma? In a study with 41 newly diagnosed MM patients, WB-MRI was found superior to WBCT in detecting lesions in the skeleton [76]. In a prospective study, Zamagni et al. compared MRI of the spine and pelvis with WBXR and PET-CT in 46 MM patients at diagnosis. Although PET-CT was superior to WBXR in detecting lytic lesions in 46% of patients (19% had negative WBXR), it failed to reveal abnormal findings in 30% of patients who had abnormal MRI in the same areas, mainly of diffuse pattern. In that study, the combination of spine and pelvic MRI with PET-CT detected both medullary and extramedullary active myeloma sites in almost all patients (92%) [77]. Nevertheless, the Arkansas group was not able to confirm any superiority of MRI over PET-CT in the detection of more focal lesions in a large number of patients (n = 303) within the total therapy three protocols [78]. Still, in 188 patients who had at least one focal lesion in MRI, MRI was superior to PET-CT regarding the detection of higher number of focal lesions (p = 0.032). Furthermore, in this study, the presence of diffuse marrow pattern was not taken into consideration as an abnormal MRI finding [78]. Compared to sestamibitechnetium-99 m (MIBI) scan, WB-MRI detected more lesions in the vertebrae and the long bones, produced similar results in the skull, and was inferior in the ribs [79]. One important question in this point is the value of WB-MRI, which is not available everywhere, over the MRI of the spine and pelvis. In 100 patients with MM and MGUS who underwent WB-MRI, 10% presented with focal lesions merely in the extra-axial skeleton. These lesions would have been ignored if only MRI of the spine and pelvis had been performed [80].
Other advantages of MRI over WBXR and CT include the discrimination of myeloma from normal marrow [41, 81]; this finding can help in the differential diagnosis between myeloma and benign cause of a vertebral fracture. This is of extreme importance in cases of patients with a vertebral fracture and no other CRAB criteria and no lytic lesions. The MRI can also accurately illustrate the spinal cord and/or nerve root compression for surgical intervention or radiation therapy [29, 41]. Furthermore, the presence of soft tissue extension of MM and the presence of extramedullary plasmacytomas that are developed in approximately 10–20% of patients during the course of their disease can be precisely visualized by WB-MRI [82,83,84,85]. MRI can also help in the better evaluation of avascular necrosis of the femoral head [85] and the presence of soft tissue amyloid deposits [86]. Moreover, the tumor load can be assessed and monitored by MRI even in patients with nonsecretory and oligosecretory MM [87].
In conclusion, according to the latest IMWG guidelines, MRI is the gold standard imaging technique for the detection of bone marrow involvement in MM (grade A). MRI detects bone marrow involvement and not bone destruction. MRI of the spine and pelvis can detect approximately 90% of focal lesions in MM, and thus it can be used in cases where WB-MRI is not available (grade B). MRI is the procedure of choice to evaluate a painful lesion in myeloma patients, mainly in the axial skeleton, and to detect spinal cord compression (grade A). MRI is particularly useful in the evaluation of collapsed vertebrae, especially when myeloma is not active, where the possibility of osteoporotic fracture is high (grade B) [88].
Prognostic Value of MRI. The prognostic significance of MRI findings in symptomatic myeloma has been evaluated. The largest study in the literature included 611 patients who received tandem ASCT-based protocols. Focal lesions detected by spinal MRI and not seen on WBXR independently correlated with overall survival (OS). Resolution of the focal lesions on MRI posttreatment occurred in 60% of the patients who had superior survival. At disease progression after complete response (CR), MRI revealed new focal lesions in 26% of patients, enlargement of previous focal lesions in 28% of patients, and both features in 15% of patients [69]. In a more recent analysis of the same group on 429 patients, patients who had >7 focal lesions in MRI (n = 147) had a 73% probability of 3-year OS vs. 86% for those who had 0–7 focal lesions (n = 235) and 81% for those who had diffuse pattern of marrow infiltration (n = 47; p = 0.04). PET-CT and WBXR also produced similar results in the univariate analysis. In the multivariate analysis, from the imaging variables, only the presence of >2 osteolytic lesions in WBXR at diagnosis and the presence of >3 focal lesions in the PET-CT, 7 days post-ASCT had independent prognostic value for inferior OS (p = 0.01 and 0.03, respectively). However, we have to mention the high percentage of patients (232/429, 54%) who had no detectable osteolytic lesions by WBXR and the absence of evaluation of diffuse MRI pattern in this study [89].
The MRI pattern of marrow infiltration has also reported to have prognostic significance in newly diagnosed patients with symptomatic disease [67, 90, 91]. In the conventional chemotherapy (CC) era, Moulopoulos et al. published that the median OS of newly diagnosed MM patients was 24 months if they had diffuse MRI pattern versus 51, 52, and 56 months for those with focal, variegated, and normal patterns, respectively, (p = 0.001) [67]. This is possibly because diffuse MRI marrow pattern correlates with increased angiogenesis and advanced disease features [92, 93]. The same group also reported the prognostic value of MRI patterns in 228 symptomatic MM patients who received upfront regimens based on novel agents. Patients with diffuse pattern had inferior survival compared to patients with other MRI patterns; moreover, the combination of diffuse MRI pattern, ISS-3 stage, and high risk cytogenetics could identify a group of patients with very poor survival: median of 21 months and a probability of 3-year OS of only 35% [91]. Another study in 126 patients with newly diagnosed symptomatic myeloma who underwent an ASCT showed that the diffuse and the variegated MRI patterns had an independent predictive value for disease progression (HR: 1.922; p = 0.008) [93]. Finally, in patients with progressive or relapsed MM, an increased signal of DCE-MRI offered shorter PFS, possibly due to its association with higher MVD [58].
MRI and Response to Antimyeloma Therapy. An interesting finding is that a change in MRI pattern correlates with response to therapy. Moulopoulos et al. firstly reported in the era of CC that CR is characterized by complete resolution of the preceding marrow abnormality, while partial response (PR) is characterized by changeover of diffuse pattern to variegated or focal patterns [94]. In a retrospective study that was conducted in the era of novel agents, response to treatment was compared with changes in infiltration patterns of WB-MRI before and after ASCT (n = 100). There was a strong correlation between response to antimyeloma therapies and changes in both diffuse (p = 0.004) and focal (p = 0.01) MRI patterns. Furthermore, the number of focal lesions at second MRI was of prognostic significance for OS (p = 0.001) [95]. Another study in 33 patients who underwent an ASCT showed that WB-MRI data demonstrated progressive disease in ten patients (30%) and response to high-dose therapy in 23 (70%). Eight (80%) of the ten patients with progressive disease revealed intramedullary lesions, and two patients (20%) had intra- and extramedullary lesions. WB-MRI had a sensitivity of 64%, specificity of 86%, positive predictive value of 70%, negative predictive value of 83%, and accuracy of 79% for detection of remission [96]. This study supports that one of the disadvantages of MRI is that it often provides false-positive results because of persistent nonviable lesions. Thus, PET-CT might be more suitable than MRI for determination of remission status [97]. Indeed in a large study of 191 patients, PET-CT revealed faster change of imaging findings than MRI in patients who responded to therapy [98]. It seems that the PET-CT normalization after treatment can offer more information compared to MRI for the better definition of CR [99].
To improve the results of MRI for the most accurate detection of remission, the DW-MRI has been recently used. In a first preliminary report, ADC values in active myeloma were significantly higher than marrow in remission [50]. Furthermore, the mean ADC increased in 95% of responding patients and decreased in all (n = 5) nonresponders (p = 0.002). An increase of ADC by 3.3% was associated with response, having a sensitivity of 90% and specificity of 100%. Furthermore, there was a negative correlation between changes of ADC and changes of biochemical markers of response (r = −0.614; p = 0.001) [100]. Large prospective clinical studies are definitely justified by these results.
The Value of MRI in the Definition of Smoldering/Asymptomatic Myeloma. The presence of lytic lesions by WBXR is included in the definition of symptomatic myeloma, based on studies showing that patients with at least one lytic lesion in WBXR have a median time to progression (TTP) of 10 months [101]. However, in patients with no osteolytic lesions in WBXR, the MRI reveals abnormal marrow appearance in 20–50% of them [66, 67, 102,103,104]; these patients are at higher risk for progression. Moulopoulos et al. reported that patients with SMM and abnormal MRI studies required therapy after a median of 16 months vs. 43 months for those with normal MRI (p < 0.01) [102]. Hillengass and colleagues evaluated WB-MRI in 149 SMM patients. Focal lesions were detected in 42 (28%) patients, while >1 focal lesion was present in 23 patients (15%) who had high risk of progression (HR = 4.05, p < 0.001). The median TTP was 13 months, and the progression rate at 2 years was 70%. On multivariate analysis, presence of >1 focal lesion remained a significant predictor of progression after adjusting for other risk factors including bone marrow plasmacytosis, serum and urine M-protein levels, and suppression of uninvolved immunoglobulins. In the same study, the diffuse marrow infiltration on MRI was also associated with increased risk for progression (HR = 3.5, p < 0.001) [103]. Kastritis and colleagues also showed in 98 SMM patients that abnormal marrow pattern in the MRI of the spine, which was present in 21% of patients, was associated with high risk of progression with a median TTP to symptomatic myeloma of 15 months (p = 0.001) [104].
An important issue is whether patients who have two or more small focal lesions (<5 mm) should be considered as patients with symptomatic myeloma and how to manage them. The Heidelberg group analyzed very recently data of 63 SMM patients who had at least two WB-MRIs performed for follow-up before progression into symptomatic disease. The definition of radiological progression according to MRI findings included one of the following: (1) development of a new focal lesion, (2) increase of the diameter of an existing focal lesion, and (3) detection of novel or progressive diffuse MRI pattern. The second MRI was performed 3–6 months after the performance of the first MRI. Evaluation of response according to IMWG criteria was also performed. Progressive disease according to MRI was observed in approximately 50% of patients, while 40% of patients developed symptomatic MM based on the CRAB criteria. In the multivariate analysis, MRI-PD was an independent prognostic factor for progression. Patients with stable MRI findings had no higher risk of progression, even when focal lesions were present at the initial MRI [105]. Prospective clinical trials should be conducted to confirm the above findings.
MRI Findings in Monoclonal Gammopathy of Undetermined Significance (MGUS). MGUS by definition is characterized by the absence of osteolytic lesions. However, MGUS patients have higher incidence of osteoporosis and vertebral fractures compared to normal population [106, 107]. In a small study which included 37 patients with MGUS or SMM, MRI abnormalities were detected in 20% of them. These patients had a higher time to progression (TTP) to symptomatic myeloma compared to patients with a normal MRI who did not progress after a median follow-up of 30 months [108]. A prospective study in 331 patients with MGUS or SMM revealed that the detection of multiple (>1) focal lesions by MRI conferred an increased risk of progression [109]. In another large study, which included only MGUS patients (n = 137) who underwent a WB-MRI at diagnosis, a focal infiltration pattern was detected in 23% of them. Independent prognostic factors for progression to symptomatic myeloma included the presence and number of focal lesions and the value of M-protein [110].
MRI and Solitary Plasmacytoma of the Bone (SPB). The diagnosis of SBP includes the presence of a solitary bone lesion, with a confirmed infiltration by plasma cells in the biopsy of the lesion, absence of clonal plasma cells in the trephine bone marrow biopsy, and no CRAB criteria. Although definitive radiotherapy usually eradicates the local disease, the majority of patients will develop MM because of the growth of previously occult lesions which have not been detected by WBXR [83]. Moulopoulos et al. published that spinal MRI revealed additional focal lesions in 4/12 SBP patients. After treatment with radiotherapy to the painful lesion, three patients developed systemic disease within 18 months from diagnosis [82]. Furthermore, Liebross et al. observed that among SBP patients with spinal disease, 7/8 staged by WBXR alone developed MM compared to only 1/7 patients who also had spinal MRI [111].
8.7 PET-CT
PET-CT Detection of Bone Involvement in Myeloma. FDG-PET-CT is a functional imaging method, which combines demonstration of hypermetabolic activity in intramedullary and extramedullary sites (PET) with evidence of osteolysis (CT). Several studies have shown that PET-CT is more sensitive compared to WBXR for the detection of osteolytic lesions in MM [77, 112,113,114]. This has been confirmed by the largest meta-analysis in the field [75]. The higher detection rate of PET-CT over WBXR for the presence of osteolytic lesions is especially important for patients with SMM. In one study with 120 patients with SMM based on the previous IMWG criteria [77], 16% of patients with normal WBXR had positive PET-CT results. The median time to progression (TTP) for PET-CT-positive patients was 1.1 years vs. 4.5 for patients with negative PET-CT, while the probability of progression at 2 years for PET-CT-positive patients was 58% [115]. The largest study in the field involved 188 with suspected SMM examined with PET-CT. PET-CT was positive in 39% of patients. The probability of progression to symptomatic MM within 2 years was 75% for patients with a positive PET-CT under observation versus only 30% for patients with a negative PET-CT. This probability was higher if hypermetabolic activity was combined with underlying osteolysis (2-year progression rate: 87%). The median TTP was 21 months vs. 60 months for PET-CT-positive and PET-CT-negative patients, respectively [116]. The results of these two studies support the integration of changes in imaging requirements in the new IMWG diagnostic criteria for MM; detection of osteolytic lesions by PET-CT is a criterion for symptomatic MM [14].
Compared to MRI, as mentioned previously, PET-CT performs equally well in detecting focal lesions, but MRI is better in detecting diffuse disease [76, 77, 114].
Value of PET-CT for Better Definition of Complete Response to Antimyeloma Therapy. Data obtained from PET-CT in 40 MM patients, including average SUV and FDG kinetic parameters K1, influx, and fractal dimension, correlated significantly with percentage of bone marrow infiltration on trephine biopsies (PC %) [117]. Furthermore, PET-CT efficiently detected extramedullary disease in patients both at diagnosis and at relapse [118]. Consequently, PET-CT was tested for better definition of CR in 282 MM patients. It was performed at diagnosis and every 12–18 months afterward. At diagnosis, 42% of MM patients had >3 focal lesions; in 50% of these patients SUV max was >4.2. After treatment, PET-CT was negative in 70% of patients, while 53% of patients achieved CR according to IMWG criteria. Approximately 30% of patients at CR had positive PET-CT. More importantly, PET-CT negativity was an independent predictor for prolonged PFS and OS in CR patients; median PFS was 50 months for PET-CT-positive and 90 months for PET-CT-negative CR patients [119]. PET-CT, therefore, provides more accurate definition of CR, and it has been suggested that it should be incorporated to CR criteria [120].
Prognostic Significance of PET-CT. Several studies have confirmed the value of PET-CT as an independent factor for survival in MM patients both at diagnosis and posttreatment [99, 121,122,123,124,125]. In 192 newly diagnosed patients who underwent ASCT, the presence of extramedullary disease and SUVmax >4.2 on PET-CT performed at diagnosis, as well as the persistence of FDG uptake post-ASCT were independent variables, adversely affecting PFS [121]. In the largest study in the field, 429 patients who were treated with total therapy protocols in Arkansas were evaluated with both MRI and PET-CT at diagnosis and 7 days post-ASCT. From the imaging variables, in the multivariate analysis, only the detection of >2 osteolytic lesions by WBXR at diagnosis and the detection of >3 focal lesions by PET-CT, 7 days post-ASCT, were independent prognostic factors for inferior OS. Limitation of this study was the exclusion of diffuse MRI pattern from the analysis [89]. Despite this limitation, studies reported to-date support the role of PET-CT after therapy, deeming it the best imaging technique for the follow-up of myeloma patients. Indeed, in a recent study which has been reported only in an abstract form, 134 patients who were eligible for treatment with ASCT were randomized to receive 8 cycles of bortezomib-lenalidomide-dexamethasone (VRD) followed by 1-year maintenance with lenalidomide or 3 cycles of VRD followed by ASCT plus 2 cycles of VRD consolidation and 1-year lenalidomide maintenance. PET-CT and WB-MRI were performed after induction and before maintenance. Both techniques were positive at diagnosis in more than 90% of patients. After induction therapy and before maintenance, more patients continued to have positive MRI than PET-CT (93% vs. 55%, and 83% vs. 21%, respectively), possibly due to earlier reduction of activity of PET-CT lesions. Both after induction and before maintenance, normalization of PET-CT and not of MRI could predict for PFS, while only normalization of PET-CT before maintenance could predict for OS (30-month OS rate: 70% in PET-CT-positive patients vs. 94.6% in patients with negative PET-CT negative; p = 0.01) [126].
At this point, it is crucial to mention that one of the major limitations of PET-CT is the lack of standardization and the controversies regarding SUV level of positivity. Recently, an Italian panel of experts introduced novel criteria for the interpretation of PET-CT images [127]. Large, multicenter, studies with prospective evaluation of these new criteria will reveal their clinical impact.
Other PET-CT Indications and Limitations. PET-CT may be used for the work-up of patients with SBP at diagnosis [128]. However, it is not clear whether PET-CT or MRI is more suitable in this setting since restaging PET-CT after radiotherapy has a number of false-positive findings [129]. PET-CT also has a role in patients with nonsecretory or oligo-secretory myeloma for the detection of active lesions in the body [130]. Major limitations of PET-CT include high cost, lack of availability in many centers and countries, and false-positive results due to inflammation of other underlying pathology.
8.8 Management of Multiple Myeloma Bone Disease
Bisphosphonates (BPs) are the mainstay in the management of MM bone disease. They are artificial analogues of pyrophosphates. In comparison with natural pyrophosphates, bisphosphonates are resistant to phosphatase-induced hydrolysis [131]. Bisphosphonates cause osteoclast suppression. They bind to calcium containing molecules such as hydroxyapatite [132]. Osteoclast-induced bone resorption causes exposure of hydroxyapatite. Bisphosphonates bind to the exposed molecules of hydroxyapatite. This fact leads to increased concentration of bisphosphonates within the lytic lesions [132,133,134]. There are two main groups of bisphosphonates, each with a differently proposed mechanism of action [132]. Non-nitrogen-containing bisphosphonates induce osteoclast apoptosis via their cytotoxic ATP analogues. On the other hand, nitrogen-containing bisphosphonates downregulate osteoclast activity by inhibiting the HMG-CoA reductase pathway. Etidronate and clodronate (CLO) are non-nitrogen-containing bisphosphonates. Zoledronic acid (ZOL), ibandronate, pamidronate (PAM), and risedronate are nitrogen-containing bisphosphonates. All bisphosphonates have similar physicochemical properties; however, their anti-resorbing activity is different. Their activity is drastically increased when an amino group is entered into the aliphatic carbon chain. Thus, pamidronate is 100- and 700-fold more potent than etidronate, both in vitro and in vivo, while zoledronic acid and ibandronate show 10,000- to 100,000-fold greater potency than etidronate [135]. Bisphosphonates also appear to affect the microenvironment in which tumor cells grow and may have direct antitumor activity [136,137,138,139,140,141]. Possible mechanisms include the reduction of IL-6 secretion by bone marrow stromal cells or the expansion of gamma/delta T cells with possible anti-MM activity. The aim of bisphosphonates use is the reduction of SREs in patients with myeloma bone disease [23].
According to the latest IMWG guidelines, bisphosphonates should be initiated in MM patients, with (grade A) or without (grade B) detectable osteolytic bone lesions in conventional radiography, who are receiving antimyeloma therapy, as well as patients with osteoporosis (grade A) or osteopenia (grade C) due to myeloma. The beneficial effect of zoledronic acid in patients without detectable bone disease by MRI or PET-CT is not known. Oral clodronate, intravenous pamidronate, and intravenous zoledronic acid have been licensed for the management of myeloma bone disease. Etidronate and ibandronate were found to be ineffective for the treatment of bone disease in myeloma patients [142, 143]. Several studies have evaluated the effects of bisphosphonates (BPs) on SREs and bone pain in patients with MM [144].
8.8.1 Etidronate
Etidronate was found to be ineffective in two placebo-controlled studies in myeloma patients [142, 145].
8.8.2 Ibandronate
Ibandronate is ineffective in reducing SREs or improving bone pain in patients with MM [143].
8.8.3 Clodronate
The oral BP, clodronate, reduced the proportion of patients with MM who experienced progression of osteolytic lesions by 50% compared with placebo (24% vs. 12%; P = 0.026) 24 and reduced the time to first and the rate of nonvertebral fracture (6.8% vs. 13.2% for placebo; P = 0.04) in patients with newly diagnosed MM [13]. Two major, placebo-controlled, randomized trials have been performed in MM. Lahtinen et al. reported reduction of the development of new osteolytic lesions by 50% in myeloma patients who received oral CLO for 2 years that was independent of the presence of lytic lesions at baseline [146]. In the other study, although there was no difference in overall survival (OS) between CLO and placebo patients, patients who received CLO and did not have vertebral fractures at baseline appeared to have a survival advantage (59 vs. 37 months). Both vertebral and nonvertebral fractures as well as the time to first nonvertebral fracture and severe hypercalcemia were reduced in the CLO group after 1 year of follow-up, and at 2 years, the patients who received CLO had better performance status and less myeloma-related pain than patients treated with placebo [147].
8.8.4 Pamidronate
PAM is an aminobisphosphonate, which has been administered either orally or intravenously. In one trial, patients with advanced disease and at least one lytic lesion were randomized to placebo or intravenous PAM [148]. Administration of PAM resulted in a significant reduction in skeletal-related events (SREs; 24%) vs. placebo (41%; p < 0.001). Patients receiving PAM also experienced reduced bone pain and no deterioration in quality of life (QoL) during the 2-year study. By contrast, administration of oral PAM failed to reduce SREs relative to placebo [149]. However, patients treated with oral PAM experienced fewer episodes of severe pain. The overall negative result of this study was attributed to the low absorption of orally administered BPs [149]. A recent study for patients with newly diagnosed MM demonstrated that PAM 30 mg monthly had comparable time with SREs and SRE-free survival time as compared with PAM 90 mg monthly. After a minimum of 3 years, patients receiving PAM 30 mg showed a trend toward lower risks of osteonecrosis of the jaw (ONJ) and nephrotoxicity compared with the higher dose. However, the study was not powered to show SRE differences between the two PAM dosages but only to show QoL differences [150].
8.8.5 Zoledronic Acid (ZOL)
In a non-inferiority randomized phase II trial published by Berenson et al., escalating doses of ZOL were tested in comparison with 90 mg of PAM, in 280 patients, 108 of them affected by MM (the other had metastatic breast cancer to bone). Both ZOL (at doses of 2 and 4 mg) and PAM significantly reduced SREs in contrast to 0.4 mg ZOL [151]. This phase II trial failed to show any superiority of ZOL compared with PAM in terms of SREs, but it was not powered to show differences between the groups.
Bisphosphonates Head to Head. There are only two large randomized studies comparing two different BPs. A phase III, randomized, double-blind study was performed to compare the effects of zoledronic acid with pamidronate for patients with myeloma and lytic bone disease or with metastatic breast cancer to bone [152, 153]. In the myeloma cohort, there was no difference between the two treatment arms regarding incidence and time to first SRE. However, N-terminal cross-linking telopeptide of collagen type I (NTX) levels, a sensitive marker of bone resorption, normalized more often in the zoledronic acid arm compared with pamidronate-treated patients. More recently, the Medical Research Council (MRC) of the UK compared zoledronic acid (4 mg intravenous every 3–4 weeks or at doses according to creatinine clearance [CrCl] rates) and oral clodronate (1600 mg orally daily) for patients with newly diagnosed, symptomatic MM, who were treated with antimyeloma therapy (n = 1960 evaluable for efficacy). Zoledronic acid reduced the incidence of SREs both in myeloma patients with or without bone lesions as assessed using conventional radiography, compared with clodronate [154, 155]. After a median follow-up of 3.7 years, 35% of patients receiving clodronate had experienced SREs vs. 27% of patients receiving zoledronic acid (p = 0.004). More importantly, zoledronic acid reduced mortality and extended median survival. Further, subset analysis showed this treatment extended survival by 10 months over clodronate for patients with osteolytic disease at diagnosis, whereas myeloma patients without bone disease at diagnosis as assessed using conventional radiography had no survival advantage with zoleronic acid [155]. These results confirm preclinical studies suggesting indirect and direct antimyeloma effects of zoledronic acid [156]. Possible mechanisms for the antimyeloma effects of zoledronic acid include direct cytotoxic effect on the tumor cells, the reduction of IL-6 secretion by bone marrow stromal cells, the expansion of gamma/delta T cells with possible anti-MM activity, anti-angiogenic effects, and inhibitory effects in the adhesion molecules. In specific subsets of patients, other BPs have also been associated with improved survival: patients receiving second-line antimyeloma chemotherapy and treated with pamidronate experienced a borderline improvement in OS over placebo [148], whereas clodronate had an OS advantage in patients without vertebral fractures at presentation relative to placebo [147]. Nevertheless, a Cochrane database meta-analysis showed that zoledronic acid was the only BP associated with superior OS compared with placebo (hazard ratio, 0.61; 95% CI, 0.28–0.98), but not compared with other BPs [157].
Patients with Asymptomatic Myeloma (AMM). Intravenous PAM (60–90 mg monthly for 12 months) in patients with AMM reduced bone involvement at progression but did not decrease the risk and increase the time to progression [158]. Similarly, intravenous ZOL (4 mg monthly for 12 months) reduced the SRE risk at progression but did not influence the risk of progression of AMM patients [159].
Several studies have reported the value of MRI (presence of >1 focal lesion and presence of diffuse pattern of marrow infiltration) in detecting patients with AMM at high risk for progression [102, 103]. Since there is no data supporting PFS advantage with bisphosphonates in AMM, bisphosphonates should not be recommended except for a clinical trial of high-risk patients.
Patients with MGUS. MGUS patients are at high risk for developing osteoporosis and pathological fractures [160, 161]. Three doses of ZOL (4 mg intravenously every 6 months) increased bone mineral density (BMD) by 15% in the lumbar spine and by 6% in the femoral neck in MGUS patients with osteopenia or osteoporosis [162]. Oral alendronate (70 mg/weekly) also increased BMD of the lumbar spine and total femur by 6.1% and 1.5%, respectively, in 50 MGUS patients with vertebral fractures and/or osteoporosis [163].
Patients with Solitary Plasmacytoma (SPB). Patients with solitary plasmacytoma and no evidence of MM do not require therapy with bisphosphonates. However, these patients should have a whole-body MRI since in a study of 17 patients diagnosed with a solitary plasmacytoma, all showed additional focal lesions or a diffuse infiltration on MRI, leading to a classification as stage I MM (76%), stage II MM (12%), or stage III MM (12%) using the Durie-Salmon PLUS system [164].
Route of Administration. Strict adherence to dosing recommendations is required for bisphosphonate therapy to effectively reduce and delay SREs in patients with MM. Each patient prescribed bisphosphonate therapy should be instructed about the crucial importance of adherence to the dosing regimen. Although a few randomized, placebo-controlled clinical studies suggest that long-term compliance with oral bisphosphonates such as CLO is satisfactory in MM patients [13, 146], compliance with oral bisphosphonate therapy is generally suboptimal [165]. Further, the MRC-IX data strongly support the use of intravenous ZOL over CLO in all outcomes measured, including reduction of SREs and improvement in OS [154, 155, 166]. According to the latest IMWG guidelines, intravenous administration of BPs is the preferred choice (grade A). However, oral administration remains an option for patients who cannot receive regular hospital care or in-home nursing visits (grade D) [144].
Treatment Duration. Intravenous bisphosphonates should be administered at 3- to 4-week intervals to all patients with active MM (grade A). ZOL improves OS and reduces SREs over CLO in patients who received treatment for more than 2 years; thus, it should be given until disease progression in patients not in complete remission (CR) or a very good partial remission (VGPR) and further continued at relapse (grade B). There is not similar evidence for PAM. PAM may be continued in patients with active disease at the physician’s discretion (grade D), and PAM therapy should be resumed after disease relapse (grade D). For patients in CR/VGPR, the optimal treatment duration of BPs is not clear. According to the IMWG, BPs should be given for at least 12 months and up to 24 months and then at the physician’s discretion (grade D; panel consensus).
According to the latest IMWG guidelines and due to higher reported rates of ONJ with extended duration of therapy, ZOL or PAM should be discontinued after 1–2 years in patients who have achieved CR or VGPR (grade D; panel consensus) [144].
8.8.6 Adverse Events
Even though bisphosphonate therapy is well tolerated in patients with MM, clinicians should be alert for symptoms and signs suggesting adverse events (AEs), and patients and healthcare professionals should be instructed on how to prevent and recognize AEs. Potential AEs associated with bisphosphonate administration include hypocalcemia and hypophosphatemia, gastrointestinal events after oral administration, inflammatory reactions at the injection site, and acute-phase reactions after IV administration of aminobisphosphonates. Renal impairment and ONJ represent infrequent but potentially serious AEs with bisphosphonate use.
Hypocalcemia. Hypocalcemia is usually relatively mild and asymptomatic with bisphosphonate use in most MM patients. The incidence of symptomatic hypocalcemia is much lower in MM patients compared to patients with solid tumors. Although severe hypocalcemia has been observed in some patients [167], it is usually preventable via the administration of oral calcium and vitamin D3. Patients should routinely receive calcium (600 mg/day) and vitamin D3 (400 IU/day) supplementation since 60% of MM patients have vitamin D deficiency or insufficiency [168, 169]. In vitamin D-deficient patients, there is an increase in bone remodeling. This fact shows that MM patients should be calcium and vitamin D sufficient [170]. Calcium supplementation should be used with caution in patients with renal insufficiency.
Renal Impairment. Bisphosphonate infusions are associated with both dose- and infusion rate-dependent effects on renal function. The potential for renal damage is dependent on the concentration of bisphosphonate in the bloodstream, and the highest risk is observed after administration of high dosages or rapid infusion. Both ZOL and PAM have been associated with acute renal damage or increases in serum creatinine [152, 171]. Patients should be closely monitored for compromised renal function by measuring CrCl before administration of each IV bisphosphonate infusion. Current guideline recommendations [144] state that the dosages of zoledronic acid and clodronate, when administered intravenously, should be reduced for patients who have preexisting renal impairment (CrCl 30–60 mL/min), but there are no clinical studies demonstrating the efficacy of this approach. For patients with CrCl between 30–60 mL/min, zoledronic acid dose should be adjusted. Zoledronic acid has not been studied for patients presented with severe renal impairment (CrCl <30 mL/min), and it is not recommended for patients with severe renal impairment (CrCl <30 mL/min). We suggest that pamidronate may be given at a dose of 90 mg infused over 4–6 h for myeloma patients with osteolytic disease and renal insufficiency. Furthermore, serum creatinine and CrCl should be measured before each infusion of pamidronate or zoledronic acid, while BPs should not be administered in short infusion times (<2 h for pamidronate and less than 15 min for zoledronic acid). Bisphosphonate therapy can be resumed, after withholding zoledronic acid or pamidronate for patients who develop renal deterioration during therapy, when serum creatinine returns to within 10% of baseline [144].
Osteonecrosis of the Jaw. It is an uncommon complication of intravenous bisphosphonates. It is potentially serious, and its main characteristic is the presence of exposed bone in the mouth. Incidence may vary from 2 to 10% [172, 173]. Longer exposure increases the cumulative incidence of ONJ. One of the main risk factors for the development of ONJ is the invasive dental procedures [172]. Other risk factors include poor oral hygiene, age, and duration of myeloma. Zoledronic acid was associated with a higher incidence of ONJ in retrospective evaluations [174]. In approximately one half of patients, ONJ lesions will heal [175], but approximately one half of patients who restart bisphosphonate therapy after having stopped it will develop recurrence of ONJ. According to recent IMWG guidelines [176], preventive strategies should be adopted to avoid ONJ. A dental examination is necessary before beginning of the bisphosphonate’s course. Patients should also be alerted regarding dental hygiene (grade C; panel consensus). All existing dental condition should be treated before initiation of bisphosphonate therapy (grade C; panel consensus). After bisphosphonate treatment initiation, unnecessary invasive dental procedures should be avoided, and dental health status should be monitored on annual basis (grade C). Patients’ dental health status should be monitored by a physician and a dentist (grade D; panel consensus). Dental problems should be managed conservatively if possible (grade C). If invasive dental procedures are necessary, there should be temporary suspension of bisphosphonate treatment (grade D). The panel consensus suggests the interruption of bisphosphonates before and after dental procedures for a total of 180 days (90 days before and 90 days after procedures such as tooth extraction, dental implants, and surgery to the jaw). Bisphosphonates do not need to be discontinued for routine dental procedures including root canal. Initial treatment of ONJ should include discontinuation of bisphosphonates until healing occurs (grade C). The physician should consider the advantages and disadvantages of continued treatment with bisphosphonates, especially in the relapsed/refractory MM setting (grade D). Preventive measures during bisphosphonate treatment have the potential to reduce the incidence of ONJ about 75% [177]. Prophylactic antibiotic treatment may prevent ONJ occurrence after dental procedures [178]. Management of patients depends on ONJ stage. Stage I (asymptomatic exposed bone, no soft tissue infection) can be managed conservatively with oral antimicrobial rinses. Stage II (exposed bone and associated pain/swelling and/or soft tissue infection) requires culture-directed long-term and maintenance antimicrobial therapy, analgesic management, and, occasionally, minor bony debridement. Stage III disease (pathological fracture and exposed bone or soft tissue infection not manageable with antibiotics) requires surgical resection in order to reduce the volume of necrotic bone in addition to the measures described in stage II [179]. When ONJ occurs, initial therapy should include discontinuation of bisphosphonates until healing occurs [132]. The administration of medical ozone (O3) as an oil suspension directly to the ONJ lesions that are below ≤2.5 cm may be another possible therapeutic strategy for those patients who fail to respond to conservative treatment. In such patients, there are reports suggesting that ONJ lesions resolved with complete reconstitution of oral and jaw tissue, with 3–10 applications [180, 181]. In addition, treatment with hyperbaric oxygen has been reported to be helpful.
8.9 Future Treatment Options
8.9.1 RANKL/RANK Pathway Regulators: Targeting the Osteoclast
RANKL Antagonists. Preclinical models of MM demonstrated that RANKL inhibition can prevent bone destruction from MM. RANKL inhibition with recombinant RANK-Fc protein not only reduced MM-induced osteolysis but also caused a marked decline in tumor burden [182, 183]. Similar results were obtained using recombinant OPG for the treatment of MM-bearing animals [184]. These data gave the rationale for using RANKL inhibition in the clinical setting.
Denosumab, a fully human monoclonal antibody, has showed high affinity and specificity in binding RANKL and inhibits RANKL-RANK interaction, mimicking the endogenous effects of OPG. In knock-in mice with chimeric (murine/human) RANKL expression, denosumab showed inhibition of bone resorption [185].
In a phase I trial, 54 patients with breast cancer (n = 29) or MM (n = 25) with radiologically confirmed bone lesions received a single dose of either denosumab or pamidronate. Denosumab decreased bone resorption within 24 h of administration, as reflected by levels of urinary and serum NTX. That was similar in magnitude but more sustained than with intravenous pamidronate [186]. These results were confirmed in another phase I trial, in which denosumab was given at multiple doses [187].
In a phase II trial, the ability of denosumab (120 mg given monthly as a subcutaneous injection) to affect bone resorption markers and monoclonal protein levels in MM patients who relapsed after response to prior therapy and in patients with response to most recent therapy and who had stable disease for at least 3 months was evaluated. No patients experienced complete or partial response (≥50% reduction in M-protein), but seven patients had maximum reduction of ≥25% in serum M-protein. Bone resorption markers were reduced by more than 50% with denosumab [188].
In another phase II trial, Fizazi et al. evaluated the effect of denosumab in patients with bone metastases and elevated urinary NTX levels despite ongoing intravenous bisphosphonate therapy. Patients were stratified by tumor type (total 111 patients: 9 patients with multiple myeloma, 50 patients with prostate cancer, 46 patients with breast cancer, and 6 patients with another solid tumor) and screening NTX levels and randomly assigned to receive subcutaneous denosumab 180 mg every four or every 12 weeks or continue intravenous bisphosphonates every 4 weeks. Denosumab normalized urinary NTX levels more frequently than the continuation of intravenous bisphosphonate (64% vs. 37%, respectively, p = 0.01), while fewer patients receiving denosumab experienced on-study SREs than those receiving intravenous bisphosphonate (8% vs. 17%) [189]. This study showed that denosumab inhibits bone resorption and prevents SREs even in patients who are refractory to bisphosphonate therapy.
A meta-analysis of major phase 3 studies comparing denosumab vs. zoledronic acid including mainly patients with solid tumors showed that denosumab was superior in terms of delaying the time to first on-study SRE by 8 months and reducing the risk of the first SRE by 17%. No difference between the two drugs was reported regarding disease progression and overall survival. Hypocalcaemia was more common in denosumab arm, while ONJ was similar with the two drugs [190].
Denosumab appears to have little toxicity, mainly asthenia, and multiple phase III trials of denosumab in patients with bone metastasis are ongoing. However, it is crucial to mention that RANKL is involved in dendritic cell survival and that the anti-RANKL strategy may have an effect on the immune system and a possible increase in infection rate, especially in cancer patients who have already had severe immunodeficiency. For MM patients, while denosumab was comparable to zoledronic acid with respect to the occurrence of SREs, inferior survival occurred in denosumab compared to zoledronic acid-treated patients, but this was a subset analysis from a large phase III trial that involved mostly solid tumor patients with metastatic bone disease [191]. Interpretation is limited based on the small numbers of MM patients who were enrolled on the trial and imbalance in baseline disease characteristics.
To address this survival discrepancy in the phase 3 RCT, a confirmatory phase 3 trial that included 1718 newly diagnosed myeloma patients, randomized to denosumab (758 patients) and zoledronic acid (758 patients), stratified by type of first-line therapy and previous SRE, was recently reported at the IMW 2017 [Raje et al. OP-46]. Primary endpoint was non-inferiority of denosumab (vs ZA) for time to first SRE while on study. Several secondary endpoints were evaluated including the superiority of denosumab and overall survival (OS). At a median follow-up of 17.4 months, median time to first on-study SRE was similar between both groups (23 months). 43.8% pts. on denosumab and 44.6% on ZA had a first on-study SRE (P = 0.01), confirming the non-inferiority of denosumab to ZA in delaying time to first on-study SRE (HR = 0.98[0.85,1.14]). More interestingly, a pre-specified exploratory endpoint, the PFS favored the denosumab arm (HR = 0.82[0.68,0.99]), P = 0.036. Denosumab met the primary endpoint of the study demonstrating the non-inferiority to ZA in delaying time to first SRE. The safety profile of denosumab is established. Though the lack of OS difference suggests a shorter follow-up of the study, it is reassuring to know that the inferiority in survival from earlier RCT was not demonstrated and will need further follow-up.
8.9.2 Activin-A Inhibitors
Sotatercept (ACE-011) is a fusion protein of the extracellular domain of the high-affinity activin receptor IIA (ActRIIA) and human immunoglobulin G (IgG) Fc domain with potent inhibitory effect on activin, enhancing the deposition of new bone tissue and preventing bone loss. In the preclinical setting, RAP-011, a murine counterpart of sotatercept, prevented the formation of osteolytic lesions in a murine MM model by stimulating bone formation through osteoblasts, while having no effect on osteoclast activity [192].
In a phase 1 study, in healthy postmenopausal volunteers, single-dose sotatercept was associated with increased serum levels of the bone formation marker bone-specific alkaline phosphatase (bALP) and decreased bone resorption markers CTX and tartrate-resistant acid phosphatase isoform 5b (TRACP-5b), reflecting a decrease in bone resorption and an increase in bone formation [193]. No safety concerns were noted in this study.
In a multicenter phase 2 trial, patients with osteolytic bone lesions due to MM were randomized to receive either four 28-day cycles of sotatercept or placebo as subcutaneous injection with concomitant anticancer therapy consisting of oral melphalan, prednisolone, and thalidomide (MPT). Sotatercept treatment demonstrated clinically significant increases in biomarkers of bone formation, decreases in bone pain, and antitumor activity as well as increase in hemoglobin levels [192], but further research is needed to support these findings. Moreover, increased activin-A secretion was induced by lenalidomide and was canceled by the addition of an activin-A-neutralizing antibody. This effectively restored osteoblast function and subsequently inhibited myeloma-related osteolysis without abrogating the cytotoxic effects of lenalidomide on malignant cells [194] and thus supporting the combination of lenalidomide with an anti-activin-A molecule.
8.10 Future Agents Targeting the Osteoclast
The pathophysiology of myeloma bone disease is complex. Interactions between myeloma cells, stromal cells, osteoclasts, and osteoblasts create vicious cycles that lead to the development of osteolytic disease and support the myeloma cell growth and survival. The better understanding of this biology has revealed several other pathways that enhance osteoclastogenesis, including the PI3K/AKT/mTOR pathway, the extracellular signal-regulated kinase 1/2 pathway, the nuclear export protein CRM1/XPO1 signaling, the MAPK pathways, the parathyroid hormone-related protein, chemokines and their receptors such as the C-C chemokine receptor type 1 and 2 (CCR1 and -2), the C-C motif ligand 3 (CCL-3; previously known as macrophage inflammatory protein 1a) pathways, and others [23, 195,196,197,198,199,200,201,202]. This knowledge has led to the development of novel drugs that may be used in the near future for the management of lytic bone disease in myeloma patients. AKT pathway is upregulated in marrow monocytes from MM patients, leading to a sustained high expression of RANK in osteoclast precursors. AKT inhibition blocks this upregulation of RANK expression and the subsequent osteoclast formation. In the clinical setting, the novel AKT inhibitor LY294002 blocked the formation of myeloma masses in the bone marrow cavity and dramatically reduced osteoclast formation and osteolytic lesions in SCID mice, suggesting a potential role in the management of MM patients with bone disease in the future [196]. AZD6244 is a mitogen-activated or extracellular signal-regulated protein kinase (MEK) inhibitor. It has been reported in preclinical models that AZD6244 blocked osteoclast formation in a dose-dependent manner and inhibited bone resorption targeting a later stage of osteoclast differentiation [197]. Novel, oral, irreversible selective nuclear export inhibitors (SINEs) that target CRM1 have shown strong antimyeloma activity, and they inhibit the MM-induced osteolysis. SINEs have direct anti-osteoclastic function through the blockade of RANKL-induced NF-kB and NFATc1, with almost no impact on osteoblasts, supporting their clinical development for myeloma-related bone disease [198]. MLN3897 is a novel antagonist of the chemokine receptor CCR1 that demonstrated reduction of osteoclast formation and function by inhibiting the AKT signaling and the CCL-3 pathway in preclinical models [203].
8.11 Wnt Pathway Regulators: Helping the Osteoblast
DKK-1 Antagonists. DKK-1 plays an important role in the dysfunction of osteoblasts observed in MM. The production of this soluble Wnt inhibitor by MM cells inhibits osteoblast activity, and its serum level reflects the extension of focal bone lesions in MM [68, 149]. Serum DKK-1 is increased not only in symptomatic MM patients at diagnosis and but also in relapsed MM, correlating with advanced disease features and the presence of lytic lesions, while serum DKK-1 levels of asymptomatic patients at diagnosis and plateau do not differ from control values [26, 204].
BHQ880, an IgG antibody, the first-in-class, fully human anti-Dkk-1 neutralizing antibody, seems to promote bone formation, and thus it has been shown to inhibit tumor-induced osteolytic disease in preclinical studies [190]. Inhibiting Dkk-1 with BHQ880 in the 5T2MM murine model of myeloma reduced the development of osteolytic bone lesions and in vivo growth of MM cells [205]. A phase I/II study of BHQ880 in combination with zoledronic acid in relapsed or refractory myeloma patients is ongoing as well as phase II studies in patients with high-risk smoldering MM or untreated MM and renal insufficiency. Results are highly anticipated.
Sclerostin Antagonists. Sclerostin is another Wnt inhibitor, specifically expressed by osteocytes, which inhibits osteoblast-driven bone formation and induces mature osteoblast apoptosis [206]. Sclerostin deficiency leads to the development of rare bone sclerosing disorders, including sclerosteosis and van Buchem disease. On the other hand, elevated sclerostin is implicated in the mechanisms of bone loss in metabolic bone diseases, such as postmenopausal osteoporosis and thalassemia-associated osteoporosis [207, 208]. Elevated circulating sclerostin levels correlate with advanced disease features and abnormal bone remodeling in symptomatic myeloma [27]. In particular, MM patients who presented with fractures at diagnosis had very high levels of circulating sclerostin compared with all others (p < 0.01), while sclerostin serum levels correlated negatively with bALP (r = −0.541; p < 0.0001) and positively with CTX (r = 0.524; p < 0.0001) [27]. Romosozumab (AMG 785; CDP7851), an investigational humanized monoclonal antibody that inhibits the activity of sclerostin, has been used in phase II clinical studies in postmenopausal women with low bone mineral density (BMD), demonstrating significant increases in lumbar spine BMD after 12 months [209]. Studies in MM are planned to start soon.
8.12 Antimyeloma Agents
8.12.1 Bortezomib
Bortezomib is the first proteasome inhibitor with established activity against myeloma, with subsequent effects on osteoclasts that leads to reduced bone resorption [210, 211]. For patients with relapsed/refractory MM, bortezomib reduces circulating RANKL, osteoclast function, and bone resorption, as assessed by TRACP-5b and CTX serum levels, respectively [212]. Furthermore, bortezomib increases osteoblast activity and bone formation both in vitro and for patients with relapsed/refractory MM [213, 214]. More specifically, bortezomib increased bone formation markers such as bALP; this increase was observed both among responders and nonresponders to bortezomib suggesting a direct effect of bortezomib on osteoblastic activity [215]. Another proteasome inhibitor, carfilzomib, has been reported to increase bALP in patients with relapsed/refractory MM that responded to therapy [216]. Bortezomib in combination with zoledronic acid increased BMD in a subset of MM patients at first relapse even in the presence of dexamethasone [217]. However, when bortezomib was given in combination with other antimyeloma drugs, such as melphalan and thalidomide (VMDT regimen), no increase in bALP and osteocalcin was observed suggesting that in such combinations bortezomib seems to lose its beneficial effect on osteoblasts [218]. Even in post-autologous stem cell transplantation patients with low myeloma burden, bortezomib in combination with thalidomide and dexamethasone as consolidation therapy failed to produce a significant bone anabolic effect [219]. Nevertheless, in this specific cohort of patients who did not receive BPs during consolidation, bone resorption was reduced, and there were no SREs in responding patients. In a subanalysis of a phase III study in newly diagnosed patients (VISTA trial), bortezomib in combination with melphalan and prednisone (VMP) reduced substantially DKK-1 in responding patients, while the MP regimen increased DKK-1 even in responders [220]. In the same study, there was evident bone formation effect in conventional radiography in subset of VMP patients but not in MP patients [220].
These findings suggest that proteasome inhibition and especially bortezomib, in addition to its antineoplastic effects on tumor cells, may directly stimulate osteoblast differentiation and function and lead to increased bone formation and increased BMD, at least in responders. However, it is unclear if bortezomib alone is sufficient to reverse bone disease in MM patients and heal lytic lesions as evidence of the effect of bortezomib on clinical end points specific to the bone, such as SREs is limited, possibly as a result of relatively short follow-up periods. Prospective trials that specifically investigate end points related to bone formation are needed.
8.13 Immunomodulatory Agents
Immunomodulatory agents (IMiDs), such as thalidomide, lenalidomide, and pomalidomide, are highly active agents in the treatment of both newly diagnosed and relapsed/refractory MM. These agents also alter interactions between bone marrow microenvironment and malignant plasma cells and modify abnormal bone metabolism in MM [23].
Thalidomide. Thalidomide almost completely blocks RANKL-induced osteoclast formation in vitro. In relapsed/refractory MM patients, intermediate dose of thalidomide (200 mg/day) in combination with dexamethasone produced a significant reduction of serum markers of bone resorption [C-telopeptide of collagen type I (CTX) and tartrate-resistant acid phosphatase isoform-5b (TRACP-5b)] and also of sRANKL/OPG ratio [221].
Lenalidomide. Lenalidomide also inhibited osteoclast formation, by targeting PU.1, a critical transcription factor for the development of osteoclasts, and downregulating cathepsin K. The downregulation of PU.1 in hematopoietic progenitor cells resulted in a complete shift of lineage development toward granulocytes. Lenalidomide also reduced the serum levels of sRANKL/OPG ratio in MM patients [222].
Pomalidomide. Pomalidomide, like thalidomide, blocks RANKL-induced osteoclastogenesis in vitro, even at concentrations of one μM, which is similar or even lower than that achieved in vivo after the therapeutic administration of this agent. Pomalidomide downregulates transcription factor PU.1, affecting the lineage commitment of osteoclast precursors toward granulocytes instead of mature osteoclasts [223].
8.14 Other Novel Agents
Panobinostat is a histone deacetylase inhibitor, which has shown significant preclinical antimyeloma activity and is currently in phase III trials for relapsed MM. Recently, a potent synergistic antiproliferative effect of panobinostat with zoledronic acid was described in three myeloma cell lines and may result in clinical trials in myeloma patients [224].
Bruton’s tyrosine kinase (BTK) has been reported to play an important role in myeloma cell homing to bone and the subsequent myeloma-induced bone disease [225]. Several BTK inhibitors have been developed including ibrutinib, which was recently approved for the treatment of mantle cell lymphoma. This new category of drugs has entered into clinical trials in myeloma patients and may be used in the future in patients with bone disease.
Other novel antimyeloma agents have also shown effects on bone disease in preclinical models. Antibodies against B cell activating factor (anti-BAFF) have produced direct antimyeloma effects and reductions in tartrate-resistant acid phosphatase-positive osteoclasts and in lytic lesions in anti-BAFF-treated animals [226]. Similarly, SCIO-469, a selective p38a MAPK inhibitor, inhibited MM growth and prevented bone disease in the 5T2MM and 5T33MM animal models [227].
8.15 Kyphoplasty and Vertebroplasty
Several studies have demonstrated that balloon kyphoplasty (BKP) or vertebroplasty is well-tolerated and effective procedures that provide pain relief and improve functional outcomes in patients with painful neoplastic spinal fractures. A single randomized study of 134 patients with bone metastases due to solid tumors and MM demonstrated that treatment of VCFs with BKP was associated with clinically meaningful improvements in physical functioning, back pain, QoL, and ability to perform daily activities relative to nonsurgical management. These benefits persisted throughout the 12-month study [228]. A meta-analysis of 7 nonrandomized studies of patients with MM or osteolytic metastasis revealed that BKP was associated with reduced pain and improved functional outcomes, benefits that were maintained up to 2 years post-procedure (N = 306). BKP also improved early vertebral height loss and spinal deformity, but these effects were not long-term [229]. Similarly, a retrospective review of 67 patients with MM-related vertebral compression fractures (VCFs) demonstrated that vertebroplasty provided clinically meaningful improvements in physical functioning, pain, and mobility throughout 12 months of follow-up [230]. Several small nonrandomized studies of BKP or BKP and vertebroplasty generated comparable results [231,232,233]. However, the role of vertebroplasty for myeloma patients remains debatable in the absence of prospective data [232, 234], as two randomized trials failed to show any benefit of vertebroplasty in patients with osteoporotic fractures vs. conservative therapy [235, 236]. Furthermore, a meta-analysis of 59 studies (56 case series) showed that BKP appears to be more effective than vertebroplasty in relieving pain secondary to cancer-related VCFs and is associated with lower rates of cement leakage [237].
8.16 Radiation Therapy
Several studies, the majority of which were retrospective and included relatively small patient cohorts, demonstrated that radiotherapy provided pain relief, decreased analgesic use, promoted recalcification, reduced neurologic symptoms, and improved motor function and QoL in patients with MM [238,239,240]. In addition, the total administered dose should be limited and the field of therapy restricted, especially when the aim of treatment is pain relief rather than treatment or prevention of pathologic fractures. A single 8- to 10-Gy fraction is generally recommended. Indeed, single fractions are increasingly preferred to fractionated treatment. No difference in rapidity of onset or duration of pain relief was observed between a single 8-Gy fraction and a fractionated 2-week course of 30 Gy in a randomized study of 288 patients with widespread bony metastases, including 23 patients with MM [241].
MM accounts for 11% of the most prevalent cancer diagnoses causing spinal cord compression (SCC) [242]. In the largest retrospective series to date, radiotherapy alone improved motor function in 75% of patients with MM and SCC. One-year local control was 100%, and 1-year survival was 94% [243].
8.17 Surgery
Surgery is usually directed toward preventing or repair of axial fractures, unstable spinal fractures, and SCC in myeloma patients. Decompression laminectomy is rarely required in MM patients, but radioresistant MM or retropulsed bone fragments may require surgical intervention [244]. In a relatively large study, 75 MM patients were treated surgically (83 interventions) for skeletal complications of the disease. Most of the lesions were in the axial skeleton or the proximal extremities apart from one distal lesion of the fibula, and most surgery was performed in the spine (35 patients). Surgical treatment in these patients was mostly limited to a palliative approach and was well tolerated [245].
References
Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009;23:1152–7.
Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300.
Parker SL, Davis KJ, Wingo PA, et al. Cancer statistics by race and ethnicity. CA Cancer J Clin. 1998;48:31–48.
Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
Terpos E, Dimopoulos MA. Myeloma bone disease: pathophysiology and management. Ann Oncol. 2005;16:1223–31.
Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17:1278–86.
Coleman RE. Skeletal complications of malignancy. Cancer. 2007;80:1588–94.
Roodman GD. Novel targets for myeloma bone disease. Expert Opin Ther Targets. 2008;12:1377–87.
Croucher PI, Apperley JF. Bone disease in multiple myeloma. Br J Haematol. 1998;103:902–10.
Cocks K, Cohen D, Wisloff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–8.
Bruce NJ, McCloskey EV, Kanis JA, et al. Economic impact of using clodronate in the management of patients with multiple myeloma. Br J Haematol. 1999;104:358–64.
McCloskey EV, MacLennan IC, Drayson MT, et al. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. Br J Haematol. 1998;100:317–25.
Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Silbermann R, Roodman GD. Current controversies in the management of myeloma bone disease. J Cell Physiol. 2016; doi:10.1002/jcp.25351. [Epub ahead of print].
Bataille R, Chappard D, Marcelli C, et al. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88(1):62–6.
Abe M, Hiura K, Wilde J, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104(8):2484–91.
Tanaka Y, Abe M, Hiasa M, et al. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res. 2007;13(3):816–23.
Pearse RN, Sordillo EM, Yaccoby S, et al. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A. 2001;98:11581–6.
Terpos E, Szydlo R, Apperley JF, et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102:1064–9.
Sugatani T, Alvarez UM, Hruska KA. Activin A stimulates IkappaB-alpha/NFkappaB and RANK expression for osteoclast differentiation, but not AKT survival pathway in osteoclast precursors. J Cell Biochem. 2003;90:59–67.
Terpos E, Kastritis E, Christoulas D, et al. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol. 2012;23:2681–6.
Christoulas D, Terpos E, Dimopoulos MA. Pathogenesis and management of myeloma bone disease. Expert Rev Hematol. 2009;2:385–98.
Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94.
Colucci S, Brunetti G, Oranger A, et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011;1:e27.
Politou MC, Heath DJ, Rahemtulla A, et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119:1728–31.
Terpos E, Christoulas D, Katodritou E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131:1466–71.
Oshima T, Abe M, Asano J, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–5.
Dimopoulos M, Terpos E, Comenzo RL, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23:1545–56.
Terpos E, Moulopoulos LA, Dimopoulos MA. Advances in imaging and the management of myeloma bone disease. J Clin Oncol. 2011;29:1907–15.
Pianko MJ, Terpos E, Roodman GD, et al. Whole-body low-dose computed tomography and advanced imaging techniques for multiple myeloma bone disease. Clin Cancer Res. 2014;20:5888–97.
Ippolito D, Besostri V, Bonaffini PA, et al. Diagnostic value of whole-body low-dose computed tomography (WBLDCT) in bone lesions detection in patients with multiple myeloma. Eur J Radiol. 2013;82:2322–7.
Horger M, Claussen CD, Bross-Bach U, et al. Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol. 2005;54:289–97.
Kropil P, Fenk R, Fritz LB, et al. Comparison of whole- body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur Radiol. 2008;18:51–8.
Gleeson TG, Moriarty J, Shortt CP, et al. Accuracy of whole-body low-dose multidetector CT (WBLDCT) versus skeletal survey in the detection of myelomatous lesions, and correlation of disease distribution with whole-body MRI (WBMRI). Skelet Radiol. 2009;38:225–36.
Princewill K, Kyere S, Awan O, et al. Multiple myeloma lesion detection with whole body CT versus radiographic skeletal survey. Cancer Investig. 2013;31:206–11.
Wolf MB, Murray F, Kilk K, et al. Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur J Radiol. 2014;83:1222–30.
Cretti F, Perugini G. Patient dose evaluation for the whole-body low-dose multidetector CT (WBLDMDCT) skeleton study in multiple myeloma (MM). Radiol Med. 2016;121(2):93–105.
Borggrefe J, Giravent S, Campbell G, et al. Association of osteolytic lesions, bone mineral loss and trabecular sclerosis with prevalent vertebral fractures in patients with multiple myeloma. Eur J Radiol. 2015;84:2269–74.
Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100:1254–66.
Moulopoulos LA, Dimopoulos MA. Magnetic resonance imaging of the bone marrow in hematologic malignancies. Blood. 1997;90:2127–47.
Libshitz HI, Malthouse SR, Cunningham D, et al. Multiple myeloma: appearance at MR imaging. Radiology. 1992;182:833–7.
Weininger M, Lauterbach B, Knop S, et al. Whole-body MRI of multiple myeloma: comparison of different MRI sequences in assessment of different growth patterns. Eur J Radiol. 2008;69:339–45.
Attariwala R, Picker W. Whole body MRI: improved lesion detection and characterization with diffusion weighted techniques. J Magn Reson Imaging. 2013;38:253–68.
Muller MF, Edelman RR. Echo planar imaging of the abdomen. Top Magn Reson Imaging. 1995;7:112–9.
Wang Y. Description of parallel imaging in MRI using multiple coils. Magn Reson Med. 2000;44:495–9.
Nonomura Y, Yasumoto M, Yoshimura R, et al. Relationship between bone marrow cellularity and apparent diffusion coefficient. J Magn Reson Imaging. 2001;13:757–60.
Terpos E, Koutoulidis V, Fontara S, et al. Diffusion-weighted imaging improves accuracy in the diagnosis of MRI patterns of marrow involvement in newly diagnosed myeloma: results of a prospective study in 99 patients. Blood. 2015;126:4178 (ASH abstract).
Xu X, Ma L, Zhang JS, et al. Feasibility of whole body diffusion weighted imaging in detecting bone metastasis on 3.0T MR scanner. Chin Med Sci J. 2008;23:151–7.
Messiou C, Giles S, Collins DJ, et al. Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol. 2012;85:e1198–203.
Messiou C, Collins DJ, Morgan VA, et al. Optimizing diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol. 2011;21:1713–8.
Hillengass J, Bäuerle T, Bartl R, et al. Diffusion-weighted imaging for non-invasive and quantitative monitoring of bone marrow infiltration in patients with monoclonal plasma cell disease: a comparative study with histology. Br J Haematol. 2011;153:721–8.
Lemke A, Stieltjes B, Schad LR, et al. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging. 2011;29:766–76.
Giles SL, deSouza NM, Collins DJ, et al. Assessing myeloma bone disease with whole-body diffusion-weighted imaging: comparison with x-ray skeletal survey by region and relationship with laboratory estimates of disease burden. Clin Radiol. 2015;70:614–21.
Sachpekidis C, Mosebach J, Freitag MT, et al. Application of (18)F-FDG PET and diffusion weighted imaging (DWI) in multiple myeloma: comparison of functional imaging modalities. Am J Nucl Med Mol Imaging. 2015;5:479–92.
Pawlyn C, Fowkes L, Otero S, et al. Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia leu. 2015;2015:338. doi:10.1038/leu.2015.338.
Horger M, Weisel K, Horger W, et al. Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results. AJR Am J Roentgenol. 2011;196:W790–5.
Hillengass J, Wasser K, Delorme S, et al. Lumbar bone marrow microcirculation measurements from dynamic contrast-enhanced magnetic resonance imaging is a predictor of event-free survival in progressive multiple myeloma. Clin Cancer Res. 2007;13:475–81.
Hillengass J, Landgren O. Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging “early myeloma”. Leuk Lymphoma. 2013;54:1355–63.
Huang SY, Chen BB, HY L, et al. Correlation among DCE-MRI measurements of bone marrow angiogenesis, microvessel density, and extramedullary disease in patients with multiple myeloma. Am J Hematol. 2012;87:837–9.
Zechmann CM, Traine L, Meissner T, et al. Parametric histogram analysis of dynamic contrast-enhanced MRI in multiple myeloma: a technique to evaluate angiogenic response to therapy? Acad Radiol. 2012;19:100–8.
Zwick S, Brix G, Tofts PS, et al. Simulation-based comparison of two approaches frequently used for dynamic contrast-enhanced MRI. Eur Radiol. 2010;20:432–42.
Fraioli F, Punwani S. Clinical and research applications of simultaneous positron emission tomography and MRI. Br J Radiol. 2014;87:20130464.
Sachpekidis C, Hillengass J, Goldschmidt H, et al. Comparison of (18)F-FDG PET/CT and PET/MRI in patients with multiple myeloma. Am J Nucl Med Mol Imaging. 2015;5:469–78.
Baur-Melnyk A, Buhmann S, Durr HR, et al. Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol. 2005;55:56–63.
Moulopoulos LA, Varma DG, Dimopoulos MA, et al. Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology. 1992;185:833–40.
Moulopoulos LA, Gika D, Anagnostopoulos A, et al. Prognostic significance of magnetic resonance imaging of bone marrow in previously untreated patients with multiple myeloma. Ann Oncol. 2005;16:1824–8.
Durie BGM. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer. 2006;42:1539–43.
Walker R, Barlogie B, Haessler J, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25:1121–8.
Ludwig H, Frühwald F, Tscholakoff D, et al. Magnetic resonance imaging of the spine in multiple myeloma. Lancet. 1987;2:364–6.
Ghanem N, Lohrmann C, Engelhardt M, et al. Whole-body MRI in the detection of bone marrow infiltration in patients with plasma cell neoplasms in comparison to the radiological skeletal survey. Eur Radiol. 2006;16:1005–14.
Lecouvet FE, Malghem J, Michaux L, et al. Skeletal survey in advanced multiple myeloma: radiographic versus MR imaging survey. Br J Haematol. 1999;106:35–9.
Tertti R, Alanen A, Remes K. The value of magnetic resonance imaging in screening myeloma lesions of the lumbar spine. Br J Haematol. 1995;91:658–60.
Narquin S, Ingrand P, Azais I, et al. Comparison of whole-body diffusion MRI and conventional radiological assessment in the staging of myeloma. Diagn Interv Imaging. 2013;94:629–36.
Regelink JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. 2013;162:50–61.
Baur-Melnyk A, Buhmann S, Becker C, et al. Whole-body MRI versus whole-body MDCT for staging of multiple myeloma. AJR Am J Roentgenol. 2008;190:1097–104.
Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–5.
Waheed S, Mitchell A, Usmani S, et al. Standard and novel imaging methods for multiple myeloma: correlates with prognostic laboratory variables including gene expression profiling data. Haematologica. 2013;98:71–8.
Khalafallah AA, Snarski A, Heng R, et al. Assessment of whole body MRI and sestamibi technetium-99m bone marrow scan in prediction of multiple myeloma disease progression and outcome: a prospective comparative study. BMJ Open. 2013;3:e002025.
Bauerle T, Hillengass J, Fechtner K, et al. Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imaging. Radiology. 2009;252:477–85.
Baur A, Stabler A, Bruning R, et al. Diffusion-weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology. 1998;207:349–56.
Moulopoulos LA, Dimopoulos MA, Weber D, et al. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol. 1993;11:1311–5.
Dimopoulos MA, Moulopoulos LA, Maniatis A, et al. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96:2037–44.
Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–30.
Lafforgue P, Dahan E, Chagnaud C, et al. Early-stage avascular necrosis of the femoral head: MR imaging for prognosis in 31 cases with at least 2 years of follow-up. Radiology. 1993;187:199–204.
Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3:155–64.
Carlson K, Aström G, Nyman R, et al. MR imaging of multiple myeloma in tumour mass measurement at diagnosis and during treatment. Acta Radiol. 1995;36:9–14.
Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015;33(6):657–64. doi:10.1200/JCO.2014.57.9961.
Usmani SZ, Mitchell A, Waheed S, et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy. Blood. 2013;121:1819–23.
Lecouvet FE, Vande Berg BC, Michaux L, et al. Stage III multiple myeloma: clinical and prognostic value of spinal bone marrow MR imaging. Radiology. 1998;209:653–60.
Moulopoulos LA, Dimopoulos MA, Kastritis E, et al. Diffuse pattern of bone marrow involvement on magnetic resonance imaging is associated with high risk cytogenetics and poor outcome in newly diagnosed, symptomatic patients with multiple myeloma: a single center experience on 228 patients. Am J Hematol. 2012;87:861–4.
Moulopoulos LA, Dimopoulos MA, Christoulas D, et al. Diffuse MRI marrow pattern correlates with increased angiogenesis, advanced disease features and poor prognosis in newly diagnosed myeloma treated with novel agents. Leukemia. 2010;24:1206–12.
Song MK, Chung JS, Lee JJ, et al. Magnetic resonance imaging pattern of bone marrow involvement as a new predictive parameter of disease progression in newly diagnosed patients with multiple myeloma eligible for autologous stem cell transplantation. Br J Haematol. 2014;165(6):777–85. doi:10.1111/bjh.12820.
Moulopoulos LA, Dimopoulos MA, Alexanian R, et al. Multiple myeloma: MR patterns of response to treatment. Radiology. 1994;193:441–6.
Hillengass J, Ayyaz S, Kilk K, et al. Changes in magnetic resonance imaging before and after autologous stem cell transplantation correlate with response and survival in multiple myeloma. Haematologica. 2012;97:1757–60.
Bannas P, Hentschel HB, Bley TA, et al. Diagnostic performance of whole-body MRI for the detection of persistent or relapsing disease in multiple myeloma after stem cell transplantation. Eur Radiol. 2012;22:2007–12.
Derlin T, Peldschus K, Münster S, et al. Comparative diagnostic performance of 18F-FDG PET/CT versus whole-body MRI for determination of remission status in multiple myeloma after stem cell transplantation. Eur Radiol. 2013;23:570–8.
Spinnato P, Bazzocchi A, Brioli A, et al. Contrast enhanced MRI and 18F-FDG PET-CT in the assessment of multiple myeloma: a comparison of results in different phases of the disease. Eur J Radiol. 2012;81:4013–8.
Bartel TB, Haessler J, Brown TL, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–76.
Giles SL, Messiou C, Collins DJ, et al. Whole-body diffusion-weighted MR imaging for assessment of treatment response in Myeloma. Radiology. 2014;271(3):785–94.
Dimopoulos MA, Moulopoulos A, Smith T, et al. Risk of disease progression in asymptomatic multiple myeloma. Am J Med. 1993;94:57–61.
Moulopoulos LA, Dimopoulos MA, Smith TL, et al. Prognostic significance of magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 1995;13:251–6.
Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606–10.
Kastritis E, Terpos E, Moulopoulos L, et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia. 2013;27:947–53.
Merz M, Hielscher T, Wagner B, et al. Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia. 2014;28(9):1902–8.
Pepe J, Petrucci MT, Nofroni I, et al. Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134:485–90.
Van de Donk NW, Palumbo A, Johnsen HE, et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014;99(6):984–96.
Vande Berg BC, Michaux L, Lecouvet FE, et al. Nonmyelomatous monoclonal gammopathy: correlation of bone marrow MR images with laboratory findings and spontaneous clinical outcome. Radiology. 1997;202:247–51.
Dhodapkar MV, Sexton R, Waheed S, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood. 2014;123:78–85.
Hillengass J, Weber MA, Kilk K, et al. Prognostic significance of whole-body MRI in patients with monoclonal gammopathy of undetermined significance. Leukemia. 2014;28:174–8.
Liebross RH, Ha CS, Cox JD, et al. Solitary bone plasmacytoma: outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:1063–7.
Bredella MA, Steinbach L, Caputo G, et al. Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol. 2005;184:1199–204.
Lütje S, de Rooy JW, Croockewit S, et al. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol. 2009;88:1161–8.
Breyer RJ 3rd, Mulligan ME, Smith SE, et al. Comparison of imaging with FDG PET/CT with other imaging modalities in myeloma. Skeletal Radiol. 2006;35:632–40.
Zamagni E, Nanni C, Gay F, et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia. 2015;30(2):417–22. doi:10.1038/leu.2015.291.
Siontis B, Kumar S, Dispenzieri A, et al. Positron emission tomography-computed tomography in the diagnostic evaluation of smoldering multiple myeloma: identification of patients needing therapy. Blood Cancer J. 2015;5:e364.
Sachpekidis C, Mai EK, Goldschmidt H, et al. (18)F-FDG dynamic PET/CT in patients with multiple myeloma: patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin Nucl Med. 2015;40:e300–7.
Tirumani SH, Sakellis C, Jacene H, et al. Role of FDG-PET/CT in extramedullary multiple myeloma: correlation of FDG-PET/CT findings with clinical outcome. Clin Nucl Med. 2016;41:e7–e13.
Zamagni E, Nanni C, Mancuso K, et al. PET/CT improves the definition of complete response and allows to detect otherwise unidentifiable skeletal progression in multiple myeloma. Clin Cancer Res. 2015;21:4384–90.
Paiva B, van Dongen JJ, Orfao A, et al. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125:3059–68.
Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–95.
Patriarca F, Carobolante F, Zamagni E, et al. The role of positron emission tomography with 18F-fluorodeoxyglucose integrated with computed tomography in the evaluation of patients with multiple myeloma undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1068–73.
Fonti R, Pace L, Cerchione C, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in the prediction of outcome of patients with multiple myeloma: a comparative study. Clin Nucl Med. 2015;40:303–8.
Lapa C, Lückerath K, Malzahn U, et al. 18 FDG-PET/CT for prognostic stratification of patients with multiple myeloma relapse after stem cell transplantation. Oncotarget. 2014;5(17):7381–91.
Cascini GL, Falcone C, Console D, et al. Whole-body MRI and PET/CT in multiple myeloma patients during staging and after treatment: personal experience in a longitudinal study. Radiol Med. 2013;118(6):930–48.
Moreau P, Attal M, Karlin L, et al. Prospective evaluation of MRI and PET-CT at diagnosis and before maintenance therapy in symptomatic patients with Multiple Myeloma included in the IFM/DFCI 2009 trial. Blood. 2015;126:395 (ASH abstract).
Nanni C, Zamagni E, Versari A, et al. Image interpretation criteria for FDG PET/CT in multiple myeloma: a new proposal from an Italian expert panel. IMPeTUs (Italian Myeloma criteria for PET USe). Eur J Nucl Med Mol Imaging. 2016;43:414–21.
Fouquet G, Guidez S, Herbaux C, et al. Impact of initial FDG-PET/CT and serum-free light chain on transformation of conventionally defined solitary plasmacytoma to multiple myeloma. Clin Cancer Res. 2014;20:3254–60.
Alongi P, Zanoni L, Incerti E, et al. 18F-FDG PET/CT for early post-radiotherapy assessment in solitary bone plasmacytomas. Clin Nucl Med. 2015;40:e399–404.
Lonial S, Kaufman JL. Non-secretory myeloma: a clinician's guide. Oncology (Williston Park). 2013;27:924–8.
Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961.
Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303.
Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, et al. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner. 1986;1:27.
Rowe DJ, Etre LA, Lovdahl MJ, et al. Relationship between bisphosphonate concentration and osteoclast activity and viability. In Vitro Cell Dev Biol Anim. 1999;35:383.
Terpos E, Berenson J, Raje N, et al. Management of bone disease in multiple myeloma. Expert Rev Hematol. 2014;7(1):113–25.
Mundy GR, Yoneda T. Bisphosphonates as anticancer drugs. N Engl J Med. 1998;339:398.
Yin JJ, Selander K, Chirgwin JM, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197.
Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357.
Aparicio A, Gardner A, Tu Y, et al. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia. 1998;12:220.
Shipman CM, Rogers MJ, Apperley JF, et al. Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997;98:665.
Dhodapkar MV, Singh J, Mehta J, et al. Anti-myeloma activity of pamidronate in vivo. Br J Haematol. 1998;103:530.
Daragon A, Humez C, Michot C, et al. Treatment of multiple myeloma with etidronate: results of a multicentre double-blind study. Eur J Med. 1993;2(8):449–52.
Menssen HD, Sakalova A, Fontana A, et al. Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol. 2002;20(9):2353–9.
Terpos E, Morgan G, all DMA e. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31(18):2347–57.
Belch AR, Bergsagel DE, Wilson K, et al. Effect of daily etidronate on the osteolysis of multiple myeloma. J Clin Oncol. 1991;9(8):1397–402.
Lahtinen R, Laakso M, Palva I, et al. Randomised, placebo-controlled multicentre trial of clodronate in multiple myeloma. Lancet. 1992;340(8832):1049–52.
McCloskey EV, Dunn JA, Kanis JA, et al. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol. 2001;113(4):1035–43.
Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16(2):593–602.
Brincker H, Westin J, Abildgaard N, et al. Failure of oral pamidronate to reduce skeletal morbidity in multiple myeloma: a double-blind placebo-controlled trial. Danish-Swedish co-operative study group. Br J Haematol. 1998;101(2):280–6.
Gimsing P, Carlson K, Turesson I, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol. 2010;11(10):973–82.
Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91(7):1191–200.
Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7(5):377–87.
Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–44.
Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989–99.
Morgan GJ, Davies FE, Gregory WM, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: MRC Myeloma IX trial. Blood. 2012;119(23):5374–83.
Croucher PI, De Hendrik R, Perry MJ, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18(3):482–92.
Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;5:CD003188.
D'Arena G, Gobbi PG, Broglia C, et al. Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leuk Lymphoma. 2011;52:771–5.
Musto P, Petrucci MT, Bringhen S, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–95.
Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84:685–93.
Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood. 2010;116:2651–5.
Berenson JR, Yellin O, Boccia RV, et al. Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res. 2008;14:6289–95.
Pepe J, Petrucci MT, Mascia ML, et al. The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int. 2008;82:418–26.
Fechtner K, Hillengass J, Delorme S, et al. Staging monoclonal plasma cell disease: comparison of the Durie-Salmon and the Durie-Salmon PLUS staging systems. Radiology. 2010;257:195–204.
Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–31.
Morgan GJ, Child JA, Gregory WM, et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743–52.
Roux S, Bergot C, Fermand JP, et al. Evaluation of bone mineral density and fat-lean distribution in patients with multiple myeloma in sustained remission. J Bone Miner Res. 2003;18:231–6.
Badros A, Goloubeva O, Terpos E, et al. Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br J Haematol. 2008;142:492–4.
Laroche M, Lemaire O, Attal M. Vitamin D deficiency does not alter biochemical markers of bone metabolism before or after autograft in patients with multiple myeloma. Eur J Haematol. 2010;85:65–7.
Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8.
Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488–93.
Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–7.
Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91(7):968–71.
Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol. 2006;134(6):620–3.
Badros A, Terpos E, Katodritou E, et al. Natural history of osteonecrosis of the jaw in patients with multiple myeloma. J Clin Oncol. 2008;26(36):5904–9.
Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23(22):4925–35.
Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117–20.
Montefusco V, Gay F, Spina F, et al. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma. 2008;49(11):2156–62.
Migliorati CA, Casiglia J, Epstein J, et al. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136(12):1658–68.
Ripamonti CI, Cislaghi E, Mariani L, et al. Efficacy and safety of medical ozone (O(3)) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: preliminary results of a phase I-II study. Oral Oncol. 2011;47(3):185–90.
Agrillo A, Filiaci F, Ramieri V, et al. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur Rev Med Pharmacol Sci. 2012;16(12):1741–7.
Yaccoby S, Pearse RN, Johnson CL, et al. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol. 2002;116(2):278–90.
Croucher PI, Shipman CM, Lippitt J, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98(13):3534–40.
Vanderkerken K, De Leenheer E, Shipman C, et al. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63(2):287–9.
Kostenuik P, Nguyen H, McCabe J, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases bone density in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24(2):182–95.
Body JJ, Facon T, Coleman RE, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12(4):1221–8.
Yonemori K, Fujiwara Y, Minami H, et al. Phase 1 trial of denosumab safety, pharmacokinetics, and pharmacodynamics in Japanese women with breast cancer-related bone metastases. Cancer Sci. 2008;99(6):1237–42.
Vij R, Horvath N, Spencer A, Kitagawa K, et al. An open-label, Phase 2 trial of denosumab in the treatment of relapsed (R) or plateau-phase (PP) multiple myeloma (MM). Presented in the 49th ASH Annual Meeting and Exposition; 2007 Dec 8–11, Atlanta, GA.
Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–71.
Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–92.
Raje N, et al. Evaluating results from the multiple myeloma patient subset treated with denosumab or zoledronic acid in a randomized phase 3 trial. Blood Cancer J. 2016;6:e378.
Chantry AD, Heath D, Mulivor AW, et al. Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res. 2010;25(12):2633–46.
Ruckle J, Jacobs M, Kramer W, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744–52.
Scullen T, Santo L, Vallet S, et al. Lenalidomide in combination with an activin A-neutralizing antibody: preclinical rationale for a novel anti-myeloma strategy. Leukemia. 2013;27(8):1715–21.
Oranger A, Carbone C, Izzo M, et al. Cellular mechanisms of multiple myeloma bone disease. Clin Dev Immunol. 2013;2013:289458.
Cao H, Zhu K, Qiu L, et al. Critical role of AKT protein in myeloma-induced osteoclast formation and osteolysis. J Biol Chem. 2013;288(42):30399–410.
Breitkreutz I, Raab MS, Vallet S, et al. Targeting MEK1/2 blocks osteoclast differentiation, function and cytokine secretion in multiple myeloma. Br J Haematol. 2007;139(1):55–63.
Tai YT, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2013;28(1):155–65.
Cafforio P, Savonarola A, Stucci S, et al. PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res. 2013;29(1):55–66.
Moreaux J, Hose D, Kassambara A, et al. Osteoclast-gene expression profiling reveals osteoclast-derived CCR2 chemokines promoting myeloma cell migration. Blood. 2011;117(4):1280–90.
Choi SJ, Oba Y, Gazitt Y, et al. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108(12):1833–41.
Roussou M, Tasidou A, Dimopoulos MA, et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia. 2009;23(11):2177–81.
Vallet S, Raje N, Ishitsuka K, et al. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110(10):3744–52.
Terpos E, Christoulas D, Papatheodorou A, et al. Dickkopf-1 is elevated in newly-diagnosed, symptomatic patients and in relapsed patients with multiple myeloma; correlations with advanced disease features: a single-center experience in 284 patients. Presented in the 15th Congress of the European Hematology Association; 2010 June 10–13, Barcelona, Spain.
Steinman RM, Bonifaz L, Fujii S, et al. The innate functions of dendritic cells in peripheral lymphoid tissues. Adv Exp Med Biol. 2005;560:83–97.
Moester MJ, Papapoulos SE, CW L¨w, et al. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010;87(2):99–107.
Polyzos SA, Anastasilakis AD, Bratengeier C, et al. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—the six-month effect of risedronate and teriparatide. Osteoporos Int. 2012;23(3):1171–6.
Voskaridou E, Christoulas D, Plata E, et al. High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res. 2012;44(12):909–13.
Lewiecki EM. Sclerostin: a novel target for intervention in the treatment of osteoporosis. Discov Med. 2011;12(65):263–73.
von Metzler I, Krebbel H, Hecht M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21(9):2025–34.
Boissy P, Andersen TL, Lund T, et al. Pulse treatment with the proteasome inhibitor bortezomib inhibits osteoclast resorptive activity in clinically relevant conditions. Leuk Res. 2008;32(11):1661–8.
Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135(5):688–92.
Giuliani N, Morandi F, Tagliaferri S, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110(1):334–8.
Zangari M, Esseltine D, Lee CK, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131(1):71–3.
Heider U, Kaiser M, Muller C, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77(3):233–8.
Zangari M, Aujay M, Zhan F, et al. Alkaline phosphatase variation during carfilzomib treatment is associated with best response in multiple myeloma patients. Eur J Haematol. 2011;86(6):484–7.
Terpos E, Christoulas D, Kokkoris P, et al. Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Ann Oncol. 2010;21(7):1561–2.
Terpos E, Kastritis E, Roussou M, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22(12):2247–56.
Terpos E, Christoulas D, Kastritis E, et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post-ASCT. Leukemia. 2013;28(4):928–34.
Delforge M, Terpos E, Richardson PG, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with Bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol. 2011;86:372–84.
Terpos E, Mihou D, Szydlo R, et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005;19(11):1969–76.
Breitkreutz I, Raab MS, Vallet S, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22(10):1925–32.
Anderson G, Gries M, Kurihara N, et al. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107(8):3098–105.
Bruzzese F, Pucci B, Milone MR, et al. Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ROS and modulating mevalonate and p38-MAPK pathways. Cell Death Dis. 2013;4:e878.
Bam R, Ling W, Khan S, et al. Role of Bruton’s tyrosine kinase in myeloma cell migration and induction of bone disease. Am J Hematol. 2013;88(6):463–71.
Neri P, Kumar S, Fulciniti MT, et al. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13(19):5903–9.
Vanderkerken K, Medicherla S, Coulton L, et al. Inhibition of p38alpha mitogen-activated protein kinase prevents the development of osteolytic bone disease, reduces tumor burden, and increases survival in murine models of multiple myeloma. Cancer Res. 2007;67(10):4572–7.
Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–3596.
Bouza C, Lopez-Cuadrado T, Cediel P, et al. Balloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysis. BMC Palliat Care. 2009;8:12.
McDonald RJ, Trout AT, Gray LA, et al. Vertebroplasty in multiple myeloma: outcomes in a large patient series. AJNR Am J Neuroradiol. 2008;29:642–8.
Huber F, McArthur N, Tanner M, et al. Kyphoplasty for patients with multiple myeloma is a safe surgical procedure: results from a large patient cohort. Clin Lymphoma Myeloma. 2009;9:375–80.
Zou J, Mei X, Gan M, et al. Kyphoplasty for spinal fractures from multiple myeloma. J Surg Oncol. 2010;102:43–7.
Dalbayrak S, Onen M, Yilmaz M, et al. Clinical and radiographic results of balloon kyphoplasty for treatment of vertebral body metastases and multiple myelomas. J Clin Neurosci. 2010;17:219–24.
Chew C, Craig L, Edwards R, et al. Safety and efficacy of percutaneous vertebroplasty in malignancy: a systematic review. Clin Radiol. 2011;66:63–72.
Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–68.
Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79.
Bhargava A, Trivedi D, Kalva L, et al. Management of cancer-related vertebral compression fracture: comparison of treatment options: a literature meta-analysis. J Clin Oncol (Meeting Abstracts). 2009;27:e20529.
Rades D, Hoskin PJ, Stalpers LJ, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys. 2006;64:1452–7.
Hirsch AE, Jha RM, Yoo AJ, et al. The use of vertebral augmentation and external beam radiation therapy in the multimodal management of malignant vertebral compression fractures. Pain Physician. 2011;14:447–58.
Balducci M, Chiesa S, Manfrida S, et al. Impact of radiotherapy on pain relief and recalcification in plasma cell neoplasms: long-term experience. Strahlenther Onkol. 2011;187:114–9.
Price P, Hoskin PJ, Easton D, et al. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol. 1986;6:247–55.
Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80:824–31.
Rades D, Veninga T, Stalpers LJ, et al. Outcome after radiotherapy alone for metastatic spinal cord compression in patients with oligometastases. J Clin Oncol. 2007;25:50–6.
Wedin R. Surgical treatment for pathologic fracture. Acta Orthop Scand Suppl. 2001;72(2p):1–29.
Utzschneider S, Schmidt H, Weber P, et al. Surgical therapy of skeletal complications in multiple myeloma. Int Orthop. 2011;35:1209–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Terpos, E., Kanellias, N. (2018). Practical Considerations for Bone Health in Multiple Myeloma. In: Usmani, S., Nooka, A. (eds) Personalized Therapy for Multiple Myeloma. Springer, Cham. https://doi.org/10.1007/978-3-319-61872-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-61872-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61871-5
Online ISBN: 978-3-319-61872-2
eBook Packages: MedicineMedicine (R0)